Abstract
Despite large unmet medical needs in the field for several decades, CNS drug discovery and development has been largely unsuccessful. Biomarkers, particularly those utilizing neuroimaging, have played important roles in aiding CNS drug development, including dosing determination of investigational new drugs (INDs). A recent working group was organized jointly by CINP and Japanese Society of Neuropsychopharmacology (JSNP) to discuss the utility of biomarkers as tools to overcome issues of CNS drug development.
The consensus statement from the working group aimed at creating more nuanced criteria for employing biomarkers as tools to overcome issues surrounding CNS drug development. To accomplish this, a reverse engineering approach was adopted, in which criteria for the utilization of biomarkers were created in response to current challenges in the processes of drug discovery and development for CNS disorders. Based on this analysis, we propose a new paradigm containing 5 distinct tiers to further clarify the use of biomarkers and establish new strategies for decision-making in the context of CNS drug development. Specifically, we discuss more rational ways to incorporate biomarker data to determine optimal dosing for INDs with novel mechanisms and targets, and propose additional categorization criteria to further the use of biomarkers in patient stratification and clinical efficacy prediction. Finally, we propose validation and development of new neuroimaging biomarkers through public-private partnerships to further facilitate drug discovery and development for CNS disorders.
Keywords: CNS drug development, neuroimaging biomarkers, public-private-partnerships, patient stratification, clinical efficacy prediction
Significance Statement
Recent central nervous system (CNS) drug discovery and development has been largely unsuccessful. Biomarkers, particularly those utilizing neuroimaging, have played important roles in aiding CNS drug development, including dosing determination of investigational new drugs (INDs). The consensus statement from working group organized jointly by CINP and Japanese Society of Neuropsychopharmacology (JSNP) propose a new paradigm containing five distinct tiers to further clarify the use of biomarkers in patient stratification and clinical efficacy prediction and establish new strategies to develop new neuroimaging biomarkers through public-private-partnerships (PPPs) that combine disease knowledge, cutting-edge technologies, chemical libraries, medicinal chemistry and funding to achieve novel breakthroughs.
Introduction
Most current medications for psychiatric disorders stem from mechanistic optimizations of agents serendipitously discovered approximately 60 years ago. While these discoveries have led to the development of next generation drugs, including the antipsychotics and antidepressants widely prescribed today, much remains to be desired in this arena; although newer drugs show fewer serious side effects than first-generation compounds, many current medications are plagued by lingering safety and efficacy issues (Becker et al., 2015). In an effort to overcome these, current drug discovery strategies have, by necessity, evolved to focus on novel molecular targets that influence neural systems not previously targeted by legacy drugs.
Although symptom-improving drugs have been developed for several intractable CNS disorders (e.g., acetylcholinesterase inhibitors for Alzheimer’s disease (AD)), current drug discovery efforts have shifted to the development of disease-modifying agents that interfere with the neurodegenerative processes that may underlie disorders whose etiologies are not fully understood (Becker et al., 2015).
However, despite the wide array of new drug targets, success rates in developing new CNS drugs have not increased for many years. Clinical trials of recently discovered agents frequently fail, mostly owing to a lack of efficacy (Griebel and Holsboer, 2012; Dunlop and Brandon, 2015). As a result, global pharmaceutical companies have ceased or reduced their efforts in this space.
To identify avenues to overcome these problems, the Collegium Internationale Neuro-Psychopharmacologicum (CINP) convened a summit meeting (CNS Drug Innovation Summit Meeting) in Tokyo in April, 2015 to discuss options for facilitating more efficacious drug discovery and clinical development activities for CNS disorders, activities ultimate aimed at increasing success rates in current and future clinical trials. Based on discussion during the meeting, 3 working groups including researchers in academia and industry were organized jointly by CINP and Japanese Society of Neuropsychopharmacology (JSNP) to put forth potential solutions. At the following meetings, 2 factors were noted as major barriers to improving success rate of CNS drug development.
(1) Difficulties in designing appropriate clinical plans for clinical proof-of-concept (POC) studies: To conduct successful clinical POC studies, appropriate setting of optimal dose(s) and patient stratification are critically important factors. Without control of these variables, one could not reasonably conclude that an on-target investigational new drug (IND) is ineffective or, worse, invalid. Moreover, both dosing and patient stratification should be determined based on the concept or mechanism the drug target stands on. While this is seemingly evident, methodologies to satisfy these issues have not been clearly established.
(2) Difficulties predicting clinical efficacy: Development of biomarkers, which can substitute for clinical endpoints, is increasingly critical for predicting clinical efficacy. Considering, however, the limited biomarkers currently available for most CNS disorders, it is often difficult to confidently predict clinical outcomes in small-scale efforts preceding larger, more expensive trials. As an implicit corollary, the lack of reliable and objective biomarkers is an additional hurdle for pharmaceutical companies engaging in challenging clinical POC studies.
It has become increasingly evident that continued development and implementation of biomarkers will closely follow successes in overcoming the above-mentioned barriers. In attempting to improve and accelerate this process, we first analyzed the current challenges to (and utilization of) biomarkers in the current drug discovery landscape (section 2) and used this as a starting point a newly proposed process for clinical POC studies based on real-world observation (section 3). Then we propose the development and validation of new biomarkers to achieve successful clinical POC studies through public-private partnerships (PPPs) (section 4). In this CINP/JSNP working group report, we focus heavily on neuroimaging biomarkers due to their widely acknowledged utility as a noninvasive tool in CNS disorders (Wong et al., 2009).
Roles of Neuroimaging Biomarkers in Drug Discovery and Development of CNS Disorders
Morgan et al. (2012) previously described a 3-pillar model for biomarker utilization in successful clinical development, consisting of the following: (1) drug exposure at the site of action for the desired length of time; (2) drug binding to the intended target; (3) evidence of functional modulation of the target organ resulting from the drug pharmacological activity (for example pharmacological functional magnetic resonance imaging (phMRI), a method for analysis of the drug-induced functional changes in the neural circuits (Wandschneider et al., 2016). In their review, it was mentioned that a clinical development candidate that satisfies all 3 pillars will (1) have increased likelihood of surviving through Phase II into Phase III, and (2) enable efficient and effective development through POC and Phase II.
