Abstract
Background and Aims This study investigates the structural diversity of the secondary xylem of 54 species of Acacia from four taxonomic sections collected across five climate regions along a 1200 km E–W transect from sub-tropical [approx. 1400 mm mean annual precipitation (MAP)] to arid (approx. 240 mm MAP) in New South Wales, Australia. Acacia sensu stricto (s.s.) is a critical group for understanding the effect of climate and phylogeny on the functional anatomy of wood.
Methods Wood samples were sectioned in transverse, tangential and radial planes for light microscopy and analysis.
Key Results The wood usually has thick-walled vessels and fibres, paratracheal parenchyma and uniseriate and biseriate rays, occasionally up to four cells wide. The greater abundance of gelatinous fibres in arid and semi-arid species may have ecological significance. Prismatic crystals in chambered fibres and axial parenchyma increased in abundance in semi-arid and arid species. Whereas vessel diameter showed only a small decrease from the sub-tropical to the arid region, there was a significant 2-fold increase in vessel frequency and a consequent 3-fold decrease in the vulnerability index.
Conclusions Although the underlying phylogeny determines the qualitative wood structure, climate has a significant influence on the functional wood anatomy of Acacia s.s., which is an ideal genus to study the effect of these factors.
Keywords: Acacia Mill. s.s., crystals, parenchyma, vessels, rays, fibres, climate, vulnerability index
INTRODUCTION
The redefined genus Acacia Mill. sensu stricto (s.s.) formerly Acacia subg. Phyllodineae DC. (syn. Racosperma Mart.), is the second largest genus in the Leguminosae and the largest of the subfamily Mimosoideae. With recent advances in legume systematics and following an extensive debate (Smith and Figueiredo, 2011; Thiele et al., 2011; Miller and Seigler, 2012; Kyalangalilwa et al., 2013), Acacia sensu lato has been divided into a number of genera, and the genus Acacia s.s. now consists primarily of Australian species. Comprising an estimated 986–1045 species (when including undescribed taxa), Acacia s.s. is the largest genus of woody species on the Australian continent (Lewis, 2005; Murphy et al., 2010; Thiele et al., 2011).
As with many genera in the Leguminosae, Acacia has the ability to fix atmospheric nitrogen symbiotically via rhizobium root-nodule bacteria, thus contributing to nitrogen capital in many natural Australian ecosystems (Brockwell et al., 2005; Thrall et al., 2007). Australian aboriginal peoples utilized all parts of the plant for a range of purposes including food (Cribb and Cribb, 1987; Low, 1991; Latz, 1995), tools (Clarke, 2012) and medicine (Clarke, 2008, 2012). A number of Australian Acacia species are used worldwide for timber, dyes, adhesives, paper pulp, food, forage and as ornamental and garden plants (McDonald et al., 2001; Midgley and Turnbull, 2003).
The species of Acacia s.s. (hereafter referred to as Acacia) are distributed across the continent of Australia and form the dominant element of many vegetation types. Growing across a broad range of climates and edaphic environments, Acacia is morphologically heterogeneous, with a wide diversity of growth form, vegetative and floral morphology, and anatomy (Boughton, 1986, 1990; Whinder et al., 2013). Climate and particularly precipitation have been identified as the major factors determining the broad geographic patterns of distribution and abundance of the species of Acacia (Hnatiuk and Maslin, 1988; Maslin and Pedley, 1988). Foliar nervation in Acacia was observed to be strongly influenced by climate, with microneurous phyllodes (numerous, close and parallel) abundant in arid locations and oligoneurous (few, distant) in mesic environments. In addition, an increase in phyllode sclerophylly in particular was noted for arid rather than more mesic environments (Sommerville et al., 2012).
Acacia s.s. has been grouped into seven taxonomic sections based on phyllode presence and venation, and floral morphology (Pedley, 1978). The majority of species are included in only four sections: Botrycephalae, Juliflorae, Phyllodineae and Plurinerves (Pedley, 1978). Phyllodes take on the photosynthetic role of leaves in all sections, with the exception of the Botrycephalae, which possess bipinnate leaves (Gardner et al., 2008). Recent phylogenetic work has suggested that Pedley’s sections are artificial (Miller et al., 2003; Murphy et al., 2003, 2010). However, there is genetic support for the sections, with species assigned to Botrycephalae grouped in a single clade, and other clades consisting of an obvious majority of one section (Murphy et al., 2010). Sections Juliflorae and Plurinerves reflect these groupings through habitat and adaptations to climate, having been shown to be more xeromorphic than the Phyllodineae in a study of phyllode morphology (Boughton, 1986). Pending the creation of a comprehensive phylogeny and practical methods for identifying members of each major clade, the sections described by Pedley (1978) remain in popular use by botanists and are used here.
Diversity within wood anatomy is a product of the requirement for mechanical support of above-ground tissue, the need for water, carbon and nutrient storage and water transport (Chave et al., 2009), and selective pressure in the environment. Particularly important components of the environment are precipitation and temperature, because tracheary elements have a crucial role in water transport from roots to leaves (Carlquist, 1975; Baas, 1976; Scholz et al. 2013). These effects are most often apparent at family and genus rather than species level (Bailey, 1966; Baas, 1976; Baas et al., 1983), but the large number of species of Acacia lends itself to an intrageneric study. Trees and shrubs of Acacia populate a wide range of habitats, with precipitation and temperature regimes varying from arid, semi-arid, alpine, temperate and sub-tropical to tropical. The impact of climatic and edaphic factors upon Acacia wood micromorphology, particularly quantitative vessel characteristics, should be more apparent than in genera from more uniform habitats and will provide broader support for Carlquist’s hypothesis on climatological effects on anatomy (Carlquist, 2001). A significant climatic and latitudinal relationship within genera from other orders has been reported by Noshiro and Baas (2000). In addition, Kribs (1935, 1937) and Baretta-Kuipers (1981) have shown that specialization to climate in the Leguminosae, and the eudicotyledons in general, also affects wood characters other than those of the vessels. The climatic range for families, genera or species and absence of specific climatic data are often limiting and problematic in wood studies (Carlquist, 1977, 1982), but the wide distribution and large number of species of Acacia help to overcome some of these limitations.
Many past publications on Acacia wood anatomy have been on species from Africa which are now not included in the genus and known to be distantly related. Much of the earlier work on the Acacia ‘genus’ now relates to other genera such as Acaciella Britton & Rose, Senegalia Raf., Vachellia Wright & Arn. and Mariosousa Seigler & Ebinger (Maslin, 2008; Kyalangalilwa et al., 2013). Ford (1984) carried out a preliminary study of Australian Acacia wood anatomy, limiting his work to the vessel characteristics of 15 species. More comprehensive studies by Baas et al. (2004) found support for ecological trends in vessel characters as generalized by Ford (1984). Whinder et al. (2013) pursued this theme with 12 temperate Acacia species from New South Wales, and included vessel, fibre, axial parenchyma, ray and crystal characteristics in their study. The other main ecological wood anatomy study of Australian Acacia melanoxylon R.Br., by Wilkins and Papassotiriou (1989), compared a wider range of characteristics with latitude and the associated general climate, with limited samples allowing for only broad conclusions to be drawn.
Recent studies have been made on the influence of climate on Acacia phyllode–invertebrate interactions (Bairstow et al., 2010), phyllode anatomy (Sommerville et al., 2012) and calcium oxalate accumulation (Brown et al., 2013). Unlike Acacia, Eucalyptus wood anatomy has been studied extensively in the past (Baker, 1919; Dadswell et al., 1934) due to a greater economic significance as timber and a source of paper fibres. Despite the ecological significance of Acacia on the Australian continent, the genus has received relatively little attention from wood anatomists except as part of broader anatomical studies (Dadswell and Eckersley, 1935; Evans et al., 2006).
Here we assess the wood anatomy of 54 species in the four main taxonomic groups (sections) of Acacia s.s. along a climate gradient of increasing temperature and decreasing precipitation from sub-tropical forest to arid desert, consisting of a transect from east to west of >1200 km across New South Wales, Australia. The four taxonomic sections of Acacia are representative of the entire genus. The climate regions in New South Wales based on the Köppen–Geiger classification (Stern et al., 2000) are representative of large areas of the continent. Furthermore, Hnatiuk and Maslin (1988) identified New South Wales as a centre of species richness for Acacia s.s.
We hypothesize that there are clear climatic and phylogenetic influences on a selected range of qualitative and quantitative wood characters, previously shown to vary in more limited studies. Trends in wood anatomy based on taxonomic section will also provide some insight into the systematic relevance of Pedley’s (1978) classification of Acacia.
MATERIALS AND METHODS
Collection sites
New South Wales, Australia, holds the majority of the eastern centre of Acacia species richness, surpassed only by Western Australia (Maslin, 1997). A wide spectrum of climates exists in the state owing to continental, orographic and coastal effects, making the region suitable for the sampling requirements of this study. Samples were collected from trees and shrubs in their natural habitats, across five different climate regions (Fig. 1). These regions range from xeric to mesic, and were grouped (with corresponding modified Köppen–Geiger classification) as: arid [desert – hot (persistently dry)]; semi-arid I [grassland – warm (persistently dry)]; semi-arid II ([grassland – hot (summer drought)]; temperate [temperate – no dry season (warm–hot summer)]; and sub-tropical (sub-tropical – no dry season) (Stern et al., 2000) (Table 1). Sample discs from usually three mature individuals of each species (two discs for six species) were taken close to the ground from either the single main stem or one of several vertical branches where basal branching was a feature, and were placed in 70 % ethanol.
Fig. 1.
Mean annual precipitation (MAP) (mm) map of New South Wales, Australia with collection sites (open circles) (modified from Bureau of Meteorology, Australia, 2011).
Table 1.
Average altitude (m a.s.l.), monthly mean minimum and maximum temperature (°C) and mean annual precipitation (MAP) (mm) for the five climate regions
| Climate region | Altitude (m a.s.l.) | Mean annual minimum temperature (°C) | Mean annual maximum temperature (°C) | MAP (mm) |
|---|---|---|---|---|
| Arid | 170 | 5 | 35 | 237 |
| Semi-arid I | 186 | 4 | 33 | 392 |
| Semi-arid II | 347 | 3 | 33 | 653 |
| Temperate | 676 | 2 | 27 | 954 |
| Sub-tropical | 61 | 8 | 28 | 1436 |
Where possible, the species sampled were evenly selected from the four taxonomic sections of Acacia, the Botrycephalae, Juliflorae, Phyllodineae and Plurinerves (Table 2). Full and even coverage of each taxonomic section within each climate region was not achieved, mainly because the more mesic Botrycephalae is scarce in the semi-arid I region, and absent from the arid region, where there are also fewer species from the other three sections (Maslin and Pedley, 1988).
Table 2.
