Abstract
Background and Aims In plants, extensive intra-specific variation exists in the allocation of resources between vegetative growth and reproduction, reflecting different functional strategies. A simple method for the classification of intra-specific variation in these strategies would enable characterization of evolutionary and ecological processes.
Methods C–S–R theory can be applied to classify functional strategies (competitive C; stress tolerant, S; ruderal, R) in different plant species. Using a diverse set of arabidopsis (Arabidopsis thaliana) accessions grown under common conditions, it was tested whether a simple approach designed for allocating C–S–R strategies at the species level can also be used to analyse intra-specific variation.
Key Results Substantial intra-specific variation between arabidopsis accessions was found along the S–R axis. There was a positive correlation of temperature at the geographical origin with the dimension of S and a negative correlation with the dimension of R. Flowering time in a natural annual cycle and leaf dry matter content were identified as the main determinants of this adaptation, with plants originating from warmer climates having a higher leaf dry matter content and flowering earlier in a common garden.
Conclusions It was shown that functional strategies reflect adaptation to climate, with consequences for important traits such as fecundity and total plant dry weight. The approach could be used in genome-wide association studies to determine the genetic basis of functional strategies in wild species or crops.
Keywords: Arabidopsis thaliana, C–S–R theory, life history, resource allocation, ruderality, senescence, stress tolerance, world-wide leaf economics spectrum
INTRODUCTION
Plants exhibit substantial intra-specific genetic variation in the developmental processes that determine resource allocation. A trade-off between the allocation of resources for vegetative growth and reproduction has been described in arabidopsis: genotypes with large plant sizes produce fewer fruits and seeds (Aarssen and Clauss, 1992; Levey and Wingler, 2005). More recently, the genetic basis of intra-specific variation in biomass allocation has been analysed in arabidopsis (e.g. Chardon et al., 2014) and in crops (e.g. Luo et al., 2015). The allocation of biomass between seed formation and vegetative growth determines harvest index and has therefore been optimized by crop breeding. In addition, intra-specific variation underlies evolutionary adaptations to environmental change and the response of plants to stress. For example, arabidopsis accessions with earlier flowering dates have lower water-use efficiencies (McKay et al., 2003; Kenney et al., 2014). In some crops, such as oilseed rape, resource allocation in response to stress has not been optimized, which can lead to pod abortion. This suggests that there is a need not only to focus on resource allocation to the seeds under optimal conditions, but also to optimize plant strategies in response to stress (Bennett et al., 2012).
Analysis of intra-specific genetic variation in resource allocation has mainly focused on individual traits and their combinations, but not on overall plant functional strategy. At the species level, the C–S–R theory was developed to assign functional types according to three primary strategies: competitive (C), stress tolerant (S) and ruderal (R) (Grime, 1977). The theory assumes that competitive plants evolved under low stress and low disturbance, stress-tolerant plants under high stress and low disturbance, and ruderal plants under low stress and high disturbance. Grime (1977) defined stress as the shortage of resources (light, water and nutrients) and sub-optimal temperature resulting in low rates of growth, whereas disturbance results from physical damage to the vegetation. The characteristics underlying the functional strategies include maximum growth rate, size, leaf longevity and seed production. Competitive plants are characterized by rapid maximum growth rates, large biomass resulting from an extended period of growth, relatively short-lived leaves and a small investment of resources in seed production. Stress-tolerant plants are similarly long-lived but slow-growing, have long-lived leaves and a small investment in seed production. Ruderal plants are short-lived, can show rapid maximum growth rates, have short-lived leaves and a large investment in seed production. These primary strategies represent the extremes, and C–S–R classification reflects the full spectrum of possible intermediate types. For the classification of functional strategies in different plant species, standardized screening protocols were developed by Grime et al. (1997). These protocols were subsequently simplified to allow easy assignment of functional types under field conditions (Hodgson et al., 1999). While C–S–R theory was originally applied to British plant species, Cerabolini et al. (2010) showed that the C–S–R classification system of Hodgson et al. (1999) can be applied outside Britain.
As pointed out by Bilton et al. (2010), classification of plant strategies is complicated by intra-specific trait variation. Application of standard protocols at the within-species level would allow rapid initial screening for variation in overall functional strategies which may be related to important traits such as invasiveness, fitness and resource allocation in crops (Bennett et al., 2012). It would also enable analysis of the genetic basis of functional strategies in genome-wide association studies. However, procedures for the classification of plant functional strategies by C–S–R theory have not been routinely applied for characterizing intra-specific genetic variation. Some work has been published discussing the predictions of C–S–R theory in the context of intra-specific variation in life-history traits. For example, in wild mustard (Sinapis arvensis), a shift towards stress escape by earlier flowering (i.e. increased ruderality) was found during selection under stressful conditions (Stanton et al., 2000). Volis et al. (2004) determined the competitive ability of different populations of wild barley (Hordeum spontaneum) and found that plants from more favourable environments are better competitors than those from stressful environments. Similarly, Berger and Ludwig (2014) found that Lupinus luteus plants from high rainfall habitats have competitive traits, whereas those from drought-prone habitats have more ruderal traits. In these publications, it is discussed that the findings are in agreement with C–S–R theory, but intra-specific classification of strategies was not attempted. Pierce et al. (2013) classified individual plants of species in the genus Poa, showing variation within populations, but without further investigation of ecological relevance.
