Abstract
Background and Aims It has been suggested that the dynamics of nectar replenishment could differ for flowers after being nectar robbed or visited legitimately, but further experimental work is needed to investigate this hypothesis. This study aimed to assess the role of nectar replenishment in mediating the effects of nectar robbing on pollinator behaviour and plant reproduction.
Methods Plant–robber–pollinator interactions in an alpine plant, Salvia przewalskii, were studied. It is pollinated by long-tongued Bombus religiosus and short-tongued B. friseanus, but robbed by B. friseanus. Nectar production rates for flowers after they were either robbed or legitimately visited were compared, and three levels of nectar robbing were created to detect the effects of nectar robbing on pollinator behaviour and plant reproduction.
Key Results Nectar replenishment did not differ between flowers that had been robbed or legitimately visited. Neither fruit set nor seed set was significantly affected by nectar robbing. In addition, nectar robbing did not significantly affect visitation rate, flowers visited within a plant per foraging bout, or flower handling time of the legitimate pollinators. However, a tendency for a decrease in relative abundance of the pollinator B. religiosus with an increase of nectar robbing was found.
Conclusions Nectar robbing did not affect female reproductive success because nectar replenishment ensures that pollinators maintain their visiting activity to nectar-robbed flowers. Nectar replenishment might be a defence mechanism against nectar robbing to enhance reproductive fitness by maintaining attractiveness to pollinators. Further studies are needed to reveal the potential for interference competition among bumble bees foraging as robbers and legitimate visitors, and to investigate variation of nectar robbing in communities with different bumble bee species composition.
Keywords: Bombus, bumble bee, geitonogamous mating, nectar replenishment, nectar robbing, pollinator behaviour, pollination, Salvia przewalskii
INTRODUCTION
Nectar robbers feed upon nectar by biting or piercing holes in flowers, often without providing an effective pollination service (Inouye, 1980; Irwin et al., 2010). Flowering plants with long tubular flowers or nectar spurs are most likely to be subjected to nectar robbing (Irwin and Maloof, 2002). Nectar robbers may have important ecological and evolutionary effects on host plants through direct or indirect effects (Maloof and Inouye, 2000; Irwin et al., 2001, 2010, 2015; Zhang et al., 2009). Direct effects may result from robber damage to floral reproductive structures (Traveset et al., 1998; Zhang et al., 2011), while indirect effects may result from changes to the behaviour of legitimate pollinators (Maloof, 2001; Gonzalez-Gomez and Valdivia, 2005).
Indirect effects of robbing on pollination can be categorized into two main mechanisms: interference competition with pollinators, and changes in floral traits affecting pollinator behaviour (Irwin et al., 2010). Robbers may physically constrain pollinators’ access to robbed flowers (Roubik, 1982). Moreover, changes in quantity and quality of nectar caused by nectar robbing were found to alter attractiveness to pollinators, and to change their behaviour and thus influence plant reproduction (reviewed in Irwin et al., 2010; Irwin et al., 2015). However, continuous nectar production may help maintain pollinator attractiveness to some degree by replenishing nectar lost due to robbing (Irwin et al., 2008; Fumero-Cabán and Meléndez-Ackerman, 2013). Plants may display different patterns of nectar replenishment in robbed (and unpollinated) flowers, and those that are pollinated (Ordano and Ornelas, 2004). For example, nectar replenishment might be sensitive to pollen removal or stigmatic pollen deposition, and then slow down or cease for flowers being pollinated rather than robbed (Aizen and Basilio, 1998; Ordano and Ornelas, 2004). In addition, differences in nectar removal efficiency between pollinators and robbers may also result in different nectar production rates. Disentangling whether nectar replenishment differs between flowers after being robbed or pollinated would be helpful to enrich our understanding of the effects of nectar robbing on pollinator behaviour, plant reproduction and even defence mechanisms.
Nectar robbers may often have a detrimental effect on plant reproductive success. For example, robbers may reduce nectar volume and change sucrose concentration by a potential combination of decreased nectar replenishment (McDade and Kinsman, 1980) and evaporation through the robbing incision (Pleasants, 1983; Hazlehurst and Karubian, 2016), which may then decrease pollinators’ visitation rates, thus reducing pollen export and female reproductive success (reviewed in Irwin et al., 2010). However, nectar robbers can also be indirectly beneficial to reproductive success in some cases. For example, legitimate pollinators may be forced to fly for longer distances due to smaller nectar rewards as a result of nectar robbing, hence increasing genetic variability through outcrossing (Zimmerman and Cook, 1985; Singh et al., 2014). In other instances, detrimental and beneficial effects may occur simultaneously (Zhang et al., 2007). In addition, neutral effects of nectar robbing on host plant reproductive success have also been found in some taxa (Morris, 1996; Maloof, 2001; Richardson, 2004; Hazlehurst and Karubian, 2016). The causes of negative or positive effects can be detected easily, while the reasons for a neutral effect need further investigation.