The current state of biomarker usage (taking into account the 3 pillars concept) in recently conducted clinical trials for CNS disorders is listed in Table 1. Several issues emerging from meta-analyses of these trials are discussed below.
Table 1.
Status of Biomarker Usages in CNS Disorders
| Target Disease | Compound | Sponsor Collaborator | Mechanism of Action | Pillar 1 | Pillar 2 | Pillar 3 | Nct# | References |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia | TAK-063 | Takeda | PDE10A inhibitor | PDE10A occupancy | fMRI BOLD Ketamine-induced fMRI BOLD |
NCT02370602 NCT01892189 |
Takano et al., 2016 | |
| Schizophrenia | PF-02545920 | Pfizer | PDE10A inhibitor | PDE10A occupancy | Ketamine-induced fMRI BOLD |
NCT01918202 NCT01244880 |
||
| Schizophrenia | MK-0777 /TPA023 |
Merck & Co | GABA-Aα2/3 receptor agonist | GABA-Aα occupancy | qEEG |
Atack et al., 2010
Lewis et al., 2008 |
||
| Schizophrenia | bitopertin /RG1678 /RO4917838 |
Roche | GlyT-1 inhibitor | GlyT-1 occupancy | CSF Glycine, Event-Related Potential | NCT01116830 |
Martin-Facklam et al., 2013
Hofmann et al., 2016 |
|
| Schizophrenia | GSK1018921 | GlaxoSmith Kline | GlyT-1 inhibitor | GlyT-1 occupancy | CSF Glycine, qEEG, mismatchnegativity |
NCT00945503 NCT00527020 NCT00929370 |
Gunn et al., 2011 | |
| Schizophrenia | MK-2637 | Merck & Co | GlyT-1 inhibitor | GlyT-1 occupancy | Motor evoked potential, qEEG | NCT00934466 | Joshi et al., 2015 | |
| Schizophrenia | LY2140023 /Pomaglumetad methionil |
Eli Lilly | mGlu2/3 agonist | CSF PK | Ketamine-Challenge fMRI Assay, CSF monoamine metabolites |
NCT01524237 | Lowe et al., 2012 | |
| Schizophrenia | JNJ-40411813 | J&J | mGlu2 PAM | mGlu2 receptor occupancy 5-HT2A occupancy |
Sleep EEG, Ketamine- induced psychotic symptom |
NCT01359852 NCT01358006 NCT01101659 NCT01951053 |
Ahnaou et al., 2016 | |
| Schizophrenia | AZD8529 | AstraZeneca | mGlu2 PAM | CSF PK | Ketamine-induced fMRI, EEG |
NCT00985933 NCT00986531 |
Cook et al., 2014 | |
| Depression /FXS |
RO4917523 | Roche | mGlu5 antagonist | mGlu5 receptor occupancy | fMRI |
NCT01483469 NCT01045083 |
||
| Mild-to-moderate Alzheimer’s disease | Bapineuzumab | Janssen /Pfizer |
anti-amyloid antibody | amyloid PET, CSF p-tau, vMRI, FDG PET |
NCT00575055 NCT00574132 |
Liu et al., 2015
Salloway et al., 2014 |
||
| Mild Alzheimer’s disease | Solanezumab | Eli Lilly | anti-amyloid antibody | amyloid in blood & CSF, tau in CSF, vMRI, amyloid/tau PET, FDG PET |
NCT00905372 NCT00904683 NCT01900665 |
Doody et al., 2014
Siemers et al., 2016 |
||
| Early Alzheimer’s disease | Aducanumab /BIIB037 |
Biogen | anti-amyloid antibody | amyloid PET, vMRI, FDG PET, fluid biomarkers |
NCT01677572 | |||
| Prodromal Alzheimer’s disease | Gantenerumab | Roche | anti-amyloid antibody | amyloid PET, amyloid and tau in CSF, vMRI, FDG PET |
NCT01224106 NCT01760005 |
|||
| Prodromal Alzheimer’s disease | Verubecestat /MK-8931 |
Merck & Co | BACE inhibitor | CSF PK | CSF Aβs & sAPPβ, amyloid PET | NCT01953601 | ||
| Early Alzheimer’s disease | AZD3293 | AstraZeneca /Eli Lilly |
BACE inhibitor | CSF PK | CSF Aβs & sAPPβ, amyloid PET, tau in CSF, FDG PET |
NCT02245737 |
Abbreviatoins: Aβ, amyloid beta; BACE, beta-secretase; BOLD, blood oxygenation level dependent; CSF, cerebrospinal fluid; fMRI, functional magnetic resonance imaging; FDG, fluorodeoxyglucose; FXS, fragile X syndrome; GABA, gamma-aminobutyric acid; GlyT-1, glycine transporter 1; 5-HT, 5-hydroxytryptamine; mGlu, metabotropic glutamate; PAM, positive allosteric modulator; PDE10A, phosphodiesterase 10A; PET, positron emission tomography; PK, pharmacokinetics; qEEG, quantitative electroencephalography; sAPP, soluble amyloid precursor protein; vMRI, volumetric MRI.
Psychiatric Disorders
The 3-pillar concept has gained widespread acceptance across pharmaceutical companies. For example, measurement of drug levels in the cerebrospinal fluid (CSF) (Lin, 2008; Caruso et al., 2013) and occupancy of target molecules using positron emission tomography (PET) has become commonplace, particularly for well-investigated targets like the dopamine D2 receptor (for antipsychotics) (Farde et al., 1988; Kapur et al., 2000; Arakawa et al., 2008) and serotonin transporter (for antidepressants) (Meyer et al., 2001; Suhara et al., 2003). Thus, while the implementation of pillar 2 depends on the availability of a PET tracer, the strategy for measuring occupancy has been established and the importance widely acknowledged.