Climate region, taxonomic section and site details for 54 species of Acacia s.s
| Climate region – section | Species | Plant form; height (m) | Stem diameter ± s.e. (mm) | Latitude | Longitude | Altitude (m a.s.l.) | Monthly min./max. temp. (°C) | MAP (mm) |
|---|---|---|---|---|---|---|---|---|
| Arid | ||||||||
| Juliflorae | A. aneura F. Muell. ex Benth. | Small tree; 3–5 | 36·0 ± 0·0 | 29°28′14·5′′S | 141°58′58·4′′E | 191 | 5/36 | 225 |
| Phyllodineae | A. tetragonophylla F. Muell. | Upright shrub; 2–3 | 25·5 ± 3·5 | 29°29′02·8′′S | 141°58′58·4′′E | 191 | 5/36 | 225 |
| A. victoriae Benth. | Small tree; 3–5 | 46·0 ± 0·0 | 29°29′02·8′′S | 141°58′58·4′′E | 191 | 5/36 | 225 | |
| Plurinerves | A. cambagei R.T. Baker | Small tree; 3–5 | 31·5 ± 1·5 | 29°37′40·8′′S | 141°52′18·5′′E | 185 | 5/36 | 227 |
| A. cana Maiden | Upright shrub; 2–3 | 25·5 ± 3·5 | 33°18′37·4′′S | 142°53′07·8′′E | 78 | 4/33 | 290 | |
| A. loderi Maiden | Small spreading tree; 3–5 | 26·0 ± 4·0 | 29°37′40·8′′S | 141°52′18·5′′E | 185 | 5/36 | 227 | |
| Semi-arid I | ||||||||
| Botrycephalae | A. deanei subsp. deanei (R.T. Baker) M.B. Welch et al. | Sapling; 3–5 | 111·7 ± 12·4 | 32°38′01·3′′S | 145°35′24·7′′E | 221 | 4/33 | 389 |
| Juliflorae | A. aneura F. Muell. ex Benth. | Small tree; 3–5 | 68·3 ± 14·1 | 32°35′21·5′′S | 145°31′12·0′′E | 221 | 4/33 | 389 |
| A. burkittii F. Muell. ex Benth. | Spreading multistemmed shrub; 2 | 20·0 ± 1·5 | 32°53′53·9′′S | 145°43′28·2′′E | 162 | 4/33 | 394 | |
| A. triptera Benth. | Multistemmed spreading shrub; 2 | 17·7 ± 0·9 | 32°54′21·6′′S | 145°43′33·2′′E | 162 | 4/33 | 394 | |
| Phyllodineae | A. brachybotrya Benth. | Upright shrub; 2–3 | 15·0 ± 0·0 | 32°54′03·2′′S | 145°43′29·3′′E | 162 | 4/33 | 394 |
| A. conferta A. Cunn. ex Benth. | Upright shrub; 1–2 | 17·7 ± 0·9 | 32°56′20·8′′S | 145°42′13·0′′E | 162 | 4/33 | 394 | |
| A. hakeoides A. Cunn. ex Benth. | Small tree; 2–3 | 47·7 ± 3·3 | 32°35′39·1′′S | 145°31′39·0′′E | 221 | 4/33 | 389 | |
| Plurinerves | A. colletioides Benth. | Spreading multistemmed shrub; 2–3 | 21·7 ± 3·5 | 32°35′39·1′′S | 145°31′39·0′′E | 221 | 4/33 | 389 |
| A. havilandiorum Maiden | Upright shrub; 1·0–1·5 | 15·0 ± 1·0 | 32°53′53·9′′S | 145°43′28·2′′E | 162 | 4/33 | 394 | |
| A. wilhelmiana F. Muell. | Upright shrub; 0·5–1·0 | 10·8 ± 1·0 | 32°56′16·1′′S | 145°44′20·8′′E | 162 | 4/33 | 394 | |
| Semi-arid II | ||||||||
| Botrycephalae | A. deanei subsp. deanei (R.T. Baker) M.B. Welch et al. | Sapling; 2–3 | 24·5 ± 0·5 | 30°45′15·8′′S | 149°6′22·0′′E | 261 | 3/33 | 622 |
| A. mearnsii De Willd. | Sapling; 2–3 | 39·0 ± 3·2 | 31°17′49·9′′S | 149°00′28·4′′E | 654 | 1/31 | 789 | |
| A. polybotrya Benth. | Sapling; 2–3 | 37·3 ± 1·8 | 30°51′04·3′′S | 149°27′32·4′′E | 412 | 2/32 | 713 | |
| A. spectabilis A. Cunn. ex Benth. | Single-stemmed shrub; 1·0–2·0 | 17·8 ± 3·6 | 30°47′07·1′′S | 148°58′40·1′′E | 239 | 3/34 | 595 | |
| Juliflorae | A. carolae Pedley | Upright shrub; 1–2 | 16·7 ± 0·4 | 30°51′56·9′′S | 149°32′27·6′′E | 404 | 2/32 | 710 |
| A. cheelii Blakely | Sapling; 2–3 | 56·7 ± 6·9 | 31°16′34·3′′S | 148°58′05·5′′E | 508 | 2/32 | 699 | |
| A. doratoxylon A. Cunn. | Upright shrub; 1–2 | 17·0 ± 0·6 | 30°51′12·2′′S | 149°28′47·3′′E | 412 | 2/32 | 713 | |
| Phyllodineae | A. dorothea Maiden | Sapling; 2–3 | 36·3 ± 8·4 | 31°17′20·0′′S | 149°03′25·9′′E | 654 | 1/31 | 789 |
| A. pravifolia F. Muell. | Low spreading multistemmed shrub; 0·2–0·5 | 4·5 ± 0·8 | 30°51′14·4′′S | 149°28′47·3′′E | 412 | 2/32 | 713 | |
| A. uncinata Lindl. | Upright shrub; 1–2 | 28·0 ± 3·6 | 30°47′07·1′′S | 148°58′40·1′′E | 239 | 3/34 | 595 | |
| Plurinerves | A. harpophylla F. Muell. ex Benth. | Sapling; 4–5 | 68·3 ± 2·0 | 30°24′38·9′′S | 149°00′00·0′′E | 174 | 4/34 | 583 |
| A. homalophylla A. Cunn. ex Benth. | Small tree; 2–4 | 27·5 ± 0·3 | 30°29′58·9′′S | 148°45′45·7′′E | 160 | 4/34 | 547 | |
| A. montana Benth. | Upright to spreading shrub; 1–2 | 13·0 ± 0·6 | 31°36′45·4′′S | 148°48′24·5′′E | 316 | 3/32 | 609 | |
| A. pendula A. Cunn. ex Benth. | Tree; 4–5 | 29·0 ± 0·0 | 30°23′22·6′′S | 148°41′18·6′′E | 160 | 4/34 | 547 | |
| A. stenophylla A. Cunn. ex Benth. | Tree; 4–5 | 33·6 ± 2·3 | 30°22′19·9′′S | 148°41′41·3′′E | 160 | 4/34 | 547 | |
| A venulosa Benth. | Tall shrub; 2–3 | 29·0 ± 1·0 | 30°45′15·8′′S | 149°06′22·0′′E | 261 | 3/33 | 622 | |
| Temperate | ||||||||
| Botrycephalae | A. dealbata Link. | Sapling; 2 | 13·5 ± 2·3 | 30°19′17·8′′S | 151°41′20·0′′E | 1290 | 0/24 | 877 |
| A. filicifolia Cheel. & M.B. Welch | Sapling; 2–3 | 20·7 ± 6·8 | 30°34′35·4′′S | 151°42′41·8′′E | 983 | 0/26 | 803 | |
| A. leucoclada Tindale | Sapling: 2 | 11·3 ± 2·9 | 29°53′39·5′′S | 151°08′18·6′′E | 752 | 0/29 | 811 | |
| Juliflorae | A. blakei Pedley | Sapling; 1–2 | 16·3 ± 5·4 | 30°39′58·7′′S | 151°56′02·4′′E | 903 | 1/26 | 854 |
| A. floribunda (Vent.) Willd. | Sapling; 2–3 | 27·0 ± 2·1 | 32°10′01·2′′S | 152°01′28·2′′E | 277 | 5/27 | 1256 | |
| A. longifolia (Andrews) Willd. | Upright shrub; 2 | 21·7 ± 5·4 | 32°41′46·3′′S | 151°52′17·4′′E | 17 | 7/27 | 1175 | |
| Phyllodineae | A. falcata Willd. | Upright shrub; 2 | 8·5 ± 0·5 | 32°38′16·4′′S | 151°50′12·8′′E | 60 | 6/27 | 1170 |
| A. flexifolia A. Cunn. ex Benth. | Multistemmed spreading shrub; 0·5–1·0 | 6·7 ± 0·9 | 30°24′38·9′′S | 151°00′00·0′′E | 872 | −1/28 | 809 | |
| A. neriifolia A. Cunn. ex Benth. | Sapling; 2–3 | 19·7 ± 6·7 | 30°29′07·1′′S | 151°28′37·9′′E | 981 | 0/27 | 789 | |
| Plurinerves | A. dawsonii R.T. Baker | Upright shrub; 1·0 | 6·5 ± 1·3 | 30°24′38·9′′S | 151° 0′ 00·0′′E | 872 | −1/28 | 809 |
| A. implexa Benth. | Sapling, tree; 2–3 | 22·0 ± 2·0 | 32°10′01·2′′S | 152°01′28·2′′E | 277 | 5/27 | 1256 | |
| A. viscidula Benth. | Upright shrub; 1·0 | 7·7 ± 0·7 | 32°10′01·2′′S | 152°01′28·2′′E | 830 | 0/28 | 837 | |
| Sub-tropical | ||||||||
| Botrycephalae | A. irrorata subsp. velutinella Tindale | Sapling; 2–3 | 28·0 ± 0·6 | 29°19′54·5′′S | 153°14′13·9′′E | 44 | 8/28 | 1410 |
| A. oshanesii F. Muell. Maiden | Sapling; 3–4 | 33·7 ± 4·2 | 29°57′09·4′′S | 153°08′12·1′′E | 94 | 7/28 | 1487 | |
| A. terminalis Salisb. | Upright shrub; 1–2 | 9·5 ± 1·8 | 29°56′19·7′′S | 153°09′36·7′′E | 94 | 7/28 | 1487 | |
| Juliflorae | A. aulacocarpa A. Cunn. ex Benth. | Sapling; 3–4 | 43·0 ± 2·6 | 29°19′50·2′′S | 153°14′15·7′′E | 44 | 8/28 | 1410 |
| A. concurrens Pedley | Sapling; 3–4 | 38·0 ± 2·3 | 29°19′50·2′′S | 153°14′15·7′′E | 44 | 8/28 | 1410 | |
| A. leiocalyx (Domin) Pedley | Sapling; 3–4 | 34·0 ± 1·5 | 29°19′50·2′′S | 153°14′15·7′′E | 44 | 8/28 | 1410 | |
| Phyllodineae | A. fimbriata A. Cunn. ex Benth. | Upright tall shrub; 3–4 | 22·7 ± 4·8 | 29°56′24·4′′S | 153°09′09·4′′E | 94 | 7/28 | 1487 |
| A. myrtifolia (Sm.) Willd. | Upright shrub; 1–2 | 14·2 ± 1·6 | 29°18′45·7′′S | 153°14′53·5′′E | 44 | 8/28 | 1410 | |
| A. suaveolens (Sm.) Willd. | Upright shrub; 1–2 | 8·2 ± 0·9 | 29°18′15·1′′S | 153°14′59·3′′E | 44 | 8/28 | 1410 | |
| Plurinerves | A. baeuerlenii Maiden & R.T. Baker | Upright shrub; 1–2 | 6·3 ± 0·3 | 29°18′45·7′′S | 153°14′53·5′′E | 44 | 8/28 | 1410 |
| A. complanata A. Cunn. ex Benth. | Upright shrub; 2–3 | 25·0 ± 2·1 | 29°56′19·7′′S | 153°09′36·7′′E | 94 | 7/28 | 1487 | |
| A. elongata Siber ex DC. | Upright shrub; 1–2 | 12·0 ± 1·9 | 29°19′50·2′′S | 153°14′15·7′′E | 44 | 8/28 | 1410 |
Mean annual precipitation (MAP) ranged from 237 mm in the arid region to 1436 mm in the sub-tropical region (Table 1). The five climate regions ranged in monthly mean minimum temperature from 2 ºC in the temperate region to 8 ºC in the sub-tropical region and in monthly mean maximum temperature from 28 ºC in the temperate region to 35 ºC in the arid region.
The altitude of collection ranged from 44–94 m above sea level (a.s.l.) in the sub-tropical region, 78–191 m a.s.l. in the arid region, 162–221 m a.s.l. in the semi-arid I region, 261–654 m a.s.l. in the semi-arid II region and from 17 to a maximum of 1290 m a.s.l. in the temperate region (Table 2). A total of 165 specimens from 54 species were collected from five contiguous climate regions.
Slide preparation
At the Jodrell Laboratory at Kew, blocks of approx. 1 cm3, or the whole stem if a similar size, were cut from each sample and boiled in water for a period of time to soften. Transverse (TS), tangential longitudinal (TLS) and radial longitudinal (RLS) sections were cut to 20–30 μm thick using a Reichert sliding microtome (C Reichert Optische Werke AG, Vienna, Austria). These sections were stained in Safranin (1 % in 50 % ethanol) for 2 min and Alcian Blue (1 % aqueous) for 1 min, rinsed in distilled water, then dehydrated using a graded series of 50, 70, 95 and 100 % ethanol. Final rinses were made in Histoclear (National Diagnostics, Hull, UK) prior to permanent mounting onto slides in Euparal (Fisher Scientific, Loughborough, UK). Slides were cured in an oven at 60 °C for 12 weeks. These permanent wood sections are held in the reference microscope-slide collection in the Jodrell Laboratory, Royal Botanic Gardens, Kew, UK.