We investigated a naturally occurring plant–robber interaction in Salvia przewalskii to identify the impact of nectar robbing on plant reproduction and pollinator behaviour. First we investigated the dynamics of nectar production in flowers after either robbers or legitimate pollinators visited, as well as the mating system of the plant species. Three levels of nectar robbing were artificially created, namely 0, 50 and 92 % (natural condition) of flowers within an individual plant were robbed. We focused on the effects of nectar robbers on three aspects of plant–robber–pollinator interactions in S. przewalskii: (1) the visitation rate of legitimate pollinators; (2) the foraging efficiency and number of flowers visited within a plant per bout of legitimate pollinators; and (3) the female reproductive success of S. przewalskii.
MATERIALS AND METHODS
Study species and site
Salvia przewalskii Maxim. var. przewalskii is a perennial herb, mainly distributed in north-west Yunnan, west Sichuan, west Gansu and west Tibet provinces of China. It inhabits hillsides, sub-alpine meadows, forest margins, roadsides and thickets. The flowering season is usually from August to September. Individual plants produce multiple stems (1–20) arising from a single large root. Each mature stem is topped by a terminal racemose inflorescence bearing up to 60 flowers; stems also may have some side inflorescences with 5–20 flowers each. Flowers are zygomorphic, nectar-rich, purple or red-purple, with long tubular corollas about 34–38 mm long, with a hooded upper lip (Wu and Li, 1977). The style is exserted and protrudes out of the upper lip, thus contributing to a well-developed approach herkogamy. Most flowers last for two and a half days before they begin to wilt. Each S. przewalskii flower contains four ovules; hence, the flower can produce 0–4 seeds (Wu and Li, 1977).
In the summer of 2013, we conducted the study at a hillside slope in Shangri-La County (27°51′2″N, 99°43′18″E; 3305 m), in the north-west of Yunnan province, China. The study site was about 30 × 130 m2 and included approx. 9000 S. przewalskii plants. During our study, S. przewalskii was mainly co-flowering with Origanum vulgare (Lamiaceae), Ajuga forrestii (Lamiaceae) and Verbascum thapsus (Scrophulariaceae).
Our pilot investigations revealed that two bumble bees, short-tongued Bombus friseanus (9·87 ± 0·44 mm) and long-tongued B. religiosus (16·13 ± 0·76 mm), were the primary visitors at our study site. These species were the main legitimate pollinators, foraging for nectar by entering flowers through the corolla opening. Bombus friseanus also forages sometimes as a nectar robber, making holes at the base of the tubular corolla and removing nectar without pollinating. A field survey indicated that about 92 % of open individual flowers and 40 % of closed individual flowers were robbed during the season.
Mating system of S. przewalskii
To study the mating system of S. przewalskii, three flowers from one inflorescence of 20 plants (one inflorescence of each plant) were randomly selected when they were in bud. We applied three pollination treatments: (1) bagged without emasculation to detect spontaneous autogamy; (2) self-pollinated with pollen from the same plant to check for selfing ability; and (3) emasculated and pollinated with pollen grains from other plants from at least 30 m away to measure outcrossing ability. All the flowers were hand-pollinated twice during their 2 d longevity (once each day). The inflorescences were bagged before anthesis and until the selected flowers wilted. One flower from another inflorescence of the same plant was also randomly selected for open pollination and served as a control (n = 20). Seed set was calculated as seed number of each fruit divided by the number of ovules. To detect differences in seed set among the four treatments, we used one-way analysis of variance (ANOVA). Tamhane’s T2 (for one-way ANOVA with unequal group variances; calculated by SPSS 22) was used for multiple comparisons when a significant difference was revealed.
Pollination efficiency
To compare the natural pollination efficiency of the two bumble bees, B. religiosus and B. friseanus, we examined pollen deposition on stigmas of previously unvisited flowers after the first visit of the pollinator (Dafni, 2005). Twenty flowers were used for each bumble bee species. The stigma was stained and mounted in 0·2 % auramine O and the total number of pollen grains deposited on the stigma was counted by using an epifluorescence microscope (Nikon E-600). A t-test was used to test the difference between stigmatic pollen loads per visit by B. religiosus and B. friseanus.
Nectar removal efficiency
To examine the foraging efficiency of visitors and robbers, the nectar volume of newly opened flowers (n = 30) was measured and compared with nectar remaining in other flowers after they received the first visit by a legitimate pollinator (n = 30) or a robber (n = 30). The nectar volume was measured with 2 µL capillary micropipettes. One-way ANOVA was conducted to detect the differences in nectar removal efficiency among the three groups.
Dynamics of nectar production
To explore the dynamics of the nectar production of S. przewalskii, we measured the cumulative nectar volume every hour. We bagged 200 unrobbed and closed flowers from 45 inflorescences to exclude flower visitors at 10·00 h the day before opening. We opened the bags and monitored the visitation by pollinators or robbers when flowers were accessible. The flowers were quickly bagged again upon receiving a single bumble bee’s visit. These flowers were cut off to measure the nectar volume at different times (1, 2, 3, 4, 5 and 24 h) after pollination or nectar robbing. The nectar volume was measured with 2 and 5 µL capillary micropipettes. For each time interval, we compared the nectar volume between pollinated and robbed flowers by using a t-test.