However, a number of INDs employing new mechanisms of action (ex: positive allosteric modulators) (Conn et al., 2014) loom on the horizon. For some agents with new mechanisms or modes of action, the relationship between drug efficacy and target occupancy has not been well established or remains unclear. Therefore, there is an increasing need for dose selection rationales based on changes in neuronal circuitry (i.e., pillar 3) to confirm that target occupancy relates to changes in neural function. As for any new approach, significant issues require addressing, including (1) the absence of consensus regarding methodology, (2) the absence of fully validated or standardized methods, and (3) variations in the definition of pillar 3, often owing to differing biomarker criteria that results in significant company-to-company variations in patient stratification, dosing, and efficacy endpoints.
In part because of these issues, we believe it is necessary to redefine the existing pillars to further clarify the use of biomarkers as well as to establish new strategies for decision-making in the context of CNS drug development.
Neurodegenerative Disorders
In the clinical development of disease modifiers for neurodegenerative diseases, AD in particular (Salloway et al., 2014; Siemers et al., 2016), there is no precedent for the application of biomarkers under pillar 2 (although use in enzyme inhibition mechanism like β-secretase inhibitors is theoretically possible) with biomarkers falling under pillar 3 being substituted to various ends. However, these parameters may be too broad to adequately categorize biomarkers with different and/or overlapping utilities.
To illustrate this point, consider the following: an amyloid-lowering strategy has long been the mainstream approach in AD-modifying drug development (Hardy and Selkoe, 2002; Golde, 2005; Tanzi, 2005). Amyloid PET imaging is a well-established method to investigate the accumulated amyloid in the brain (Klunk et al., 2004; Jagust et al., 2009; Clark et al., 2012), an approach that doubles as an effective screening tool for enrollment of appropriate patients into clinical trials. Recently, a small POC trial of an amyloid-targeting antibody showed promise as both a potential biomarker and therapeutic that offered cognitive benefits (Ratner, 2015; ALZFORUM). In the ensuing clinical trial, all of the enrolled subjects were confirmed amyloid positive by amyloid PET imaging. The concomitant use of brain imaging and fluidic biomarkers illustrates how pillar 3 biomarker may maintain dual roles in patient enrollment and efficacy prediction of targeted pharmacological action. As such, more detailed categorization of pillar 3 biomarkers into subclasses may be preferable for early and efficient decision-making during the drug development phase.
Redesign of Biomarker Classification to Improve the Success Rate of CNS Drugs
As discussed above, success rates of CNS drugs in clinical POC studies would almost certainly benefit from optimal dose selection, patient stratification, and efficacy prediction in a small-scale trial. Information derived from both target occupancy data and consequent functional change(s) in the brain can improve the accuracy of optimal dose selection to achieve maximal efficacy. Functional changes in the brain can be measured by multiple methods, including phMRI and electroencephalography (EEG); however, these methods can sometimes detect confounding and/or nonspecific reactions within the brain. Because of this, we propose to redefinition of pillar 3 to better clarify purpose.
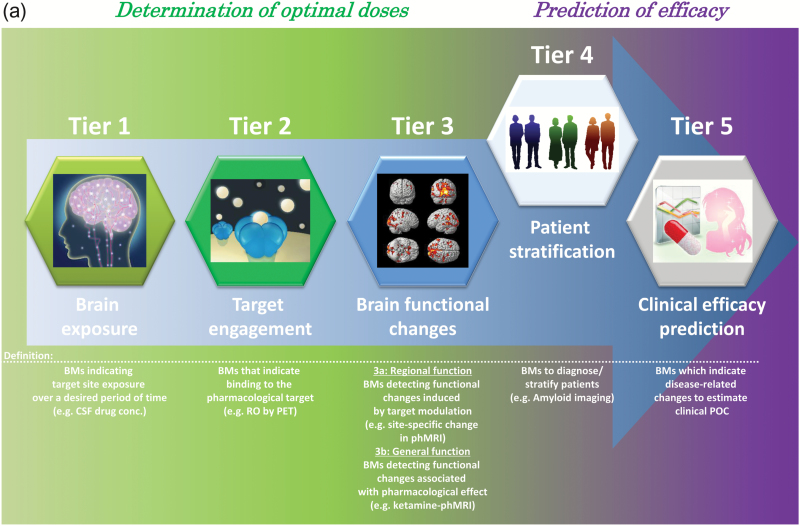
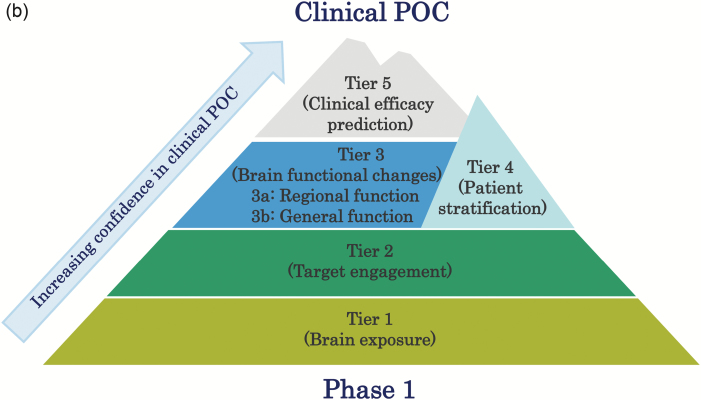
Furthermore, while the 3 pillars paradigm remains a useful tool for estimating clinical success, a more precise use of biomarkers, including biomarkers for patient stratification and efficacy prediction, can further improve the success rates in CNS drugs development trials. In this report, we propose redesign and expansion of the existing classification system into one constituting 5 unique tiers relating to different aspects of biomarker utility (Figure 1a-b). In the proposed system, the increased specificity of additional tiers allows for improved estimation of drug action (and subsequent systemic reaction), resulting in an increasingly descriptive toolkit for ensuing clinical POC studies.
Figure 1.