Characters studied
The characters investigated were chosen based on the IAWA List (Wheeler et al., 1989). In certain cases, these characters have been adapted to allow more quantitative information to be obtained, following the methods set out in Gasson et al. (2010). Further modification has been made to ensure comparability of measurements from each sectioned sample.
Quantitative characters.
(1) Vessel diameter at the widest tangential point (μm) – 25 measurements per specimen, from the fourth growth ring, TS; (2) vessel frequency (mm–2) – five measurements per specimen, from the fourth growth ring, TS; (3) ray frequency (mm–1) – five measurements per specimen, from the fourth growth ring, TS; (4) ray height (cells) – 25 measurements per specimen, TLS; (5) ray width (cells) – 25 measurements per specimen, TLS.
Qualitative characters.
Five characters were found to vary widely across the species and climate regions, and are considered in this study: (1) vessel grouping; (2) paratracheal axial parenchyma pattern; (3) fibre wall thickness; (4) ray width; and (5) prismatic crystal abundance and location.
Four other qualitative characters, distinctness of growth ring boundaries, porosity, presence of apotracheal parenchyma and ray composition, varied so little they were not used.
Data collection
Qualitative data for vessels were collected from the TS, whereas rays were observed using TLS at × 100 magnification with a Leica light microscope (Leica, Wetzlar, Germany). Quantitative data on vessel frequency, ray height, ray width and ray frequency were collected using a Leica microscope with an attached Axiovision digital camera and associated software. A Leitz DMRB light microscope (Leica) and attached Olympus digital camera (Olympus, Southend-on-Sea, UK) with dedicated ‘Analysis’ software (Olympus Soft Imaging Solutions, Münster, Germany) was used to measure vessel diameter. Measurements were taken from the fourth growth ring, ensuring that they were from wood of the same cambial age.
Data analysis
A vulnerability index (VI) was calculated according to Carlquist (1977, 2001) as follows:
where Vd is vessel diameter and Vf is vessel frequency in transverse section.
Minitab 16 (Minitab, State College, PA, USA) was used for analyses of variance (ANOVA) using a general linear model with climate and taxonomic section nested within climate, as fixed factors, and species nested within taxonomic section and climate, as a random factor. Data were log10-transformed to ensure homogeneity of variance and normal distribution. Regression analysis was used to determine the relationship of the climate variables, MAP and mean annual maximum and minimum temperature, to vessel characters.
Climate variables
Climate data for MAP and maximum and minimum temperatures for each collection site were determined using DIVA-GIS (Hijmans et al., 2012).
RESULTS
Table 3 summarizes the qualitative wood characters and is organized to reflect decreasing aridity from arid through semi-arid I to II to temperate to sub-tropical regions. This climate gradient runs from west to east in New South Wales (Fig. 1). There is an overall trend of arid and semi-arid I species having thicker fibre walls than species from the progressively wetter regions. Further differences are the poor development of vasicentric parenchyma in the cline confluent–aliform–vasicentric–scanty in semi-arid I, and the presence of some prismatic crystals in ray cells in semi-arid II. However, whereas chambered crystals in axial parenchyma are more or less ubiquitous in Acacia wood, crystals in ray cells can be hard to find. Acacia aneura spans two climate regions, the arid and semi-arid I (Fig. 1), and fibre walls are thicker and axial parenchyma is more abundant in samples from the arid region.
Table 3.
Qualitative characters for 54 species of Acacia s.s. from four taxonomic sections collected across five climate regions
| Climate region | Section | Species | Vessel grouping | Fibre wall thickness | Paratracheal parenchyma | Ray width (cells) | Crystals |
|---|---|---|---|---|---|---|---|
| Arid | Juliflorae | A. aneura | 0, 1, 3 | 0 | 0, 1, 2, 3 | 1 | 0, 1 |
| Phyllodineae | A. tetragonophylla | 0, 1, 3 | 0 | 0, 1, 2, 3 | 0, 1 | 0, 1, 2 | |
| A. victoriae | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1, 2 | ||
| Plurinerves | A. cambagei | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1, 2 | |
| A. cana | 0, 1, 2, 3 | 0 | 0, 1, 2, 3 | 1 | 0, 1, 2 | ||
| A. loderi | 0, 1, 3 | 0 | 0, 1, 2, 3 | 0 | 0, 1, 2 | ||
| Semi-arid I | Botrycephalae | A. deanei subsp. deanei | 0, 1, 3 | 0 | 0, 1, 3 | 0 | 0, 1 |
| Juliflorae | A. aneura | 0, 1, 3 | 0, 1 | 3 | 1 | 0, 1 | |
| A. burkittii | 0, 1, 3 | 0, 1 | 0, 1, 3 | 1 | 0, 1 | ||
| A. triptera | 3 | 0 | 3 | 1 | 0, 1 | ||
| Phyllodineae | A. brachybotrya | 1, 3 | 0 | 3 | 0 | 0, 1 | |
| A. conferta | 0, 1, 3 | 0, 1 | 0, 1, 3 | 2 | 0, 1 | ||
| A. hakeoides | 0, 1, 3 | 0 | 0, 1, 2, 3 | 0 | 0, 1 | ||
| Plurinerves | A. colletioides | 1, 3 | 0, 1 | 0, 1, 3 | 1 | 0, 1 | |
| A. havilandiorum | 1, 3 | 0 | 3 | 1 | 0, 1 | ||
| A. wilhelmiana | 3 | 0 | 0, 1, 2 | 1 | 0, 1 | ||
| Semi-arid II | Botrycephalae | A. deanei subsp. deanei | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1, 2 |
| A. mearnsii | 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1 | ||
| A. polybotrya | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1 | ||
| A. spectabilis | 0, 1, 3 | 0, 1, 2 | 0, 1, 2, 3 | 0 | 0, 1 | ||
| Juliflorae | A. carolae | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 1 | 0, 1 | |
| A. cheelii | 3 | 0, 1 | 0, 3 | 2 | 0, 1 | ||
| A. doratoxylon | 3 | 0, 1 | 0, 1, 3 | 1 | 0,1 | ||
| Phyllodineae | A. dorothea | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1 | |
| A. pravifolia | 3 | 0, 1 | 0, 1 | 0, 1, 2 | 0 | ||
| A. uncinata | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1 | ||
| Plurinerves | A. harpophylla | 1, 3 | 0 | 0, 1, 3 | 2 | 0, 1 | |
| A. homalophylla | 0, 1, 3 | 0 | 0, 1, 2, 3 | 0 | 0, 1, 2 | ||
| A. montana | 0, 1, 3 | 0, 1 | 0, 1, 3 | 1 | 0, 1 | ||
| A. pendula | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1, 2 | ||
| A. stenophylla | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 0 | 0, 1 | ||
| A. venulosa | 0, 1, 3 | 0, 1 | 0, 1, 2, 3 | 1 | 0, 1 | ||
| Temperate | Botrycephalae | A. dealbata | 1, 3 | 0, 1, 2 | 0, 2 | 0 | 0, 1, 2 |
| A. filicifolia | 3 | 0, 1 | 0, 2 | 0 | 1, 2 | ||
| A. leucoclada | 0, 1, 2, 3 | 0, 1, 2 | 0, 2, 3 | 0 | 1 | ||
| Juliflorae | A. blakei | 3 | 1, 2 | 0, 2 | 0 | 0, 1, 2 | |
| A. floribunda | 3 | 0, 1, 2 | 0, 2 | 1 | 0, 1, 2 | ||
| A. longifolia | 0, 1, 2, 3 | 0, 1, 2 | 0, 2 | 1 | 1, 2 | ||
| Phyllodineae | A. falcata | 1, 3 | 1, 2 | 0, 1, 2 | 1 | 3 | |
| A. flexifolia | 3 | 0, 1 | 0, 2 | 1 | 0, 1 | ||
| A. neriifolia | 3 | 1, 2 | 0, 2 | 0, 1 | 0, 1 | ||
| Plurinerves | A. dawsonii | 3 | 0, 1 | 0, 2 | 1 | 0, 1 | |
| A. implexa | 2, 3 | 1, 2 | 0, 2, 3 | 1 | 0, 1 | ||
| A. viscidula | 1, 3 | 1, 2 | 0, 1, 2 | 1 | 3 | ||
| Sub-tropical | Botrycephalae | A. irrorata | 3 | 1 | 0, 1, 2, 3 | 2 | 0, 1 |
| A. oshanesii | 0, 3 | 1, 2 | 0, 1, 2, 3 | 0 | 0, 1, 2 | ||
| A. terminalis | 3 | 1 | 0, 1, 3 | 1 | 3 | ||
| Juliflorae | A. aulacocarpa | 3 | 0, 1, 2 | 0, 1, 2, 3 | 1 | 0 | |
| A. concurrens | 3 | 1, 2 | 0, 1, 3 | 1 | 0, 1 | ||
| A. leiocalyx | 3 | 1, 2 | 0, 1, 3 | 1 | 0, 1, 2 | ||
| Phyllodineae | A. fimbriata | 0, 3 | 0, 1, 2 | 0, 1, 2, 3 | 0 | 1 | |
| A. myrtifolia | 1, 3 | 1, 2 | 0, 1, 3 | 1 | 3 | ||
| A. suaveolens | 3 | 1 | 0, 1 | 1 | 3 | ||
| Plurinerves | A. baeuerlenii | 0, 1, 3 | 1 | 0, 1, 3 | 1 | 3 | |
| A. complanata | 1, 3 | 1, 2 | 0, 1, 3 | 1 | 3 | ||
| A. elongata | 1, 3 | 1, 2 | 0, 1, 3 | 1 | 1 |
Vessel grouping: clusters common (0), radial multiples of four or more common (1), 90 % or more solitary (2), primarily in groups of two or three (3).
Fibre walls: very thick (0), thin to thick (1), very thin (2).
Paratracheal axial parenchyma: confluent (0), aliform (1), vasicentric (2), scanty (3).
Ray width: exclusively uniseriate (0), uniseriate to biseriate (1), 1–4 cells (2).
Prismatic crystals: in chambered axial parenchyma (0), in chambered fibres (1), in non-chambered ray cells (2), not found (3).
There are no Botrycephalae present in the arid region. The section Botrycephalae consists of species with bipinnate leaves and not phyllodes, and is ill adapted to drier environments. As a consequence, only one species of Botrycephalae was studied from semi-arid I, A. deanei subsp. deanei, and this species also occurs in semi-arid II which has almost twice the annual precipitation. Within this species, the fibre walls are thicker in the semi-arid I samples than in those from the semi-arid II region (Table 3).
Six species were collected from the arid region (Fig. 2). The most striking characteristic of species in this region is that all six have very thick-walled fibres, although two (A. victoriae and A. cambagei) (Fig. 2B, E, F) do have some fibres that are slightly less thick walled. None of the species has predominantly solitary vessels. Prismatic crystals are always present, often abundant, and in addition to their usual location in chambered axial parenchyma and fibres they are found in some ray cells in five out of the six species. Vessels occur in a range of groupings, particularly in pairs, short radial multiples and clusters, with the exception of A. cana, which has a high proportion of solitary vessels.
