Abstract
Background and Aims Nepenthes attracts wide attention with its spectacularly shaped carnivorous pitchers, cultural value and horticultural curiosity. Despite the plant’s iconic fascination, surprisingly little anatomical detail is known about the genus beyond its modified leaf tip traps. Here, the wood anatomical diversity of Nepenthes is explored. This diversity is further assessed with a phylogenetic framework to investigate whether the wood characters within the genus are relevant from an evolutionary or ecological perspective, or rather depend on differences in developmental stages, growth habits, substrates or precipitation.
Methods Observations were performed using light microscopy and scanning electron microscopy. Ancestral states of selected wood and pith characters were reconstructed using an existing molecular phylogeny for Nepenthes and a broader Caryophyllales framework. Pairwise comparisons were assessed for possible relationships between wood anatomy and developmental stages, growth habits, substrates and ecology.
Key Results Wood anatomy of Nepenthes is diffuse porous, with mainly solitary vessels showing simple, bordered perforation plates and alternate intervessel pits, fibres with distinctly bordered pits (occasionally septate), apotracheal axial parenchyma and co-occurring uni- and multiseriate rays often including silica bodies. Precipitation and growth habit (stem length) are linked with vessel density and multiseriate ray height, while soil type correlates with vessel diameter, vessel element length and maximum ray width. For Caryophyllales as a whole, silica grains, successive cambia and bordered perforation plates are the result of convergent evolution. Peculiar helical sculpturing patterns within various cell types occur uniquely within the insectivorous clade of non-core Caryophyllales.
Conclusions The wood anatomical variation in Nepenthes displays variation for some characters dependent on soil type, precipitation and stem length, but is largely conservative. The helical-banded fibre-sclereids that mainly occur idioblastically in pith and cortex are synapomorphic for Nepenthes, while other typical Nepenthes characters evolved convergently in different Caryophyllales lineages.
Keywords: Ancestral state reconstruction, carnivorous plants, Caryophyllales, helically banded idioblasts, Nepenthes, pitcher plants, silica grains, wood anatomy
INTRODUCTION
Nepenthes L. is a genus of carnivorous woody plants including around 140 species, many of which have been described in just the last 5 years (McPherson, 2012; http://www.ipni.org/, accessed 24 March 2016). Its centre of distribution is in the Malay Archipelago, but extends into Australia, Cambodia, India, Laos, Madagascar, Sri Lanka, Thailand and Vietnam (Cheek and Jebb, 2001; Meimberg and Heubl, 2006). This distribution supports diverse growth habits of robust lianas up to 20 m tall to compact, woody rosette plants of only a few centimetres high (McPherson, 2009). Nepenthes are most widely recognized and identified by their impressive, liquid-filled pit-fall traps (Cheek and Jebb, 2001), whose main function is to lure, retain and digest insect prey. Some species have developed alternative feeding strategies, acquiring nitrogen from fallen leaf litter or the faeces of small mammals and birds (Moran et al., 2003; Chin et al., 2010; Greenwood et al., 2011). In spite of its iconic fascination to the horticulture, tourism and research community, surprisingly little is known about the anatomical detail of the genus beyond its predatory structures. More information about the anatomical plant body of Nepenthes, whose dioecious character minimizes colonization potential (Baker, 1955), is desired in response to growing concern over the physiological pliability needed for plants with low ability to move along with a progressively changing climate gradient (Shaw and Etterson, 2012; IPCC, 2014; Merckx et al., 2015; Schwallier et al., 2016).
The Intergovernmental Panel on Climate Change (IPCC) predicts that the climate of Southeast Asia will face unprecedented extremes in precipitation within this century (IPCC, 2014). Consequently, information about the drought tolerance of CITES-protected species that grow as narrow endemics in very wet environments, such as highland Nepenthes, is especially pertinent. Although no experimental studies on drought stress resistance have been carried out in the genus, it is to be expected that such narrow endemics are vulnerable to lethal levels of embolism formation in their water-conducting cells when facing mild levels of drought stress (Choat et al., 2012). In combination with experimental studies, observations on wood anatomy could be integrated in mechanistic models to estimate survival in future climate scenarios, which is especially relevant to the narrowly endemic Nepenthes species that have range-confining abiotic and biotic variables (Clarke et al., 2009; Bonhomme et al., 2011; Greenwood et al., 2011; Rembold et al., 2012; Merckx et al., 2015; van der Ent et al., 2015; Schwallier et al., 2016).
Anatomical studies of non-pitcher-forming leaves, roots and stems of Nepenthes are available for only a very small number of species (Heinricher, 1906; Metcalfe and Chalk, 1950; Pant and Bhatnagar, 1977; Carlquist, 2010). One of the more interesting anatomical features observed in the genus are helical idioblasts (or ‘spiral elements’) in the leaves (Solereder, 1908; Metcalfe and Chalk, 1950), pith, cortex and rhizome rays (Metcalfe and Chalk, 1950; Carlquist, 2010) and in the stem cortex (Metcalfe and Chalk, 1950). The most seminal wood anatomical study of the genus investigated only three species, N. ampullaria, N. lowii and N. × kinabaluensis (Carlquist, 1981). With this, Carlquist reasoned that further investigation of additional species would be unlikely to show more anatomical diversity, yet observation of just one additional species, N. alata, almost 30 years later (Carlquist, 2010), unveiled novel characters. In addition to this, Nepenthes species inhabit various elevations, climates and substrates throughout their distribution range (McPherson, 2012; Moran et al., 2013), all of which could be reflected in variation of wood anatomy (Carlquist, 1966, 1975; Baas, 1976; Baas et al., 1983; Lens et al., 2011; Kidner et al., 2016). More thorough investigation of wood species spanning across the ecological and phylogenetic diversity is therefore desired.
Previous wood anatomical studies in other Caryophyllales families have revealed evolutionary informative characters, elucidating important taxonomical clarifications and insights in key innovations (Carlquist, 2010). The phylogenetic position of Nepenthaceae within the non-core Caryophyllales is supported by both nuclear and plastid gene sequences (Cuénoud et al., 2002; Brockington et al., 2009; Schäferhoff et al., 2009) in a monophyletic clade together with three other carnivorous plant families: Droseraceae, Drosophyllaceae and Dioncophyllaceae. This clade is characterized by a specific leaf habit with juvenile rosette forms elongating during maturation (Albert et al., 1992). Relationships within this carnivorous clade were poorly resolved in the first phylogenetic studies, but more recent multigene analyses indicate a potential sister group relationship between Nepenthaceae and Droseraceae, still with poor support (Schäferhoff et al., 2009; Soltis et al., 2011).
Here, we present a detailed wood anatomical survey of 40 Nepenthes species covering a wide range of altitude, life form and climatic/edaphic preferences, thereby increasing our anatomical knowledge of the genus significantly. In addition to these novel wood descriptions, our observations are confronted with an existing phylogenetic framework at the genus level and beyond to assess the evolutionary history of selected wood characters. Furthermore, we explore whether differences in developmental stages of the stem, growth habit and abiotic preferences have an impact on stem anatomical variation as has been demonstrated in various woody angiosperms (Carlquist, 1966, 1975; Baas, 1976; Baas et al., 1983; van den Oever et al., 1981; Noshiro and Baas, 2000; Lens et al., 2004, 2005, 2008a, 2011; Olson et al., 2014; Kidner et al., 2016).
MATERIALS AND METHODS
In total, wood samples of 40 Nepenthes species were collected, representing all major sub-clades within the genus based on present phylogenetic knowledge (Heubl et al., 2006; Alamsyah and Ito, 2013; Merckx et al., 2015; Schwallier et al., 2016). Specimens were sourced from living plants as follows: five species were collected in the field from Borneo, one in the field from Madagascar and nine from the living collection of the Hortus botanicus in Leiden. Twenty-five samples were harvested from dried herbaria material of Naturalis Biodiversity Center (n = 20) and the Sabah Parks Herbarium (n = 5) (Table 1).
Table 1.