Redefinition of “5-Tiers” for future CNS-drug development. Each Tier can provide different degrees of evidence of biomarkers (BMs) for appropriate clinical POC studies, the efficacy of a drug, and accumulating tier-specific evidence (receptor occupancy [RO]; pharmacological functional MRI [phMRI]) portends drug action efficacy in a way that is comprehensive than previous paradigms and will lead to improved clinical POC (Fig 1a). Thus, each Tier can be considered as a milestone when climbing difficult-but-manageable peaks such as Mt. Fuji (Fig 1b).
Tier 1: Brain Exposure over the Application Period
Sufficient drug exposure is a prerequisite for drug action; however, accurate measurement of CNS drug exposure to target sites in the brain can be quite challenging. The majority of CNS drugs penetrate into brain via blood circulation; thus, PK/PD modeling using plasma exposure has been afforded a certain level of significance. Similarly, microdosing of labeled drugs and intracerebral microdialysis of CSF or interstitial fluid have also been employed in assessing drug pharmacokinetics (Lin, 2008; Burt et al., 2016).
However, it should be noted that these methods have certain limitations; blood PK/PD modeling cannot infallibly predict precise CNS exposure of a given drug, and microdosing of a labeled drug does not measure its free fraction. In addition, there are ethical issues attached to sampling interstitial fluid from healthy volunteers, and CSF drug concentration can differ significantly from those at target brain regions due to route of administration and variance arising from circulation within the ventricular compartment.
Tier 2: Target Engagement Biomarkers
Measuring occupancy via target-specific PET probes is a well-established and accurate way to detect target engagement (Hargreaves, 2002). Occupancy data also provide some degree of confidence as to the brain exposure of a particular drug.
PET imaging has historically been successful in this regard, especially for orthosteric antagonists or enzyme inhibitors with clear relationships between target occupancy and pharmacological efficacy (Hargreaves, 2002; Le et al., 2008). However, it is difficult to apply PET imaging studies to other types of drugs, such as agonists, partial agonists, and allosteric modulators, because of complicated binding modes and low occupancies required to produce pharmacological effects (Grimwood and Hartig, 2009; O’Brien and Conn, 2016). Therefore, alternative approaches to indirectly measure target engagement based on functional or pharmacodynamic changes are discussed under Tier 3.
Tier 3: Biomarkers Detecting Brain Functional Changes
Investigation of drug-induced brain functional changes remains important, especially when specific PET tracers are not available or when drugs such as agonists and allosteric modulators are evaluated. Fluorodeoxy glucose (FDG)-PET, phMRI, and EEG are commonly used to capture drug-induced changes in neural function and cerebral metabolism. Despite their ubiquity, these methods occasionally produce nonspecific signals unrelated to the modulatory effects of the drug. Drug-induced functional changes to the brain can be divided into 2 segments: (1) functional changes specific to brain regions where drug target molecules are highly expressed, and (2) alterations observed beyond the normal distribution of a drug target molecule, both of which can be considered a pharmacological effect of drug administration. Because evidentiary weighting may differ between 1 and 2, we propose that Tier 3 be further divided into Tier 3a and Tier 3b.
Tier 3a: Biomarkers detecting regional functional changes related to target
Signal specificity should be carefully considered by assessing, among other factors, distribution of the drug target molecule and the molecular mechanism of the drug. A region-specific functional change exhibiting a direct correlation with the distribution of the drug target molecule would naturally provide higher levels of confidence than alterations in other brain regions. For example, TAK-063, a phosphodiesterase 10A (PDE10A) inhibitor, has been reported to increase reginal blood flow in only the brain regions where PDE10A is abundantly expressed (Tomimatsu et al., 2016), indicating that functional change induced by TAK-063 may be mediated through PDE10A inhibition.
Tier 3b: Biomarkers detecting general functional changes associated with pharmacological effect
Functional changes in neural circuits may play a key role in pathogenesis of various neuropsychiatric disorders. As such, drug-mediated functional changes observed in offsite through neural circuitry may provide additional relevant information for said drug’s method of action. Indications of this subclass of biomarker can be detected in healthy volunteers at earlier stages of clinical development and prove useful in bridging preclinical and clinical studies. For example, perturbation of neural circuits associated with some neuropsychiatric disorders by agents like ketamine or scopolamine can be conducted in rodents, nonhuman primates, and healthy volunteers.
Tier 4: Patient Stratification Biomarkers
Current diagnosis of neuropsychiatric disorders is defined by international guidelines and classification systems (ICD-11/DSM-5) and is based primarily on patient symptoms. Accordingly, biological heterogeneity among patients can contribute significantly to lack of efficacy in Phase II trials. To improve clinical success rates, it is essential to select subsets of patients who share biological characteristics optimal for testing candidate compounds. Empirical evidence supports this notion: a retrospective analysis of AstraZeneca’s R&D projects from 2005 to 2010 revealed that projects with high confidence in patient selection demonstrated a greater likelihood of success in Phase IIb (Cook et al., 2014).
Amyloid imaging for AD provides an example illustrating this aspect of patient stratification. By imaging amyloid in patients, AD and non-AD dementia can be discriminated (Weiner et al., 2015). Therefore, it is reasonable to select patients displaying amyloid deposits when evaluating potential AD-preventive drugs.
Patient stratification biomarkers may also play a role in mechanism-based drug discovery. For example, α-synuclein accumulation is observed in both Parkinson’s disease and dementia with lewy bodies (Barker and Williams-Gray, 2016), while TAR DNA-binding protein 43 kDa (TDP-43) accumulation is observed in some population of patients with both frontotemporal lobar degeneration and amyotrophic lateral sclerosis (Neumann et al., 2006). These overlapping molecular signatures may illuminate common pathophysiological pathways between different disorders, facilitating drug development aimed at common biological components of differing diseases.