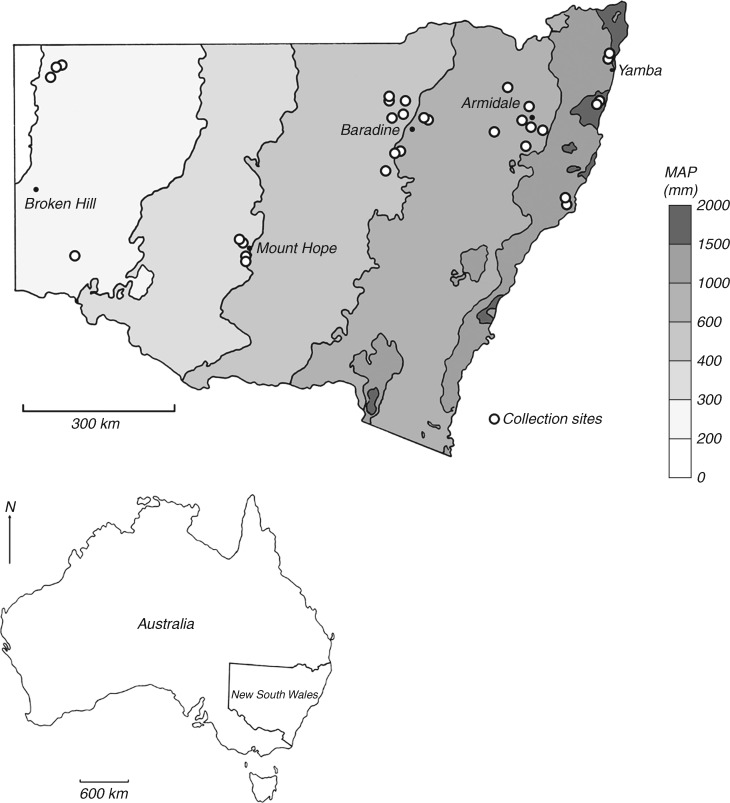
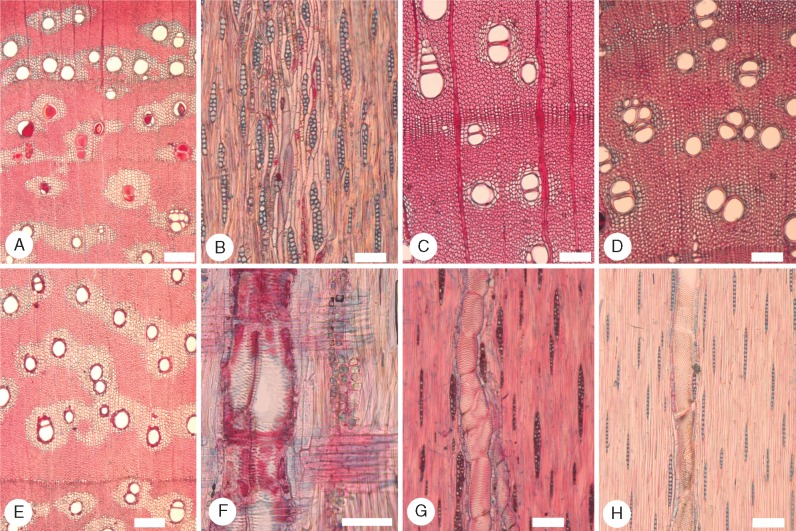
Fig. 2.
Species of Acacia sampled in the arid region. (A–C) Transverse sections (TS); (D and E) tangential longitudinal sections (TLS); (F) radial longitudinal section (RLS). (A and D) Acacia aneura (Juliflorae) LH44. Large idioblasts, almost the diameter of small vessels, each containing a prismatic crystal, either modified axial parenchyma or fibres, certainly originating from a single fusiform initial. Note also the uniseriate rays and very thick-walled fibres. (B, E and F) Acacia cambagei (Plurinerves) LH27. Abundant aliform and confluent parenchyma, marginal parenchyma packed with chambered prismatic crystals, rays short and mainly biseriate, homocellular. (C) Acacia loderi (Plurinerves) LH15 TS. Growth ring boundary with prismatic crystals, mainly gelatinous fibres, vessels in radial multiples, many containing gum or resin staining either blue or red. Scale bars = 100 μm.
All ten species in the semi-arid I region (Fig. 3) have very thick-walled fibres, although in four species these are also in combination with thick-walled fibres. The majority of species have a combination of paratracheal parenchyma patterns, although four species, A. aneura, A. triptera, A. brachybotrya and A. havilandiorum, have predominantly only scanty paratracheal parenchyma. Although crystals were found in axial parenchyma and fibres, they were not found in any ray cells. This is a surprising observation, since crystals were found in ray cells in at least one species in all the other climate regions.
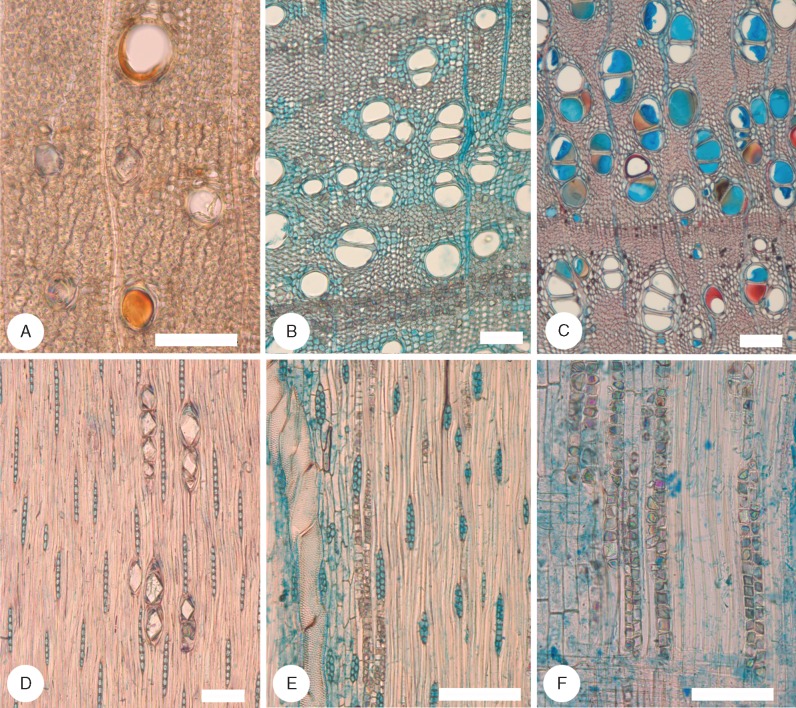
Fig. 3.
Species of Acacia sampled in the semi-arid region I. (A–C) Transverse sections (TS); (D and E) tangential longitudinal sections (TLS); (F) radial longitudinal section (RLS). (A, D and F) Acacia deanei subsp. deanei (Botrycephalae) ST32. Vessels mainly in radial multiples, some containing gum, rays mainly 2–4 cells wide, homocellular, prismatic crystals abundant in chambered axial parenchyma. (B and E) Acacia hakeoides (Phyllodineae) ST29. Vessels mainly in groups, thick-walled fibres most with prominent lumina, rays mainly biseriate, abundant prismatic crystals. (C) Acacia wilhelmiana (Plurinerves) ST10. Well-defined aliform and confluent axial parenchyma, very thick-walled fibres. Scale bars = 100 μm.
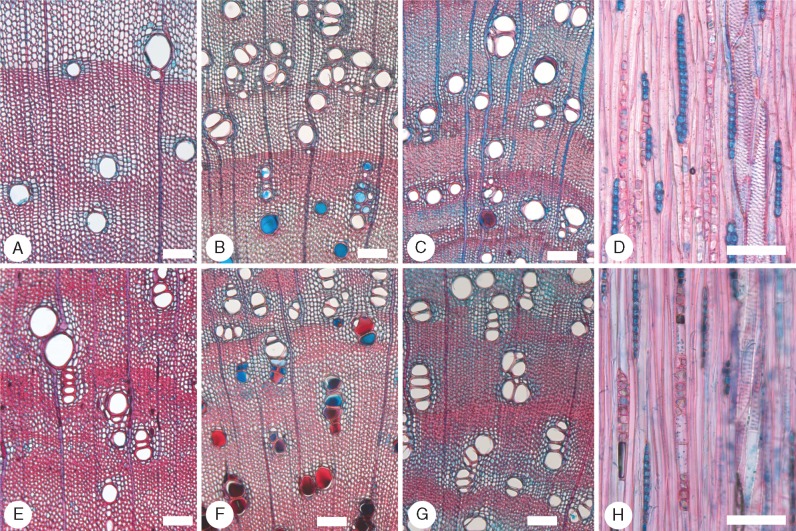
In the semi-arid II (Figs 4 and 5) and temperate regions (Fig. 6) we find the first species with some thinner walled fibres (A. spectabilis and A. implexa), although A. spectabilis (Fig. 5C, G) has the full range from very thick to thin walled. Two species have very thick-walled fibres (A. harpophylla and A. homalophylla) and the rest range from very thick to thick walled. None of the species shows a tendency to solitary vessels, and most have a full range of paratracheal parenchyma patterns. Four species have some crystals in ray cells: A. deanei subsp. deanei, A. homalophylla, A. implexa and A. pendula. The presence of crystals in ray cells is erratic, since they were not found in the semi-arid I samples of A. deanei subsp. deanei.
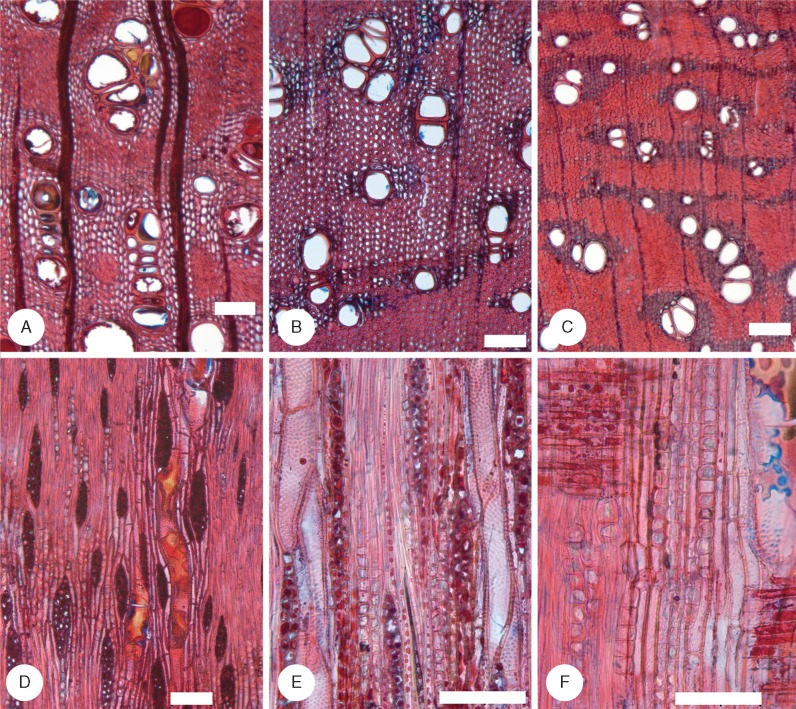
Fig. 4.
Species of Acacia sampled in the semi-arid region II. (A–D) Transverse sections (TS); (E–H) tangential longitudinal sections (TLS). (A) Acacia carolae (Juliflorae) EKg16. Vessels in groups, very thick-walled fibres, axial parenchyma not abundant. (B and F) Acacia deanei subsp. deanei (Botrycephalae) LH49. Vessels mainly grouped, fibres very thick walled, axial parenchyma aliform and confluent, vessels with alternate pitting, axial parenchyma with chambered prismatic crystals, rays 2–4 cells wide. (C and G) Acacia dorothea (Juliflorae) EKg43. Fibres thin to thick walled, axial parenchyma mainly vasicentric, rays 1–4 cells wide. (D) Acacia harpophylla (Plurinerves) LH29. Abundant aliform and confluent axial parenchyma, gelatinous fibres. (E) Acacia homalophylla (Plurinerves) LH34. Rays 1–2 cells wide, prismatic crystals abundant in chambered axial parenchyma. (H) Acacia pendula (Plurinerves) LH36. Rays 2–4 cells wide, prismatic crystals abundant in chambered axial parenchyma. Scale bars = 100 μm.