Effect of nectar robbing on pollinator behaviour
To test the effects of nectar robbing on pollinator behaviour, we artificially established three groups with different levels of nectar robbing (see also Fumero-Cabán and Meléndez-Ackerman, 2013): (1) all flowers within a plant were manipulated to prevent all nectar robbing (0 % nectar robbing); (2) all flowers within a plant were unmanipulated and exposed to nectar robbers (approx. 100 % nectar robbing); and (3) half of the flowers within a plant were protected from nectar robbing (approx. 50 % nectar robbing). The plants were randomly selected in a 15 × 15 m area and ten plants were assigned to each treatment. To prevent nectar robbing, we used a piece of clear cellophane tape to cover the base of the corolla. This manipulation was finished by 08·00 h, before flower visitors were active. The field experiment continued for 3 weeks, by which time most flowering had finished. For all studied plants, flowers that opened within the period of our field investigations were marked with a thread so we could exclude other flowers in our analyses on fruit and seed set. Those flowers were labelled until fruits were fully mature.
We then observed the behaviour of flower visitors toward S. przewalskii flowers and tested whether legitimate pollinators behaved differently toward plants with different levels of nectar robbing. Observations were conducted over three periods, 10·00 to 12·00 h, 12·00 to 16·00 h and 14·00 to 16·00 h, on three separate days (20, 23 and 26 August) with good weather conditions. Each plant was observed for two 10 min periods on each observation day, and every studied plant therefore was observed for 1 h in total. To ensure that the data in each treatment were collected during the same period on observation days, we designed an observation schedule so that observations on each treatment had the same replicates at any period during field investigations. We recorded the flower handling time of each legitimate pollinator (B. friseanus and B. religiosus) and nectar robber (B. friseanus) on single flowers, noted the number of flowers visited within a plant by each legitimate pollinator (B. friseanus and B. religiosus) for a single foraging bout, and measured the visitation rate by all legitimate pollinators to a plant within an observation period (estimated by number of pollinators per plant per 10 min). In addition, we recorded the visitation rate for each of the two legitimate pollinators to detect whether the effects of nectar robbing affected the activity of the two bumble bees separately.
We used two-way ANOVA to test the effects of treatment (different levels of nectar robbing) and bumble bee species (including foraging behaviour) on flower handling time and flowers visited within a plant for a foraging bout of B. friseanus and B. religiosus in a fixed model. Post-hoc tests (Tukey HSD) were used for multiple comparisons among different treatments and bumble bee species when there was a significant difference. We used a generalized linear model to test the effects of treatment (different levels of nectar robbing) and observation date on visitation rate by all legitimate pollinators in a fixed model under Poisson distribution. In addition, the number of flowers of each studied plant in the observation period was added to the model as a covariate factor to mitigate the effects of flower number on visitation rate.
Effect of nectar robbing on female reproductive success
For each treatment group, we calculated fruit set by dividing all fruits by the number of flowers produced by the plant during the field experiment period. The difference in fruit set among treatment groups was estimated by one-way ANOVA. Additionally, 20 fruits of each plant were randomly collected from the three treatment groups to compare the difference in average number of seeds set by using one-way ANOVA. All the data in this study were analysed with SPSS 22 statistical software. All values are presented as mean ± s.e.
RESULTS
Mating system
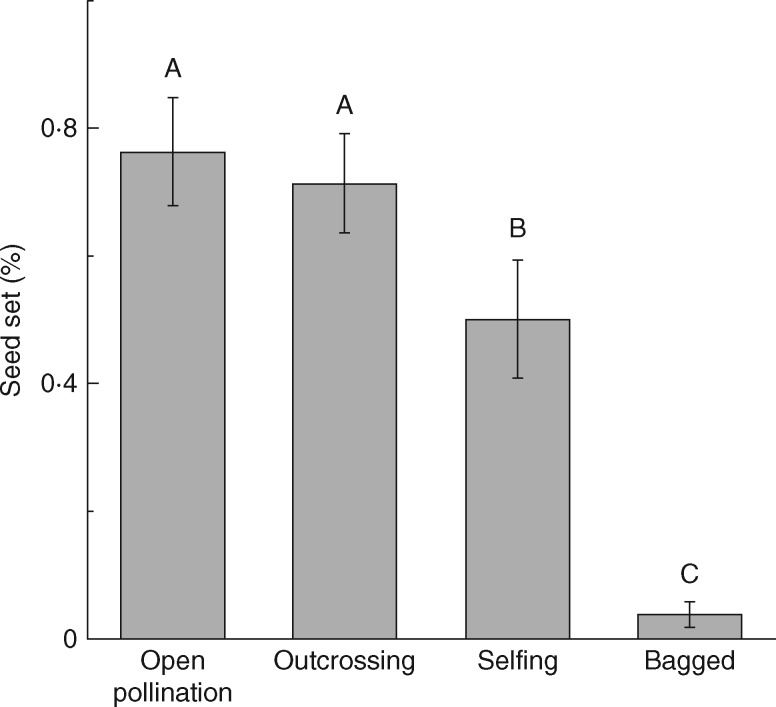
Hand pollination indicated that seed set under different treatments differed significantly (F = 19·83, d.f. = 3, 76, P < 0·001). Multiple comparisons showed that seed set of self-pollinated flowers (0·55 ± 0·09) was lower than flowers with outcross pollination (0·71 ± 0·08) and open pollination (0·76 ± 0·08). In addition, bagged flowers did not set seed at all (Fig. 1).