Tier 5: Clinical Efficacy Prediction Biomarkers
In addition to patient stratification, establishing biomarkers that predict efficacy (i.e., exhibit a high degree of correlation with clinical symptoms) is needed to make a clear go/no-go decision in early phases of clinical studies. Indeed, Cook et al. (2014) have also reported that Phase IIa projects with an efficacy prediction biomarker had twice as much likelihood of stage-up compared with projects without such biomarkers.
Although amyloid imaging is highly useful as a diagnostic marker for AD, correlation between amyloid accumulation and clinical symptoms remains controversial (Liu et al., 2015). On the other hand, signal density in tau imaging has been reported to correlate with cognitive dysfunction and hippocampal atrophy in patients (Maruyama et al., 2013; Ossenkoppele et al., 2016). Thus, tau imaging may have the potential to be both a patient stratification marker and an efficacy prediction biomarker.
Tier 5 biomarkers require both imaging and clinical data derived from limited samples used for further decision-making.
Proposed Neuroimaging Biomarkers to Be Developed by PPPs
A number of biomarker candidates would benefit from development within PPPs. These include validated, standardized biomarkers labeling subsets of neurons (e.g., parvalbumin-positive GABA interneurons) or aggregated proteins (e.g., α-synuclein) as well as markers aimed at gauging the activity within particular neural circuits. In contrast, development of PET tracers for novel drug target molecules may not always be suited for PPPs due to conflicts of interest and confidentiality issues. Both the EU and US have established some precompetitive PPPs to improve CNS drug discovery and development, including the identification and validation of biomarkers. For example, the Innovative Medicines Initiative (Brasy and Potter, 2014; Gottwald et al., 2016) program Novel Methods Leading to New Medications in Depression and Schizophrenia (NEWMEDS) has validated the use of PET tracers to measure changes in extracellular concentrations of some neurotransmitters (Finnema et al., 2015).
Biomarkers Specifically Labeling Particular Cell Types or Molecules
Markers labeling glutamatergic and GABAergic systems
Disruption of the brain’s excitatory/inhibitory balance has increasingly been implicated in the pathophysiology and etiology of several neuropsychiatric disorders (including schizophrenia, autistic spectrum disorders, and prodromal neurodegenerative dementias) (Rubenstein and Merzenich, 2003; Lewis et al., 2012). Given the broad cellular subtypes involved in maintaining this balance (including NMDA receptor-positive cells and certain types of GABA- and parvalbumin-positive interneurons), imaging agents for glutamatergic and GABAergic transmissions, including radioligands for NMDA, AMPA, and GABA receptors and GABA transporters, could serve as early diagnostic markers associated with neuromodulatory and neuroprotective treatments in these disorders. Additionally, it would be important to develop or validate a magnetic resonance spectroscopy method to measure glutamate, glutamine, and GABA to comprehensively understand the molecular underpinnings of this balance.
Neuroinflammatory markers
Growing evidence suggests a prominent role for neuroinflammation in the pathology of neuropsychiatric disorders. In particular, several studies have implicated microglia, the resident immune cells of the CNS, in the development and progression of schizophrenia, mood disorders, and neurodegenerative disorders (Réus et al., 2015). Translocator protein (TSPO) has been studied as a biomarker of reactive gliosis and inflammation in a variety of neuropathological conditions, and increased levels of this factor have been suggested as a marker for activated microglia (Sandiego et al., 2015). Therefore, TSPO PET imaging may be useful for investigating both the role of neuroinflammation in various diseases and for stratifying patients with diseases for which neuroinflammatory pathophysiology is suspected. Moreover, despite some controversy, accumulating evidence supports the existence of aggressive M1-like and protective M2-like phenotypes of microglia (Nakagawa and Chiba, 2015). TSPO is believed to be a marker for M1-like microgliosis, while other signaling molecules are linked to the establishment of other microglial phenotypes. Imaging of purinergic receptors via PET imaging could be a useful tool to monitor microglial activation, as both P2X7 and P2Y12 are evidently involved in M1-like and M2-like microgliosis (Moore et al., 2015; Iwata et al., 2016), respectively.
Oligodendrocyte markers
Dysfunction of oligodendrocytes or demyelination due to loss of oligodendrocytes has been observed in neuropsychiatric disorders such as schizophrenia and multiple sclerosis (Prineas et al., 1984; Hof et al., 2003). Status markers labeling oligodendrocytes or oligodendrocyte precursor cells are useful tools for understanding diseases in which oligodendrocyte abnormalities are involved and for stratifying these patients. Development of PET tracers that bind molecules specifically expressed in oligodendrocytes (S1P5) or oligodendrocyte precursor cells (GPR17) would also be useful.
Markers for aggregated proteins
Among markers for aggregated proteins, amyloid imaging has been extensively explored for diagnostic purposes in AD, while tau imaging has been employed in studying tauopathies. Other examples being actively explored include PET tracers for α-synuclein (for α-synucleionopathies) and TDP-43 (for TDP-43 proteinopathies).
Validation and Standardization of Methods to Measure Brain Function
FDG-PET, functional MRI (fMRI) and EEG have all been used to measure brain function, via measurement of different biological signals. These approaches can distinguish neural network aberrancies induced by psychotomimetic drugs such as ketamine and scopolamine. These changes may represent translatable biomarkers, as these alterations frequently resemble abnormalities observed in certain pathological conditions (Molchan et al., 1994; Jones et al., 2012; Hegedűs et al., 2015; Joules et al., 2015). However, the above-mentioned methods are not fully validated and standardized, introducing the potential for contradictory results. To avoid this, uniform guidelines to validate and standardize are necessary in a clinical setting.
Example of development of imaging biomarkers by PPPs
Given that the TSPO has been observed in higher density in activated microglia across various brain diseases, TSPO PET tracer can be used in a wide range of diseases in which neuroinflammation is implicated (Yasuno et al., 2008; Takano et al., 2010). To date, several TSPO PET tracers have been developed, but the use of existing radioligands has been complicated by the existence of low- and high-affinity binders (Kreisl et al., 2010) that has been ascribed to a single nucleotide polymorphism (rs6971) (Owen et al., 2012). The resulting heterogeneity has led to inconsistent results and has complicated interpretation of this data (Kreisl et al., 2013; Bloomfield et al., 2016; Coughlin et al., 2016). The development of a novel PET tracer of TSPO that is unaffected by genetic variability would be of great use in determining drug intervention timing (e.g., illness phase specific pharmacotherapy) for neuropsychiatric disorders in which inflammatory processes are involved.