Overview of selected anatomical wood characters of Nepenthaceae
| Nepenthes species | Vessel diameter (μm) | Vessel density (mm–1) | Vessel element length (μm) | Gums in vessels | Fibre- tracheid length (μm) | Fibre- tracheids septate | Fibre- tracheids thick- walled | Distinct axial parenchyma bands | Width of axial parenchyma bands (no. of cells) | Scanty paratracheal axial parenchyma | Rays exclusively uniseriate | Ray width (no. of cells) | Height uniseriate rays (μm) | Height multiseriate rays (μm) | Density uniseriate rays (mm–1) | Density multiseriate rays (mm–1) | Ray compo- stition | Silica bodies in rays | Medullary bundles | Pith lignification | Helical idioblasts in pith | Helical idioblasts in multiseriate rays | Helical idioblasts in cortex |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. ampullaria† | 25–60–105 | 14–27–40 | 250–360–470 | + | 300–500–700 | ± | + | ± | 1–8 | + | – | 1 (2–4) | 170–760–2100 | 310–1090–1900 | 12–14 | 0–2 | Usp | + | – | + | + | – | / |
| N. bicalcarata* | 40–98–160 | 8–14–25 | 300–460–590 | – | 450–620–780 | – | + | – | / | – | + | / | 400–800–1800 | / | 6–10 | 0 | US | – | – | + | + | / | / |
| N. bokorensis*† | 40–68–100 | 14–24–44 | 200–325–500 | + | 410–590–820 | – | – | + | 1–2 | + | – | 1 (2–4,10) | 170–330–550 | 400–1140–3300 | 3–7 | 2–5 | US | ± | – | + | – | – | / |
| N. burbidgeae1* | 50–78–100 | 22–25–30 | 200–320–500 | – | 500–650–750 | – | – | + | 1 | – | + | / | 250–620–1000 | / | 12–14 | 0 | U | ± | + | + | + | / | + |
| N. burbidgeae2* | 50–76–120 | 13–19–24 | 250–380–525 | – | 625–770–900 | – | – | + | 1 | – | – | 1 (2–3) | 300–690–1500 | 700–1070–1700 | 11–14 | 0–1 | Us | + | + | + | + | – | / |
| N. chaniana*† | 35–47–70 | 16–24–36 | 250–340–540 | + | 415–545–670 | – | + | + | 2 | + | – | 1 (2) | 250–390–750 | 560–935–1210 | 7–16 | 0–2 | US | ± | – | – | + | – | + |
| N. distillatoria* | 20–46–75 | 28–36–40 | 200–285–375 | – | 350–525–710 | – | + | – | / | – | – | 1 (2,10) | 170–490–1250 | 750–1350–1950 | 8–12 | 0–1 | US | ± | – | ± | + | – | / |
| N. edwardsiana* | 30–51–70 | 22–43–52 | 270–380–550 | – | 300–515–710 | – | – | – | / | – | – | 1 (2) | 260–580–1250 | 405–810–2250 | 8–13 | 0–3 | Us | – | – | ± | – | – | / |
| N. gracilis | 40–94–170 | 9–16–23 | 250–332–550 | – | 400–549–750 | + | – | + | 1–4 | + | – | 1,2–5 (6–10) | ND | ND | ND | ND | Usp | + | – | + | + | / | + |
| N. gracillima* | 25–37–60 | 20–31–40 | 240–315–405 | – | 250–430–600 | – | + | ± | 1 | – | – | 1 (2–3,10) | 150–290–600 | / | 12–16 | 0 | Us | ± | – | ± | + | / | / |
| N. gymnamphora | 50–104–150 | 28–33–39 | 250–370–500 | + | 400–565–750 | – | – | ± | 1–4 | + | – | 1 (2) | 400–755–1400 | 600–1365–2100 | 9–13 | 1–2 | Usp | – | – | ± | ± | + | / |
| N. hemsleyana* | 25–37–50 | 20–24–28 | 210–360–450 | – | 310–445–600 | ± | + | + | 1–2 | + | – | 1 (2) | 210–690–1200 | 600–940–1250 | 10–13 | 0–2 | Us | ± | – | ± | + | – | / |
| N. hirsuta* | 15–38–50 | 32–45–60 | 280–390–500 | – | 300–850–625 | – | + | ± | 1 | – | + | / | 150–370–700 | / | 9–12 | 0 | uS | + | – | + | + | / | + |
| N. kerrii*† | 30–53–75 | 16–31–44 | 200–295–400 | – | 300–465–800 | – | – | – | / | ± | + | / | 245–710–1210 | / | 8–14 | 0 | US | – | – | + | + | / | + |
| N. khasiana | 20–61–90 | 45–52–64 | 200–260–340 | + | 350–515–700 | ± | – | ± | 1 | – | – | 1 (2–3,14) | 170–485–710 | 230–765–1800 | 11–14 | 3–5 | Us | – | – | ± | + | + | / |
| N. lamii* | 35–60–105 | 16–37–48 | 200–420–710 | – | 300–505–740 | + | – | – | / | ± | – | 1 (5–8) | 200–580–1350 | 450–640–900 | 7–15 | 0–1 | Us | ± | – | ± | – | – | / |
| N. lowii | 45–80–115 | 21–22–30 | 250–450–730 | – | 270–465–600 | ± | – | + | 1–7 | + | + | 1 (2–5) | 120–470–950 | 250–660–1800 | 4–10 | 0–3 | US | – | – | + | + | + | + |
| N. macfarlanei* | 35–57–90 | 24–34–48 | 260–490–720 | – | 300–605–950 | – | + | – | / | – | – | 1 (2–3) | 400–805–1950 | 100–1500–2200 | 14–20 | 0–1 | Us | – | + | ++ | + | – | / |
| N. madagascariensis | 25–54–120 | 16–29–40 | 200–265–360 | + | 300–450–710 | – | – | ± | 4–6 | + | – | 1 (6–12) | 180–520–1600 | 300–1365–3900 | 3–10 | 1–6 | Usp | ± | – | – | + | – | / |
| N. maxima*† | 25–43–95 | 18–33–46 | 205–340–570 | – | 360–565–750 | – | – | ± | 3–4 | – | – | 1 (2–3) | 175–470–1150 | 550–1270–3400 | 7–12 | 2–6 | uS | ± | – | ± | – | – | / |
| N. mirabilis | 30–78–150 | 20–23–34 | 210–370–710 | – | 350–520–700 | ± | – | ± | 1–4 | + | – | 1 (2) | 300–1090–2200 | 450–1390–3200 | 9–13 | 0–2 | Usp | ± | – | ± | + | – | + |
| N. muluensis* | 25–54–100 | 20–28–36 | 250–390–500 | – | 360–460–610 | – | + | – | / | – | + | / | 150–400–900 | / | 6–14 | 0 | Us | – | – | ++ | + | / | + |
| N. neoguineensis* | 40–88–125 | 20–27–40 | 250–320–425 | – | 320–440–600 | – | ± | – | / | ± | + | / | 300–520–950 | / | 9–12 | 0 | Us | – | – | + | + | / | / |
| N. pervillei* | 50–73–110 | 26–32–40 | 300–405–510 | – | 500–645–900 | ± | – | – | / | – | – | 1 (2) | 175–395–1100 | 450–535–600 | 7–15 | 0–1 | US | ± | – | + | + | – | / |
| N. pilosa* | 50–94–140 | 22–26–32 | 250–400–600 | – | 600–680–850 | – | – | – | 2–3 | – | + | / | 350–770–1100 | / | 10–15 | 0 | U | + | + | + | + | / | + |
| N. rafflesiana† | 25–78–125 | 18–22–29 | 275–390–540 | + | 400–555–740 | – | + | + | 1–10 | + | – | 1 (2–4) | 200–500–1100 | 1000–1475–2300 | 7–10 | 2–3 | Usp | + | – | ± | + | + | / |
| N. rajah | 50–65–90 | 8–13–20 | 150–256–400 | + | 450–580–700 | ± | – | + | 2–6 | – | – | 1 (2) | 150–183–350 | 150–192–250 | 10–13 | 0–1 | Usp | ± | – | ± | + | + | + |
| N. reinwardtiana* | 40–52–70 | 28–36–44 | 210–350–540 | – | 320–525–700 | – | + | – | / | – | – | 1 (2–4) | 150–510–790 | 400–955–2100 | 9–13 | 1–4 | uS | + | + | + | + | – | / |
| N. rhombicaulis† | 30–45–75 | 32–38–50 | 260–375–490 | – | 400–515–850 | ± | – | + | 1–5 | + | – | 1 (5–7) | 150–535–1200 | 700–970–1400 | 7–12 | 0–4 | Us | ± | – | ± | + | – | / |
| N. sanguinea | 15–34–50 | 20–32–48 | 240–340–480 | + | 375–520–710 | – | + | ± | 1–2 | – | – | 1 (2–6) | 200–390–655 | 250–870–1600 | 5–10 | 0–5 | US | – | + | – | + | – | / |
| N. smilesii | 20–38–50 | 40–55–64 | 155–215–325 | – | 275–420–530 | – | ± | + | 1–4 | – | – | 1 (2) | 170–366–575 | 190–555–1650 | 5–12 | 0–3 | Us | ± | – | + | ± | – | / |
| N. stenophylla* | 60–84–100 | 14–23–32 | 200–340–450 | – | 650–740–850 | ± | – | – | 2–3 | – | + | / | 350–812–1700 | / | 10–15 | 0 | U | + | + | ++ | + | / | + |
| N. tentaculata* | 25–48–100 | 16–30–36 | 270–355–500 | – | 300–460–700 | – | + | – | / | – | + | / | 200–460–850 | / | 8–12 | 0 | Us | – | – | – | + | / | + |
| N. thorelii | 20–37–55 | 40–48–56 | 200–305–460 | – | 300–425–550 | – | ± | ± | 1–2 | – | + | / | 160–260–410 | / | 11–16 | 0 | uS | – | – | ± | + | / | / |
| N. tobaica | 25–54–75 | 32–41–54 | 245–360–500 | – | 450–600–750 | – | + | – | / | – | + | / | 100–1030–2600 | / | 14–18 | 0 | Usp | ± | + | ++ | + | / | / |
| N. tomoriana | 25–40–65 | 36–46–64 | 260–355–500 | – | 380–585–850 | – | + | ± | 1–2 | ± | – | 1 (2–14) | 120–515–1200 | 350–1190–2500 | 11–14 | 0–2 | Us | – | – | ± | + | – | / |
| N. veitchii* | 75–107–130 | 28–35–48 | 200–304–450 | – | 500–640–700 | ND | – | + | 1–2 | – | + | / | ND | ND | 10–15 | 0 | U | ± | + | + | ± | / | / |
| N. ventricosa† | 40–65–105 | 15–20–30 | 200–315–500 | – | 355–490–605 | – | – | + | 1–2 | + | + | / | 100–295–625 | / | 3–7 | 0 | US | – | – | ± | + | / | + |
| N. villosa | 30–53–75 | 36–49–61 | 290–380–490 | + | 400–625–900 | – | – | + | 1–2 | + | – | 1, 2–5 | 190–425–760 | 270–950–3400 | 4–7 | 3–6 | Usp | – | – | ± | + | – | + |
| N. sp. (Thai origin) | 30–57–90 | 32–46–58 | 250–330–460 | – | 275–435–550 | ± | + | + | 1–2 | + | – | 1 (2–3) | 250–383–750 | 520–725–900 | 6–11 | 0–1 | Us | + | – | + | / | – | ± |
Values reported between en rules are mean values with flanking min and max.
Ray composition reported as (1) upright (U), (2) most upright, few square (Us), (3) most square, few upright (uS), (4) mixed upright and square (US) or (5) mainly upright with few square and procumbent cells (Usp).
Pith lignification reported as (1) slightly lignified with few, thin-walled cells (–), (2) slightly lignified with many, thin-walled cells in outer zone of pith (±), (3) markedly lignified with thin–thick-walled cells intermediate between axial parenchyma cells and fibres in large portion of outer pith (+) or (4) markedly lignified with thick-walled cells intermediate between axial parenchyma and fibres throughout the entire pith ( ++).
Cortex was not available for analysis in species marked with /.
Characters not determinable in categories are marked with ND.
Juvenile wood indicated with *, and greenhouse-grown specimens are indicated with †.
Wood from living plants was harvested at the base of mature plants. To increase our sampling, we also used herbarium material, which is most often collected further up from the plant base. More juvenile herbarium branches/twigs, therefore, were the only available stems in these samples (Supplementary Data Table S1). Categorization of wood juvenilism was assessed for each species (Table 1) based on the amount of wood formed in each of the specimens. Since wood formation is never pronounced within Nepenthes, we considered a sample to be mature when there were at least 20 rows of wood cells, which clearly delimited the herbarium samples from the more mature field/greenhouse samples. Our observations in sampling the entire stem of the mature N. mirabilis, N. rafflesiana and N. reinwardtiana showed a strikingly similar wood anatomy from the base towards the stem apex where upper pitchers were growing (>100 cm from base), which validated inclusion of juvenile samples in our assessment. Nepenthes campanulata and N. clipeata, the only two small herbaceous species within the genus that never form tendrils, are rare in cultivation and had to be excluded from the study because sampling would have killed the plants.