Using global data for plant functional traits, theories explaining plant functional and life-history strategies at the global level have been developed, such as the world-wide leaf economics spectrum (Wright et al., 2004), the global spectrum of plant form and function (Díaz et al., 2016) and the fast–slow continuum (Salguero-Gómez et al., 2016). The world-wide leaf economics spectrum ranges from a potential for a quick return of investment [associated with low leaf mass per area (LMA), high rates of photosynthesis per area and short life span] to slow return of investment (associated with high LMA, low rates of photosynthesis and long life span). One commonality of the world-wide leaf economics spectrum and in C–S–R theory is, for example, the importance assigned to the trait LMA, or its inverse, specific leaf area (SLA). A connection between the world-wide leaf economics spectrum and C–S–R theory reflected in SLA was, for example, described for an arid steppe community in Morocco (Frenette-Dussault et al., 2012) and for grasses in northern Italy (Pierce et al., 2007). More recently, leaf traits were used to calculate C–S–R strategies across biomes (Pierce et al., 2016). Considerable intra-specific variation in leaf economics traits such as SLA was found for environmental gradients (e.g. Albert et al., 2010a; De Frenne et al., 2011) and in meta-analyses (Read et al., 2014; Siefert et al., 2015); however, because of the strong response of this trait to some environmental conditions (Poorter et al., 2009, 2010), the described variation may be due to phenotypic plasticity rather than genetic differences, thus reflecting acclimation rather than adaptation.
The validity of methods for the classification of functional strategies can be assessed by analysing the relationship between the assigned type and other functional and life-history traits that were not directly included in the classification process. Leaf senescence is a key trait in the life history of plants as it is required for the recycling of nutrients, such as nitrogen, to support the growth and photosynthesis of new leaves and reproductive organs. Exploiting natural variation in arabidopsis, it was shown that there is a trade-off between investment in vegetative and reproductive development, probably determined by leaf senescence (Levey and Wingler, 2005) and that there is a genetic link between the timing of leaf senescence and flowering (Wingler et al., 2010; Chardon et al., 2014). While Masclaux-Daubresse and Chardon (2011) showed that, during the reproductive stage, there is no clear association between leaf senescence and the efficiency of nitrogen allocation to arabidopsis seeds, a positive interaction was found between leaf senescence and percentage seed carbon content (Chardon et al., 2014). This finding can possibly be explained by rosette leaf senescence supplying nitrogen for photosynthesis in the green inflorescence, instead of direct nitrogen allocation to the seeds. Leaf life span is an important factor in the leaf economics spectrum (Wright et al., 2004; Shipley et al., 2006) and was included as a trait in screening protocols for C–S–R classification (Grime et al., 1997), but is not a trait that can easily be determined in the field, and it is not included in the method of Hodgson et al. (1999). If a clear relationship between leaf senescence and C–S–R classification can be established, it may not be necessary to quantify the start and rate of senescence per se to establish its importance for functional strategy.
Arabidopsis has been classified as a stress-tolerant ruderal (Grime et al., 1988; Hodgson et al., 1999), but shows considerable variation in life-history traits. In this study, we make use of natural variation in arabidopsis to test if intra-specific variation in functional strategies can be determined using a simple system for allocating functional types developed by Hodgson et al. (1999). We explore how the assigned types are related to other life-history traits, such as leaf senescence and fecundity. In addition, we investigate the relationship between functional strategies and climate at the geographic origin of the arabidopsis accessions.
MATERIALS AND METHODS
Plant material
The experiments were performed with 16 accessions of Arabidopsis thaliana (L.) from a nested core collection representing genetic and morphological diversity (McKhann et al., 2004), but Ita-0 was replaced with Sy-0 (see Supplementary Data Table S1 for accessions used, and Fig. 1 for geographic origin). Seeds were stratified for 4–5 d at 4 °C in 0·3 % agarose before sowing onto compost (Miracle-Gro All Purpose Enriched Compost). The plants were then grown under controlled conditions for determination of most traits, but outside in a common garden to determine annual flowering dates. For cultivation under controlled conditions, the plants (eight plants per accession) were grown in individual pots at 20 °C and a 16 h photoperiod at a photon flux density of 130 μmol m–2 s–1. For cultivation in a common garden, the plants were grown over two generations under outdoor conditions on a roof in Central London, UK (latitude = 51·523, longitude = –0·133). This allowed plants to set and shatter seed naturally before flowering was determined in the second generation during the following year. The vernalization-dependent accessions Sy-0 and Blh-1 were vernalized before further cultivation. After germination at 20 °C for 2 d, the seedlings were vernalized for 8 weeks (5 °C and a 12 h photoperiod at a photon flux density of 50 μmol m–2 s–1). Before planting outside, all accessions were grown under controlled conditions (20 °C and a 12 h photoperiod at a photon flux density of 130 μmol m–2 s–1) to reach a comparable developmental stage (12 d after vernalization for Sy-0 and Blh-1; 27 d after sowing for the other accessions). From 30 June 2014, the plants were grown outside. Once flowering started, the pots (two replicate pots with four plants each) were covered with transparent and breathable bread bags to avoid cross-pollination and the spread of seeds into other pots. The plants were allowed to set and shatter seeds. Vernalization of the second generation occurred naturally over the winter. Excessive seeds and seedlings were removed from the soil in January 2015, and flowering (opening of the first flower) and duration of the flowering period were recorded throughout the spring of 2015 (see Table S1 for flowering dates).
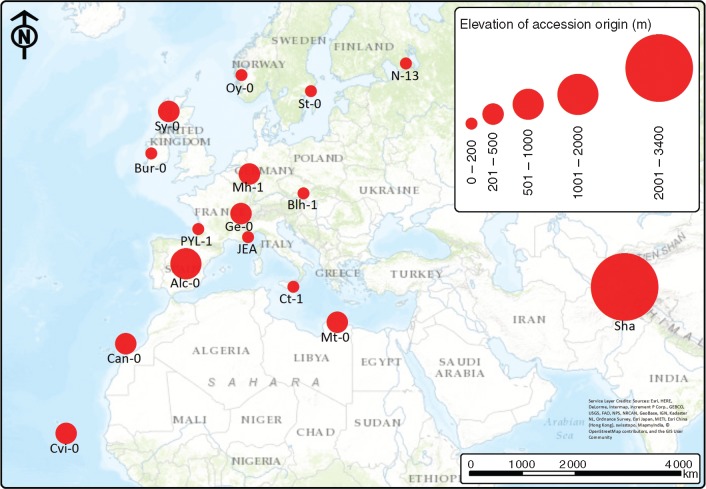
Fig. 1.