Fig. 1.
Mean seed set (± s.e.) of Salvia przewalskii under four different pollination treatments: open pollination, outcrossing, selfing and bagged. Different letters indicate that differences are significant at P < 0·05.
Pollination and nectar removal efficiency
Stigmatic pollen load per visit did not differ between B. religiosus (1·2 ± 0·34) and B. friseanus (1·25 ± 0·39) (t = –0·097, d.f. = 38, P = 0·59). The volumes of nectar remaining after one visit did not differ significantly among B. religiosus (1·02 ± 0·32 μL) and B. friseanus (1·17 ± 0·37 μL) as legitimate pollinators and B. friseanus (0·94 ± 0·29 μL) as a nectar robber (F = 0·14, d.f. = 2, 87, P = 0·87).
Dynamics of nectar production
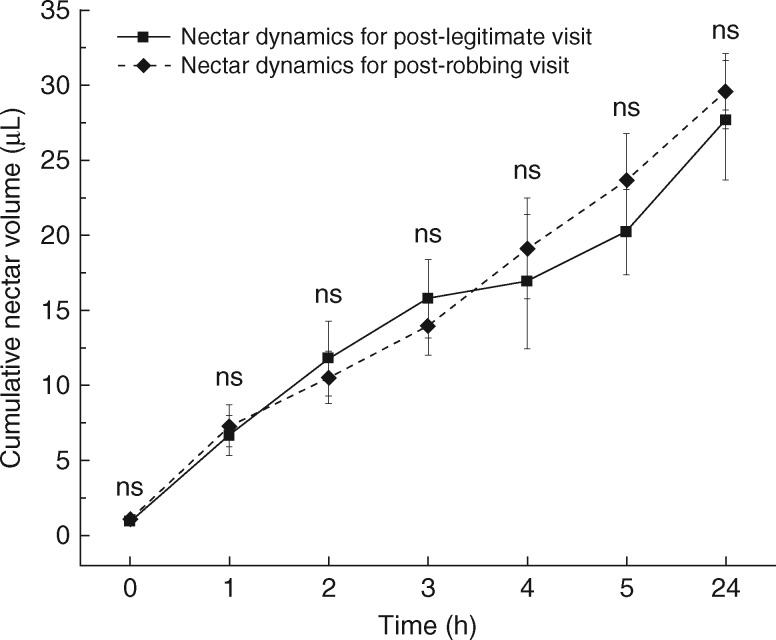
Nectar was continuously produced during the whole of anthesis. However, the nectar was replenished quite quickly during the first 5 h after a visit by a legitimate pollinator or nectar robber (Fig. 2). After that, it was replenished slowly and continued to 24 h after flower opening. There was no significant difference in the temporal pattern of nectar production between robbed and pollinated flowers (0 h, d.f. = 58, t = 0·294, P = 0·77; 1 h, d.f. = 32, t = 0·330, P = 0·74; 2 h, d.f. = 20, t = 0·429, P = 0·67; 3 h, d.f. = 26, t = 0·554, P = 0·58; 4 h, d.f. = 21, t = 0·408, P = 0·69; 5 h, d.f. = 33, t = 0·832, P = 0·41; 24 h, d.f. = 39, t = 0·402, P = 0·69) (Fig. 2).
Fig. 2.
Comparison of nectar production rate at different stages for flowers after receiving a single visit by legitimate visitors or nectar robbers. Data are presented as cumulative nectar volume (μL ± s.e.), and ns indicates that there was no significant difference between flowers of two groups at each stage.