Wood sections of 25 μm in thickness were made using a sledge microtome (Reichert, Germany). Preparation of sections and macerations follows Lens et al. (2005). Sections were observed using a Leica DM2500 light microscope and photographed with a Leica DFC-425C digital camera (Leica Microscopes, Wetzlar, Germany). Wood surfaces for scanning electron microscopy (SEM) observations were platinum–palladium coated with a sputter coater (Quorum Q150TS Quorum Technologies, Laughton, UK) and observed with a Jeol JSM-7600F field emission scanning electron microscope (JEOL Ltd, Tokyo, Japan). For this study, we use the wood anatomical terminology of the IAWA list of microscopic features for hardwood identification (IAWA Committee, 1989). In alignment with this, fibre-tracheids are defined as long, imperforate cells with more than one row of distinctly bordered pits in tangential and radial walls. The combination of mainly solitary vessels and imperforate cells with many, large bordered pits, led Carlquist (1981) to call these imperforate cells tracheids under the assumption that they are able to conduct water if a sufficient number of vessels embolize (Carlquist, 1984). As hydraulic studies have not been carried out in the genus, we prefer to name the imperforate cells fibre-tracheids. In this publication, we focus on wood characters, but comment also on pith and cortex characters. Since the material of the stem samples had been dried, we were often unable to section the entire stem. In most species, the cortex part in our sections was limited to only a few cell layers at best, making it possible to screen for helical idioblasts, but not for the presence of cortical bundles or the occurrence of deep-seated periderm.
Nepenthes sequences of the nuclear ribosomal marker nrITS and the plastid marker trnK-matK were derived from previous studies and NCBI GenBank (Supplementary Data Table S2). A Caryophyllales alignment was obtained from Soltis et al. (2011) based on 17 genes representing nucleus, plastid and mitochondrion genomes. Sequences were aligned automatically using MAFFT v.7.237 with default parameters (Katoh et al., 2002) as implemented in AliView v.1.14 (Larsson, 2014). Character trait mapping and phylogenetic analyses were performed in two separate analyses, within Nepenthes and across selected genera within the Caryophyllales, using BEAST v.1.8.2 (Heled and Drummond, 2008; Drummond et al., 2012) on the CIPRES portal (Miller et al., 2010). Nepenthes trees have been deposited in TreeBASE (no. 19543; see http://www.treebase.org/), and the Caryophyllales trees of Soltis et al. (2011) can also be found in TreeBASE (no. 11267).
For the Nepenthes analysis, nrITS and trnK-matK were analysed independently rather than concatenated due to the extensive levels of hybridization between Nepenthes species (Clarke and Wong, 1997; McPherson, 2009). With two separate trees, we were able to include more species for which wood anatomical data were collected at the highest possible support rather than using concatenated trees, which require heavy pruning. For the independent analyses of nrITS and trnK-matK matrices, speciation patterns were described using a Birth–Death tree prior (Gernhard, 2008). Test for the best fit substitution model was performed using PartitionFinder v1.1.1, only testing for models implemented in the BEAST software bundle. For the resulting TN93, equal base frequencies and gamma were selected for nrITS. For HKY, estimated base frequencies and gamma were selected for in trnK-matK. Markov chain Monte Carlo (MCMC) algorithms were run for 10 million generations, sampling parameters every 1000 generations. Tracer v.1.6 (Rambaut et al., 2014) was used to assess effective sampling sizes (ESS) for all parameters and to decide the percentage of burn-in for tree constructions. Two independent runs per marker were carried out in BEAST, and combined using LogCombiner v.1.8.2 (part of the BEAST software bundle). The combined set of posterior topologies was summarized as a maximum clade credibility (MCC) tree using TreeAnnotator v.1.8.2 (also part of the BEAST software bundle).
Because BEAST co-estimates tree topology and branch length uncertainties together with the trait model, trees were first produced using all Nepenthes species with marker data to maximize topology results and then pruned of species lacking wood data in Mesquite v.2.75 (Maddison and Maddison, 2011) to create a set of empirical trees to use for the wood anatomy trait optimization. The main reason for pruning taxa post-analysis rather than prior to the analysis is because the choice of outgroup could be influential on the ingroup topology, resolution and support levels. Three wood characters – axial parenchyma distribution, presence of septate fibres and silica presence in ray cells – and two pith characters – pith lignification and presence of medullary bundles – were added as five separate trait partitions to be optimized together with the topology as described above. The empirical trees created with the full species data set were selected for in TreeAnnotator as the ‘target tree’ so that the inferred topology was based on the most robust data set available. Character trees were visualized in FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
For the Caryophyllales level ancestral state reconstructions, the analyses were set up as described above but instead included wood anatomical characters more informative at the genus level, i.e. presence of silica bodies, type of perforation plate border, presence of successive cambia and spiral thickening and location referenced from the literature (Supplementary Data Table S3). A trait was considered present if it was recorded in at least one species within each genus. To fit with character optimization, this alignment was pruned to include only genera with woody species that had wood characters described for at least two of the four characters of interest. The molecular phylogeny of Soltis et al. (2011) included 31 of the 33 families of Caryophyllales, 24 of which were eventually included in our analysis. Based on model test results, substitution models were set to GTR with estimated base frequencies and gamma being selected, while the remaining settings were identical to the previously described Nepenthes wood anatomy character optimization.
Pairwise comparisons of measured wood anatomical characters against precipitation variables, juvenile wood samples, referenced maximum stem length and occurrence on different soil types were made using the Pearson correlation coefficient. To estimate the potential of drought exposure, we extracted BIOCLIM variables (http://www.worldclim.org/) at 2·5 arc-min spatial raster cell resolution for annual precipitation and mean temperature of the driest month from a total of 930 localities for the species for which we have studied wood samples. Locality data were downloaded from the Global Biodiversity Information Facility (GBIF; 13 February 2015) and from the L, NY, US, KEP, NBC, SI and SING herbaria records. Extractions were made in QGIS v2.8 (http://www.qgis.org/en/site/). Referenced maximum stem length and soil type (whether occurring on ultramafic soil or not) were extracted from the descriptive texts of McPherson (2009) and the International Union for Conservation of Nature (IUCN, 2015) (Supplementary Data Table S4).
RESULTS
Wood description
All values for the Nepenthes genus-wide wood description are provided as averages, with minimum and maximum values in parentheses. Detailed species-specific observations can be found in Table 1.
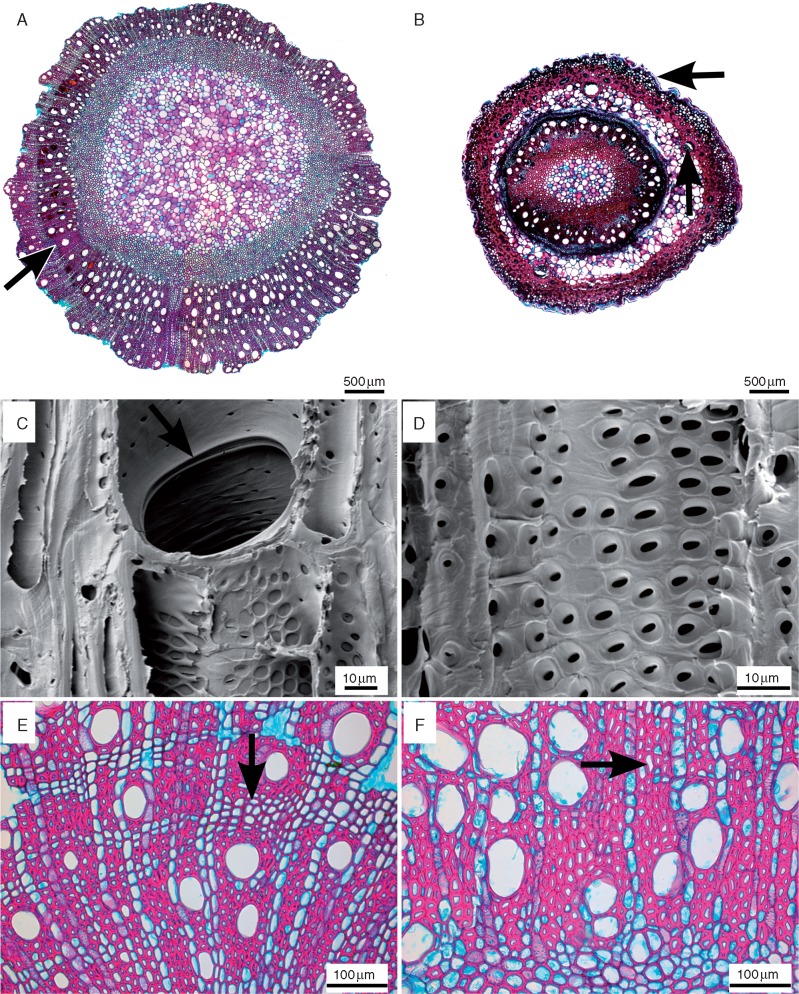
The diagnostic summary of the genus is as follows: growth ring boundaries absent in all species, with the exception of an indistinct growth ring in N. khasiana (Fig. 1A) and N. rajah. Wood diffuse porous. Vessels almost exclusively solitary with simple perforation plates (Fig. 1C); vessel elements (15)–35–110–(170) μm in tangential diameter, (150)–215–490–(730) μm in length and (8)–12–55–(64) mm–2. Intervessel pits alternate (Fig. 1D), pits 5–7 μm in horizontal diameter. Gums occasionally present in N. ampullaria, N. bokorensis, N. chaniana, N. gymnamphora, N. khasiana, N. madagascariensis, N. rafflesiana, N. rajah, N. sanguinea and N. villosa. Sculpturing patterns on inside vessel walls absent. Fibre-tracheids thin- and thick-walled combination or thick-walled, (250)–415–770–(950) μm long with distinctly bordered pits of 5–6 μm in horizontal diameter in both tangential and radial vessels; scarce septate fibres in N. ampullaria, N. hemsleyana, N. khasiana, N. lamii, N. lowii, N. mirabilis, N. pervillei, N. rajah, N. rhombicaulis, N. tentaculata and the as yet unnamed Thai N. sp. Axial parenchyma diffuse-in-aggregates, sometimes forming incomplete short bands of 1–2–(3–8) cells wide in N. ampullaria, N. gracillima, N. gymnamphora, N. hirsuta, N. khasiana, N. madagascariensis, N. maxima, N. mirabilis, N. sanguinea, N. thorelii and N. tomariana; clear banding pattern of 1–2–(3–10) cells wide observed in N. bokoriensis, N. burbidgeae, N. chaniana, N. hemsleyana, N. lowii, N. rafflesiana, N. rajah, N. rhombicaulis, N. smilesii (Fig. 1E), N. veitchii, N. ventricosa, N. villosa and the as yet unnamed Thai N. sp. Axial parenchyma strands of 2–3–(4) cells; N. ampullaria and N. lowii additionally included fusiform axial parenchyma; little axial parenchyma observed in N. tobaica; scarcely scanty paratracheal in several species. Rays exclusively uniseriate in N. bicalcarata, N. burbidgeae, N. hirsuta, N. kerrii, N. muluensis, N. neoguinensis, N. pilosa, N. stenophylla, N. tentaculata, N. tobaica and N. veitchii; 3–18 rays mm–1, (100)–185–1090–(2600) μm long. Uniseriate and multiseriate rays present in the other species (Fig. 2A); multiseriate rays usually 2–(3–4) seriate, occasionally up to 14-seriate in N. bokorensis and N. tomariana; (0)–1–6 rays mm–1, (150)–190–1500–(3900) μm long. Rays usually composed of upright or square cells, sometimes in combination with procumbent cells. Silica in ray cells was found in most species studied (Fig. 2C, D) and additionally in the axial parenchyma of N. rafflesiana. Helical idioblasts scarcely present in the multiseriate rays of N. gymnamphora, N. khasiana, N. lowii, N. rafflesiana and N. rajah.