Origin of the arabidopsis accessions used in this study. The size of the circle reflects elevation.
C–S–R classification
The C–S–R classification was performed as in Hodgson et al. (1999) using the following parameters: SLA, leaf dry matter content (LDMC) of fully hydrated leaves, leaf dry weight, canopy height, lateral spread, flowering start and flowering period. This method requires information on during which month of the year flowering starts, which cannot be determined in growth chambers. Exact dates are not required for the classification, and Hodgson et al. (1999) recommend that the months during which flowering occurs can be obtained from the literature. However, this information is not readily available for specific arabidopsis accessions from a wide range of geographic origins. Flowering start and flowering period were therefore determined under a natural annual cycle in a common garden as described above, while the other parameters were determined after growth under controlled conditions. Leaf parameters were determined in the largest leaves (including petioles), SLA after 46 d, and leaf dry weight and LDMC after 61 d. Triangulation was performed as in Hunt et al. (2004); the relative contribution of the three C–S–R dimensions was calculated (adding up to one in total) and ternary plots were created, with the apices of the triangle representing the value of one and opposite sides zero.
Determination of other parameters
Additional parameters were recorded for the plants grown under controlled conditions to characterize related traits: the first day of flowering, the total plant dry mass and the number of seeds per plant. To calculate the seed number, the number of seeds per fruit was counted for three fruits per plant and multiplied by the number of fruits per plant. In addition, senescence was monitored as the decline in maximum photosystem II (PSII) efficiency (Fv/Fm) in whole rosettes using a FluorCam 700MF imaging fluorometer (Photon Systems Instruments, Brno, Czech Rebublic) as described in Wingler et al. (2004). The plants were incubated in the dark for at least 20 min before the measurements. Quadratic equations (y = ax2 + bx + c) were fitted to the Fv/Fm values plotted against time. All curves, other than for the accession Ge-0 (not included in further analyses), provided a good fit (R2 between 0·92 and 1·00).
Geographic origin and climate data
The geographic origin of the accessions was obtained from The Arabidopsis Information Resource (TAIR; www.arabidopsis.org) and the Arabidopsis 1001 Genome project (http://1001genomes.org/). In cases of missing data or discrepancies between the described site and the co-ordinates given, co-ordinates were reconstructed from the site descriptions (Table S1, and visualized in Fig. 1). Climate data for each site were obtained from WorldClim at 30 arc-second resolution (www.worldclim.org, Version 1.4), producing gridded data at approx. 1 km resolution. Temperature and precipitation data are based on monthly means, with minimum temperature given for the coldest and maximum temperature for the warmest month. Climate data were imported into ArcMap 10.2 (ESRI, USA), and values for each climate layer obtained at accession co-ordinate locations.
Statistics
Eight replicate plants were grown per accession. The data were averaged for each accession before further analysis, apart from flowering dates under outdoor conditions where first and last flowering dates were recorded. To compare the accessions, analysis of variance (ANOVA) followed by Tukey’s pairwise comparison was performed in SPSS22 (IBM). For continuous parameters, Pearson and Spearman rank correlation analysis were performed (using SPSS22, IBM); for the dimensions of C, S and R, only non-parametric Spearman rank correlation analysis was included. Multiple regression analysis was performed in Minitab 16.
RESULTS
C–S–R classification of arabidopsis accessions
To test if C–S–R classification can be applied for the characterization of intra-specific variation in functional strategies, we grew 16 accessions from a nested core collection representing genetic and morphological diversity (McKhann et al., 2004), but replacing Ita-0 with Sy-0 (see Table S1 for accessions used). ANOVA showed significant differences between the accessions, but also considerable overlap in the trait values (Supplementary Data Table S2). C–S–R classification was performed as in Hodgson et al. (1999). Flowering start and period were determined in a common garden experiment, growing the plants under outdoor conditions in Central London where the accessions started flowering between the end of February (Cvi-0) and mid-April (Table S1).
As expected, the majority of arabidopsis accessions were classified as stress-tolerant ruderal (SR). However, there was a wide spread of ruderal to stress-tolerant characteristics (Fig. 2; Table S3).
Fig. 2.
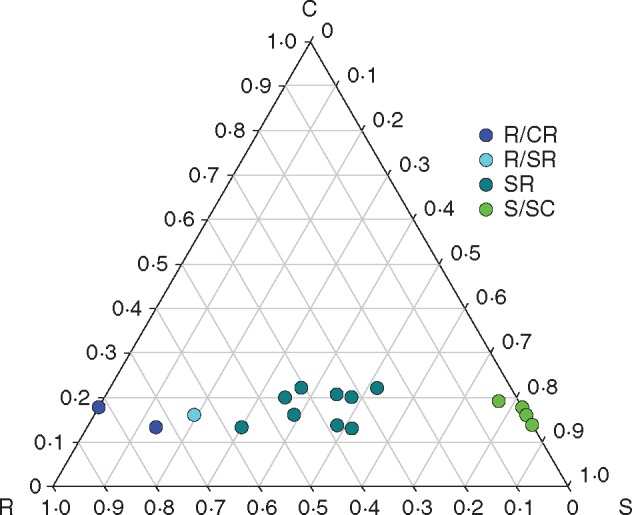
C–S–R classification of arabidopsis accessions. The ternary graph shows the relative contribution to each of the dimensions calculated as in Hunt et al. (2004). Values for each accession are given in Supplementary Data Table S3.