Effect of nectar robbing on pollinator behaviour and female reproductive success
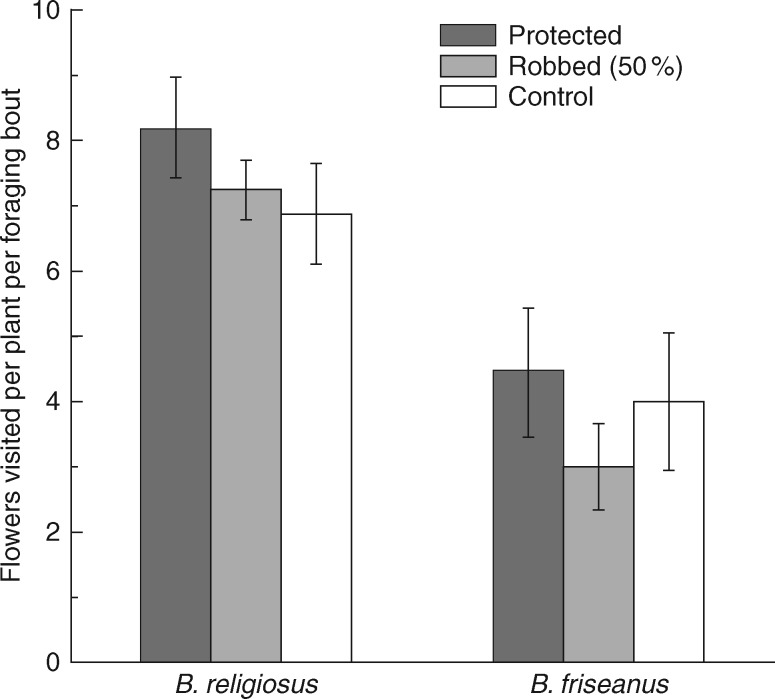
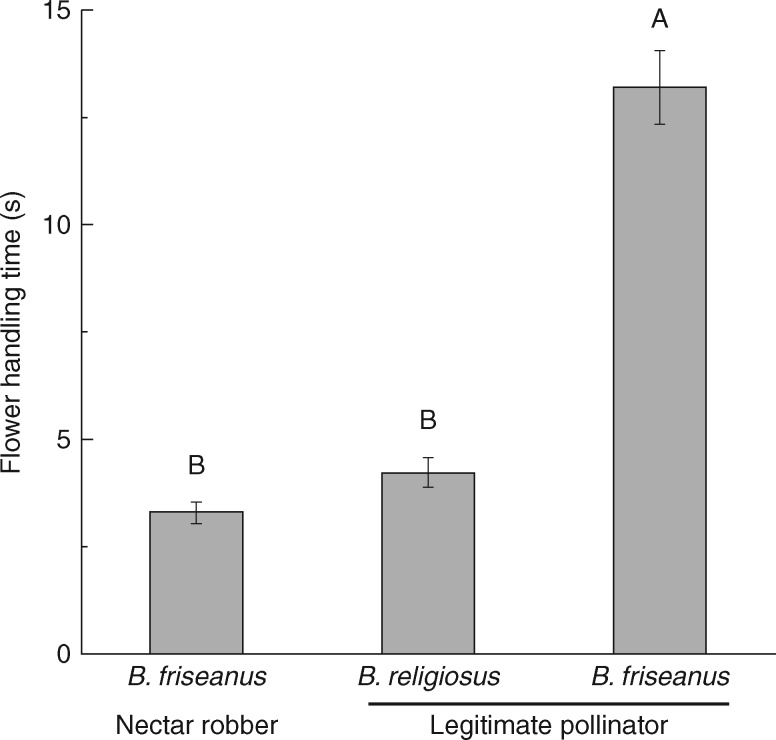
Bombus friseanus, the legitimate pollinator and nectar robber, used only one foraging strategy within a single bout. They did not legitimately visit a flower when accessing the study area as a robber, and vice versa. When robbing flowers, the bumble bees made holes on unrobbed flowers (primary robbing) and also used holes that had already been made (secondary robbing) in a single foraging bout. Two-way ANOVA indicated that flower handling time and flowers visited per plant by a pollinator within a foraging bout were significantly influenced by bumble bee species, but not by treatment (level of nectar robbing) (Table 1). The legitimate pollinators, B. religiosus, visited more flowers per plant within a foraging bout than B. friseanus (Table 1; Fig. 3). Flower handling time of B. religiosus (4·22 ± 0·34 s) was significantly shorter than that of B. friseanus when foraging legitimately (13·19 ± 0·86 s). However, handling time of B. friseanus was significantly shorter when nectar robbing, on flowers with or without a hole (3·29 ± 0·25 s) (Fig. 4). Visitation rates of all bumble bees to a plant within each observation period were not significantly affected by either treatment (level of nectar robbing) or observation date (Table 2). The visitation rate of each of the two legitimate pollinators was also not significantly affected by either treatment or observation date (Table 2). In addition, out of a total of 262 visits by legitimate pollinators, B. religiosus visited 79·00 % of plants that had not been nectar robbed, 71·61 % of those with 50 % nectar robbing and 70·39 % of plants under natural pollination.
Table 1.
Effects of levels of nectar robbing and bumble bee species on flower handling time and flowers visited per plant for a pollinator within a foraging bout by two-way ANOVA under a fixed model
| Flower handling time |
Flowers visited per plant per foraging bout |
|||||
|---|---|---|---|---|---|---|
| F | d.f. | P | F | d.f. | P | |
| Levels of nectar robbing | 0 ·458 | 2 | 0 ·64 | 1 ·301 | 2 | 0 ·28 |
| Bumble bee species | 89 ·59 | 1 | <0 ·0001 | 32 ·25 | 1 | <0 ·0001 |
| Nectar robbing rate × bumble bee species | 0 ·21 | 2 | 0 ·81 | 0 ·39 | 2 | 0 ·68 |
Fig. 3.