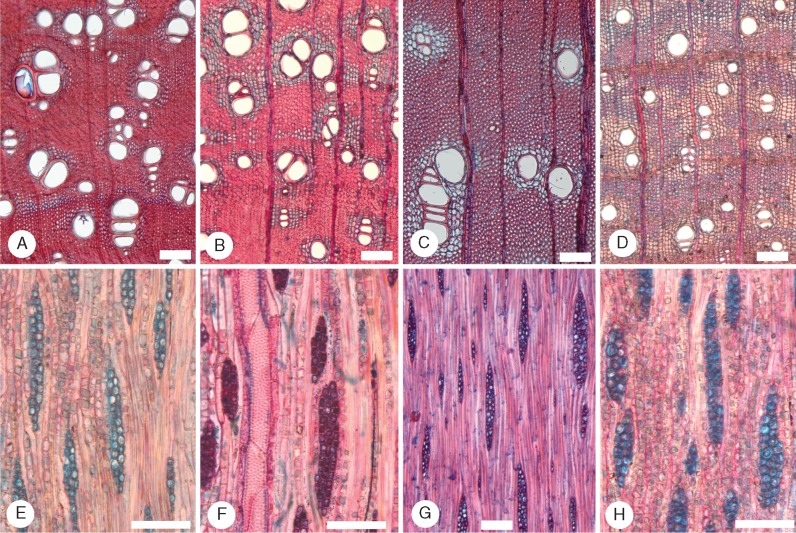
Fig. 5.
Species of Acacia sampled in the semi-arid region II. (A and C–E) Transverse sections (TS); (B, G and H) tangential longitudinal sections (TLS); (F) radial longitudinal section (RLS). (A, B, E and F) Acacia stenophylla (Plurinerves) LH38. In (A), some vessels contain gum, whereas in (E), slightly closer to the pith, the axial parenchyma cells immediately surrounding the vessels contain gum. Axial parenchyma is vasicentric, aliform and confluent. In (B) rays are 1–2 cells wide. In (F), axial parenchyma strands with chambered prismatic crystals, homocellular rays. (C and G) Acacia spectabilis (Botrycephalae) LH52. Growth ring boundary well defined. Axial parenchyma vasicentric to aliform, not abundant, fibres gelatinous and very thick walled, rays 1–2 cells wide. (D and H) Acacia venulosa (Plurinerves) LH50. Growth ring boundary well defined, thick- to very thick-walled fibres, rays uniseriate. Scale bars = 200 μm in (A) and (E), 100 μm in (B), (C), (D) and (F–H).
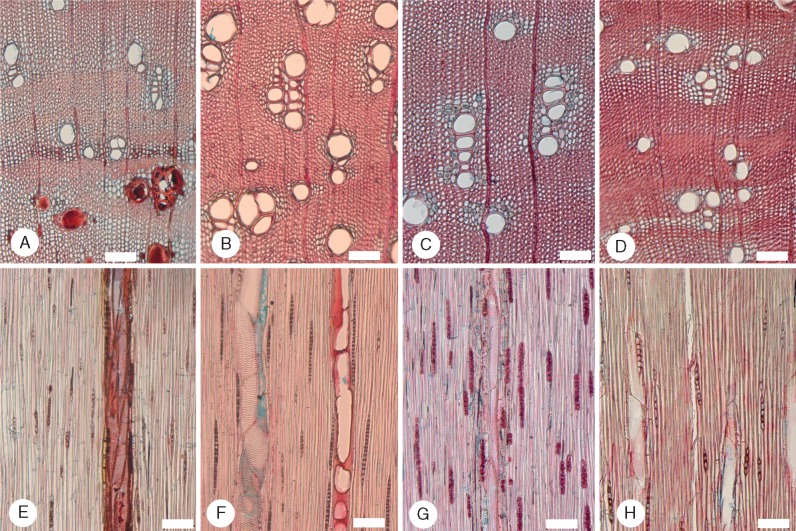
Fig. 6.
Species of Acacia sampled in the temperate region. (A–C and E–G) Transverse sections (TS); (D and H) tangential longitudinal sections (TLS). (A) Acacia implexa (Plurinerves) FW79. Well-defined growth ring boundary, vessels mostly solitary, fibres thin to thick walled. (B) Acacia viscidula (Plurinerves) FW64. Growth rings well defined, vessels mainly grouped, some occluded with gum, axial parenchyma pattern indistinct. (C and D) Acacia blakei (Juliflorae) FW67. Growth rings well defined, thick- and very thick-walled fibres, many gelatinous, axial parenchyma mainly aliform and confluent; rays uniseriate, axial parenchyma and possibly fibres with chambered prismatic crystals. (E) Acacia longifolia (Juliflorae) FW75. Paratracheal parenchyma somewhat indistinct, thick- to very thick-walled fibres. (F) Acacia falcata (Phyllodineae) FW71. Growth ring boundary well defined, vessels grouped, many occluded with gum, aliform, confluent and initial parenchyma. (G) Acacia falcata (Phyllodineae) FW68. Vessels grouped, none with gum. (H) Acacia implexa (Plurinerves) FW79. Uniseriate rays, prismatic crystals in chambered axial parenchyma or fibres. Scale bars = 100 μm.
In the 12 species from the temperate region (Fig. 6) there is a much wider range of fibre wall thicknesses from very thick to thin. None of the species have entirely very thick walls. Species with confluent and vasicentric parenchyma predominate, and only two species warranted scoring for some scanty paratracheal as well as confluent and vasicentric parenchyma. Only A. implexa, A. leucoclada and A. longifolia have any tendency towards solitary vessels. Crystals are found in ray cells in five species, and were not found at all in A. viscidula.
Only two of the 12 species of the sub-tropical region (Fig. 7) have very thick-walled fibres, and both species, A. aulacocarpa and A. fimbriata, have these in combination with a wide range of wall thicknesses. Most species have predominantly thick-walled fibres. All the species have vessels in groups and radial multiples with relatively few solitary vessels. Crystals were found in chambered fibres and/or axial parenchyma cells in seven species and ray cells in two of these (A. oshanesii and A. leiocalyx), but were not found at all in five species.
Fig. 7.
Species of Acacia sampled in the sub-tropical region. (A–D) Transverse sections (TS); (E–H) tangential longitudinal sections (TLS). (A and E) Acacia elongata (Plurinerves) LH65. Some vessels occluded with gum, thick-walled fibres, axial parenchyma aliform and confluent, rays uniseriate. (B and F) Acacia fimbriata (Phyllodineae) LH61. Vessels in groups, very thick-walled and gelatinous fibres, axial parenchyma limited in extent and mainly scanty paratracheal to vasicentric, rays uniseriate. (C and G) Acacia complanata (Plurinerves) LH101. Vessels mainly in radial multiples, fibres thick walled, axial parenchyma scanty paratracheal to aliform, rays uniseriate. (D and H) Acacia bauerlenii (Plurinerves) LH70. Fibres thick walled, axial parenchyma scanty paratracheal to aliform and confluent, rays uniseriate. Prismatic crystals are not apparent. Scale bars = 100 μm.
There is a trend of increasing fibre wall thickness with increasing aridity (Table 4). All arid and semi-arid I region species have thick-walled fibres, whereas sub-tropical and temperate species have only a proportion with thick-walled fibres but all also have thin-walled fibres. While very thin-walled fibres were absent from the arid and semi-arid region I species, very thin-walled fibres were present in a large proportion of the temperate and sub-tropical species (Fig. 8).
Table 4.
Comparison of qualitative characters (fibres, crystals and rays) expressed as percentages for 54 species of Acacia s.s. from four taxonomic sections collected across five climate regions
| Factor | % with fibre wall thickness |
% with crystals |
% with ray width (cells) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Very thick | Thin to thick | Very thin | Absent | Axial parenchyma | Fibres | Rays | Uniseriate | Uniseriate to biseriate | 1–4 cells | |
| Climate region | ||||||||||
| Arid | 100 | 29 | 0 | 0 | 100 | 100 | 86 | 71 | 43 | 0 |
| Semi-arid I | 100 | 40 | 0 | 0 | 100 | 100 | 0 | 30 | 60 | 10 |
| Semi-arid II | 94 | 88 | 12 | 0 | 100 | 94 | 24 | 65 | 29 | 18 |
| Temperate | 58 | 100 | 75 | 42 | 58 | 83 | 42 | 42 | 67 | 0 |
| Sub-tropical | 17 | 100 | 67 | 42 | 42 | 50 | 17 | 17 | 75 | 8 |
| Section | ||||||||||
| Botrycephalae | 73 | 91 | 36 | 18 | 73 | 91 | 36 | 82 | 9 | 9 |
| Juliflorae | 77 | 85 | 46 | 15 | 92 | 92 | 31 | 8 | 85 | 8 |
| Phyllodineae | 71 | 79 | 29 | 21 | 71 | 71 | 14 | 64 | 50 | 14 |
| Plurinerves | 70 | 65 | 25 | 15 | 80 | 85 | 35 | 35 | 60 | 5 |
Fig. 8.
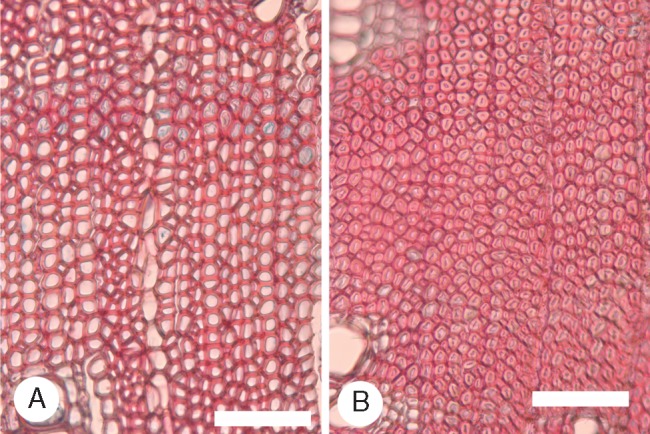
Acacia myrtifolia (Phyllodineae, sub-tropical region), normal and gelatinous fibres in transverse section (TS). (A) LH64. Most fibres thin to thick walled with prominent lumina, some with gelatinous inner walls. (B) All fibres thick to very thick walled with gelatinous inner walls and hardly any lumen. Scale bars = 100 μm.
Crystals were found in the axial parenchyma and fibres of all species of the arid, semi-arid I and II regions, with a decline in number of species with crystals in temperate and sub-tropical regions as precipitation increased (Table 4). Crystals were also present in some ray cells in a high proportion of arid region species, but a much lower proportion in the other regions, with none found in semi-arid I, an unexpected result (Table 4). No overall trend in ray width was observed with decreasing precipitation, although the arid and semi-arid II region species had a high proportion of uniseriate rays. No clear differences in the fibre wall thickness, crystals and ray width were observed across the four taxonomic sections. The largest proportion of species with multiseriate rays (1–4 cells) was in the Botrycephalae, the Juliflorae having a high proportion of species with uniseriate rays.