Summary and Future Directions
To improve the success rate of INDs in the CNS field, we have proposed the expansion and reorganization of existing biomarker utility measures into a 5-tiered indices covering the following functional facets: Tier 1 (brain exposure), Tier 2 (target binding), Tier 3 (brain functional changes), Tier 4 (patient stratification), and Tier 5 (clinical efficacy prediction).
Further rollout of biomarkers is imperative for improvement in clinical development, particularly in the field of psychiatry. Failures of INDs in the CNS field are largely due to small overall effect and/or failure to attain primary endpoints set for clinical trials. For patients diagnosed by the current ICD-10/DSM-5, the general assumption is that patients suffering from schizophrenia, bipolar disorder, and major depression can be composed of biologically distinct subpopulations with heterogeneous pathophysiology. Thus, INDs targeting a selective mechanism could be beneficial to only a fraction of the entire patient population. Currently available drugs for schizophrenia share one selective mechanism, the blockade of the dopamine D2 receptor (Farde et al., 1988). Although blocking D2 receptors is widely effective in schizophrenic populations, patient subgroups exhibit a wide range of responses to these drugs (Demjaha et al., 2012). If we apply neuroimaging data prospectively to exclude treatment-resistant patients (vis-à-vis Tier 4), the effect size for a given compound could increase. Because neuroimaging biomarkers that predict clinical efficacy might depend on biological pathways disturbed in patients, Tier 5 (clinical efficacy prediction) criteria could be tightly linked to Tier 4. As discussed above, neuroimaging biomarkers monitoring the status of excitatory/inhibitory balance, neuroinflammation, and oligodendrocytes also represent potential candidates to benefit from the use of Tier4/5 biomarkers. It is important to remember that the cost and effort involved in neuroimaging biomarkers renders them unsuitable for large-scale clinical trials; therefore, biomarkers that are less costly and easier to measure than neuroimaging biomarkers may be needed in later trials.
Although it remains largely outside the scope of this working group’s report, PET may also be used to predict safety/tolerability; microdosing of labeled therapeutics could indicate drug predisposition for accumulation in certain organs, allowing advanced prediction of possible side effects (Roberts et al., 2015; Papadimitriou et al., 2016; Burt et al., 2016).
In summary, neuroimaging biomarkers are ever-more-powerful tools for evaluating the potential of INDs. To better support this mission, we propose redefinition of existing criteria to further the use of biomarkers as shepherds of clinical development, while implementing a fourth (patient stratification) and fifth (clinical efficacy prediction) tier to this index. Our ultimate objective is to improve the success rate of INDs and eventually to achieve true “precision medicine” in CNS disorders. This includes addressing emerging problems, including symptom- or mechanism-specific biomarkers used for diagnosis and stratification. We also propose to pursue generation and development of new neuroimaging biomarkers through PPPs that combine disease knowledge, cutting-edge technologies, chemical libraries, medicinal chemistry, and funding to achieve novel breakthroughs. Considering their potential to accelerate drug discovery in the CNS field, PPPs should also include regulatory agencies, such as U.S. Food and Drug Administration, Eurpean Medicines Agency, and Pharmaceuticals and Medical Devices Agency so as to standardize application of neuroimaging biomarkers and their related general biomarkers in clinical trials of INDs and frame how they may be used to stratify target patients and reach primary and co-primary endpoints.
Statement of Interest
Drs. Chaki and Omura are employees of Taisho Pharmaceutical Co., Ltd. Drs. Kimura and Furusawa are employees of Takeda Pharmaceutical Co., Ltd. Drs. Matsumoto and Miyoshi are employees of Astellas Pharmaco Inc. Drs. Ogura and Yamamoto are employees of Eisai Co., Ltd. Dr. Negishi is an employee of Mochida Pharmaceutical Co., Ltd. Dr. Saijo is an employee of Mitsubishi Tanabe Pharma Co. Dr. Watanabe is an employee of Daiichi Sankyo Co., Ltd. Dr. Nakatani is an employee of Chugai Pharmaceutical Co., Ltd. Dr. Liou is an employee of Ono Pharmaceutical Co., Ltd. Dr. Walton is an employee of Astellas Research Institute of America LLC.
Acknowledgments
We acknowledge Dr. Maiko Ono for use of her artwork (Figure 1a).
References
- Aducanumab. Alzforum website. http://www.alzforum.org/therapeutics/aducanumab Accessed July 22, 2016.
