Abstract
Background and Aims In a cost–benefit framework, plant carnivory is hypothesized to be an adaptation to nutrient-poor soils in sunny, wetland habitats. However, apparent exceptions to this cost–benefit model exist, although they have been rarely studied. One of these exceptions is the carnivorous subshrub Drosophyllum lusitanicum, which thrives in Mediterranean heathlands on dry sandstone soils and has relatively well-developed, xeromorphic roots. Here, the roles of leaf (carnivory) and root (soil) nutrient uptake in growth promotion of this particular species were assessed.
Methods In a greenhouse experiment, plants were fed with laboratory-reared fruit flies (Drosophila virilis) and received two concentrations of soil nutrients in a factorial design. Above-ground plant growth and final above- and below-ground dry biomass after 13 weeks were recorded. Nutrient uptake via roots was also evaluated, using stable nitrogen isotope analysis.
Key Results Insect feeding resulted in significantly higher growth and above- and below-ground biomass compared with soil fertilization. No additional benefits of fertilization were discernable when plants were insect-fed, indicating that roots were not efficient in nutrient absorption.
Conclusions The first evidence of strong reliance on insect prey feeding in a dry-soil carnivorous plant with well-developed roots is provided, suggesting that carnivory per se does not preclude persistence in dry habitats. Instead, the combination of carnivory and xeromorphic root features allows Drosophyllum to thrive on non-waterlogged soils. New evidence is added to recent research emphasizing the role of root systems of carnivorous plants in explaining their distribution, partly challenging the cost–benefit hypothesis.
Keywords: Carnivorous plant root, dry-soil carnivorous plant, insect prey, pyrophyte, soil nutrient uptake, stable isotope analysis
INTRODUCTION
Intensively studied by Darwin (1875) in his treatise Insectivorous plants, plant carnivory is arguably the most captivating adaptation to nutrient-poor soils (Adamec, 1997; Ellison and Gotelli, 2001; Król et al., 2012). The uptake and assimilation of nutrients via modified leaf structures has evolved at least nine times independently across the angiosperms (Givnish, 2015), with ∼600 extant species of carnivorous plants in the world’s flora (Król et al., 2012; Givnish, 2015). The nutrition of carnivorous plants has been studied in various species, with a strong focus on sundews (Drosera spp.: Darwin, 1878; Karlsson and Pate, 1992; Adamec, 2002; Thorén et al., 2003; Millett et al., 2012), butterworts (Pinguicula spp.: Karlsson and Carlsson, 1984; Karlsson et al., 1991; Hanslin and Karlsson, 1996) and pitcher plants (Nepenthes/Sarracenia spp.: Schulze et al., 1997; Moran et al., 2001; Gotelli and Ellison, 2002; Butler and Ellison, 2007; Farnsworth and Ellison, 2008). These studies have supported the hypothesis that carnivorous plants benefit from captured prey insects by acquiring mineral nutrients, mainly nitrogen and phosphorus (Ellison, 2006; Farnsworth and Ellison, 2008).
Most carnivorous plants are restricted to nutrient-poor, wet soils in sunny habitats (Ellison and Gotelli, 2001; Brewer et al., 2011; Pavlovič and Saganová, 2015). These environmental associations led Givnish et al. (1984) to propose a cost–benefit model for the evolution of plant carnivory and its general restriction to sunny, infertile wetlands. According to this model, the net benefit of carnivory, i.e. the photosynthetic gain in terms of leaf production minus the cost of producing and maintaining specialized prey-trapping structures, is predicted to be largest when soil nutrient availability is the major limiting factor to plant growth but light and soil water are readily available. Several studies have since investigated nutrition in carnivorous plants, demonstrating that species vary widely in their capacity to assimilate mineral nutrients from soil (Adamec, 1997, 2010; Schulze et al., 1997; Ellison, 2006; Król et al., 2012). Support for the cost–benefit model comes in particular from studies showing that reliance on prey nutrients decreases with increasing soil nutrient availability (e.g. Benzing, 1987; Karlsson and Pate, 1992; Millett et al., 2012) or shade (Givnish et al., 1984; Schulze et al., 2001).
More recently, extensions or alternatives to the cost–benefit model have been proposed (Benzing, 2000; Brewer et al., 2011; Abbott and Brewer, 2016). Brewer et al. (2011), for instance, hypothesized that the characteristic, weakly developed and low-porosity roots, rather than low soil fertility per se, might explain the general restriction of carnivorous plants to boggy, waterlogged soils and their disadvantage in drier, non-waterlogged soils. However, carnivorous plant species that thrive in dry habitats and appear to contradict the predictions of the cost–benefit model have received far less attention in the literature, despite potentially providing significant novel insights into the evolution of plant carnivory (Givnish et al., 1984; Givnish, 2015). One prominent example is the subshrub Drosophyllum lusitanicum (Drosophyllaceae). This species (Drosophyllum hereafter) is the only extant species of the family Drosophyllaceae (Heubl et al., 2006) and is endemic to the western Iberian Peninsula and northern Morocco (Garrido et al., 2003). Across its range, Drosophyllum is restricted to fire-prone Mediterranean heathlands on acid, nutrient-poor, dry soils that are subject to a moderate summer drought (Adlassnig et al., 2006; Paniw et al., 2015).