Flowers visited per plant for a bumble bee within a foraging bout (± s.e.) by Bombus religiosus and B. friseanus when they legitimately visited plants under treatments, with 0, 50 and 92 % nectar-robbed flowers within a plant.
Fig. 4.
Flower handling time (± s.e.) of Bombus religiosus (the legitimate visitors) and B. friseanus as nectar robber and legitimate visitor. Bars with different letters differ significantly at the P < 0·05 level.
Table 2.
Generalized linear model analysis of the effects of levels of nectar robbing and observation date on visitation rate (number of pollinators per plant per 10 min) of all bumble bees, B. religiosus and B. friseanus
| Total number |
B. religiosus |
B. friseanus |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | d.f. | Sig. | χ2 | d.f. | Sig. | χ2 | d.f. | Sig. | |
| Levels of nectar robbing | 0·158 | 2 | 0·924 | 0·468 | 2 | 0 ·791 | 0·982 | 2 | 0·612 |
| Observation date | 3·941 | 2 | 0·139 | 3·838 | 2 | 0 ·147 | 0·282 | 2 | 0·868 |
| No. of flowers per plant | 0·046 | 1 | 0·831 | 0·013 | 1 | 0 ·91 | 0·076 | 1 | 0·783 |
In this model, number of flowers per plant was set as a covariate factor.
Neither fruit set nor seed set differed significantly among treatments (F = 0·52, d.f. = 2, 142, P = 0·59 for fruit set and F = 0·43, d.f. = 2, 142, P = 0·65 for seed set).
DISCUSSION
In S. przewalskii, outcrossing resulted in more seeds than selfing. The plant was pollinated by a long-tongued bumble bee (B. religiosus) and the short-tongued B. friseanus, but was only robbed by B. friseanus. The robbers made holes on fresh flowers (primary robbing) and also used previously made holes (secondary robbing), but never shifted to legitimate foraging in a single foraging bout. Neither flower handling time nor flowers visited per plant was significantly influenced by nectar robbing, but they were by bumble bee species. In addition, neither visitation rates of both legitimate pollinators nor plant female reproductive success was significantly affected by nectar robbing. No direct or indirect effects of nectar robbing were detected on plant female reproductive success in our study (see also Rojas-Nossa et al., 2016). We found that nectar replenishment of S. przewalskii was quite fast and plentiful, and, moreover, nectar secretion rate did not differ among flowers that were robbed or legitimately visited. We conclude that nectar replenishment can help to maintain the neutral effects of nectar robbing in this system.
Effects of nectar robbing on plant fitness are always mediated by nectar availability and quality (Irwin et al., 2010), and the dynamics of nectar replenishment may play an important role in plant–robber–pollinator interactions (Ordano and Ornelas, 2004; Fumero-Cabán and Meléndez-Ackerman, 2013). For example, timely nectar replenishment may help to relieve the effects of nectar robbing on pollinator attraction (Irwin et al., 2008; Fumero-Cabán and Meléndez-Ackerman, 2013). Considering that nectar production might be costly (Pyke 1991), plants may have different nectar production strategies in response to nectar robbing and pollination. A difference could be mediated by whether pollen was removed or deposited on the stigma (Nepi et al., 2001); a plant might cease nectar production in response to proximate pollination events (Ordano and Ornelas, 2004). However, several experimental studies indicated that nectar secretion was not correlated with stigmatic pollen load (Aizen and Basilio, 1998; Ordano and Ornelas, 2004). Our study also did not find a difference in nectar secretion rate between robbed and legitimately visited flowers, indicating that nectar replenishment of S. przewalskii was not dependent on the type of visit by the bumble bees. The robbed flowers might even increase the nectar secretion rate if evaporation of nectar occurred through the robbing incision (Pleasants, 1983) to match the evaporation rate. Moreover, robbers and legitimate visitors had similar nectar removal efficiency; nectar remaining in a flower was almost removed completely after a single visit. Therefore, legitimate visitors might not distinguish whether a flower was robbed or legitimately visited if nectar is replenished quickly and at a similar rate, making flowers continuously attractive to legitimate pollinators. Nectar replenishment might be a defence mechanism against nectar robbing to enhance reproductive fitness by maintaining attractiveness to pollinators.