Fig. 1.
Wood anatomical sections of Nepenthaceae. Transverse light microscope sections (A, B, E, F) along with radial (C) and tangential (D) scanning electron microscopy surfaces of Nepenthes wood. (A) Nepenthes khasiana, mature stem (bark detached) showing wood with an indistinct growth ring (arrow). (B) Nepenthes muluensis, entire juvenile stem with pronounced cuticle (horizontal arrow) and lignified areas in both the outer stem area (cortex) and the inner stem part (wood and outer pith region); the vertical arrow points to the vascular bundle in the cortex. (C) Nepenthes tobaica, bordered, simple perforation plate with rim (arrow). (D) Nepenthes smilessi, alternate intervessel pits. (E) Nepenthes smilessi, tendency to form banded axial parenchyma (arrow). (F) Nepenthes edwardsiana, diffuse-in-aggregates axial parenchyma (arrow).
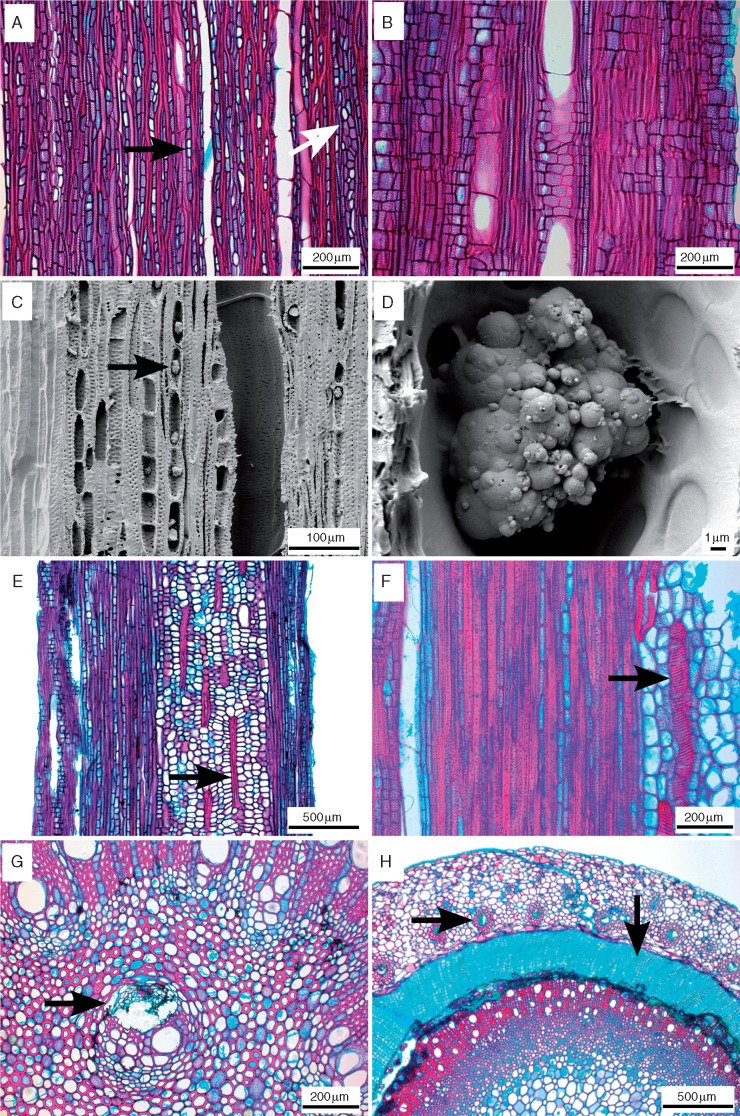
Fig. 2.
Light microscope sections of tangential (A), radial (B, E) and transverse (F, G, H) views, and scanning electron microscope images (C, D) of tangential surfaces of Nepenthes wood. (A) Nepenthes khasiana, overview showing dense uniseriate (black arrow) and narrow multiserate rays (white arrow). (B) Nepenthes gymnamphora, overview of rays with mainly square to upright ray cells. (C, D) Nepenthes ampullaria, abundant silica grains in ray cells (arrow). (E) Nepenthes reinwardtiana, thick-walled, helically banded sclereids within the pith (arrow). (F) Nepenthes burbidgeae, detail of thick-walled, helical idioblast in pith (arrow). (G) Nepenthes tobaica, medullary bundle (arrow). (H) Nepenthes ventricosa, cortical vascular bundles inside cortex (horizontal arrow), deep-seated periderm with cork cylinder (vertical arrow).
Stem parts outside the wood cylinder
Pith is composed of wider parenchyma cells in the centre, surrounded by an outer zone of more narrow, lignified cells. The level of pith lignification varies (Table 1). Nepenthes chaniana, N. madagascariensis, N. sanguinea and N. tentaculata are barely lignified with few, thin-walled lignified cells. The majority of species have either slight pith lignification with many thin-walled lignified cells (n = 14) or markedly lignified pith with thin- to thick-walled cells (n = 15). The latter cells are intermediate between parenchyma cells and fibres, and are usually septate. This intermediary cell type is also present in the four most markedly lignified, thick-walled pith cells of N. macfarlanei, N. muluensis, N. stenophylla and N. tobaica. Helically banded fibre-sclereids (Fig. 2E, F) are present in the pith in all species except N. bokorensis, N. edwardsiana, N. lamii and N. maxima. Medullary collateral bundles are present in the pith of N. burbidgeae, N. macfarlanei, N. pilosa, N. reinwardtiana, N. sanguinea, N. stenophylla, N. tobaica (Fig. 2G) and N. veitchii. Concentric amphivasal cortical bundles were present for N. ventricosa (Fig. 2H). Helical idioblasts were present in the cortex of all species for which we could section parts of the cortex (n = 14), and can be very thin- to very thick-walled, depending on the species. Silica grains were also observed in the secondary phloem of the species for which secondary phloem was sectioned. Crystal druses were found in pith cells of N. rhombicaulis.
For only one species, N. ventricosa, were we able to observe the deep-seated origin of the periderm, showing a pronounced cork cylinder (Fig. 2H); the outer part of the other samples that were available to us – except for the juvenile twig of N. muluensis (Fig. 1B) – was too much destroyed from the drying process to make sectioning possible. Therefore, we cannot state whether the deep-seated periderm formation is typical of the entire genus. Likewise, the presence of the outer lignified zone in the cortex in N. muluensis (Fig. 1B) cannot be generalized for Nepenthes as a whole.