Relationship between C–S–R classification and climate
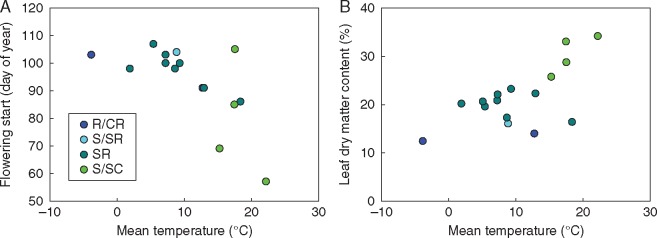
It is striking that the three accessions that clustered at the stress-tolerant end of the spectrum (Fig. 2) all originated from hot locations (Mt-0 from Libya, Cvi-0 from the Cape Verde Islands and Ct-1 from Sicily) (Fig. 1; Table S1). Correlation analysis (Table 1) revealed statistically significant correlations between temperature (minimum, maximum and mean annual temperature) at the site of origin and the dimensions of S (positive) and R (negative). For the dimension of R, negative correlations with temperature were statistically significant for all months of the year, and the most significant correlation was found for the maximum temperature during the growing season in May (Spearman rank correlation: r = –0·719, P = 0·002). For S, correlations were also significant for most of the year (but not in February, March, November and December), with the most significant correlation found for the minimum temperature in August (Spearman rank correlation: r = 0·664, P = 0·005). No correlations were found with precipitation. Analysing the relationship between climate at the site of origin and individual traits revealed that plants from warmer climates flowered earlier under outdoor conditions and had a higher LDMC (Fig. 3; Supplementary Data Table S4). SLA was not correlated with any of the climate variables.
Table 1.
Spearman rank correlation analysis between climatic parameters at the site of origin of the accessions and the C–S–R dimension
| C dimension | S dimension | R dimension | Precipitation | Min. temp. | Max. temp. | |
|---|---|---|---|---|---|---|
| S dimension | r = 0·183 | |||||
| P = 0·496 | ||||||
| R dimension | r = –0·255 | r = –0·855 | ||||
| P = 0·340 | P = 0·000 | |||||
| Precipitation | r = 0·348 | r = –0·249 | r = 0·402 | |||
| P = 0·187 | P = 0·353 | P = 0·122 | ||||
| Min. temp. | r = –0·227 | r = 0·535 | r = –0·564 | r = –0·387 | ||
| P = 0·397 | P = 0·033 | P = 0·023 | P = 0·139 | |||
| Max. temp. | r = –0·246 | r = 0·559 | r = –0·611 | r = –0·743 | r = 0·607 | |
| P = 0·358 | P = 0·024 | P = 0·012 | P = 0·001 | P = 0·013 | ||
| Mean temp. | r = –0·269 | r = 0·535 | r = –0·618 | r = –0·626 | r = 0·905 | r = 0·821 |
| P = 0·313 | P = 0·033 | P = 0·011 | P = 0·009 | P = 0·000 | P = 0·000 |
Fig. 3.
Correlation between the mean annual temperature at the site of origin and the start of flowering (A) and leaf dry matter content (B). Spearman rank correlation analysis for mean temperature vs. flowering start: r = –0·432, P = 0·095; and for mean temperature vs. leaf dry matter content: r = 0·538, P = 0·031. Pearson correlation analysis for mean temperature vs. flowering start: r = –0·605, P = 0·013; and for mean temperature vs. leaf dry matter content: r = 0·652, P = 0·006. See also Table S4 for correlations.
Relationship between fecundity, plant size and C–S–R functional type
To determine if the classification provides a realistic functional characterization of the accessions used, how the C–S–R functional strategies relate to fecundity was tested. As expected, there was a positive correlation between the number of seeds produced and the dimension of R, validating the approach (Spearman rank correlation: r = 0·565, P = 0·035; Table S4). In addition, total plant dry weight was positively correlated with the dimension of C (Spearman rank correlation: r = 0·862, P = 0·000). Multiple regression models were used to test the contribution of parameters not directly included in the C–S–R classification (total dry mass of plant, days to flowering under laboratory conditions, rosette diameter and the number of seeds per plant) to the three dimensions (Table 2). The model for prediction of the dimension of C was highly statistically significant, with total dry mass (positively), flowering start under controlled conditions in the laboratory (negatively) and number of seeds (negatively) contributing significantly to the prediction. The model for the dimension of R provided a moderate fit, with the number of seeds contributing positively to the prediction.
Table 2.
Multiple regression models describing the relationship between the C–S–R dimensions and the parameters: total dry mass of plant (TDM), flowering start under laboratory conditions (FlL), rosette diameter (RD) and the number of seeds per plant (Seeds)
| C dimension | S dimension | R dimension | |
|---|---|---|---|
| Equation | = –2·048 + 1·163 × TDM – 0·002 × FlL + 0·0016 × RD – 0·002 × Seeds | = 0·135 – 0·852 × TDM – 0·040 × FlL + 0·042 × RD – 0·021 × Seeds | = –2·324 – 0·338 × TDM + 0·026 × FlL – 0·035 × RD + 0·025 × Seeds |
| Fit of model | R2 = 0·86, P = 0·001 | R2 = 0·53, P = 0·108 | R2 = 0·60, P = 0·062 |
| Total dry mass of plant (TDM; g) | P = 0·002 | P = 0·875 | P = 0·943 |
| Flowering start lab (FlL; days) | P = 0·036 | P = 0·030 | P = 0·087 |
| Rosette diameter (RD; mm) | P = 0·457 | P = 0·319 | P = 0·342 |
| No. of seeds (Seeds) | P = 0·001 | P = 0·038 | P = 0·011 |
P-values for variables that contribute significantly to the regression models are highlighted in bold font.