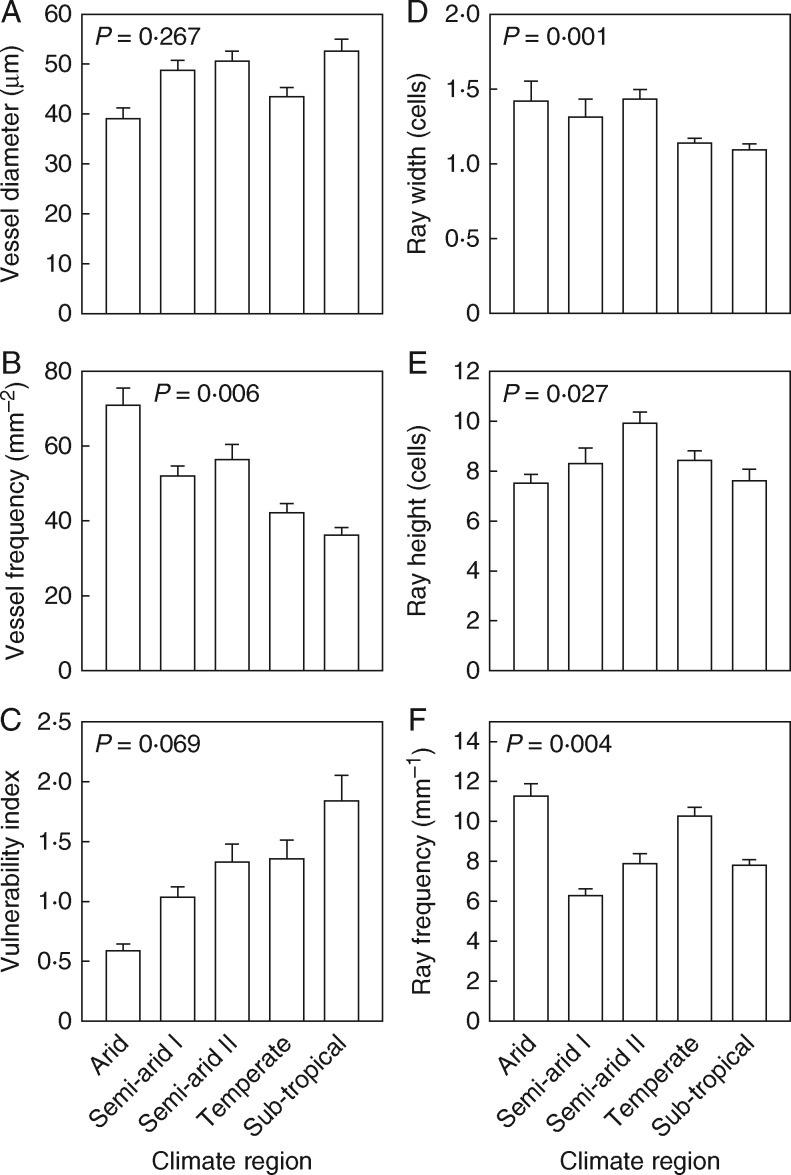
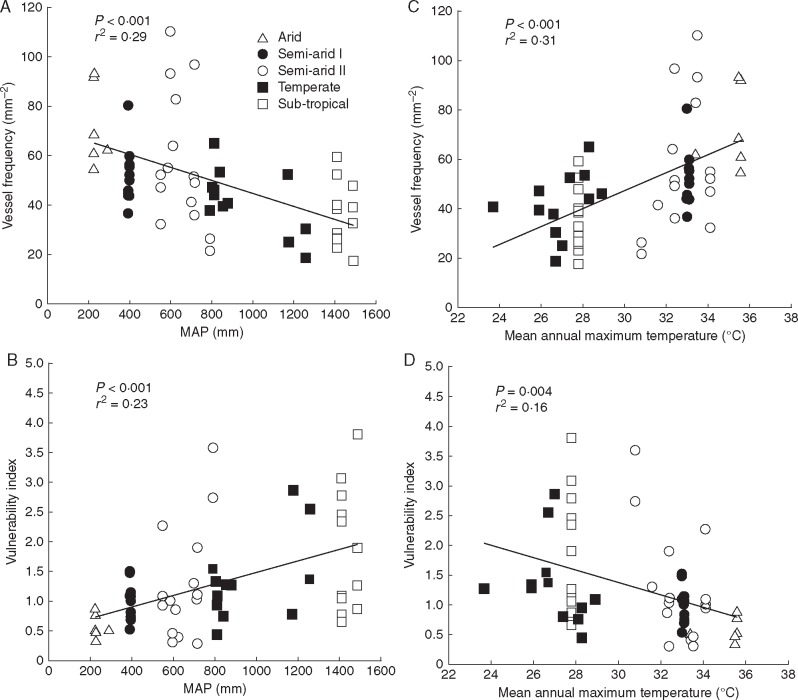
Analysis of variance found that vessel frequency, ray height, ray width and ray frequency were significantly different between species (P < 0·001). There was a weak trend of decreasing vessel diameter with increasing aridity (P = 0·267) (Fig. 9A), with the narrowest vessels in the arid region and the widest in the sub-tropical region. The difference, however, is relatively small, ranging from approx. 40 to 50 μm. Vessel frequency, in contrast, increased significantly (P = 0·006) with decreasing precipitation from approx. 36 mm–2 in the sub-tropical region to 68 mm–2 in the arid region (Fig. 9B). The VI showed a near significant trend (P = 0·069), and followed a consistent increase across the climate regions, with a > 3-fold increase in vulnerability between arid and sub-tropical species (Fig. 9C).
Fig. 9.
(A) Vessel diameter (μm), (B) vessel frequency (mm–2), (C) vulnerability index, (D) ray width (cells), (E) ray height (cells) and (F) ray frequency (mm–1) for Acacia s.s. from five climate regions. Values are means ± s.e.
Ray width varied by approx, 33 % across climate regions, with a significant (P = 0·001) effect of climate. The arid and semi-arid species have wider rays than temperate and sub-tropical species (Fig. 9D). The pattern for ray height showed no climate trend, with a minimum in the arid and sub-tropical species, and a maximum for those in the semi-arid II region (Fig. 9E). Ray frequency was, however, significantly (P = 0·004) influenced by climate, being lowest for the semi-arid I and sub-tropical species, and highest for those in the arid and temperate regions (Fig. 9F), but with no clear trend with aridity.
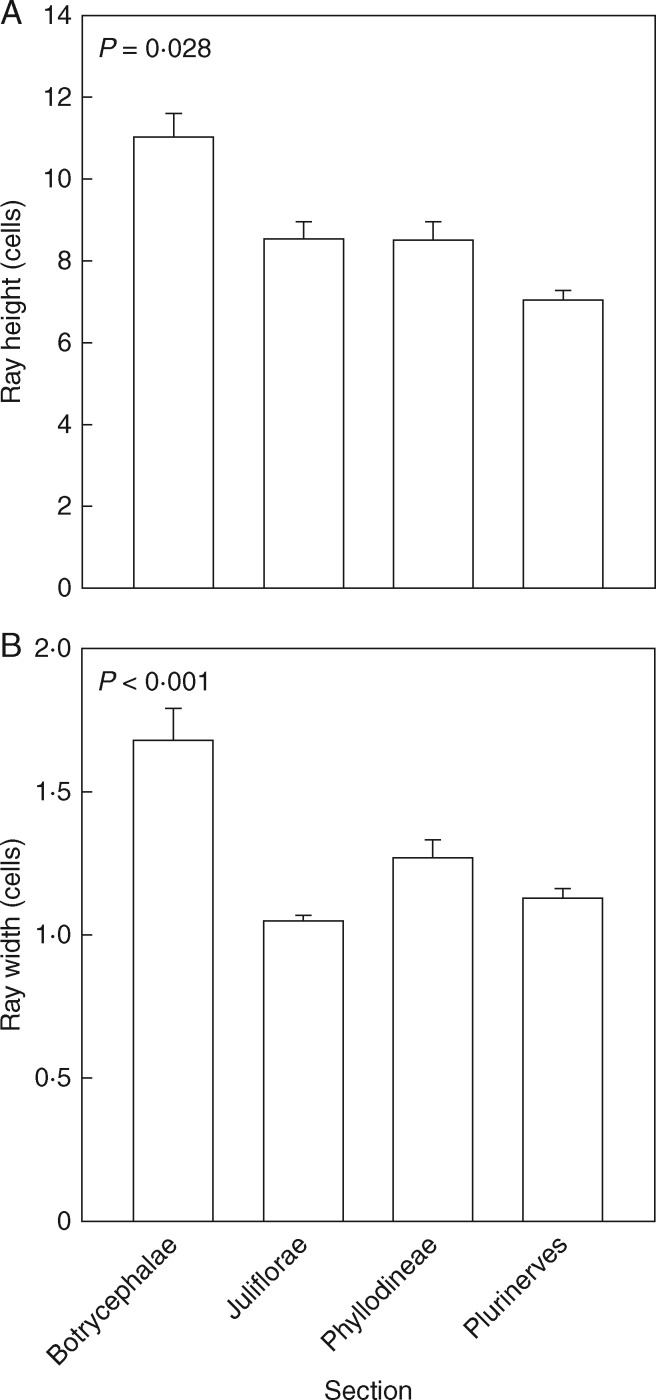
No significant differences were found between the sections for vessel diameter, vessel frequency, VI and ray frequency (data not shown). Ray width, however, increased significantly (P < 0·001) from an average of 1·1 cells in the Juliflorae to 1·7 cells in the Botrycephalae, with the Phyllodineae and Plurinerves being intermediate. Ray height (P = 0·028) increased from an average of 7.1 cells in the Plurinerves to 11 cells in the Botrycephalae (Fig. 10A, B). A feature of the pinnate Botrycephalae section is that their rays are wider and higher than the other three phyllodinous sections.
Fig. 10.
(A) Ray height (cells) and (B) ray width (cells) for four sections (Botrycephalae, Juliflorae, Phyllodineae and Plurinerves) of Acacia s.s. Values are means ± s.e.
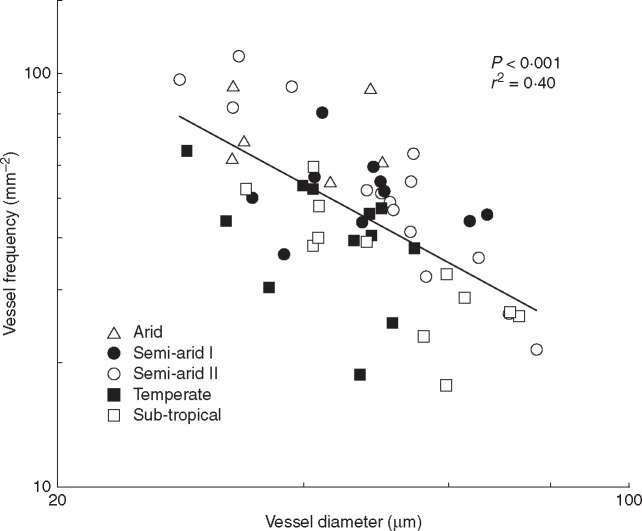
There was a significant negative logarithmic relationship between vessel frequency and vessel diameter (P < 0·001) with an upper limit to both, constrained by trade-offs between increasing frequency and a consequent decline in vessel diameter to accommodate the greater number of vessels (Fig. 11). Significant linear relationships (P < 0·001) were found between MAP and vessel frequency and the VI; vessel frequency decreased with mean annual precipitation while the VI increased (Fig. 12A, B). A significant positive relationship was found between mean annual maximum temperature and vessel frequency (P < 0·001) and a negative relationship with VI (P = 0·004) (Fig. 12C, D). Vessel diameter was not significantly correlated with either of these climate variables. Mean annual minimum temperature was not correlated with vessel diameter, vessel frequency or VI (data not shown).
Fig. 11.
Logarithmic relationship between vessel diameter (μm) and vessel frequency (mm–2) for Acacia s.s. from five climate regions. The r2 correlation coefficient and P-value are shown. Each point is the mean value for a species, n = 2–3.
Fig. 12.
Relationships between (A and C) vessel frequency (mm–2) and (B and D) vulnerability index, and between (A and B) mean annual precipitation (mm) and (C and D) mean annual maximum temperature (°C), for Acacia s.s. from five climate regions. The r2 correlation coefficient and P-value are shown. Each point is the mean value for a species, n = 2–3.
DISCUSSION
The wood of Acacia s.s. can be summarized as being diffuse porous, with more or less randomly arranged vessels which are solitary, in radial pairs and short multiples. The vessels are associated with a varying amount of paratracheal parenchyma, usually more abundant than scanty paratracheal. A cline forms through scanty paratracheal, vasicentric, aliform and confluent parenchyma. The fibres are non-septate, of varying thickness and often gelatinous. The rays are narrow and short, predominantly one or two cells wide or slightly wider (up to about four cells). Prismatic crystals are of varying abundance in chambered axial parenchyma and fibres, and in ray cells in some species. Overall, this combination of characters is quite distinctive, and the variations seem to be more closely correlated with environment (MAP and maximum temperature) than taxonomy or phylogenetic position.
The coding of axial parenchyma patterns in Table 3 probably overemphasizes differences between species. The abundance and pattern, especially the relative proportions of the various paratracheal ‘types’, can vary considerably along a radius in many species, and has been shown, for example, to be very variable in Inga when several samples of the same species have been examined (Gasson, 1997). This variation reflects changes in radial growth rate, and in Acacia s.s. this is more likely in arid and semi-arid regions where precipitation is very unevenly distributed within and between the years. The Bureau of Meteorology, Australia, precipitation variability index [(90th percentile – 10th percentile)/50th percentile] varies from a low to moderate variability of 0·5–0·75 in the sub-tropical region to a moderate to high variability of 1·0–1·25 in the arid region (Bureau of Meteorology, 2015).