Correlations with developmental stem stages, growth habit and abiotic preferences
Complete pairwise comparison data and results are presented in Supplementary Data Tables S4 and S5, with supported correlations described below. Juvenile wood specimens had higher pith lignification than mature specimens (r = 0·27, n = 39, P < 0·05) and had lower ray width (r = 0·29, n = 39, P < 0·05). Species referenced to grow on ultramafic soil had an average multiseriate ray height shorter than species not referenced to grow on this soil type (r = 0·31, n = 39, P < 0·05). Species with longer referenced stem lengths had larger multiseriate ray height maximums (r = 0·27, n = 39, P < 0·05). Maximum vessel diameter and ray width were greater when precipitation in the driest month of the year was higher (r = 0·27, n = 39, P < 0·05 and r = –0·26, n = 39, P < 0·05, respectively). Multiseriate ray height average and maximum were higher with greater annual precipitation (r = 0·28, n = 39, P < 0·05 and r = 0·30, n = 39, P < 0·05, respectively)
Reconstruction of wood and pith ancestral states
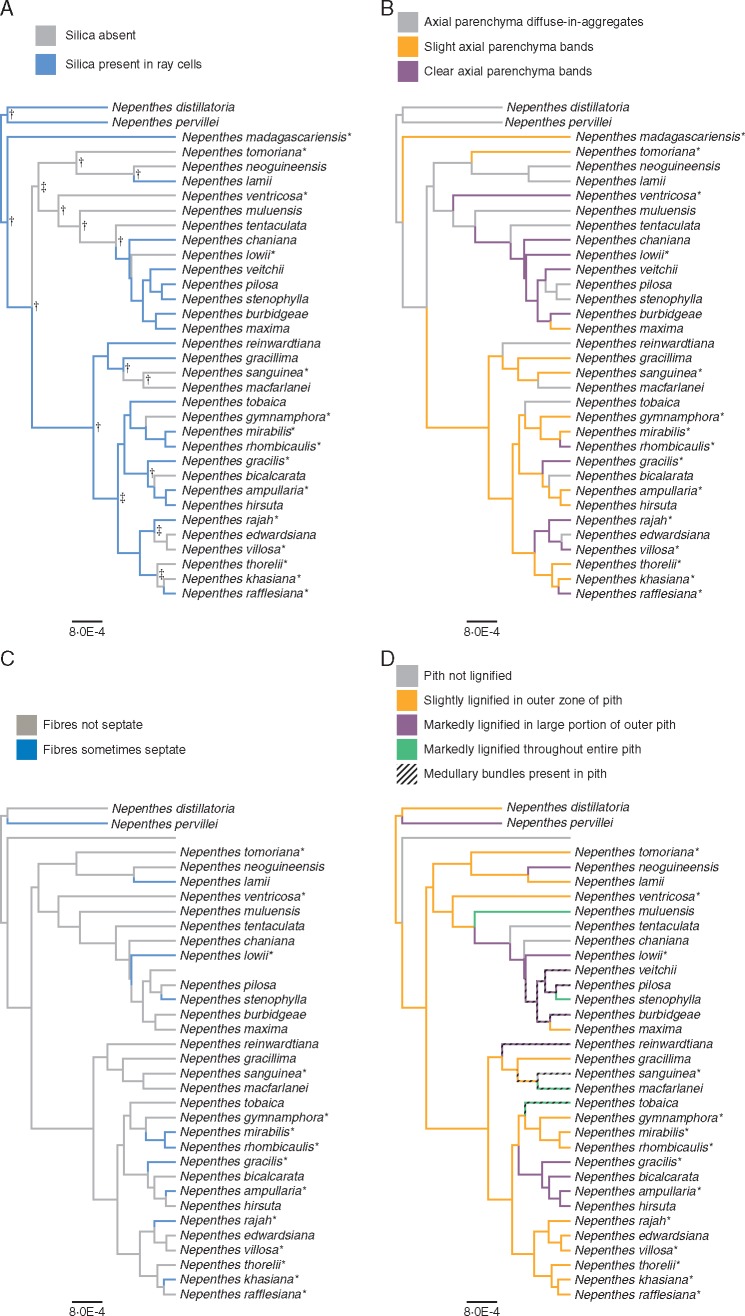
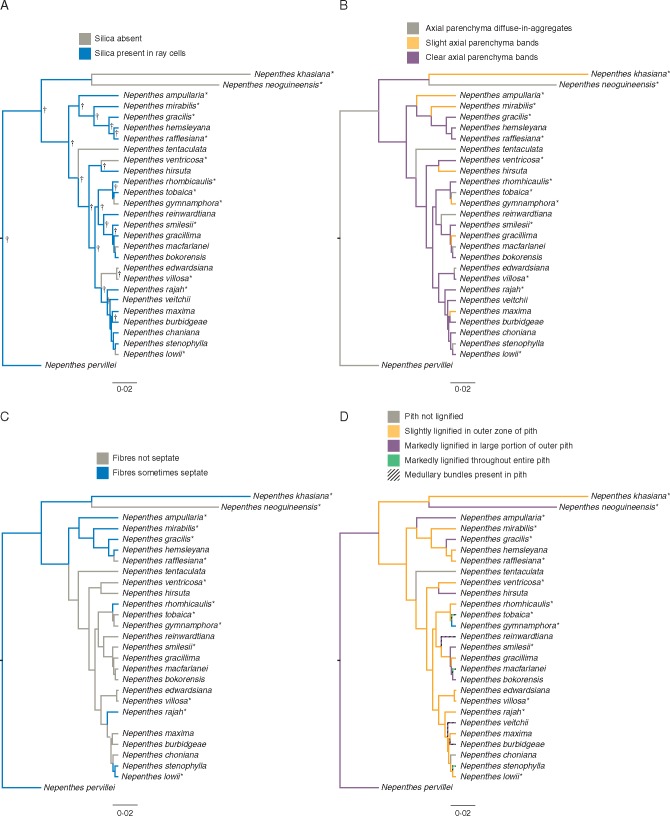
The wood and pith characters optimized on the Nepenthes phylogeny are presented in Figs 3 and 4. Posterior support values generated by the BEAST analyses are indicated on Figs 3 and 4 as icons when Bayesian posterior probabilities (bpps) were ≥0·80 and ≥0·90. Although the major bifurcations of Figs 3 and 4 are well supported, it should be noted that polytomies exist in the phylogeny of Nepenthes, and the resulting low phylogenetic resolution might affect interpretation of the evolution of particular character states. There is no single wood character that defines one entire sub-clade. Silica grains (Figs 3A and 4A), for example, are lost seven times throughout the trnK-matK phylogeny. Markedly lignified pith (Figs 3D and 4D) is present in a number of independent clades in both trnK-matK and ITS. Likewise, the presence of occasional septate fibres (Figs 3C and 4C) is scattered throughout the phylogeny. Seven of the eight species with medullary bundles also have a marked lignification of the pith (Figs 3D and 4D). Clear axial parenchyma bands (Figs 3B and 4B) and medullary bundle presence in the pith (Figs 3D and 4D) are derived features that evolved multiple times independently.
Fig. 3.
Wood and pith anatomical characters optimized on the empirical trees of the full Nepenthes trnK-matK produced in BEAST. Wood characters include (A) silica presence, (B) axial parenchyma distribution and (C) presence of septate fibres. The pith characters (D), lignification and medullary bundle presence, are combined in one map with black diagonal bands laid over lignification-keyed colour. Mature wood specimens are indicated with *. Posterior support values generated by BEAST analyses indicated for bpp ≥0·90 with † and for bpp threshold 0·80 with ‡. The scale bar is in units of substitutions/site.
Fig. 4.
Wood and pith anatomical characters optimized on the empirical trees of the full Nepenthes nrITS produced in BEAST. Wood characters include (A) silica presence, (B) axial parenchyma distribution and (C) presence of septate fibres. The pith characters (D), lignification and medullary bundle presence, are combined in one map with black diagonal bands laid over lignification-keyed colour. Mature wood specimens are indicated with *. Support values generated by BEAST analyses are indicated for threshold bpp ≥0·90 with †. The scale bar is in units of substitutions/site.
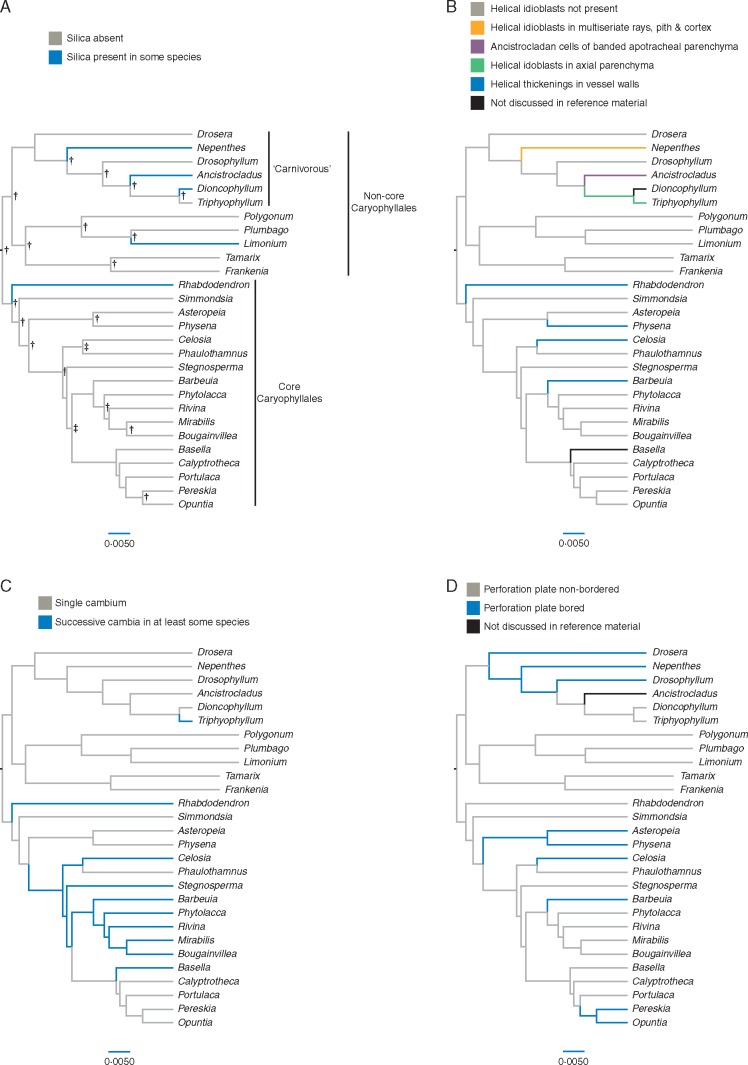
Character optimizations for a selection of woody genera in Caryophyllales are presented in Fig. 5. Posterior support values generated by the BEAST analyses are indicated on Fig. 5 as icons when bpps were ≥0·80 and ≥0·90. The most striking evolutionary trend is the diversity of helical sculpturing patterns in the carnivorous clade, with helical idioblasts in pith and cortex (and occasionally the rays) of Nepenthes (Fig. 5B). Other typical Nepenthes features, such as the presence of silica grains, have evolved convergently within the order (Fig. 5A). Successive cambia (Fig. 5C) and non-bordered vessel perforation plates (Fig. 5D) have evolved in numerous Caryophyllales families independently as well.
Fig. 5.
Four wood characters mapped on the Caryophyllales order sensuSoltis et al. (2011) with characters optimized on a maximum likelihood tree based on 19 genes from the plastid, nuclear and mitochondrial genomes produced in BEAST. Genera included in the mapping have woody species and referenced anatomical observations. The ‘carnivorous’ clade includes the non-carnivorous genera Ancistrocladus (which has ‘Ancistrocladan cells’; Carlquist, 2010) and Triphyophyllum. Support values generated by BEAST analyses are indicated for bpp ≥0·90 with †, and for bpp threshold 0·80 with ‡. The scale bar is in units of substitutions/site.
DISCUSSION
Wood anatomical diversity in Nepenthes
We present the most extensive wood anatomical survey of Nepenthes to date. The species sampled represent the full diversity in growth habit, ecology and phylogenetic position, providing a better understanding of the wood anatomical diversity in the genus (Table 1). Because of the strict conservation rules and monopodial growth habit for Nepenthes, we incorporated many juvenile specimens, but found that only pith lignification and maximum ray width were correlated with juvenility (Table S5).
Our observations confirm earlier wood descriptions by Metcalfe and Chalk (1950) and Carlquist (1981, 2010), with all species having diffuse porous wood with solitary vessels (Fig. 1A, B), simple, bordered perforation plates (Fig. 1C) and alternate intervessel pits of 5–7 μm (Fig. 1D). We also found dimorphic vessel elements with an equal number of longer, narrow vessel elements along with shorter and wider ones in the maceration slides (cf. Carlquist, 1981, 2010). Further, fibres have distinctly bordered pits in tangential and radial walls, and the axial parenchyma is diffuse in aggregates (Fig. 1F) with a tendency to form narrow bands (1–4 cells) (Fig. 1E) for most species, with exceptions of much wider bands in the mature wood samples of N. ampullaria (up to eight cells wide) and N. rafflesiana (up to ten cells wide). Rays are typically uniseriate and multiseriate (up to 14 cells wide; Fig. 2A) and consist of a combination of upright and square cells (Fig. 2B), although most juvenile samples only showed uniseriate rays.