Relationship between the senescence-dependent decline in Fv/Fm and C–S–R functional type
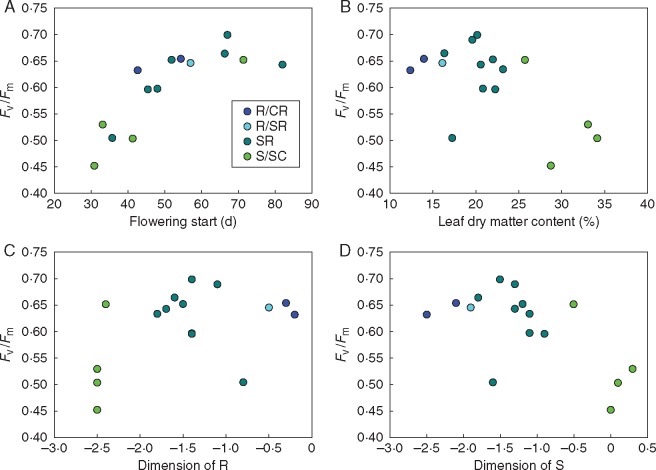
To determine how leaf senescence is related to position along the S–R axis, we monitored the decline in maximum PSII efficiency (Fv/Fm) in the whole leaf rosettes over a period from day 37 to day 72. At day 37, before the start of senescence, Fv/Fm did not correlate with any other measured parameters or the C–S–R dimensions (Table S4). Later in development (day 58), when senescence started, Fv/Fm was positively correlated with flowering start under controlled conditions (Fig. 4A), showing that late flowering is linked to whole-plant senescence. At this time point, Fv/Fm was also negatively correlated with LDMC (Fig. 4B) and with the dimension of S (Fig. 4D). The three accessions with the most stress-tolerant characteristics had a low Fv/Fm which is surprising as they would be expected to senesce later. However, this relationship broke down during later stages (days 62 and 72). Instead, Fv/Fm during late development was positively correlated with dry mass of the largest leaf and negatively with SLA (Table S4). Quadratic equations were fitted to the Fv/Fm values plotted against time. A moderate negative correlation between the coefficient a and the dimension of R (Spearman rank correlation r = – 0·549, P = 0·034) shows that a stronger downwards curvature of the Fv/Fm curves was associated with increasing ruderality, indicating faster development and a shorter lifespan (Table S4).
Fig. 4.
Correlation between whole-rosette maximum photosystem II efficiency (Fv/Fm) measured at the start of senescence (day 58) and flowering start under controlled conditions (A), leaf dry matter content (B), the dimension of R (C) and the dimension of S (D). Spearman rank correlation analysis for Fv/Fm vs. flowering start under controlled conditions: r = 0·811, P = 0·000; Fv/Fm vs. leaf dry matter content: r = –0·512, P = 0·043; Fv/Fm vs. dimension of R: r = 0·373, P = 0·155; Fv/Fm vs. dimension of S: r = –0·530, P = 0·035. Pearson correlation analysis for Fv/Fm vs. flowering start under controlled conditions: r = 0·793, P = 0·001; Fv/Fm vs. leaf dry matter content: r = –0·589, P = 0·016. See also Table S4 for correlations.
DISCUSSION
The analysis presented here shows that it is possible to classify intra-specific natural variation in arabidopsis functional strategies (Fig. 2). Previous classifications at the species level are therefore simplifications that do not represent the spectrum of intra-specific natural variation. The position of arabidopsis accessions along the S–R axis is dependent on climate at the geographic site of origin (Table 1), with flowering time and LDMC contributing to the adaptation to temperature (Fig. 3). Further analyses demonstrated that functional types also reflect traits not included in the classification, such as fecundity and total plant size (Table 2; Table S4) and photosynthetic efficiency during leaf senescence (Fig. 4).
Differences in functional strategy demonstrate adaptation to geographic origin
As the accessions were all grown under the same conditions for C–S–R classification, the properties are likely to be adaptations to the climate at the geographic origin. Accessions from hotter climates clustered towards the stress-tolerant end of the spectrum (Fig. 2; Table 1), which suggests that plants from hotter climates have a constitutive stress tolerance strategy that affects their growth even when cultivated under favourable conditions. It was shown that the Cvi accession has a constitutive high-temperature phenotype (Sanchez-Bermejo et al., 2015) which is consistent with its position towards the S corner of the triangle (Fig. 2). It is also possible that the growth conditions (mild temperature, high nutrient supply in the compost and ample supply of water) may have caused stress in the accessions from hot, dry climates, which is in agreement with the generally poor growth of the Mt-1 accession under controlled conditions in the laboratory. Adaptation of functional traits to climate at the site of origin has, for example, been demonstrated for elevational gradients (Montesinos-Navarro et al., 2011; Wingler et al., 2015; Vigidal et al., 2016), and we show here that such adaptations extend beyond individual traits and reflect overall strategy.
Laboratory-based (Hendry and Grime, 1993; Grime et al., 1997) and field-based (Hodgson et al., 1999) classification methods have been developed. If these methods are used for the classification of plants from a small geographic area, the impact of climate differences in the natural habitat on the traits is likely to be small. However, on a wider geographic scale, as in this study (Fig. 1), determination of the traits in the natural habitat in the field would not have made it possible to differentiate between acclimation and genetic adaptation. In addition, when analysing more subtle differences between genotypes within a species, it is more important to minimize environmental variation. We show here that the method of Hodgson et al. (1999) that was developed for classification of plants growing in their natural habitat can also be used to investigate adaptation to climate by cultivating plants under common conditions.
Demonstrating ecological adaptation of C–S–R functional strategy within a species to climate, our findings address criticisms of the C–S–R theory raised by Wilson and Lee (2000) that predictions made based on the theory are often untestable and that it is of limited suitability in population ecology. A simplified method of C–S–R classification using three leaf parameters (SLA, LDMC and leaf area) was developed by Pierce et al. (2013), and it was shown that this method can be applied to classify species across biomes (Pierce et al., 2016). Correlation of C–S–R strategies with environmental parameters differed between biomes; a positive correlation of mean annual temperature with S and a negative correlation with R was, for example, found in the desert biome (Pierce et al., 2016). Another recently developed method for C–S–R classification demonstrated that differences in the rates of photosynthesis can be used to classify ruderal and stress-tolerant species (Novakovskiy et al., 2016).
Leaf dry matter content and flowering start are important traits that determine functional strategy dependent on geographic origin
Large intra-specific natural variation in functional traits has also been described by Siefert et al. (2015), who show that traits relating to leaf economics, such as nutrient content, SLA and LDMC, show higher intra-specific variability than size-related traits. However, this meta-analysis does not differentiate between phenotypic plasticity and genetic adaptation.