Unlike most other carnivorous plant species, many Drosophyllum individuals maintain their complex, sticky mucilage on leaves to capture prey even under unfavourable conditions in the dry summer months (Adlassnig et al., 2006; Adamec 2009). Another difference between Drosophyllum and most other carnivorous plant species is that the root system of the former is relatively well developed, consisting of a branched tap-root with xeromorphic features (Carlquist and Wilson, 1995; Adlassnig et al., 2005, 2006; Adamec, 2009). Despite being one of the few carnivorous plant species with deep, large root systems, no research has been done on the putative role of roots for soil nutrient uptake in this species (Adlassnig et al., 2005, 2006). The taxonomic uniqueness and habitat particularity of Drosophyllum make the species a valuable system for investigating the importance of leaves versus roots in nutrient acquisition and growth promotion of carnivorous plants in dry habitats.
Here, we studied plant nutrition in Drosophyllum plants through leaves (prey insects) and roots (soil nutrients) and the effect of nutrient uptake from the two sources on above-ground growth and above-ground (leaf) and below-ground (root) biomass allocation. Given the scarcity of fine lateral roots in this species (Adamec, 2009), we hypothesized that leaf nutrient uptake from trapped insects will determine plant growth, with a low contribution, if any, of soil nutrient uptake from roots, despite their considerable size and depth (Adlassnig et al., 2005). To test this hypothesis, we performed a full-factorial greenhouse experiment in which we fed juvenile plants growing on a substrate mixture of siliceous sand and peat moss via leaves (fruit flies) and/or soil (Hoagland’s nutrient solution). We recorded above-ground growth as well as final dry biomass of above-ground (leaves) and below-ground (roots) plant parts and compared them between treatments. Since the Hoagland’s nutrient solution used had an anomalously high δ15N value (see Materials and methods), we measured δ15N values in the above-ground (leaves) and below-ground (roots) tissue of plants from the different treatments to ascertain the ability of the plants to absorb mineral nutrients from the roots.
MATERIALS AND METHODS
Growth of plants and experimental design
We grew Drosophyllum plants in the University of Cádiz greenhouse from seeds collected in July 2014 from 80 individuals randomly chosen at five sites (16 individuals per site). We mixed all seeds to provide a homogeneous pool and, on 2 February 2015, we randomly took 200 seeds from the pool and exposed them to dry heat (100 °C) for 5 min to break seed dormancy (Correia and Freitas, 2002). We then sowed these seeds in seedling trays with a 1:1 mixture of siliceous sand and peat moss and selected the first 120 emerged seedlings for the experiment. The seedlings emerged 20–26 d after sowing and were then individually transplanted into 0·5-L clay pots containing the same mixture of siliceous sand and peat moss. This low-fertility soil mixture is commonly used in nutrient addition experiments for carnivorous plants (e.g. Butler and Ellison, 2007) and approximates the low-fertility conditions of Mediterranean heathland soils (Ojeda et al., 2010). The pH of this substrate, measured in a saturated soil paste, was ∼4·5, similar to the pH of Mediterranean heathland soils (Ojeda et al., 2010).
We grew the 120 potted seedlings in the greenhouse at ambient temperature, but never exceeding 25 °C, and keeping relative humidity around 70–90 % throughout the whole experiment, resembling ambient conditions of natural Drosophyllum populations during the spring growing season (M. Paniw, unpubl. res.). During the night, the lowest temperature recorded in the greenhouse was 15 °C. Pots were kept moist via a sprinkling system mounted above the pots that sprayed decalcified water during daytime for 30 s at 2-h intervals. We used decalcified water because soil Ca is toxic to most carnivorous plants (Adlassnig et al., 2005), including Drosophyllum (Adlassnig et al., 2006). We maintained the temperature regime and periodic sprinkling throughout the study. In addition, before initiating the nutrient addition experiment, we watered the pots three times a week with 50 mL of decalcified water. On 12 March 2015, 14 d after being transplanted, the seedlings were large enough (five to seven leaves of length 5·0 ± 0·3 cm, mean ± s.d.) to start the feeding experiment, which extended for 11 weeks until 27 May 2015, lasting a total of 91 d after seedling emergence.
The experiment was performed in a full-factorial design with insect feeding [two treatment levels: insect feeding (F) and no insect feeding (NF)] and soil fertilization [three treatment levels: high (H), low (L) and zero (O)] as fixed factors. The 120 potted seedlings were randomly divided into two equal-sized groups, one of which, the F treatment, was supplied with fruit flies (Drosophila virilis; ∼0·3 mg dry weight per fly) and the other, the NF treatment, was not. Each plant of the F treatment received three flies per leaf in the first 2 weeks of the experiment, increasing the number of flies by two more per leaf each additional week until the sixth week, when the number of flies per leaf increased to four more each week. The D. virilis fruit flies used throughout the experiment were reared in a carbohydrate-rich medium under standard culture conditions and were kept frozen in vials at −20 °C prior to usage.
Plants of the F and NF groups were further split into three subgroups (20 plants each) for the soil fertilization treatments: three times per week for the duration of the experiment, plants in each subgroup received 50 mL of 1/10 strength nutrient solution (H treatment), 50 mL of 1/20 strength nutrient solution (L treatment) or 50 mL of distilled water (O treatment). We used a balanced nutrient mixture (Hoagland’s No. 2 Basal Salt Mixture; Sigma-Aldrich, St Louis, MO, USA) to avoid potential deficiencies of some nutrients caused by abundance of another. Similar dilutions have been used in feeding experiments for other carnivorous plant species (e.g. Butler and Ellison, 2007). Plants in the NF–O treatment combination, receiving neither flies nor soil nutrients, were considered as control. Each time before treatment application, pots were haphazardly shuffled on the greenhouse bench to avoid a location effect.