The rays tend to be mainly uniseriate and/or biseriate in the arid and temperate regions, with up to 19 % of species with wider rays (up to about four cells) in the semi-arid and sub-tropical regions. These wider rays are most common in Botrycephalae and Phyllodineae, reasonably common in Plurinerves and rare in Juliflorae. A similar pattern was reported by Wilkins and Papassotiriou (1989) who compared a range of wood characters across populations of Acacia melanoxylon R.Br. by latitude, which is associated with decreasing temperature and lower transpiration demand. They found that the vessel element length, proportion of fibres and proportion of multiseriate rays increased with latitude, whereas vessel frequency and diameter, crystal abundance and the proportion of uniseriate rays, vessels and parenchyma decreased.
Prismatic crystals were almost always present, with abundance related to precipitation, being most abundant in the arid region. Although there is a reasonably clear pattern of crystal abundance in relation to climate region, this is not reflected in the taxonomic sections. As the axial cells (parenchyma and fibres) become saturated with calcium oxalate crystals, the plant appears to sequester the rest into ray cells, an observation we base on crystals in ray cells being generally less common in less arid environments. Brown et al. (2013) reported a similar gradient of increasing presence of calcium oxalate crystals in the phyllodes (foliar tissue) of Acacia spp., from the section Juliflorae, collected across the same climatic gradient. Dendrochronological studies on 14 African acacias (Senegalia and Vachellia) and the cambium of Citharexylum myrianthum (Verbenaceae) have shown a strong link between lower precipitation and seasonal water deficits, and oxalate crystal accumulation. Crystals have been found to be more numerous in species from arid regions, with accumulation occurring at the cessation of growth periods (Gourlay et al., 1994; Gourlay, 1995; Marcati and Angyalossy, 2005). Fahn et al. (1985) found crystals to be more numerous not only in wood from desert areas, but also in the tropics, when compared with temperate species. In contrast, this study and that of Brown et al. (2013) showed a continuous trend of decreasing presence of crystals in the arid region to near absence in the sub-tropical region.
There has been much speculation about the role of calcium oxalate crystals in wood, not only as to their fire-retardant properties and defence against predators (Prior and Cutler, 1992). Calcium also plays a key role in cellular metabolism, and oxalate crystals have been found to form in response to high levels of the element in the growth medium (Volk et al., 2002; Nakata, 2003). The increasing presence of oxalate crystals in the arid and semi-arid regions is likely to be a consequence of the accumulation of soluble salts such as CaCO3 and CaSO4, and a greater occupation of the exchange sites in the arid soils of the Australian continent (Mengel and Kirkby, 2001; McKenzie et al., 2004). The lower occurrence of crystals in the wetter regions, particularly the sub-tropical region (approx. 1300 mm MAP), may arise as a consequence of the low soil Ca levels in the heavily leached soil profiles in this region (Conn and Gilliham, 2010).
Thick-walled and gelatinous fibres are a common feature of the Mimosoideae (Evans et al., 2006), and this study indicates that both are frequently found in Acacia s.s. Gelatinous fibres are usually associated with tension wood, particularly on the upper side of branches (Panshin et al., 1964; Jane, 1970), but in this study they occurred frequently on any radius. The increase in fibre wall thickness with aridity may have a physiological function and could be related to fibre strength compensating for the mechanical weakness caused by greater vessel frequency (Baas et al., 2004). Martínez-Cabrera et al. (2009) found a strong positive relationship between wood density and fibre wall thickness and aridity. Increased mechanical strength and wood density in arid climates may help resist embolism formation arising from frequent low water potentials (Hacke et al., 2001; Martínez-Cabrera et al., 2009). The likelihood of deformation of vessel walls under cavitation has been hypothesized to be mitigated by the greater strength of the thick-walled fibres (Jacobsen et al., 2005). That this is a role for thick-walled fibres in Acacia s.s. can be questioned given the abundance of paratracheal parenchyma, and the lack of fibres in direct contact with vessels in species from arid and semi-arid regions, as also noted by Whinder et al. (2013) for temperate species of Acacia. All the species studied have paratracheal parenchyma, which provides further support for questioning of this phenomenon. A similar conclusion was reached by Martínez-Cabrera et al. (2009) who found that fibre–vessel contacts decreased as wood density decreased and did not explain the observed correlation between wood density and implosion resistance. The trend for abundant fibres in wood and increasing fibre wall thickness with increasing aridity does, however, parallel the observations of increasing sclerophylly observed for phyllodes from mesic to arid environments (Hnatiuk and Maslin, 1988; Maslin and Pedley, 1988). An alternative speculation is that the inner gelatinous wall retains water and could have a role in maintaining adequate stem capacitance and hence the water balance of trees or shrubs in arid or semi-arid regions (Sonsin et al., 2012).
Rays and paratracheal parenchyma in sapwood provide storage sites for starch and sugars, and as conduits for sugars that could be involved in embolism repair using remobilized sugars (Nardini et al., 2011). In a comprehensive study of 800 tree species in China, Zheng and Martínez-Cabrera (2013) speculated that there was a positive relationship between abundance of axial parenchyma, water conduction and water storage capacity, with water withdrawn from parenchyma lessening the decline in water potential during periods of low water availability. Borchert and Pockman (2005) also provided support for this where species with abundant paratracheal axial parenchyma had high capacitance. The frequent occurrence of paratracheal parenchyma in these specimens does suggest a putative role in water storage or embolism repair given the xerophytic nature of the Australian Acacia s.s. and their origins within a dry environment (Maslin and Pedley, 1988).
The negative logarithmic relationship between vessel diameter and frequency indicates a clear limitation by one character on the other. While it is similar to that reported for broader studies of tracheary elements in both angiosperms and conifers, this study shows that the relationship can also hold within a genus (Sperry et al., 2008; Olson et al., 2014). At high vessel frequencies, there are packing constraints, with a large number of narrow vessels able to fit in the stem cross-section. At lower vessel frequencies, wider vessels can be accommodated but have a lower resistance to hydraulic failure under high water stress conditions (Carlquist, 2001; Tyree and Zimmerman, 2002). There are clear differences between the climate regions for these two quantitative characters, and both would have significant effects on water flow though the xylem. Although vessel diameter decreases by only 20 % from the sub-tropical to arid region, the Hagen–Poiseuille equation predicts that conductivity, which increases with the fourth power of the vessel diameter, will decrease by 65 % as a consequence. Olson et al. (2014) reported a positive correlation between vessel diameter and plant height. This relationship may apply for Acacia, but is not within the scope of this study because the range of plant forms (shrubs, saplings and trees) within the climate regions would remove this as a possible cause of the negative relationship with aridity.
Vessel frequency increased almost 2-fold in arid species compared with sub-tropical species, and would therefore compensate partly for the reduced conductivity of narrower vessels, as well as retaining a lower cavitation vulnerability. The arid and semi-arid species have a greater redundancy with the larger number of smaller vessels that increase flow through parallel pathways and increased distribution of water to surrounding woody tissues (Baas et al., 1983; Tyree and Zimmerman, 2002; Jones, 2014). The semi-arid and arid species, and the species from taxonomic sections commonly found in these regions, also possess xeromorphic features which reduce water loss and physiological mechanisms to cope with dry habitats (Boughton, 1986, 1990; Warwick and Thukten, 2006). These will also compensate for the reduced water flow to the foliage arising from possession of narrower vessels.
Mean annual precipitation is a significant climatic variable influencing plant distribution and abundance. In this study it is reflected to a limited extent by vessel diameter and to a greater extent vessel frequency and VI despite the overlying influence of other habitat factors such as the physical and chemical properties of the soil, aspect and topography. A decrease in vessel diameter in drier climates means that vessels are less vulnerable to cavitation and more likely to recover from embolism (Ewers, 1985).
Sommerville et al. (2012) reported on phyllode venation of Acacia species across a range of annual precipitations, and found greater vessel diameters but lower vessel frequencies were more common in species from areas receiving higher MAP. A pattern of increasing phyllode hydraulic conductivity in drier areas, despite the reduced diameter, was reported as a consequence of the greater frequency. A similar pattern was also expressed in the anatomy of the wood with the concomitant increase in vessel frequency in drier regions. An increase in hydraulic conductance of the phyllode in drier areas was proposed as possibly preventing large decreases in water potential which may in turn limit xylem cavitation.
In conclusion, Australian species of Acacia s.s. exhibit considerable diversity in wood anatomical features, and we have demonstrated that they form an ideal monophyletic group to study the effect of both climate and phylogeny on water transport and functional anatomy of wood. Climate has a strong effect on the development of the water transport pathways in wood as well as formation of fibres and the accumulation of oxalate crystals. Further study will be made of the ultrastructural aspects of Acacia vessel anatomy, and the development of fibres and crystal-containing cells relating these to climatic and edaphic factors.
ACKNOWLEDGEMENTS
Field collections were made by Dr Kerri Clarke, Caroline Downer, and Drs Nigel Warwick and Jim Charley, under appropriate Scientific Licences granted by NSW National Parks & Wildlife Service and Forests NSW. Microscope sections were prepared by Emily King (EKg), Luke Hailey (LH), Sarah Tytherleigh (ST) and Francis Whinder (FW). We are particularly grateful to Dr Stuart Cairns of the University of New England for providing statistical advice on the analyses of variance. We also acknowledge the two reviewers whose suggestions helped us improve the paper. Field collection for this work was made possible by the generous benefaction of Dr J.L. Charley. This work is a collaboration between N.W.M.W. and P.E.G. at the Jodrell Laboratory in 2010 and 2013, which was made possible by the Special Studies Program of the University of New England.
LITERATURE CITED
- Baas P. 1976. Some functional and adaptive aspects of vessel member morphology In: Baas P, Bolton AJ, Catling DM, eds. Wood structure and biological and technological research. Leiden Botanical Series No 3. The Hague: Leiden University Press, 157–181. [Google Scholar]
- Baas P, Ewers FW, Davis SD, Wheeler EA.. 2004. The evolution of xylem physiology In: Hemsley AR, Poole I, eds. The evolution of plant physiology. London: Academic Press, 295–273. [Google Scholar]
- Baas P, Werker E, Fahn A.. 1983. Some ecological trends in vessel characters. IAWA Bulletin 4: 141–159. [Google Scholar]
- Bailey IW. 1966. The significance of the reduction of the vessels in Cactaceae. Journal of the Arnold Arboretum 47: 288–292. [Google Scholar]
- Bairstow KA, Clarke KL, McGeoch MA, Andrew NR.. 2010. Leaf miner and plant galler species richness on Acacia: relative importance of plant traits and climate. Oecologia 163: 437–448. [DOI] [PubMed] [Google Scholar]
- Baker RT. 1919. The hardwoods of Australia. Sydney: New South Wales Government Printer. [Google Scholar]
- Baretta-Kuipers T. 1981. Wood anatomy of the Leguminosae: its relevance to taxonomy In: Polhill RM, Raven PH, eds. Advances in legume systematics 2. Kew: Royal Botanic Gardens, 677–705. [Google Scholar]
- Borchert R, Pockman WT.. 2005. Water storage capacitance and xylem tension in isolated branches of temperate and tropical trees. Ecology 75: 1437–1449. [DOI] [PubMed] [Google Scholar]
- Boughton VH. 1986. Phyllode structure, taxonomy and distribution in some Australian Acacias. Australian Journal of Botany 34: 663–674. [Google Scholar]
- Boughton VH. 1990. Aspects of phyllode anatomy in some Australian phyllodinous Acacias, with particular regard to stickiness. Australian Journal of Botany 38:131–151. [Google Scholar]
- Brockwell J, Searle SD, Jeavons AC, Waayers M.. 2005. Nitrogen fixation in acacias: an untapped resource for sustainable plantations, farm forestry and land reclamation. ACIAR Monograph No. 115.
- Brown SL, Prychid CJ, Warwick NWM.. 2013. Does aridity influence the morphology, distribution and accumulation of calcium oxalate crystals in Acacia (Leguminosae: Mimosoideae)? Plant Physiology and Biochemistry 73: 219–228. [DOI] [PubMed] [Google Scholar]
- Bureau of Meteorology, Australian Government. 2011. Average rainfall, annual http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall/index.jsp/. (last accessed 17 February 2016).