- Ahnaou A, de Boer P, Lavreysen H, Huysmans H, Sinha V, Raeymaekers L, Van De Casteele T, Cid JM, Van Nueten L, Macdonald GJ, Kemp JA, Drinkenburg WH. (2016) Translational neurophysiological markers for activity of the metabotropic glutamate receptor (mGluR2) modulator JNJ-40411813: sleep EEG correlates in rodents and healthy men. Neuropharmacology 103:290–305. [DOI] [PubMed] [Google Scholar]
- Arakawa R, Ito H, Takano A, Takahashi H, Morimoto T, Sassa T, Ohta K, Kato M, Okubo Y, Suhara T. (2008) Dose-finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 197:229–235. [DOI] [PubMed] [Google Scholar]
- Atack JR, Wong DF, Fryer TD, Ryan C, Sanabria S, Zhou Y, Dannals RF, Eng WS, Gibson RE, Burns HD, Vega JM, Vessy L, Scott-Stevens P, Beech JS, Baron JC, Sohal B, Schrag ML, Aigbirhio FI, McKernan RM, Hargreaves RJ. (2010) Benzodiazepine binding site occupancy by the novel GABAA receptor subtype-selective drug 7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine (TPA023) in rats, primates, and humans. J Pharmacol Exp Ther 332:17–25. [DOI] [PubMed] [Google Scholar]
- Barker RA, Williams-Gray CH. (2016) Review: the spectrum of clinical features seen with alpha synuclein pathology. Neuropathol Appl Neurobiol 42:6–19. [DOI] [PubMed] [Google Scholar]
- Becker RE, Seeman MV, Greig NH, Lahiri DK. (2015) What can triumphs and tribulations from drug research in Alzheimer’s disease tell us about the development of psychotropic drugs in general? Lancet Psychiatry 2:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD. (2016) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasy LS, Potter WZ. (2014) Public-private partnerships to revitalize psychiatric drug discovery. Expert Opin Drug Discov 9:1–8. [DOI] [PubMed] [Google Scholar]
- Burt T, Yoshida K, Lappin G, Vuong L, John C, de Wildt SN, Sugiyama Y, Rowland M. (2016) Microdosing and other phase 0 clinical trials: facilitating translation in drug development. Clin Transl Sci 9:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso A, Alvarez-Sánchez R, Hillebrecht A, Poirier A, Schuler F, Lavé T, Funk C, Belli S. (2013) PK/PD assessment in CNS drug discovery: prediction of CSF concentration in rodents for P-glycoprotein substrates and application to in vivo potency estimation. Biochem Pharmacol 85:1684–1699. [DOI] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Arora A, Carpenter AP, Flitter ML, Joshi AD, Krautkramer MJ, Lu M, Mintun MA, Skovronsky DM, AV-45-A16 Study Group (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 11:669–678. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Meiler J, Niswender CM. (2014) Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat Rev Drug Discov 13:692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN. (2014) Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 13:419–431. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG. (2016) In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. (2012) Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry 169:1203–1210. [DOI] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R; Alzheimer’s Disease Cooperative Study Steering Committee Solanezumab Study Group (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370:311–321. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Brandon NJ. (2015) Schizophrenia drug discovery and development in an evolving era: are new drug targets fulfilling expectations? J Psychopharmacol 29:230–238. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Halldin C, Sedvall G. (1988) Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry 45:71–76. [DOI] [PubMed] [Google Scholar]
- Finnema S, Scheinin M, Shahid M, Lehto J, Borroni E, Bang-Andersen B, Sallinen J, Wong E, Farde L, Halldin C, Grimwood S. (2015) Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology 232:4129–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald M, Becker A, Bahr I, Mueller-Fahrnow A. (2016) Public-private partnerships in lead discovery: overview and case studies. Arch Pharm Chem Life Sci 349:692–697. [DOI] [PubMed] [Google Scholar]
- Golde TE. (2005) The Abeta hypothesis: leading us to rationally-designed therapeutic strategies for the treatment or prevention of Alzheimer disease. Brain Pathol 15:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holsboer F. (2012) Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov 11:462–478. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Hartig PR. (2009) Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Ther 122:281–301. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Murthy V, Catafau AM, Searle G, Bullich S, Slifstein M, Ouellet D, Zamuner S, Herance R, Salinas C, Pardo-Lozano R, Rabiner EA, Farre M, Laruelle M. (2011) Translational characterization of [11C]GSK931145, a PET ligand for the glycine transporter type 1. Synapse 65:1319–1332. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. [DOI] [PubMed] [Google Scholar]
- Hargreaves R. (2002) Imaging substance P receptors (NK1) in the living human brain using positron emission tomography. J Clin Psychiatry 63:18–24. [PubMed] [Google Scholar]
- Hegedűs N, Laszy J, Gyertyán I, Kocsis P, Gajári D, Dávid S, Deli L, Pozsgay Z, Tihanyi K. (2015) Scopolamine provocation-based pharmacological MRI model for testing procognitive agents. J Psychopharmacol 29:447–455. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. (2003) Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 53:1075–1085. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Pizzagalli F, Boetsch C, Alberati D, Ereshefsky L, Jhee S, Patat A, Boutouyrie-Dumont B, Martin-Facklam M. (2016) Effects of the glycine reuptake inhibitors bitopertin and RG7118 on glycine in cerebrospinal fluid: results of two proofs of mechanism studies in healthy volunteers. Psychopharmacology (Berl) 233:2429–2439. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, Rasmussen K, Glasebrook A, Koester A, Song D, Jones KA, Zorn S, Smagin G, Duman RS. (2016) Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry. 80:12–22. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, Mathis CA, Alzheimer’s Disease Neuroimaging Initiative (2009) Relationships between biomarkers in aging and dementia. Neurology 73:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Reddy M, Anderson P, Salzberg MR, O’Brien TJ, Pinault D. (2012) Acute administration of typical and atypical antipsychotics reduces EEG gamma power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in gamma power. Int J Neuropsychopharmacol 15:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Sanabria-Bohórquez SM, Bormans G, Koole M, De Hoon J, Van Hecken A, Depre M, De Lepeleire I, Van Laere K, Sur C, Hamill TG. (2015) Characterization of the novel GlyT1 PET tracer [18F]MK-6577 in humans. Synapse 69:33–40. [DOI] [PubMed] [Google Scholar]