In order to ensure that the amount of nutrients provided to plants via flies or soil solution did not differ substantially, we determined the amount of nitrogen available to plants from either source. The amount of nitrogen in flies was measured as described for plant samples in the section δ15N analysis below. Throughout the nutrient-addition experiment, plants in the corresponding treatment groups were supplied weekly with ∼3·1 mg (H treatment) and 1·05 mg (L treatment) of N through the soil. The fine texture of the moss peat in the soil medium aided in retaining the nutrient solution and water. Plants in the F–O treatment received a total of ∼2·1 mg of N from insects, which corresponded to 60 % (range 52–69 %) of their total N pool. We assumed that the relative concentrations of other nutrients to N were similar between flies and fertilizer.
To track the above-ground growth of plants under different treatment combinations, we counted the number of fully developed leaves and measured the length (cm) of the longest leaf on each plant at the beginning of the experiment (d 14 after emergence) and every week or second week until the end of the experiment (11 weeks later; d 91 after emergence). We then defined size as the number of leaves × length of longest leaf (cm). This size measure is biologically significant as it approximates the available leaf area for prey capture and has been used in other studies of this species (Paniw et al., 2016). Once the experiment was terminated, we removed plants from the pots, washed them in distilled water to remove fruit flies from leaves and soil from roots, separated above-ground (shoot) and below-ground (root) material of each plant, and oven-dried them for 72 h at 65 °C to constant weight. We then weighed the shoot and root dry biomass of each plant to the nearest 0·01 mg.
δ15N analysis
Previous analyses found an average δ15N signature of 18·6 ‰ (range 18·0–19·0 ‰) in the Hoagland’s nutrient solution used in this study (Hoagland’s No. 2 Basal Salt Mixture), an anomalously high value for standard synthetic fertilizers (δ15N = −0·2 ±2·1 ‰, mean ± s.d.; Bateman and Kelly, 2007), and much higher than the δ15N signature detected in Drosophila virilis flies (range 2·8–3·0 ‰). This highly δ15N-enriched nutrient solution provided an excellent means to explore whether Drosophyllum plants were able to take up and assimilate soil nutrients through the roots. After being weighed, shoot and root dry biomass samples of all Drosophyllum plants from the nutrient addition experiment were separately placed into plastic vials (up to three samples per plant part if enough biomass was produced), ground to powder using stainless steel beads with a Mixer Mill MM400 cell disrupter (Retsch, Llanera, Spain), and analysed for percentages of N and δ15N using combustion in a Flash EA1112 elemental analyser interfaced with Finnigan Tracer Mass Isotope Ratio Mass Spectrometer. Analyses were performed at the Analytical Service Laboratory of the University of A Coruña (Spain). The δ15N results are expressed in parts per thousand (‰), where δ = [(15N/14N) −1] ×1000. All δ15N values had a precision of 0·3 ‰.
Statistical analysis
The overall effects of insect feeding (F, NF), soil fertilization (H, L, O) and their interaction on above-ground size changes over time were determined by means of a two-way repeated-measures ANOVA. The plant size variable was log-transformed prior to analysis to meet the homoscedasticity assumption. We also explored the effects of insect feeding (F, NF) and soil fertilization (H, L, O) on the final dry biomass (g) of the above-ground (shoot) and below-ground (root) portions of the plants by performing a two-way ANOVA. Shoot and root dry biomass variables were previously log-transformed to ensure the homoscedasticity assumption of multivariate ANOVA (MANOVA). Posthoc comparisons using Tukey’s honestly significant difference (HSD) tests to search for pairwise differences between the six treatment combinations were implemented separately for shoot and root dry biomass variables. An equivalent analysis for whole-plant biomass can be found in Supplementary Data Appendix S1.
In order to explore whether plants provided with soil nutrients changed their root:shoot allocation patterns, we calculated the percentage contribution of roots to the total plant dry biomass, and tested significant differences between the six treatments by using a non-parametric Kruskal–Wallis rank test. Finally, we also used the Kruskal–Wallis rank test to search for differences in the δ15N signature of the above-ground (shoot) and below-ground (root) tissue of plants between the six treatment combinations. As the Kruskal–Wallis rank test corresponds to a non-parametric one-way ANOVA, subsequent posthoc pairwise comparisons between treatment combinations were done using Bonferroni-corrected Mann–Whitney U-tests. All statistical analyses were performed in R (R Core Team, 2015).