More interestingly, we found helical idioblasts (cf. Carlquist, 2010) in all but four species investigated. These peculiar cells are mostly thin-walled or occasionally very thick-walled (Fig. 2E, F), and often occur in the pith, the cortex and rarely in multiseriate rays. Similar looking ‘spiral tracheids’ were noted previously only in the bark/cortex and tall rays of rhizomes (Heinricher, 1906) and leaves (Kny and Zimmerman, 1885; Carlquist, 1981, 2010). Furthermore, our extended study provides clear evidence for the presence of silica bodies in ray cells (Fig. 2C, D) and in the secondary phloem of most species analysed, although silica grains were previously only observed in N. alata (Carlquist, 2010). In addition, most species had some level of lignification in the pith (Figs 3D and 4D), with marked lignification occurring in a larger portion of the pith in the few remaining species. Medullary bundles (Fig. 2G) were present in the pith of eight species, often associated with the species having more lignified pith (Figs 3D and 4D). Furthermore, we found cortical vascular bundles in a ring-like arrangement surrounding the periderm producing a large phellem cylinder in N. ventricosa (Fig. 2H). In this species, the phellogen is initiated far inside the stem, but we cannot comment on whether this is a common feature for Nepenthes since the outer stem portions were often missing in our slides. Finally, we observe for the first time that fibres are occasionally septate in a number of species (Figs 3C and 4C).
Phylogenetic relevance of wood anatomical characters in Nepenthes and Caryophyllales
Silica bodies.
In the rays of 25 of the 39 Nepenthes species studied (Table 1), silica bodies were found; nine of these contained silica in huge quantities (Fig. 2C, D). Silica was not recorded in Carlquist’s (1981) initial wood study of Nepenthes, although he later reported grains in one species (Carlquist, 2010). We found a gain/loss pattern in the trait optimization of silica among species of Nepenthes (Figs 3A and 4A), which is probably related to the different edaphic conditions for which Nepenthes species have evolved to live (see the section on abiotic factors). Since silica occurs in only a limited number of flowering plant genera, it is considered to have high diagnostic value (Carlquist, 1988). Nevertheless, within our Caryophyllales analysis, the silica-bearing genera are widely scattered within the non-core group [Ancistrocladus (Gottwald and Parameswaran, 1968), Dioncophyllum (Gottwald and Parameswaran, 1968) and Nepenthes] and within the core group [Limonium (Carlquist and Boggs, 1996) and Rhabdodendrum (Carlquist, 2010)] (Fig. 5A). In addition to these, Carlquist (2003) records several additional families in the ‘non-core’ Polygonaceae that include silica in ray cells.
Helical idioblasts.
Helical thickenings in the cell walls of various types of idioblastic cells (Carlquist, 2010) appear to be characteristic of the carnivorous clade in Caryophyllales, for which Nepenthes is a typical example (Fig. 5B). Helical idioblasts, with either very thin lignified walls in a spiral arrangement or extremely thick lignified walls resembling fibre-sclereids (Fig. 2E, F) occur in the pith and cortex of nearly all Nepenthes species observed. Helical idioblasts were also occasionally found in multiseriate rays as well, although these were extremely scarce and in just a few species. The function of these peculiar cells remains unknown, but they have been associated with water storage (Kny and Zimmerman, 1885; Heinricher, 1906; Metcalfe and Chalk, 1950) or protection against insects or other predators (Carlquist, 2010). Similar idioblasts (but with ‘wide lumina’) have only been observed outside Nepenthes in the root cortex of the related genus Drosera (Oels, 1879). Ancistrocladus have idioblastic cells so unique that Carlquist (2010) coined them as ‘ancistrocladan cells’. These cells are a grouping of apotracheal parenchyma cells with banded walls that co-occur with normal axial parenchyma cells. In the same non-core clade, Triphyophyllum was reported to have helical idioblasts in the axial parenchyma (Gottwald and Parameswaran, 1968), but this was later discounted upon further investigation (Carlquist, 2010). Anacampseros, closely related to Portulacaceae and Cactaceae, also have helical idioblasts in the rays (Carlquist 2010). In summary, different types of helical idioblasts characterize the insectivorous clade of non-core Caryophyllales, but it must be stressed that these idioblasts have different ontogenetic pathways, so their homology is questionable. They are derived either from the vascular cambium (rays and axial parenchyma) or from the primary ground tissue (pith/cortex).
Single vs. successive cambia.
Our results show that single cambia are symplesiomorphic for Caryophyllales, from which acquisition of successive cambia was derived (Fig. 5C). Although this is in line with assumptions made in the past about this wood anatomical character (Rodman, 1994), it should be noted that short-lived plants might not acquire successive cambia because a single cambium provides sufficient support (Carlquist, 2010). Likewise, initiation of multiple cambia may favour the evolution from annual, herbaceous life forms to perennial, woody life forms. Since the shift from herbaceousness towards derived woodiness is characterized by massive convergent evolution (Lens et al., 2013a), it is not surprising that successive cambia have developed multiple times in Caryophyllales (Fig. 5C).
Perforation plates.
Like all Caryophyllales species, members of Nepenthes have simple perforation plates in their wood (Fig. 1C). Vestigial scalariform perforation plates in the primary xylem were observed by Carlquist (2010), who illustrated gyre tips of the primary xylem fringing the perforation plate. He also occasionally observed multiple perforations plates in Nepenthes wood, which we were unable to locate, and in Dionaea. The perforation plates of Nepenthes and its most closely related genera, Drosera and Drosophyllum, are clearly bordered (Fig. 5D). Of the families in our analyses, bordered perforation plates only occur in four other families; in Cactaceae (Pereskia and Opuntia; Carlquist 2010), Amaranthaceae (Celosia; Carlquist, 2003), Asteropeiaceae (Asteropeia; Carlquist, 2006) and in Physenaceae (Physena; Carlquist, 2006). The latter three families have a variable degree of minimally bordered to non-bordered perforation plates as well (Carlquist, 2010). Other Caryophyllales families with bordered perforation plates include Anacampserotaceae, Portulacaceae, Talinaceae, Montiaceae and some genera within Caryophyllaceae and Plumbaginaceae (Carlquist, 2010).
Influence of abiotic factors on wood anatomy
Wood anatomy is fairly conservative at the genus level (van den Oever et al., 1981; Noshiro and Baas, 2000; Lens et al., 2004), yet minor wood anatomical variation exists in widely dispersed genera covering diverse temperature and precipitation regimes, and these characters are usually associated with vessel adaptations such as vessel diameter and density, vessel element length and fine-scale intervessel pit characters (Carlquist, 1966, 1975; Baas, 1976; Lens et al., 2011, 2013b; Scholz et al., 2013). Since Nepenthes occupies a variable range of habitats, from coastal mangroves to mountain summits, and inhabits a wide spectrum of soil types, temperatures and precipitation, we investigated the influence of all these environmental factors on variation in stem anatomy.
Soil type.
For Nepenthes, soil type is one of the main factors involved in ecological preference (van der Ent et al., 2015; Schwallier et al., 2016). This is not surprising because carnivorous plants, such as Nepenthes, evolved alternative strategies for nutrient acquisition in environments where traditional resources from the soil were limited, giving them an advantage in such ecosystems. Such edaphically stressed environments include acidic kerangas (heath) and peat swamp forests on ultramafic bedrock. Ultramafic soil is extremely rich in iron, magnesium and nickel, but often poor in silica content (Brooks, 1988). Ultramafic soils are especially prevalent in the northern mountains of Malaysian Borneo (van der Ent et al., 2015), the southern Philippines, Sulawesi and other Nepenthes-inhabited islands of the Malay Archipelago. Absence of silica in some Nepenthes species could be explained in two ways. The most straightforward is a simple lack of soluble silica available in the soil where the plants were growing. A second possibility could be mechanisms blocking root uptake of silica (Parry and Kelso, 1977). We found no support for blockage of uptake of silica as our trait optimization displays an unlikely gain/loss pattern of such a scenario (Figs 3A and 4A). Interestingly, two ultramafic endemic species of Mount Kinabalu and Mount Tambuyukon, N. edwardsiana and N. villosa, lack silica in their ray cells. In N. burbidgeae, another species native to ultramafic soils, we observed abundant silica in a wood sample from the Sabah Parks Kinabalu Botanical Garden (i.e. not grown on ultramafic soil), while we could only find a small amount of silica grains in another sample collected in the wild grown on the ultramafic soil of Mount Kinabalu. This may suggest that all Nepenthes species have the ability to store silica in their wood as long as it is available in the soil. Similarly, silica was also present in the six greenhouse-grown specimens for which perlite was a component of the substrate (Table 1). Since perlite is largely made up of silicon dioxide, this would explain the availability of silica for uptake. Unfortunately, we could not trace whether the two other greenhouse-grown specimens that lack silica in their ray cells, N. kerrii and N. ventricosa, had perlite added to the soil medium. Our data, therefore, provide evidence of a possible link between edaphic factors (ultramafic bedrock) and wood anatomical variation (strongly reduced presence or even absence of silica in ray cells).
Precipitation.
Vessel maxima were wider when species lived in locations that received more precipitation (Supplementary Data Table S5). Also, multiseriate ray height (in both maximum and average measures) increased with increasing annual precipitation. Six of the species studied survive through seasonal drought stress in Cambodia, Sumatra and Thailand: N. bokorensis, N. kerrii, N. neoguineensis, N. smilesii, N. thorelii and N. tobaica (McPherson, 2009). Nepenthes bokorensis, N. smilesii and N. thorelii occur in exceptionally seasonably dry areas where the driest month averages only 20 mm, 5 mm and 6 mm of rain, respectively. We found that all of these species exposed to drought stress had pronounced pith lignification with often thick-walled lignified pith cells (Table 1). Nepenthes tobaica, for example, which grows in seasonably dry areas of Sumatra (McPherson, 2009) with a 3-fold average decrease in precipitation from the wettest to the driest month, shows marked lignification in the entire pith. Increased stem lignification may help to alleviate drought stress in avoiding water loss through the stems during drier periods (Lens et al., 2013b), which has also been found in grasses (Lens et al., 2016).
Although we were not able to section the outer stem parts for most of our samples, we observed that the periderm with a pronounced cork layer was initiated deep within the stem of N. ventricosa (Fig. 2H). Also, N. muluensis (Fig. 1B) shows a large lignified pith area, wood with thick fibre walls and a thick lignified layer at the outer part of the cortex, and a thick cuticle. The features of each of these two species could be alternative strategies to protect the stem during drought. In addition to this, half of the species studied had thick-walled fibres, reflecting a higher wood density. Although there is much noise/inconsistency in the relationship between wood density and environmental factors (Swenson and Enquist, 2007), several studies have found a link between increased wood density and increased drought stress resistance (Chave et al., 2006, 2009; Lens et al., 2013a, b).