- Joules R, Doyle OM, Schwarz AJ, O’Daly OG, Brammer M, Williams SC, Mehta MA. (2015) Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology (Berl) 232:4205–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Remington G, Houle S. (2000) Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157:514–520. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55:306–319. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW, Innis RB. (2010) Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 49:2924–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB; Biomarkers Consortium PET Radioligand Project Team (2013) A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 33:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H. (2008) Correlation between ex vivo receptor occupancy and wake-promoting activity of selective H3 receptor antagonists. J Pharmacol Exp Ther 325:902–909. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. (2008) Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry 165:1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH. (2008) CSF as a surrogate for assessing CNS exposure: an industrial perspective. Curr Drug Metab 9:46–59. [DOI] [PubMed] [Google Scholar]
- Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS, Klunk WE, Mathis CA, Salloway S, Fox NC, Hill DL, Les AS, Collins P, Gregg KM, Di J, Lu Y, Tudor IC, Wyman BT, Booth K, Broome S, Yuen E, Grundman M, Brashear HR; Bapineuzumab 301 and 302 Clinical Trial Investigators (2015) Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology 85:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S, Dean R, Ackermann B, Jackson K, Natanegara F, Anderson S, Eckstein J, Yuen E, Ayan-Oshodi M, Ho M, McKinzie D, Perry K, Svensson K. (2012) Effects of a novel mGlu₂/₃ receptor agonist prodrug, LY2140023 monohydrate, on central monoamine turnover as determined in human and rat cerebrospinal fluid. Psychopharmacology (Berl) 219:959–970. [DOI] [PubMed] [Google Scholar]
- Martin-Facklam M, Pizzagalli F, Zhou Y, Ostrowitzki S, Raymont V, Brašić JR, Parkar N, Umbricht D, Dannals RF, Goldwater R, Wong DF. (2013) Glycine transporter type 1 occupancy by bitopertin: a positron emission tomography study in healthy volunteers. Neuropsychopharmacology 38:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, Zhang MR, Trojanowski JQ, Lee VM, Ono M, Masamoto K, Takano H, Sahara N, Iwata N, Okamura N, Furumoto S, Kudo Y, Chang Q, Saido TC, Takashima A, Lewis J, Jang MK, Aoki I, Ito H, Higuchi M. (2013) Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79:1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S. (2001) Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C]DASB PET imaging study. Am J Psychiatry 158:1843–1849. [DOI] [PubMed] [Google Scholar]
- Moore CS, Ase AR, Kinsara A, Rao VT, Michell-Robinson M, Leong SY, Butovsky O, Ludwin SK, Séguéla P, Bar-Or A, Antel JP. (2015) P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm 2:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Matochik JA, Zametkin AJ, Szymanski HV, Cantillon M, Cohen RM, Sunderland T. (1994) A double FDG/PET study of the effects of scopolamine in older adults. Neuropsychopharmacology 10:191–198 [DOI] [PubMed] [Google Scholar]
- Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street SD. (2012) Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today 17:419–424. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Chiba K. (2015) Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol Ther 154:21–35. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. [DOI] [PubMed] [Google Scholar]
- O’Brien DE, Conn PJ. (2016) Neurobiological insights from mGlu receptor allosteric modulation. Int J Neuropsychopharmacol 19 pii: pyv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. (2016) Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139:1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. (2012) An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou L, Smith-Jones PM, Sarwar CMS, Marti CN, Yaddanapudi K, Skopicki HA, Gheorghiade M, Parsey R, Butler J. (2016) Utility of positron emission tomography for drug development for hart failure. Am Heart J 175:142–152. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Sternberger NH, Lennon VA. (1984) The distribution of myelin-associated glycoprotein and myelin basic protein in actively demyelinating multiple sclerosis lesions. J Neuroimmunol 6:251–264. [DOI] [PubMed] [Google Scholar]
- Ratner M. (2015) Biogen’s early Alzheimer’s data raise hopes, some eyebrows. Nat Biotechnol 33:438. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J. (2015) The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300:141–154. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Aschner M, Calligaro D, Guilarte TR, Hanig JP, Herr DW, Hudzik TJ, Jeromin A, Kallman MJ, Liachenko S, Lynch JJ, III, Miller DB, Moser VC, O’Callaghan JP, Slikker W, Jr, Paule MG. (2015) Translational biomarkers of neurotoxicity: a health and environmental sciences institute perspective on the way forward. Toxicol Sci 148:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR; Bapineuzumab 301 and 302 Clinical Trial Investigators (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF, Matuskey D, Lee JY, O’Connor KC, Huang Y, Carson RE, Hannestad J, Cosgrove KP. (2015) Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U S A 112:12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, Dowsett SA, Pontecorvo MJ, Dean RA, Demattos R. (2016) Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement 12:110–120. [DOI] [PubMed] [Google Scholar]
- Suhara T, Takano A, Sudo Y, Ichimiya T, Inoue M, Yasuno F, Ikoma Y, Okubo Y. (2003) High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Arch Gen Psychiatry 60:386–391. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y, Suhara T. (2010) Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 13:943–950. [DOI] [PubMed] [Google Scholar]
- Takano A, Stenkrona P, Stepanov V, Amini N, Martinsson S, Tsai M, Goldsmith P, Xie J, Wu J, Uz T, Halldin C, Macek TA. (2016) A human [11C]T-773 PET study of PDE10A binding after oral administration of TAK-063, a PDE10A inhibitor. Neuroimage 141:10–17. [DOI] [PubMed] [Google Scholar]
- Tanzi RE. (2005) The synaptic Abeta hypothesis of Alzheimer disease. Nat Neurosci 8:977–979. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y, Cash D, Suzuki M, Suzuki K, Bernanos M, Simmons C, Williams SC, Kimura H. (2016) TAK-063, a phosphodiesterase 10A inhibitor, modulates neuronal activity in various brain regions in phMRI and EEG studies with and without ketamine challenge. Neuroscience 339:180–190. [DOI] [PubMed] [Google Scholar]
- Wandschneider B, Koepp MJ. (2016) Pharmaco fMRI: determining the functional anatomy of the effects of medication. Neuroimage Clin 12:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, Green RC, Harvey D, Jack CR, Jagust W, Luthman J, Morris JC, Petersen RC, Saykin AJ, Shaw L, Shen L, Schwarz A, Toga AW, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative (2015) 2014 Update of the Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 11:e1–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Tauscher J, Gründer G. (2009) The role of imaging in proof of concept for CNS drug discovery and development. Neuropsychopharmacology 34:187–203. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, Nozaki S, Fujimura Y, Koeda M, Asada T, Suhara T. (2008) Increased binding of peripheral benzodiazepine receptor in Alzheimer’s Disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiat 64:835–841. [DOI] [PubMed] [Google Scholar]