RESULTS
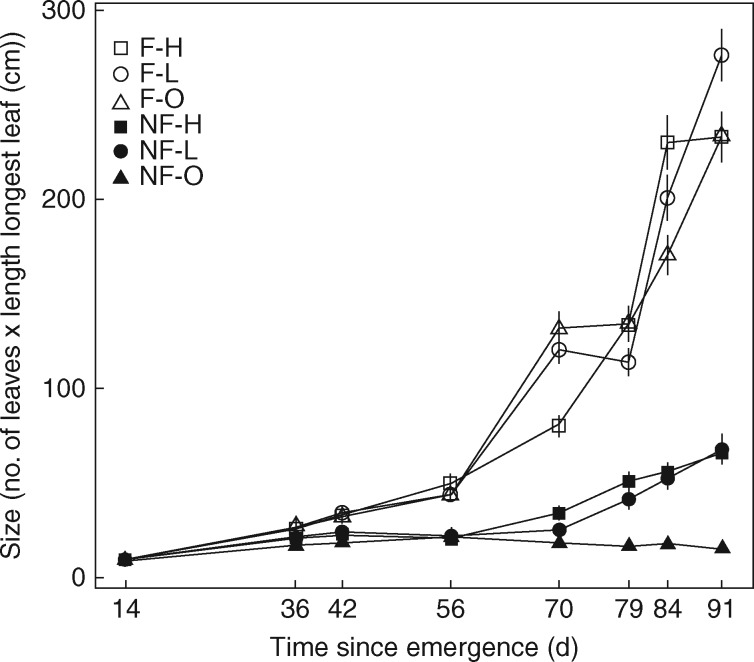
Insect-fed plants grew >4-fold as much as non-insect-fed plants during the experiment (Fig. 1) and produced a >5-fold higher dry biomass, both above and below ground (Fig. 2), regardless of soil fertility conditions.
Fig. 1.
Changes in size, defined as number of leaves × length of longest leaf (cm), of Drosophyllum plants through time (d) as a function of two treatments, insect feeding [feeding (F)/no feeding (NF)] and soil fertilization [addition of high-strength (H), low-strength (L) nutrient solution or distilled water (O)] and their interaction, resulting in six treatment groups.
Fig. 2.
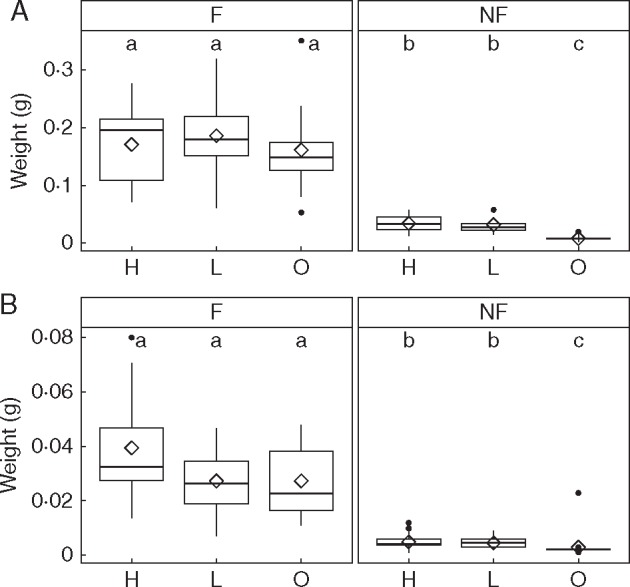
Box-plot of dry biomass of (A) above-ground (shoot) and (B) below-ground (root) parts of Drosophyllum plants measured at the end of the nutrient addition experiment as a function of two main treatments and their interactions: feeding with flies (F) or no feeding (NF) and addition of high-strength (H), low-strength (L) nutrient solution or distilled water (O). The combinations of the treatment levels resulted in six treatment groups. Different letters represent significant pairwise differences (Tukey’s HSD, P < 0·05) in group means (diamonds) between the six treatment groups. Note that the statistical comparisons were performed on the log-transformed biomass measure to ensure variance homoscedasticity.
The two-way repeated-measures ANOVA detected significant effects of the two factors, insect feeding and soil fertilization, on relative plant growth (Table 1; Fig. 1). In addition, plant size changed significantly with time (days after sowing), with plants growing significantly faster when fed with flies compared with unfed plants (Table 1; Fig. 1). Correspondingly, the two-way MANOVA showed significant effects of both factors on the final dry biomass of above-ground (shoot) and below-ground (root) portions of plants, and a significant interaction between the two factors (Table 2; Fig. 2). The significant interaction effect stemmed from soil fertilization having a slight but significant effect on final dry biomass only when plants were not supplied with fruit flies (Table 2). No significant differences in final dry biomass were detected between the H and L soil fertilization levels (Fig. 2). Insect-fed plants grew much larger, both above and below ground, than soil-fed plants, and no additive effects of soil fertilization on them were detected (Fig. 2).
Table 1.
Two-way repeated-measures ANOVA of the effects of insect feeding, soil fertilization, and their interaction on changes in above-ground size through time (days since emergence) of Drosophyllum plants
| d.f. | Mean square | F ratio | P value | |
|---|---|---|---|---|
| Between-group effect: error (plant ID) | ||||
| Insect feeding | 1 | 1·4 × 106 | 483·0 | <0·01 |
| Soil fertilization | 2 | 1·3 × 104 | 4·5 | 0·01 |
| Insect feeding × soil fertilization | 2 | 6·5 × 103 | 2·2 | 0·1 |
| Residuals | 113 | 2·9 × 103 | ||
| Within-subject effect | ||||
| Days | 6 | 2·8 × 105 | 668·7 | <0·01 |
| Insect feeding × days | 6 | 1·6 × 105 | 395·3 | <0·01 |
| Soil fertilization × days | 12 | 6·9 × 103 | 16·3 | <0·01 |
| Insect feeding × soil fertilization × days | 12 | 3·4 × 103 | 8·1 | <0·01 |
| Residuals | 678 | 419 | ||
Table 2.