Beyond the stem, leaves and roots probably play a role in drought tolerance in Nepenthes. Nepenthes pervillei, for example, develops long, pronounced roots (Adlassnig et al., 2005) to obtain water in its rocky cliff habitat (Juniper et al., 1989). In addition, two of our wild harvested Cambodian species, N. smilesii and N. thorelii, experience such severe drought in the dry season that their above-ground stem parts die off completely, relying on tuberous rootstock for regrowth when rain commences (McPherson, 2009; Mey, 2010). All of the drought-exposed Nepenthes species have relatively narrow leathery leaves to reduce evapotranspiration compared with more moist-living species (McPherson, 2012).
Most Nepenthes species, however, are regularly or even consistently exposed to wet conditions, especially the numerous higher altitude species (McPherson, 2009). Fossil and biogeographic evidence (Krutzsch, 1988; Meimberg et al., 2001) suggests that Nepenthes may have been able to occupy fairly moist ecological habitats for the duration of its evolutionary history, from the humid tropics of what is now France during the Eocene through its route to South-east Asia via the Middle East before it underwent aridification. This gives us good reason to believe that most Nepenthes species are not suited to withstand the stresses imposed from drier or drought conditions, especially if other features such as tuberous rootstock, stem lignification or leaf size and texture are not as adaptively developed as they are in the Cambodian species. From a conservation perspective, this is especially important given that Nepenthes will probably not track tolerable habitat boundaries fast enough to keep up with the sharply changing future climate (Schwallier et al., 2016).
The influence of growth habit on wood anatomy
The basic life forms of Nepenthes range from self-supporting rosette shrubs, to scramblers and woody climbers, with stems dramatically varying from just a few centimetres to > 20 m long (McPherson, 2009) (Supplementary Table S4). The mature wood anatomy of the lianoid Nepenthes species studied shares several characteristics with non-related lianoid lineages (Carlquist, 1989), including vessel dimorphism, simple perforation plates, abundant axial parenchyma and wide multiseriate rays (Table 1). We found that multiseriate rays were longer in taller lianas (Table S5), allowing them more flexibility. Another typical lianoid wood character is the presence of wide vessel diameters that can reach >200 μm in Marcgraviaceae, for example, and even 400 μm in Apocynaceae (Lens et al., 2005, 2008a). The mature wood samples representing all the vigorously climbing Nepenthes lianas (McPherson, 2009) in our study, however, had an average tangential vessel diameter of only 64 μm. The widest average vessels in our analysis were found in N. gymnamphora (104 μm, individuals growing up to 20 m) and N. veitchii (107 μm, individuals reaching up to 10 m; Table 1; McPherson, 2009). It is known that vessel widening is more pronounced towards the base of stems (Olson et al., 2014), justifying the exclusion of juvenile specimens in this comparison.
Our mature sample of N. rajah had the greatest wood production and stem diameter of all of the specimens sampled, with the extensive wood cylinder providing extra mechanical support for the plant. This species produces one of the most impressive pitcher traps in the genus, recorded to hold >3 L of water (Clarke and Wong, 1997). To accomodate this heavy trap, the plant itself is rather stout and self supporting, with a coinciding wood anatomy. Mechanical strength through pith lignification may compensate for the lack of sufficient support in juvenile stems. These younger stems have a broad pith area and narrow wood cylinder, but still need to carry heavy pitchers with their contents. The greenhouse-grown specimens investigated, which were artificially supported, had less rigidity and consequently more abundant parenchyma both inside and outside the wood cylinder, and more thin-walled fibres compared with wild-collected specimens. Underdeveloped fibres and abundant non-lignified parenchyma have previously been reported for greenhouse-grown lianas (Lens et al., 2008a). For our greenhouse specimens, it appears that the controlled environment (artificial support since seedling stage, lack of wind and other stresses including drought) influenced wood anatomy.
Other species display a marked intraspecific difference, illustrating nicely the impact of the environment on habit. In N. maxima, for example, distinct ecotypes have evolved in response to different environments. The most common form is a vigorous climbing stem up to 19 m long growing in heath or dipterocarp forests, which is very different from the reduced, diminutive form occurring in the seasonal dry savannahs of Central Sulawesi. There, the stems have a maximum self-supporting length of only 35 cm (McPherson, 2009). This shorter form additionally evolved waxy-edged leaves, which was also probably in response to the heated arid environment. The species N. lowii forms a compact rosette or short stem of only 1–2 m above the ground in exposed areas, because there is no need to produce a climbing stem to reach sunlight. In contrast, the forest ecotype of N. lowii is a vigorous climber of up to 10 m. In other words, collecting wood samples of Nepenthes in the field enables establishment of a more accurate link of the impact of growth habit and environment on the wood anatomy, which may significantly vary within Nepenthes, even at the species level.
Conclusions
With the pace of anthropogenic climate change necessitating urgent attention, focus on the links between ecology and the anatomical restrictions or pliability of plants that have deep-seated cultural, traditional and economic importance, such as Nepenthes, calls our attention. The wood anatomy of Nepenthes is generally rather uniform, but several stem anatomical adaptations in species facing drought stress or growing in ultramaphic substrate have been found. The omnipresence of helical idioblasts in the pith and cortex of Nepenthes represents a synapomorphy for the genus and supports its phylogenetic position within the carnivorous clade of Caryophyllales. Other typical Nepenthes characters, such as silica grains and bordered perforation plates, evolved convergently in different Caryophyllales lineages. The evidence on the conservative nature of most characters in our study suggests that it is unlikely that a rapid shift towards characters associated with drought stress resistance within Nepenthes, like pronounced lignification in the stems or deep root systems, will keep the pace needed in the progressively changing environmental future predicted by the Intergovernmental Panel on Climate Change (IPCC, 2014). In the Nepenthes habitat of South-east Asia, climate predictions include an increase in monsoon duration and intensity and conversely more drought exposure during the months of July–October (IPCC, 2013). Further investigation on drought stress resistance in the genus could include water transport measures in the xylem to estimate the pressure inducing 50 % loss of hydraulic conductivity (P50). In addition, minimum mid-day water potential measures (Psi min) can be performed to estimate levels of native embolism formation throughout the year in order to give an idea of the hydraulic safety margin (Psi min – P50; Choat et al., 2012). This is especially important for the high altitude species that normally thrive in very wet environments throughout the year, offering important conservation information for this iconic plant family.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Tables S1 and S2: Nepenthes specimen and NCBI GenBank accessions. Table S3: references assembled for Caryophyllales genera character optimizations. Table S4: referenced growth habit and ecology data used for pairwise comparisons. Table S5: calculations for growth habit and ecology data used for pairwise comparisons.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paul Kessler for granting us access to the plants of the Hortus botanicus of Leiden University. We acknowledge those at Sabah Parks and Naturalis Biodiversity Center, especially Menno Schiltzuizen, who organized the Mt. Kinabalu and Crocker Range expedition, which helped us to collect some important material for our study, and Rimi Repin and Rossiti Karim for facilitating permission to collect material in Sabah Parks. Permit #JHL(PB)600-3/18/1/1 Jld.10/(126) and #JKM/MBS. 1000-2/2 (180) enabled fieldwork in Malaysia. Specimens were acquired and stored at Naturalis Biodiversity Center in accordance with CITES exemption FF/75A/2015/036. We thank Jan Willem for his help in arranging this CITES material. We also thank Lena Struwe for hosting a month of laboratory space at Rutgers University. This work was supported by the Philanthropic Education Organization Sisterhood Scholar Award and multiple grants from the Alberta Mennega Foundation to R.S.