- Bureau of Meteorology, Australian Government. 2015. Rainfall variability.http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall-variability/index.jsp/. (last accessed 7 December 2015).
- Carlquist S. 1975. Ecological strategies of xylem evolution. Berkeley, CA: University of California Press. [Google Scholar]
- Carlquist S. 1977. Wood anatomy of Onagraceae: additional species and concepts. Annals of the Missouri Botanical Garden 64: 627–637. [Google Scholar]
- Carlquist S. 1982. Wood anatomy of Illicium (Illiciaceae): phylogenetic, ecological, and functional interpretations. American Journal of Botany 69: 1587–1598. [Google Scholar]
- Carlquist S. 2001. Comparative wood anatomy. Systematic, ecological, and evolutionary aspects of dicotyledon wood. Heidelberg: Springer Verlag. [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE.. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Clarke PA. 2008. Aboriginal healing practices and Australian bush medicine. Journal of the Anthropological Society of South Australia 33: 3–38. [Google Scholar]
- Clarke PA. 2012. Australian plants as Aboriginal tools. Dural: Rosenburg Publishing Pty Ltd. [Google Scholar]
- Conn S, Gilliham M.. 2010. Comparative physiology of elemental distributions in plants. Annals of Botany 105: 1081–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribb AB, Cribb JW.. 1987. Wild food in Australia. Sydney: Angus & Robertson Publishers. [Google Scholar]
- Dadswell HE, Eckersley AM.. 1935. The identification of the principal commercial Australian timbers other than Eucalypts. Division of Forest Products – Technical Paper No. 16. Melbourne: CSIR. [Google Scholar]
- Dadswell HE, Burnell M, Eckersley AM.. 1934. Identification of the light-coloured woods of the genus Eucalyptus. Division of Forest Products – Technical Paper No. 12. Bulletin No. 78. Melbourne: CSIR. [Google Scholar]
- Ewers FW. 1985. Xylem structure and water conduction in conifer trees, dicot trees and lianas. IAWA Journal 6: 309–317. [Google Scholar]
- Evans JA, Gasson PE, Lewis GP.. 2006. Wood anatomy of the Mimosoideae (Leguminosae). IAWA Journal (Suppl 5): 1–118. [Google Scholar]
- Fahn A, Werker E, Baas P.. 1985. Wood anatomy and identification of trees and shrubs from Israel and adjacent regions. Jerusalem, Israel: The Israel Academy of Science and Humanities, 116–118. [Google Scholar]
- Ford J. 1984. Vessel characteristics of the wood of some Australian species of Acacia in relation to habitat In: Sudo S, ed. Proceedings: Pacific Regional Wood Anatomy Conference October 1–7, 1984. Tsukuba, Ibaraki, Japan, 156–158. [Google Scholar]
- Gardner SK, Drinnan A, Newbigin E, Ladiges PY.. 2008. Leaf ontogeny and morphology in Acacia Mill. (Mimosaceae). Muelleria 26: 43–51. [Google Scholar]
- Gasson P. 1997. Wood and bark anatomy In: Pennington TD, ed. The genus Inga. Kew: Royal Botanic Gardens, 9–30. [Google Scholar]
- Gasson P, Miller R, Stekel DJ, Whinder F, Zieminska K.. 2010. Wood identification of Dalbergia nigra (CITES Appendix I) using quantitative wood anatomy, principal components analysis and naïve Bayes classification. Annals of Botany 105: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay ID. 1995. The definition of seasonal growth zones in some African Acacia species: a review. IAWA Journal 16: 353–359. [Google Scholar]
- Gourlay ID, Grime GW.. 1994. Calcium oxalate crystals in African Acacia species and their analysis by scanning proton microprobe (SPM). IAWA Journal 15: 137–148. [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA.. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Guarino L, Mathur P.. 2012. DIVA-GIS Version 7.5 Manual.http://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf/ (last accessed 12 February 2013).
- Hnatiuk RJ, Maslin BR.. 1988. Phytogeography of Acacia in Australia in relation to climate and species-richness. Australian Journal of Botany 36: 361–383. [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA III, Davis SD.. 2005. Do xylem fibers affect vessel cavitation resistance? Plant Physiology 139: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane FW. 1970. The structure of wood, 2nd edn London: Adam and Charles Black. [Google Scholar]
- Jones HG. 2014. Plants and microclimate. Cambridge: Cambridge University Press. [Google Scholar]
- Kribs DA. 1935. Salient lines of structural specialization in the wood rays of dicotyledons. Botanical Gazette 96: 547–557. [Google Scholar]
- Kribs DA. 1937. Salient lines of structural specialization in the wood parenchyma of dicotyledons. Bulletin of the Torrey Botanical Club 64: 177–186. [Google Scholar]
- Kyalangalilwa B, Boatwright JS, Daru BH, Maurin O, Van der Bank M.. 2013. Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Botanical Journal of the Linnean Society 172: 500–523. [Google Scholar]
- Latz PK. 1995. Bushfires and bush tucker: Aboriginal plant use in Central Australia. Alice Springs: IAD Press. [Google Scholar]
- Lewis GP. 2005. Tribe Acacieae In: Lewis G, Schrire B, Mackinder B, Lock M, eds. Legumes of the world. Kew: Royal Botanic Gardens, 187–191. [Google Scholar]
- Low T. 1991. Wild foods of Australia. Sydney: Harper Collins Publishers Pty Ltd. [Google Scholar]
- Marcati CR, Angyalossy A.. 2005. Seasonal presence of acicular calcium oxalate crystals in the cambial zone of Citharexylum myrianthum (Verbenaceae). IAWA Journal 26: 93–98. [Google Scholar]
- Martínez-Cabrera HI, Jones CS, Espino S, Schenk HJ.. 2009. Wood anatomy and wood density in shrubs: responses to varying aridity along transcontinental transects. American Journal of Botany 96: 1388–1398. [DOI] [PubMed] [Google Scholar]
- Maslin BR. 1997. Australia’s golden future. Landscape 12: 16–22. [Google Scholar]
- Maslin BR. 2008. Generic and subgeneric names in Acacia following retypification of the genus. Muelleria 26: 7–9. [Google Scholar]
- Maslin BR, Pedley L.. 1988. Patterns of distribution of Acacia in Australia. Australian Journal of Botany 36: 385–393. [Google Scholar]
- McDonald MW, Maslin BR, Butcher PA.. 2001. Utilisation of acacias In: Orchard AE, Wilson AJG, eds. Flora of Australia. Vol. 11A, Mimosaceae, Acacia. Part 1. Melbourne: ABRS/CSIRO Publishing, 30–40. [Google Scholar]
- McKenzie N, Jacquier D, Isbell R, Brown K.. 2004. Australian soil and landscapes: an illustrated compendium. Melbourne: CSIRO Publishing. [Google Scholar]
- Mengel K, Kirkby EA.. 2001. Principles of plant nutrition, 5th edn Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Midgley S, Turnbull JL.. 2003. Domestication and use of Australian acacias: case studies of five important species. Australian Systematic Botany 16: 89–102. [Google Scholar]
- Miller JT, Seigler D.. 2012. Evolutionary and taxonomic relationships of Acacia s.l. (Leguminosae: Mimosoideae). Australian Systematic Botany 25: 217–224. [Google Scholar]
- Miller JT, Andrew R., Bayer RT.. 2003. Molecular phylogenetics of the Australian Acacias of subg. Phyllodineae (Fabaceae: Mimosoideae) based on the trnK intron. Australian Journal of Botany 51: 167–177. [Google Scholar]
- Murphy DJ, Brown GK, Miller JT, Ladiges PY.. 2010. Molecular phylogeny of Acacia Mill. (Mimosoideae: Leguminosae): evidence for major clades and informal classification. Taxon 59: 7–19. [Google Scholar]
- Murphy DJ, Miller JT, Bayer RJ, Ladiges PY.. 2003. Molecular phylogeny of Acacia subgenus Phyllodineae (Mimosoideae: Leguminosae) based on DNA sequences of the internal transcribed spacer region. Australian Systematic Botany 16: 19–26. [Google Scholar]
- Nakata PA. 2003. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science 164: 901–909. [Google Scholar]
- Nardini A, Lo Gullo MA, Salleo S.. 2011. Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Science 180: 604–611. [DOI] [PubMed] [Google Scholar]
- Noshiro S, Baas P.. 2000. Latitudinal trends in wood anatomy within species and genera: case study in Cornus s.l. (Cornaceae). American Journal of Botany 87: 1495–1506. [PubMed] [Google Scholar]
- Olson ME, Anfodillo T, Rosell JA, et al. 2014. Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecology Letters 17: 988–997. [DOI] [PubMed] [Google Scholar]
- Panshin AJ, de Zeeuw C, Brown HP.. 1964. Textbook of wood technology, Vol I – Structure, identification and properties of the commercial woods of the United States. New York: McGraw-Hill. [Google Scholar]
- Pedley L. 1978. A revision of Acacia Mill. in Queensland. Austrobaileya 1: 75–234. [Google Scholar]
- Prior J, Cutler D.. 1992. Trees to fuel Africa’s fires. New Scientist 135: 35–39. [Google Scholar]
- Scholz A, Klepsch M, Karimi Z, Jansen S.. 2013. How to quantify conduit in wood? Frontiers in Plant Science 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Figueiredo E.. 2011. Conserving Acacia Mill. with a conserved type: what happened in Melbourne? Taxon 60: 1504–1506. [Google Scholar]
- Sommerville KE, Sack L, Ball MC.. 2012. Hydraulic conductance of Acacia phyllodes (foliage) is driven by primary nerve (vein) conductance and density. Plant, Cell & Environment 35: 158–168. [DOI] [PubMed] [Google Scholar]
- Sonsin JO, Gasson PE, Barros CF, Marcati CR.. 2012. A comparison of the wood anatomy of 11 species from two cerrado habitats (cerrado s.s. and adjacent gallery forest). Botanical Journal of the Linnaean Society 70: 257–276. [Google Scholar]
- Sperry JS, Meinzer FC, McCulloh KA.. 2008. Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant, Cell & Environment. 31: 632–645. [DOI] [PubMed] [Google Scholar]
- Stern H, de Hoedt G, Ernst J.. 2000. Objective classification of Australian climates. Australian Meteorological Magazine 49: 87–96. [Google Scholar]
- Thiele KR, Funk VA, Iwatsuki K, et al. 2011. The controversy over the retypification of Acacia Mill. with an Australian type: a pragmatic view. Taxon 60: 194–198. [Google Scholar]
- Thrall PH, Slattery JF, Broadhurst LM, Young AG, Bickford S.. 2007. Geographic patterns of symbiont abundance and adaptation in native Australian Acacia–rhizobia interactions. Journal of Applied Ecology 95: 1110–1122. [Google Scholar]
- Tyree M, Zimmerman M.. 2002. Xylem structure and the ascent of sap, 2nd edn Berlin: Springer-Verlag. [Google Scholar]
- Volk GM, Lynch-Holm VJ, Kostman TA, Goss LJ, Franceschi VR.. 2002. The role of druse and raphide calcium oxalate in tissue calcium regulation in Pistia stratiotes leaves. Plant Biology 4: 34–45. [Google Scholar]
- Warwick NWM, Thukten. 2006. Water relations of phyllodinous and non-phyllodinous Acacias, with particular reference to osmotic adjustment. Physiologia Plantarum 127: 393–403. [Google Scholar]
- Wheeler EA, Baas P, Gasson PE, eds. 1989. IAWA list of microscopic features for hardwood identification IAWA Journal. 10: 219–332. [Google Scholar]
- Whinder FW, Clarke KL, Warwick NWM, Gasson PE.. 2013. Structural diversity of the wood of temperate species of Acacia s.s. (Leguminosae: Mimosoideae). Australian Journal of Botany 61: 291–301. [Google Scholar]
- Wilkins AP, Papassotiriou S.. 1989. Wood anatomical variation of Acacia melanoxylon in relation to latitude. IAWA Journal 10: 201–207. [Google Scholar]
- Zheng J, Martínez-Cabrera HI.. 2013. Wood anatomy correlates with theoretical conductivity and wood density across China: evolutionary evidence of the functional differentiation of axial and radial parenchyma. Annals of Botany 112: 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]