Two-way MANOVA, using the Pillai test statistic, of the effects of insect feeding and soil fertilization factors and their interaction on above-ground (shoot) and below-ground (root) dry biomass (g)
| Pillai | Approx F | Num d.f. | Demom d.f. | P-value | |
|---|---|---|---|---|---|
| Shoot and root dry biomass (g) | |||||
| Insect feeding | 0·865 | 170·172 | 2 | 53 | <0·01 |
| Soil fertilization | 0·352 | 5·767 | 4 | 108 | <0·01 |
| Insect feeding × soil fertilization | 0·369 | 6·099 | 4 | 108 | <0·01 |
Num d.f. and demon d.f. are the numerator and denominator degrees of freedom, respectively, of the F ratio corresponding to the Pillai test.
On average, the root Drosophyllum plants made up 14·7 % (± 0·06 s.d.) of the total plant dry biomass. This result was similar to previous investigations of root:shoot ratios in Drosophyllum (Adamec, 2009). No significant differences in the relative contribution of roots to total plant biomass were found between the six treatment combinations (Kruskal–Wallis χ2 = 9·6, d.f. = 5, P = 0·1).
Soil-fertilized plants presented significantly higher δ15N signatures in both shoot and root tissues than non-fertilized plants, regardless of whether or not they were supplied with fruit flies on the leaves (Fig. 3; Kruskal–Wallis χ2 = 70·0, d.f. = 5, P < 0·05). Taking into account the high δ15N values of the nutrient solution used for soil fertilization (see Materials and methods), this result indicates that Drosophyllum plants are able to take up and assimilate dissolved soil nutrients through the roots. It should be noted that higher δ15N signatures were detected in H-fertilized than in L-fertilized plants (Fig. 3), although higher fertilization strength did not cause an increase in plant growth (Figs 1 and 2).
Fig. 3.
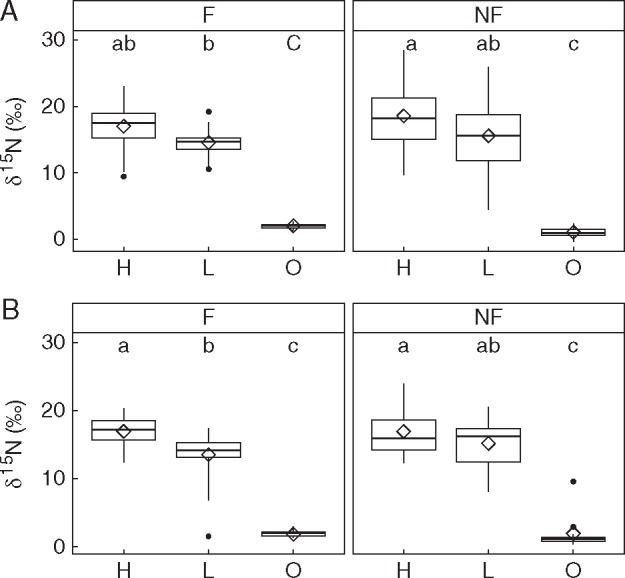
Box-plot of δ15N in (A) shoot and (B) root tissues of Drosophyllum plants measured at the end of the nutrient addition experiment as a function of two main treatments and their interactions: feeding with flies (F) or no feeding (NF) and addition of high-strength (H) or low-strength (L) nutrient solution or distilled water (O). Different letters represent significant pairwise differences (Kruskal–Wallis χ2 = 70·0, d.f. = 5, P < 0·05) in group means (diamonds) between the six treatment groups.
DISCUSSION
Carnivorous plants are predicted to benefit from prey capture under a specific set of environmental conditions, i.e. nutrient-poor, wet soils and open habitats, which offset the cost of producing trapping structures (Givnish et al., 1984; Benzing, 1987, 2000). However, our nutrient addition experiment provides the first evidence that a strong carnivorous syndrome may evolve in dry environments. Drosophyllum plants invest resources in carnivorous structures as well as in well-developed, deep roots (Adlassnig 2005, 2006) that, however, seem to play only a minor role in nutrient acquisition. Plants fed with insects in the greenhouse acquired on average >5-fold as much biomass as soil-fertilized plants, with root nutrient uptake showing no additive benefits in plant growth (Fig. 2). These results support the hypothesis that root functionality other than nutrient acquisition (e.g. securing water availability) may be a key factor determining the distribution of carnivorous plants with respect to soil moisture (Brewer et al., 2011). Indeed, unlike most other carnivorous plant species, Drosophyllum produces large, xeromorphic roots most likely as an adaptation to water uptake in non-waterlogged soils under a Mediterranean climate (Carlquist and Wilson, 1995; Adlassnig et al., 2005). Both the xeromorphic root features (for soil water acquisition) and carnivory (for nutrients) may allow this species to persist in nutrient-poor, dry Mediterranean heathlands.