LITERATURE CITED
- Adlassnig W, Peroutka M, Lambers H, Lichtscheidl IK.. 2005. The roots of carnivorous plants. Plant and Soil 274: 127–140. [Google Scholar]
- Alamsyah F, Ito M.. 2013. Phylogenetic analysis of Nepenthaceae, based on interal transcribed spacer nuclear ribosomal DNA sequences. Acta Phytotaxonomica Geobotanica 64: 113–126. [Google Scholar]
- Albert VA, Williams SE, Chase MW.. 1992. Carnivorous plants: phylogeny and structural evolution. Science 257: 1491–1495. [DOI] [PubMed] [Google Scholar]
- Baas P. 1976. Some functional and adaptive aspects of vessel member morphology In: Baas P, Bolton A, Catling D, eds. Wood structure in biological and technological research. Leiden: Leiden Univeristy Press, 157–181. [Google Scholar]
- Baas P, Werker E, Fahn A.. 1983. Some ecological trends in vessel characters. IAWA Bulletin 4: 141–59. [Google Scholar]
- Baker HG. 1955. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution 9: 347–349. [Google Scholar]
- Bonhomme V, Gounand I, Alaux C, Jousselin E, Barth D, Gaume L.. 2011. The plant-ant Camponotus schmitzi helps its carnivorous host-plant Nepenthes bicalcarata to catch its prey. Journal of Tropical Ecology 27: 15–24. [Google Scholar]
- Brockington SF, Alexandre R, Ramdial J, et al. 2009. Phylogeny of the Caryophyllales sensu lato: revisiting hypotheses on pollination biology and perianth differentiation in the core Caryophyllales. International Journal of Plant Sciences 170: 627–643. [Google Scholar]
- Brooks R. 1988. Serpentine and its vegetation A multidisciplinary approach. Portland, OR: Dioscorides Press, Inc. [Google Scholar]
- Carlquist S. 1966. Wood anatomy of compositae: a summary, with comments on factors controlling wood evolution. Aliso 6: 25–44. [Google Scholar]
- Carlquist S. 1975. Wood anatomy and relationships of Lactoridaceae. American Journal of Botany 102: 128–134. [Google Scholar]
- Carlquist S. 1981. Wood anatomy of Nepenthaceae. Bulletin of the Torrey Botanical Club 108: 324–330. [Google Scholar]
- Carlquist S. 1984. Vessel grouping in dicotyledon wood: significance and relationship to imperforate trachery elements. Aliso 10: 505–525. [Google Scholar]
- Carlquist S. 1988. Comparative wood anatomy: systematic, ecological and evolutionary aspects of dicotyledon wood. Berlin: Springer-Verlag. [Google Scholar]
- Carlquist S. 1989. Anatomy of vine and liana stems: a review and synthesis In: Putz F, Mooney H, eds. The biology of vines. Cambridge: Cambridge University Press, 53–71. [Google Scholar]
- Carlquist S. 2003. Wood anatomy of Polygonaceae: analysis of a family with exceptional wood diversity. Botanical Journal of the Linnean Society 141: 25–51. [Google Scholar]
- Carlquist S. 2006. Asteropeia and Physena (Caryophyllales): a case study in comparative wood anatomy. Brittonia 58: 301–313. [Google Scholar]
- Carlquist S. 2010. Caryophyllales: a key group for understanding wood anatomy character states and their evolution. Botanical Journal of the Linnean Society 164: 342–393. [Google Scholar]
- Carlquist S, Boggs C.. 1996. Wood anatomy of Plumbaginaceae. Bulletin of The Torrey Botanical Club 123: 135–147. [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE.. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Chave J, Muller-Landau HC, Baker TR, Easdale TA, ter Steege H, Webb CO.. 2006. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecological Applications 16: 2356–2367. [DOI] [PubMed] [Google Scholar]
- Cheek M, Jebb M.. 2001. Nepenthaceae. Flora Malesiana 15: 1–157. [Google Scholar]
- Chin L, Moran JA, Clarke C.. 2010. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytologist 186: 461–70. [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491: 752–725. [DOI] [PubMed] [Google Scholar]
- Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA.. 2009. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biology letters 5: 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C, Wong KM.. 1997. Nepenthes of Borneo. Sabah: Natural History Publications in association with Science and Technology Unit, Sabah. [Google Scholar]
- Cuénoud P, Savolainen V, Chatrou LW, Powell M, Grayer RJ, Chase MW.. 2002. Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. American Journal of Botany 89: 132–144. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A.. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ent A, Sumail S, Clarke C.. 2015. Habitat differentiation of obligate ultramafic Nepenthes endemic to Mount Kinabalu and Mount Tambuyukon (Sabah, Malaysia). Plant Ecology 216: 789–807. [Google Scholar]
- Gernhard T. 2008. The conditioned reconstructed process. Journal of Theoretical Biology 253: 769–778. [DOI] [PubMed] [Google Scholar]
- Gottwald H, Parameswaran N.. 1968. Das sekundäre xylem und die systematische stellung der Ancistrocladaceae und Dioncophyllaceae. Botanisches Jahrbuch 88: 49–69. [Google Scholar]
- Greenwood M, Clarke C, Lee CC, Gunsalam A, Clarke RH.. 2011. A unique resource mutualism between the giant Bornean pitcher plant, Nepenthes rajah, and members of a small mammal community. PLoS One 6: e21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher E. 1906. Biologie von Nepenthes: speciell der Javanischen N. melamphora. Annals du Jardin de Buitenzorg 20: 277–298. [Google Scholar]
- Heled J, Drummond A.. 2008. Bayesian inference of population size history from multiple loci. BMC Evolutionary Biology 8: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubl G, Bringmann G, Meimberg H.. 2006. Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales – revisited. Plant Biology 8: 821–830. [DOI] [PubMed] [Google Scholar]
- IAWA Committee. 1989. IAWA list of microscopic features for hardwood identification. International Association of Wood Anatomists Bulletin, new series 10: 221–332. [Google Scholar]
- IPCC. 2013. IPCC summary for policymakers in climate change 2013: the physical science basis. Stockholm: Cambridge University Press. [Google Scholar]
- IPCC. 2014. Climate Change 2014: impacts, adaptation and vulnerability. In: Field C, Barros V, Dokken D, et al. , eds. Contribution of working group II to the fifth assessment report of the Intergovernmental Panal on Climate Change Cambridge and New York: Cambridge University Press. [Google Scholar]
- IUCN. 2015. The IUCN Red list of threatened species. Version 2014.3. http://www.iucnredlist.org/.
- Juniper B, Robins R, Joel DM.. 1989. The carnivorous plants. London: Academic Press. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidner C, Groover A, Thomas DC, Emelianova K, Soliz-Gamboa C, Lens F.. 2016. First steps in studying the origins of secondary woodiness in Begonia (Begoniaceae): combining anatomy, phylogenetics, and stem transcriptomics. Biological Journal of the Linnean Society 117: 121–138. [Google Scholar]
- Kny L, Zimmerman A.. 1885. Die bedeutung der spiralzellen von Nepenthes. Berichte der Deutschen Botanischen Gesellschaft 3: 123–128. [Google Scholar]
- Krutzsch W. 1988. Paleogeography and historical phytogeography (paleochorology) in the Neophyticum. Plant Systematics and Evolution 162: 5–61. [Google Scholar]
- Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 30: 3276–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Luteyn JL, Smets E, Jansen S.. 2004. Ecological trends in the wood anatomy of Vaccinioideae (Ericaceae s.l.). Flora 199: 309–319. [Google Scholar]
- Lens F, Dressler S, Jansen S, van Evelghem L, Smets E.. 2005. Within balsaminoid Ericales: a wood anatomical approach. American Journal of Botany 92: 941–953. [DOI] [PubMed] [Google Scholar]
- Lens F, Endress ME, Baas P, Jansen S, Smets E.. 2008a. Wood anatomy of Rauvolfioideae (Apocynaceae): a search for meaningful non-DNA characters at the tribal level. American Journal of Botany 95: 1199–1215. [DOI] [PubMed] [Google Scholar]
- Lens F, Sperry J, Christman M, Choat B, Rabaey D, Jansen S.. 2011. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist 190: 709–723. [DOI] [PubMed] [Google Scholar]
- Lens F, Davin N, Smets E, del Arco M.. 2013a. Insular woodiness on the Canary Islands: a remarkable case of convergent evolution. International Journal of Plant Sciences 174: 992-1013. [Google Scholar]
- Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S.. 2013b. Embolism resistance as a key mechanism to understand adaptive plant strategies. Current Opinion in Plant Biology 16: 287–292. [DOI] [PubMed] [Google Scholar]
- Lens F, Picon-Cochard C, Delmas CEL, et al. 2016. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiology 172: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR.. 2011. Mesquite: a modular system for evolutionary analysis Version 2.75. http//mesquiteproject.org.
- McPherson SR. 2009. Pitcher plants of the Old World Vol. 1 Redfern natural history. London: Redfern Natural History Productions Ltd. [Google Scholar]
- McPherson S. 2012. The new Nepenthes. London: Redfern Natural History Productions Ltd. [Google Scholar]
- Meimberg H, Heubl G.. 2006. Introduction of a nuclear marker for phylogenetic analysis of Nepenthaceae. Plant Biology 8: 831–40. [DOI] [PubMed] [Google Scholar]
- Meimberg H, Wistuba A, Dittrich P, Heubl G.. 2001. Molecular phylogeny of Nepenthaceae based on cladistic analysis of plastid trnK intron sequence data. Plant Biology 3: 164–175. [Google Scholar]
- Merckx VSFT, Hendriks K, Arumugam N, et al. 2015. Evolution of endemism on a young tropical mountain. Nature 524: 347–350. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Chalk L.. 1950. Anatomy of the dicotyledons, vol. 11 Oxford: Claredon Press. [Google Scholar]
- Mey FS. 2010. Introduction to the pitcher plants (Nepenthes) of Cambodia. Cambodian Journal of Natural History 2: 106–117. [Google Scholar]
- Miller M, Holder MT, Vos R, et al. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees CIPRES. https://www.phylo.org/.
- Moran JA, Clarke CM, Hawkins BJ.. 2003. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. International Journal of Plant Sciences 164: 635–639. [Google Scholar]
- Moran JA, Gray LK, Clarke C, Chin L.. 2013. Capture mechanism in Palaeotropical pitcher plants (Nepenthaceae) is constrained by climate. Annals of Botany 112: 1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshiro S, Baas P.. 2000. Trends in wood anatomy within species and genera: case study in Cornus s.l. (Cornaceae). American Journal of Botany 87: 1495–1506. [PubMed] [Google Scholar]
- Oels W. 1879. Vergleichende Anatomie der Droseraceen. Dissertation, University of Breslau. London: Liegnitz. [Google Scholar]
- van den Oever L, Baas P, Zandee M.. 1981. Comparative wood anatomy of Symplocos and latitude and altitude of provenance. IAWA Bulletin new series 2: 3–24. [Google Scholar]
- Olson ME, Anfodillo T, Rosell JA, et al. 2014. Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecology Letters 17: 988–997. [DOI] [PubMed] [Google Scholar]
- Pant DD, Bhatnagar S.. 1977. Morphological studies in Nepenthes (Nepenthaceae). Phytomorphology 27: 13–34. [Google Scholar]
- Parry D, Kelso M.. 1977. The ultrastructure and analytical microscopy of silicon deposits in the roots of Saccharum officinarum (L.). Annals of Botany 4: 855–862. [Google Scholar]
- Rambaut A, Suchard M, Xie D, Drummond A.. 2014. Tracer v1.6 http://beast.bio.ed.ac.uk/Tracer.
- Rembold K, Fischer E, Striffler BF, Barthlott W.. 2012. Crab spider association with the Malagasy pitcher plant Nepenthes madagascariensis. African Journal of Ecology 51: 188–191. [Google Scholar]
- Rodman J. 1994. Cladistic and phenetic studies In: Behnke H, Mabry T, eds. Caryphyllales. Berlin: Springer-Verlag, 279–301. [Google Scholar]
- Schäferhoff B, Müller KF, Borsch T.. 2009. Caryophyllales phylogenetics: disentangling Phytolaccaceae and Molluginaceae and description of Microteaceae as a new isolated family. Willdenowia – Annals of the Botanic Garden and Botanical Museum Berlin-Dahlem 39: 209–228. [Google Scholar]
- Scholz A, Rabaey D, Stein A, Cochard H, Smets E, Jansen S.. 2013. The evolution and function of vessel and pit characters with respect to cavitation resistance across 10 Prunus species. Tree Physiology 33: 684–694. [DOI] [PubMed] [Google Scholar]
- Schwallier R, Raes N, de Boer H, Vos R, van Vugt R, Gravendeel B.. 2016. Phylogenetic analysis of niche divergence reveals distinct evolutionary histories and climate implications for tropical carnivorous plants. Diversity and Distributions 22: 97–110. [Google Scholar]
- Shaw RG, Etterson JR.. 2012. Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytologist 195: 752–765. [DOI] [PubMed] [Google Scholar]
- Solereder H. 1908. Systamatic anatomy of the dicotyledons: a handbook for laboratories of pure and applied botany. Oxford: Claredon Press. [Google Scholar]
- Soltis DE, Smith SA, Cellinese N, et al. 2011. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98: 704–730. [DOI] [PubMed] [Google Scholar]
- Swenson N, Enquist B.. 2007. Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. American Journal of Botany 94: 451–459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.