Functional type along the S–R axis was dependent on temperature at the site of origin of the arabidopsis accessions (Table 1). We did not find a correlation of SLA with temperature in arabidopsis plants grown under controlled conditions (Table S4). Summarizing results from common garden experiments, Read et al. (2014) found that three-quarters of the studies demonstrated differences in SLA in populations from different elevations or latitudes, suggesting that there is a genetic basis for intra-specific SLA variation in response to climate, but not in all species. Plants also show extensive phenotypic plasticity in SLA (Poorter et al., 2010), which can result in considerable differences between indoor- and outdoor-grown plants, in particular due to lower light intensities and often higher temperatures in growth chambers (Poorter et al., 2016).
Based on large differences in SLA between species in open and shaded habitats in northern Europe, Novakovskiy et al. (2016) recommend that low SLA should not be used as an indicator for stress-tolerant species. In addition, Wilson et al. (1999) argue that LDMC is a better predictor of resource-use strategy than SLA as it only depends on leaf composition and not a combination of composition and thickness. Our work suggests that LDMC, together with flowering start, is an important determinant of adaptation of functional strategy to temperature (Fig. 3). In the method of Hodgson et al. (1999), flowering start contributes positively to R, while LDMC contributes positively to the dimension of S and negatively to R. This is consistent with our finding that plants from warmer climates flowered earlier, had a higher LDMC and their functional strategy was shifted towards the S end of the spectrum (Table 1). LDMC in the field was correlated with temperature in the grass Phragmites australis, but only in temperate-humid regions in China (Hu et al., 2015). Albert et al. (2010b) argue that responses of LDMC to temperature along an altitudinal gradient are consistent with a bell-shaped response. These responses could, however, be due to acclimation rather than adaptation, while our findings suggest that LDMC is an ecologically meaningful variable reflecting plant adaptation to climate. Non-climate environmental factors, such as soil fertility, have a strong effect on leaf traits (Pakeman et al., 2013) but could not be considered here as information is not available for the sites of origin of the arabidopsis accessions.
Photoperiod length is an important cue controlling arabidopsis flowering time. In our experiment, the daylength when plants started flowering in an annual cycle was positively correlated with latitude of the geographic origin (Fig. 5); the Cvi-0 accession from the Cape Verde Islands flowered considerably earlier than the other accessions. Arabidopsis flowering time was also correlated with latitude in other studies when the plants were grown under controlled conditions (Kooke et al., 2016) or in a common garden (Stinchcombe et al., 2004). This correlation could be dependent on adaptation to photoperiod or temperature. Later flowering in plants from higher elevations suggests a role for temperature in flowering control (Vigidal et al., 2016). In addition, it has been proposed that vernalization is responsible for latitude-dependent flowering (Stinchcombe et al., 2004). However, in contrast to previous studies, which determined flowering time from the day of planting, we monitored the day of the year when flowering occurred under a natural annual cycle. As all plants had experienced winter, it is less likely that different vernalization requirements played a role in our experiment. Intriguingly, the flowering time in the natural annual cycle was not correlated with the time to flowering under controlled conditions without vernalization, indicating that a lack of vernalization can mask the effect of other factors, such as photoperiod and temperature.
Fig. 5.
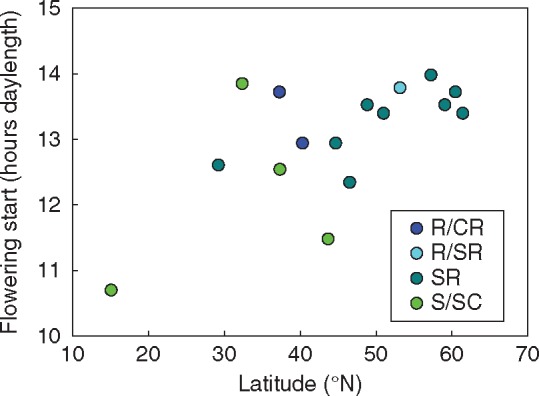
Correlation between latitude of the geographic origin and daylength at which the plants started flowering in a natural cycle under outdoor conditions. Spearman rank correlation analysis: r = 0·434, P = 0·093. Pearson correlation analysis: r = 0·638, P = 0·008. See also Table S4 for correlations.
C–S–R functional types reflect important life-history traits
Importantly, our results also show that traits that are not included as predictors in the C–S–R classification method of Hodgson et al. (1999) are reflected in the functional strategies that were assigned: as expected, the total plant dry weight was positively correlated with the dimension of C and the number of seeds with the dimension of R (Table S4). As these findings are in agreement with the assumptions of C–S–R theory, they confirm that a simple method, based on other predictors that can easily be determined, can be used for the intra-specific assignment of functional types, without having to measure all traits.
We also addressed the question of how leaf senescence may be related to C–S–R classification. According to C–S–R theory, leaf life span is long for stress-tolerant species and short for ruderals (Grime, 1977). In the context of the leaf economics spectrum, this is representing a trade-off between photosynthesis, construction costs and leaf life span (Shipley et al., 2006). As subtle differences in individual leaf longevity are difficult to assess in short-lived arabidopsis leaves, we measured the decline of whole-rosette Fv/Fm values which reflects the extent of senescence in arabidopsis plants (Wingler et al., 2004). Surprisingly, the response was opposite to what we expected, revealing a negative correlation of Fv/Fm at the start of senescence with the dimension of S and with LDMC (Fig. 4). Since whole-rosette Fv/Fm values are affected by leaf formation in addition to leaf longevity, we also determined Fv/Fm in individual leaves (position 6), but the only correlations detected were with flowering start under controlled conditions and Fv/Fm of whole rosettes at some time points (data not shown). Overall, our results indicate that rosettes that retain a high level of hydration (and thus lower LDMC) also maintain PSII efficiency longer. The negative correlation between Fv/Fm values during later stages and SLA (Table S4) indicates that accessions that invest in leaf structural components over leaf expansion have longer lived leaves, which is consistent with the world-wide leaf economics spectrum (Wright et al., 2004).