The strong reliance of Drosophyllum on prey-derived nutrients for growth highlighted by our greenhouse study is corroborated by field observations and field experiments showing great efficiency of plants of this species in attracting prey (Darwin, 1875; Bertol et al., 2015). Individuals produce complex, mucilaginous stalked glands, multicellular and vascularized with both xylem and phloem vessels (Renner and Specht, 2011). It should be emphasized that this species is, together with the part-time carnivorous Triphyophyllum peltatum, the only flypaper carnivorous species whose glandular trichomes have phloem vessels (Renner and Specht, 2011). This would allow Drosophyllum plants to add phloem sap exudates, including carbohydrates and volatile organic compounds, to the mucilage droplets, increasing their viscosity and hygroscopicity (carbohydrates; Adlassnig et al., 2006) as well as their efficiency in insect attraction (volatile organic compounds; Jürgens et al., 2009). As a result, even juvenile Drosophyllum individuals, consisting of one rosette with ten leaves, may contain >100 prey insects (Bertol et al., 2015). The strong carnivorous character of Drosophyllum stands out compared with Byblis lamellata (Byblidaceae), the only morphologically and ecologically similar carnivorous species, found in non-waterlogged, seasonally dry, siliceous sands (Conran et al., 2002). Unlike Drosophyllum, B. lamellata has simple trapping structures and does not have sessile, proteolytic enzyme-producing glands to directly digest prey insects, but may use insect mutualists that feed on trapped prey to gain nutrients by digesting their faeces (Hartmeyer, 1998).
Despite the strong reliance on prey for plant growth, our results indicate that Drosophyllum is able to take up soil nutrients from the roots, when available, and assimilate them in both root and leaf tissue (Fig. 3), although growth is far from optimal in the absence of insect prey (Figs 1 and 2). Drosophyllum is a post-fire-dwelling species (Paniw et al., 2015) with life-history adaptations to recurrent fires, which include mass post-fire recruitment from a persistent soil seed-bank (Müller and Deil, 2001). Fires release a flush of mineral nutrients to soil, including N and P, which are quickly (within 1 year) leached away (Certini, 2005; Dijkstra and Adams, 2015). By being able to assimilate nutrients from the roots, Drosophyllum plants might benefit from that transient post-fire flush in their early seedling stages, when insect capture is unlikely, due to small size. They might hence use it to assist plant growth to prey-capture levels. Similar results have been found for another fire-adapted carnivorous plant, Dionaea muscipula, and may also indicate adaptations to post-fire nutrient fluctuations (Gao et al., 2015). As lateral roots appear to be lost in mature Drosophyllum plants (Adamec, 2009), nutrient uptake via roots is likely limited to the seedling and juvenile plant stages, but future studies must determine whether mature Drosophyllum individuals can also potentially assimilate nutrients from the soil.
In practice, nutrient absorption via roots in adult Drosophyllum plants is likely limited as roots lack adaptations, such as microsymbiont associations or cluster roots, for nutrient scavenging in low-fertility soils (Carlquist and Wilson, 1995; Adlassnig et al., 2005, 2006). On the other hand, virtually all non-carnivorous plant species in heathland habitats show root adaptations for nutrient scavenging (Lambers et al., 2006). Carnivory in Drosophyllum may therefore be seen as an alternative strategy to acquire nutrients in nutrient-poor, Mediterranean heathlands, with high specialization for leaf prey capture and digestion to compensate for the lack of root adaptations. Such a trade-off or constraint-avoidance solution has been shown in wetland soils, where carnivorous genera produce shallow, low-porosity roots to prevent hypoxia, obtaining nutrients from prey instead (Karlsson and Pate, 1992; Brewer at al., 2011; Gao et al., 2015).
Despite showing little efficiency in nutrient acquisition, roots may be critical in allowing Drosophyllum to persist in dry habitats. In many Drosophyllum populations, plants consume prey insects throughout the year, even in the dry summer months (Adlassnig et al., 2006; M. Paniw and F. Ojeda, pers. comm.). It has been suggested that plants satisfy a large part of their water demand through the highly hygroscopic mucilage of leaf glands that capture water from air moisture (Adlassnig et al., 2006; Adamec, 2009). However, it is unlikely that hygroscopic mucilage is sufficient to maintain the water balance in Drosophyllum individuals, particularly in the dry summers, where average air humidity does not exceed 66·5 % (± 9·0 s.e.) (Supplementary Data Appendix S2). The xeromorphic features and relatively large size of tap roots in this species, typical of plants adapted to water-limited soils (Carlquist and Wilson, 1995), indicate that, apart from their anchoring role, roots would play an important role in maintaining the water balance in Drosophyllum plants.