The shift of B. friseanus from legitimate pollinator to nectar robber might be a result of competition for nectar resources (Irwin et al., 2010). Robbing can be a more efficient means of taking nectar than legitimate visitation (Pyke, 1982; Irwin and Maloof, 2002; Dedej and Delaplane, 2005; Irwin et al., 2010). In addition, Inouye (1980) suggested that nectar resource intake of bumble bees was significantly influenced by tongue length; nectar robbing can allow short-tongued bees to extract nectar from flowers with corollas longer than their tongues. Although the volume of nectar removed per visit for B. friseanus was similar to that for B. religiosus as legitimate pollinators, the flower handling time of B. friseanus was significantly longer. However, the handling time was significantly shorter when they shifted to nectar robbing. Newman and Thomson (2005) suggested that foraging as a legitimate pollinator should increase the energy cost compared with nectar robbing for a short-tongued bumble bee, and the significantly faster handling time of B. friseanus when nectar robbing compared with legitimate visitation supports this (Fig. 4). Bombus friseanus visits a wide range of plants in east Himalaya and was also reported to shift from legitimate pollinator to secondary nectar robber in Iris bulleyana, an alpine iris with a long corolla tube (Z. M. Ye et al., unpubl. data). Pyke (1982) reported that in the Rocky Mountains of Colorado, the short-tongued bumble bee B. occidentalis foraged on a wide range of plants and shifted from pollinator to nectar robber when foraging on flowers with long corolla tubes (see also Stout et al., 2000; Pyke et al., 2012). Our results are consistent with the findings of Pyke (1982); when foraging on flowers with long corolla tubes, robbing allows B. friseanus to forage with a higher net energy intake due to the decrease in handling time compared with legitimate visits.
Although we expected to find that nectar robbing should influence the behaviours of the bumble bees differently, visitation rates of legitimate pollinators did not differ significantly among plants with different levels of nectar robbing. This might be due to the plentiful nectar remaining in the flowers. Although the visitation rate of both bumble bees as legitimate pollinators was not significantly affected by nectar robbing, we found a clue that the composition of pollinators might be affected by nectar robbing. Bombus religiosus was recorded at a higher frequency in plants without nectar robbing compared with robbed plants, suggesting that nectar robbing might affect its activity. However, the mechanism by which nectar-robbing bumble bees influenced pollinating bumble bees remains unclear since it seems that these interactions were not affected by nectar availability in S. przewalskii. Compared with B. friseanus, B. religiosus visited more flowers within a plant in a single foraging bout, which may have increased geitonogamous mating. In our study site, the possible effects of nectar robbing on pollination of S. przewalskii might be beneficial by changing the pollinator composition. The decrease in activity of B. religiosus in robbed plants might reduce the risk of geitonogamous mating because the plant has higher reproductive success from outcrossing rather than selfing (see also Hazlehurst and Karubian, 2016).
In conclusion, our study indicated that nectar robbing did not significantly alter nectar replenishment. Neither pollinator behaviours nor female reproductive success was significantly influenced by nectar robbing. Nectar replenishment in this species can be regarded as a defence mechanism against nectar robbing to enhance plant reproduction by maintaining attractiveness to pollinators. However, careful experimental and observational research is still needed to uncover the subtleties in pollinator composition caused by nectar robbing, e.g. the potential interference of competition between bumble bee robbers and bumble bee legitimate visitors (Roubik, 1982; Irwin et al., 2010), and whether communities with different composition of bumble bee species influence or are influenced by nectar robbing (Irwin and Maloof, 2002).
ACKNOWLEDGEMENTS
We thank Yan-Wen Zhang and Kuo Liao for valuable suggestions, Mick Hanley and two anonymous reviewers for their helpful and detailed comments and suggestions on an earlier manuscript, Jian Yang, Chui-Xu Wu, Jun Yang and Wen-Kui Dai for assistance in the field, and Chao-Dong Zhu, Jian Yao and Ze-Qing Niu for identifying the bumble bees. The research was supported by the National Natural Science Foundation of China to C.-F.Y. (grant no. 31370263).
LITERATURE CITED
- Aizen MA, Basilio A.. 1998. Sex differential nectar secretion in protandrous Alstroemeria aurea (Alstroemeriaceae): is production altered by pollen removal and receipt? American Journal of Botany 85: 245–252. [PubMed] [Google Scholar]
- Dafni A, Kevan PG, Husband BC.. 2005. Practical pollination biology. Ontario: Enviroquest Ltd. [Google Scholar]
- Dedej S, Delaplane KS.. 2005. Net energetic advantage drives honey bees (Apis mellifera L). to nectar larceny in Vaccinium ashei Reade. Behavior Ecology and Sociobiology 57: 398–403. [Google Scholar]
- Fumero-Cabán JJ, Meléndez-Ackerman EJ.. 2013. Effects of nectar robbing on pollinator behavior and plant reproductive success of Pitcairnia angustifolia (Bromeliaceae). Plant Species Biology 28: 224–234. [Google Scholar]