As expected, the extent of senescence was correlated with flowering under controlled conditions in the laboratory. Similarly, previous work has shown that flowering time and senescence are correlated in arabidopsis accessions (Levey and Wingler, 2005), and quantitative trait locus (QTL) analysis revealed flowering-dependent and -independent senescence pathways in arabidopsis (Wingler et al., 2010; Chardon et al., 2014). However, the senescence-dependent decline in Fv/Fm in non-vernalized plants grown under controlled conditions was not correlated with flowering time in vernalized plants under outdoor conditions, probably because vernalization co-ordinates the flowering and senescence pathways and affects senescence to a varying extent in different accessions (Wingler et al., 2010).
Further application of intra-specific classification of functional strategies
We have demonstrated that C–S–R classification can be applied to characterize intra-specific variation in functional strategies in genetically diverse arabidopsis accessions. Genome-wide association studies have enabled investigation of the genetic basis of natural variation in traits related to resource allocation in model species, such as arabidopsis (e.g. Atwell et al., 2010; Kooke et al., 2016), in other wild species (Herrera et al., 2015) and in crops (e.g. Huang et al., 2012). As these studies usually focus on individual traits, rather than trait combinations, they do not make holistic predictions about overall plant functional strategies. Given the simplicity with which the required data can be obtained, using the approach presented here or the method developed by Pierce et al. (2013), phenotyping of functional strategies in a large number of genotypes for genome-wide association studies should be feasible. This would enable identification of the genetic basis of resource allocation strategies in wild species or crops, which could be used in breeding programmes, as discussed by Bennett et al. (2012). Common garden experiments would overcome the issue of unnatural conditions in growth chambers, such as the effect of low light on SLA (Poorter et al., 2016).
The C–S–R classification could also be applied to analysing the biological basis of invasion: He et al. (2010) found that the growth of larger plants of knapweed (Centaurea stoebe) was more severely affected by stress than that of smaller plants, indicating a trade-off between competitive and stress-tolerant strategies. Invasive North American populations overall showed increased size, but decreased stress tolerance. In addition, evolutionary adaptations in response to climate change could be investigated. For this purpose, plants could be grown under varied controlled conditions or in common gardens in different climates to test if the assigned functional type is a plastic response dependent on environmental conditions.
In conclusion, the research presented here shows that approaches for intra-specific classification of plant strategies provide useful information about plant adaptations to environmental conditions. This enables the wider use of classification of functional plant strategies in evolutionary and ecological research, in addition to applications in plant breeding.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: arabidopsis accessions used, and climatic data at the site of origin and flowering dates. Table S2: ANOVA of traits. Table S3: C–S–R dimensions of the arabidopsis accessions. Table S4: correlation analysis of functional traits, C–S–R classification, geographic origin and climate.
Supplementary Material
LITERATURE CITED
- Aarssen LW, Clauss MK.. 1992. Genotypic variation in fecundity allocation in Arabidopsis thaliana. Journal of Ecology 80: 109–114. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S.. 2010a. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Functional Ecology 24: 1192–1201. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, et al. 2010b. Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology 98: 604–613. [Google Scholar]
- Atwell S, Huang YS, Vilhjálmsson BJ, et al. 2010. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, Roberts JA, Wagstaff C.. 2012. Manipulating resource allocation in plants. Journal of Experimental Botany 63: 3391–3400. [DOI] [PubMed] [Google Scholar]
- Berger JD, Ludwig C.. 2014. Contrasting adaptive strategies to terminal drought-stress gradients in Mediterranean legumes: phenology, productivity, and water relations in wild and domesticated Lupinus luteus L. Journal of Experimental Botany 65: 6219–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilton MC, Whitlock R, Grime JP, Marion G, Pakeman RJ.. 2010. Intraspecific trait variation in grassland plant species reveals fine-scale strategy trade-offs and size differentiation that underpins performance in ecological communities. Botany 88: 939–952. [Google Scholar]
- Cerabolini BEL, Brusa G, Ceriani RM, De Andreis R, Luzzaro A, Pierce S.. 2010. Can CSR classification be generally applied outside Britain? Plant Ecology 210: 253–261. [Google Scholar]
- Chardon F, Jasinski S, Durandet M, Guerche P, Masclaux-Daubresse C.. 2014. QTL meta-analysis in Arabidopsis reveals an interaction between leaf senescence and resource allocation to seeds. Journal of Experimental Botany 65: 3949–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frenne P, Graae BJ, Kolb A, et al. 2011. An intraspecific application of the leaf-height-seed ecology strategy scheme to forest herbs along a latitudinal gradient. Ecography 34: 132–140. [Google Scholar]
- Díaz S, Kattge J, Cornelissen JHC, et al. 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Frenette-Dussault C, Shipley B, Léger J-F, Meziane D, Hingrat Y.. 2012. Functional structure of an arid steppe community reveals similarities with Grime’s C–S–R theory. Journal of Vegetation Science 23: 208–222. [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111: 1169–1194. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R.. 1988. Comparative plant ecology. A functional approach to common British species. London: Unwin Hyman. [Google Scholar]
- Grime JP, Thompson K, Hunt R. et al. 1997. Integrated screening validates primary axes of specialisation in plants. Oikos 79: 259–281. [Google Scholar]
- He W-M, Thelen GC, Ridenour WM, Callaway RM.. 2010. Is there a risk to living large? Large size correlates with reduced growth when stressed for knapweed population. Biological Invasions 12: 3591–3598. [Google Scholar]
- Herrera CM, Medrano M, Bazaga P.. 2015. Continuous within-plant variation as a source of intraspecific functional diversity: patterns, magnitude, and genetic correlates of leaf variability in Helleborus foetidus (Ranunculaceae). American Journal of Botany 102: 225–232. [DOI] [PubMed] [Google Scholar]