CONCLUSIONs
Contrary to the prediction of the cost–benefit analysis of the evolution of plant carnivory, we provide evidence that carnivory may evolve in non-waterlogged, dry soils. Therefore, roots, decoupled from nutrient-acquisition functions, may be critical in determining the distribution of carnivorous genera in response to soil moisture. Previous investigation on the nutrition of carnivorous plants has largely focused on a few genera, all found in boggy or waterlogged soils, where the ecological conditions have favoured a reduction of the root system (Brewer, 2003; Brewer et al., 2011) coupled with the maintenance of flexible nutrient acquisition strategies (e.g. Ellison and Gotelli, 2002; Millett et al., 2012), or even the ability to switch off carnivory under increasing soil nutrients (Ellison et al., 2003). Although it is certainly true that a majority of carnivorous plants are found in waterlogged soils and have reduced, shallow roots (Adlassnig et al., 2005; Brewer et al., 2011), a full understanding of the carnivorous syndrome can only be gained by considering species that have adapted to extremely low soil fertility conditions with no association with boggy habitats. Our study species, D.lusitanicum, has complex, sticky glands on their flypaper-trap leaves and is very effective in attracting prey insects (Bertol et al., 2015). At the same time, the species is also very effective at avoiding water stress, allowing it to persist on dry soils (Adlassnig et al., 2006). Using a unique system, our study supports the hypothesis that root functionality coupled with carnivory may explain the distribution of carnivorous plants better than photosynthetic cost and benefits per se. We therefore urge that more studies should be undertaken on underrepresented carnivorous taxa from non-waterlogged habitats, such as Byblis spp. in Australia or epiphytes such as Catopsis berteroniana (Adamec, 2010), to gain a more complete picture of the link between soil properties and the evolution of plant carnivory beyond bogs.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Appendix S1: analysis of whole-plant biomass. Appendix S2: seasonal relative humidity in Drosophyllum populations.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Beatriz Gasalla and José-Ramón Aracama for helping with the rearing of the fruit flies (Drosophila virilis) and Ceferino Carrera of the Servicio de Invernadero (UCA) for helping with the experiment in the University glasshouse. The Andalusian Consejería de Medio Ambiente provided the necessary permits to work with Drosophyllum lusitanicum, an endemic, red-listed species. This work was supported by the Spanish Ministerio de Ciencia e Innovación-MICINN (CGL2011-28759/BOS).
REFERENCES
- Abbott MJ, Brewer JS.. 2016. Competition does not explain the absence of a carnivorous pitcher plant from a nutrient-rich marsh. Plant and Soil 1–10. [Google Scholar]
- Adamec L. 1997. Mineral nutrition of carnivorous plants: a review. Botanical Review 63: 273–299. [Google Scholar]
- Adamec L. 2002. Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist 155: 89–100. [DOI] [PubMed] [Google Scholar]
- Adamec L. 2009. Ecophysiological investigation on Drosophyllum lusitanicum: why doesn't the plant dry out? Carnivorous Plant Newsletter 38: 71–74. [Google Scholar]
- Adamec L. 2010. Carnivorous plants: ecophysiological look at plant carnivory. Why are plants carnivorous? In: J Seckbach, Z Dubinski, eds. All flesh is grass. Plant-animal interrelationships. Cellular origin, life in extreme habitats and astrobiology. Dordrecht: Springer Science + Business Media, 457–492. [Google Scholar]
- Adlassnig W, Peroutka M, Lambers H, Lichtscheidl IK.. 2005. The roots of carnivorous plants. Plant and Soil 274: 127–140. [Google Scholar]
- Adlassnig W, Peroutka M, Eder G, Pois W, Lichtscheidl IK.. 2006. Ecophysiological observations on Drosophyllum lusitanicum. Ecological Research 21: 255–262. [Google Scholar]
- Bateman AS, Kelly SD.. 2007. Fertilizer nitrogen isotope signatures. Isotopes in Environmental and Health Studies 43: 237–247. [DOI] [PubMed] [Google Scholar]
- Benzing DH. 1987. The origin and rarity of botanical carnivory. Trends in Ecology and Evolution 2: 364–369. [DOI] [PubMed] [Google Scholar]
- Benzing DH. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Bertol N, Paniw M, Ojeda F.. 2015. Dynamics of prey capture for a flypaper-trap carnivorous plant: luring insects versus passive trap. American Journal of Botany 102: 1–6. [DOI] [PubMed] [Google Scholar]
- Brewer JS. 2003. Why don’t carnivorous pitcher plants compete with non-carnivorous plants for nutrients? Ecology 84: 451–462. [Google Scholar]
- Brewer JS, Baker DJ, Nero AS, et al. 2011. Carnivory in plants as a beneficial trait in wetlands. Aquatic Botany 94: 62–70. [Google Scholar]
- Butler JL, Ellison AM.. 2007. Nitrogen cycling dynamics in the carnivorous northern pitcher plant, Sarracenia purpurea. Functional Ecology 21: 835–843. [Google Scholar]
- Carlquist S, Wilson EJ.. 1995. Wood anatomy of Drosophyllum (Droseraceae): ecological and phylogenetic considerations. Bulletin of the Torrey Botanical Club 122: 185–189. [Google Scholar]
- Certini G. 2005. Effects of fire on properties of forest soils: a review. Oecologia 143: 1–10. [DOI] [PubMed] [Google Scholar]
- Conran JG, Lowrie A, Moyle-Croft J.. 2002. A revision of Byblis (Byblidaceae) in southwestern Australia. Nuytsia 15: 11–19. [Google Scholar]
- Correia E, Freitas H.. 2002. Drosophyllum lusitanicum, an endangered West Mediterranean endemic carnivorous plant: threats and its ability to control available resources. Botanical Journal of the Linnaean Society 140: 383–390. [Google Scholar]
- Darwin C. 1875. Insectivorous plants. London: John Murray. [Google Scholar]
- Darwin F. 1878. Experiments on the nutrition of Drosera rotundifolia. Journal of the Linnean Society, Botany (London) 17: 17–23. [Google Scholar]