- Gonzalez-Gomez PL, Valdivia CE.. 2005. Direct and indirect effects of nectar robbing on the pollinating behavior of Patagona gigas (Trochilidae). Biotropica 37: 693–696. [Google Scholar]
- Hazlehurst JA, Karubian JO.. 2016. Nectar robbing impacts pollinator behavior but not plant reproduction. Oikos 125: 1668–1676. [Google Scholar]
- Inouye DW. 1980. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia 45: 197–201. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Maloof JE.. 2002. Variation in nectar robbing over time, space, and species. Oecologia 133: 525–533. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Brody AK, Waser NM.. 2001. The impact of floral larceny on individuals, populations, and communities. Oecologia 129: 161–168. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Galen C, Rabenold JJ, Kaczorowski R, McCutcheon ML.. 2008. Mechanisms of tolerance to floral larceny in two animal-pollinated wildflowers, Polemonium viscosum and Ipomopsis aggregata. Ecology 89: 3093–104. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Bronstein JL, Manson JS, Richardson L.. 2010. Nectar robbing: ecological and evolutionary perspectives. Annual Review of Ecology, Evolution, and Systematics 41: 271–292. [Google Scholar]
- Irwin RE, Howell P, Galen C.. 2015. Quantifying direct vs. indirect effects of nectar robbers on male and female components of plant fitness. Journal of Ecology 103: 1487–1497. [Google Scholar]
- Maloof JE. 2001. The effects of a bumble bee nectar robber on plant reproductive success and pollinator behavior. American Journal of Botany 88: 1960–1965. [PubMed] [Google Scholar]
- Maloof JE, Inouye DW.. 2000. Are nectar robbers cheaters or mutualists? Ecology 81: 2651–2661. [Google Scholar]
- McDade LA, Kinsman S.. 1980. The impact of floral parasitism in two neotropical hummingbird-pollinated plant species. Evolution 34: 944–58. [DOI] [PubMed] [Google Scholar]
- Morris WF. 1996. Mutualism denied? Nectar-robbing bumble bees do not reduce female or male success of bluebells. Ecology 77: 1451–1462. [Google Scholar]
- Nepi M, Guarnieri M, Pacini E.. 2001. Nectar secretion, reabsorption, and sugar composition in male and female flowers of Cucurbita pepo. International Journal of Plant Science 162: 353–358. [Google Scholar]
- Newman DA, Thomson JD.. 2005. Effects of nectar robbing on nectar dynamics and bumblebee foraging strategies in Linaria vulgaris (Scrophulariaceae). Oikos 110: 309–320. [Google Scholar]
- Ordano M, Ornelas JF.. 2004. Generous-like flowers: nectar production in two epiphytic bromeliads and a meta-analysis of removal effects. Oecologia 140: 495–505. [DOI] [PubMed] [Google Scholar]
- Pleasants JM. 1983. Nectar production patterns in Ipomopsis aggregata (Polemoniaceae). American Journal of Botany 70: 1468–1475. [Google Scholar]
- Pyke GH. 1982. Local geographic distributions of bumblebees near Crested Butte, Colorado: competition and community structure. Ecology 63: 555–573. [DOI] [PubMed] [Google Scholar]
- Pyke GH. 1991. What does it cost a plant to produce floral nectar? Nature 350: 59.2002845 [Google Scholar]
- Pyke GH, Inouye DW, Thomson JD.. 2012. Local geographic distributions of bumble bees near Crested Butte, Colorado: competition and community structure revisited. Environmental Entomology 41: 1332–1349. [DOI] [PubMed] [Google Scholar]
- Richardson SC. 2004. Are nectar-robbers mutualists or antagonists? Oecologia 139: 246–254. [DOI] [PubMed] [Google Scholar]
- Rojas-Nossa SV, Sa´nchez JM, Navarro L.. 2016. Effects of nectar robbing on male and female reproductive success of a pollinator-dependent plant. Annals of Botany 117: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubik DW. 1982. The ecological impact of nectar robbing bees and pollinating hummingbird on a tropical srhub. Ecology 63: 354–360. [Google Scholar]
- Singh VK, Barman C, Tandon R.. 2014. Nectar robbing positively influences the reproductive success of Tecomella undulata (Bignoniaceae). PLoS One 9: e102607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Allen JA, Goulson D.. 2000. Nectar robbing, forager efficiency and seed set: bumblebees foraging on the self incompatible plant Linaria vulgaris (Scrophulariaceae). Acta Oecologica 21: 277–283. [Google Scholar]
- Traveset A, Willson MF, Sabag C.. 1998. Effect of nectar-robbing birds on fruit set of Fuchsia magellanica in Tierra del Fuego: a disrupted mutualism. Functional Ecology 12: 459–464. [Google Scholar]
- Wu ZY, Li XW.. 1977. Flora Reipublicae Popularis Sinicae, vol.65 (2), Labiatae: SALVIEAE. Beijing: Science Press. [Google Scholar]
- Zhang YW, Robert GW, Wang Y, Guo YH.. 2007. Nectar robbing of a carpenter bee and its effects on the reproductive fitness of Glechoma longituba (Lamiaceae). Plant Ecology 193: 1–13. [Google Scholar]
- Zhang YW, Yu Q, Zhao JM, Guo YH.. 2009. Differential effects of nectar robbing by the same bumble-bee species on three sympatric Corydalis species with varied mating systems. Annals of Botany 104: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Zhao JM, Yang CF, Gituru WR.. 2011. Behavioural differences between male and female carpenter bees in nectar robbing and its effect on reproductive success in Glechoma longituba (Lamiaceae). Plant Biology 13: 25–32. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Cook S.. 1985. Pollinator foraging, experimental nectar-robbing and plant fitness in Impatiens capensis. American Midland Naturalist 113: 84–91. [Google Scholar]