- Hendry GAF, Grime JP.. 1993. Methods in comparative plant ecology, a laboratory manual. London: Chapman and Hall. [Google Scholar]
- Hodgson JG, Wilson PJ, Hunt R, Grime JP, Thompson K.. 1999. Allocating C–S–R plant functional types: a soft approach to a hard problem. Oikos 85: 282–294. [Google Scholar]
- Hu Y-K, Pan X, Liu G-F, et al. 2015. Novel evidence for within-species leaf economics spectrum at multiple spatial scales. Frontiers in Plant Science 6: 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhao Y, Wei X, et al. 2012. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nature Genetics 44: 32–39. [DOI] [PubMed] [Google Scholar]
- Hunt R, Hodgson JG, Thompson K, Bungener P, Dunnett NP, Askew AP.. 2004. A new practical tool for deriving a functional signature for herbaceous vegetation. Applied Vegetation Science 7: 163–470. [Google Scholar]
- Kenney AM, McKay JK, Richards JH, Juenger TE.. 2014. Direct and indirect selection on flowering time, water-use efficiency (WUE, δ13C), and WUE plasticity to drought in Arabidopsis thaliana. Ecology and Evolution 4: 4505–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooke R, Kruijer W, Bours R, et al. 2016. Genome-wide association mapping and genomic prediction elucidate the genetic architecture of morphological traits in Arabidopsis. Plant Physiology 170: 2187–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey S, Wingler A.. 2005. Natural variation in the regulation of leaf senescence and relation to other traits in Arabidopsis. Plant, Cell & Environment 28: 223–231. [Google Scholar]
- Luo X, Ma C, Yue Y, et al. 2015. Unravelling the complex trait of harvest index in rapeseed (Brassica napus L.) with association mapping. BMC Genomics 16: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Chardon F.. 2011. Exploring nitrogen remobilization for seed filling using natural variation in Arabidopsis thaliana. Journal of Experimental Botany 62: 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T.. 2003. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology 12: 1137–1151. [DOI] [PubMed] [Google Scholar]
- McKhann HI, Camilleri C, Bérard A, et al. 2004. Nested core collections maximising genetic diversity in Arabidopsis thaliana. The Plant Journal 38: 193–202. [DOI] [PubMed] [Google Scholar]
- Montesinos-Navarro A, Wig J, Pico FX, Tonsor S.. 2011. Arabidopsis thaliana populations show clinal variation in a climatic gradient associated with altitude. New Phytologist 189: 282–294. [DOI] [PubMed] [Google Scholar]
- Novakovskiy AR, Maslova SP, Dalke IV, Dubrovskiy YA.. 2016. Patterns of allocation CSR functional types in northern Europe. International Journal of Ecology 2016: 1323614. doi:10.1155/2016/1323614. [Google Scholar]
- Pakeman RJ. 2013. Intra-specific leaf trait variation: management and fertility matter more than the climate at continental scales. Folia Geobotanica 48: 355–371. [Google Scholar]
- Pierce S, Ceriani RM, De Andreis R, Luzzaro A, Cerabolini B.. 2007. The leaf economics spectrum of Poaceae reflects variation in survival strategies. Plant Biosystems 141: 337–343. [Google Scholar]
- Pierce S, Brusa G, Vagge I, Cerabolini BEL.. 2013. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Functional Ecology 27: 1002–1010. [Google Scholar]
- Pierce S, Negreiros D, Cerabolini BEL, et al. 2016. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31: 444–457. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R.. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Walter A, Fiorani F, Schurr U.. 2010. A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. Journal of Experimental Botany 61: 2043–2055. [DOI] [PubMed] [Google Scholar]
- Poorter H, Fiorani F, Pieruschka R, et al. 2016. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytologist 212: 838–855. [DOI] [PubMed] [Google Scholar]
- Read QD, Moorhead LC, Swenson NG, Bailey JK, Sanders NJ.. 2014. Convergent effects of elevation on functional leaf traits within and among species. Functional Ecology 28: 37–45. [Google Scholar]
- Salguero-Gómez R, Jones OR, Jongejans E, et al. 2016. Fast–slow continuum and reproductive strategies structure plant life-history variation worldwide. Proceedings of the National Academy of Sciences, USA 113: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Bermejo E, Zhu W, Tasset C, et al. 2015. Genetic architecture of natural variation in thermal responses of Arabidopsis. Plant Physiology 169: 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley B, Lechowicz MJ, Wright I, Reich PB.. 2006. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87: 535–541. [DOI] [PubMed] [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, et al. 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18: 1406–1419. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Roy BA, Thiede DA.. 2000. Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution 54: 93–111. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JR, Weinig C, Ungerer M, et al. 2004. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proceedings of the National Academy, USA 101: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigidal DS, Marques ACSS, Willems LAJ, et al. 2016. Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant, Cell & Environment 39: 1737–1748. [DOI] [PubMed] [Google Scholar]
- Volis S, Mendlinger S, Ward D.. 2004. Differentiation in populations of Hordeum spontaneum Koch along a gradient of environmental productivity and predictability: intra- and interspecific competitive responses. Israel Journal of Plant Sciences 52: 223–234. [Google Scholar]
- Wilson JB, Lee WG.. 2000. C–S–R triangle theory: community-level predictions, tests, evaluation of criticisms, and relation to other theories. Oikos 91: 77–96. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG.. 1999. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist 143: 155–162. [Google Scholar]
- Wingler A. 2015. Comparison of signaling interactions determining annual and perennial plant growth in response to low temperature. Frontiers in Plant Science 5: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Marès M, Pourtau N.. 2004. Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytologist 161: 781 – 789. [DOI] [PubMed] [Google Scholar]
- Wingler A, Purdy SJ, Edwards SA, Chardon F, Masclaux-Daubresse C.. 2010. QTL analysis for sugar-regulated leaf senescence supports flowering-dependent and -independent senescence pathways. New Phytologist 185: 420–433. [DOI] [PubMed] [Google Scholar]
- Wingler A, Juvany M, Cuthbert C, Munné-Bosch S.. 2015. Adaptation to altitude affects the senescence response to chilling in the perennial plant Arabis alpina. Journal of Experimental Botany 5: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby. et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.