- Dijkstra FA, Adams MA.. 2015. Fire eases imbalances of nitrogen and phosphorus in woody plants. Ecosystems 18: 769–779. [Google Scholar]
- Ellison AM. 2006. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology 8: 740–747. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ.. 2001. Evolutionary ecology of carnivorous plants. Trends in Ecology & Evolution 16: 623–629. [Google Scholar]
- Ellison AM, Gotelli NJ.. 2002. Nitrogen availability alters the expression of carnivory in the northern pitcher plant Sarracenia purpurea. Proceedings of the National Academy of Sciences of the USA 99: 4409–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ.. 2009. Energetics and the evolution of carnivorous plants—Darwin's ‘most wonderful plants in the world’. Journal of Experimental Botany 60: 19–42. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ, Brewer JS, et al. 2003. The evolutionary ecology of carnivorous plants. Advances in Ecological Research 33: 1–74. [Google Scholar]
- Farnsworth EJ, Ellison AM.. 2008. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. Journal of Ecology 96: 213–221. [Google Scholar]
- Gao P, Loeffler TS, Honsel A, et al. 2015. Integration of trap‐and root‐derived nitrogen nutrition of carnivorous Dionaea muscipula. New Phytologist 205: 1320–1329. [DOI] [PubMed] [Google Scholar]
- Garrido B, Hampe A, Maranon T, Arroyo J.. 2003. Regional differences in land use affect population performance of the threatened insectivorous plant Drosophyllum lusitanicum (Droseraceae). Diversity and Distributions 9: 335–350. [Google Scholar]
- Givnish TJ. 2015. New evidence on the origin of carnivorous plants. Proceedings of the National Academy of Sciences of the USA 112: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD.. 1984. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist 124: 479–497. [Google Scholar]
- Gotelli NJ, Ellison AM.. 2002. Nitrogen deposition and extinction risk in the northern pitcher plant, Sarracenia purpurea. Ecology 83: 2758–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslin HM, Karlsson PS.. 1996. Nitrogen uptake from prey and substrate as affected by prey capture level and plant reproductive status in four carnivorous plant species. Oecologia 106: 370–375. [DOI] [PubMed] [Google Scholar]
- Hartmeyer S. 1998. Carnivory in Byblis revisited II: the phenomenon of symbiosis on insect trapping plants. Carnivorous Plant Newsletter 27: 110–113. [Google Scholar]
- Heubl G, Bringmann G, Meimberg H.. 2006. Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales—revisited. Plant Biology 8: 821–830. [DOI] [PubMed] [Google Scholar]
- Jürgens A, El-Sayed AM, Suckling DM. 2009. Do carnivorous plants use volatiles for attracting prey insects? Functional Ecology 23: 875–887. [Google Scholar]
- Karlsson PS, Carlsson B.. 1984. Why does Pinguicula vulgaris L. trap insects? New Phytologist 97: 25–30. [Google Scholar]
- Karlsson PS, Pate JS.. 1992. Contrasting effects of supplementary feeding of insects or mineral nutrients on the growth and nitrogen and phosphorous economy of pygmy species of Drosera. Oecologia 92: 8–13. [DOI] [PubMed] [Google Scholar]
- Karlsson PS, Nordell KO, Carlsson BÅ, Svensson BM.. 1991. The effect of soil nutrient status on prey utilization in four carnivorous plants. Oecologia 86: 1–7. [DOI] [PubMed] [Google Scholar]
- Król E, Płachno BJ, Adamec L, Stolarz M, Dziubińska H, Trębacz K.. 2012. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Annals of Botany 109: 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ.. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98: 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett J, Svensson BM, Newton J, Rydin H.. 2012. Reliance on prey‐derived nitrogen by the carnivorous plant Drosera rotundifolia decreases with increasing nitrogen deposition. New Phytologist 195: 182–188. [DOI] [PubMed] [Google Scholar]
- Moran JA, Merbach MA, Livingston NJ, Clarke CM, Booth WE.. 2001. Termite prey specialization in the pitcher plant Nepenthes albomarginata—evidence from stable isotope analysis. Annals of Botany 88: 307–311. [Google Scholar]
- Müller J, Deil U.. 2001. Ecology and structure of Drosophyllum lusitanicum (L.) Link populations in the south-west of the Iberian Peninsula. Acta Botanica Malacitana 26: 47–68. [Google Scholar]
- Ojeda F, Pausas JG, Verdú M.. 2010. Soil shapes community structure through fire. Oecologia 163: 729–735. [DOI] [PubMed] [Google Scholar]
- Paniw M, Quintana-Ascencio PF, Ojeda F, Salguero-Gómez R. 2016. Accounting for uncertainty in dormant life stages in stochastic demographic models. Oikos, doi:10.1111/oik.03696. [Google Scholar]
- Paniw M, Salguero-Gómez R, Ojeda F.. 2015. Local-scale disturbances can benefit an endangered, fire-adapted plant species in Western Mediterranean heathlands in the absence of fire. Biological Conservation 187: 74–81. [Google Scholar]
- Pavlovič A, Saganová M.. 2015. A novel insight into the cost–benefit model for the evolution of botanical carnivory. Annals of Botany 115: 1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2015. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Renner T, Specht CD.. 2011. A sticky situation: assessing adaptations for plant carnivory in the Caryophyllales by means of stochastic character mapping. International Journal of Plant Sciences 172: 889–901. [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillison AN.. 1997. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia 112: 464–471. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Schulze I, Oren R.. 2001. Quantification of insect nitrogen utilization by the venus fly trap Dionaea muscipula catching prey with highly variable isotope signatures. Journal of Experimental Botany 52: 1041–1049. [DOI] [PubMed] [Google Scholar]
- Thorén LM, Tuomi J, Kämäräinen T, Laine K.. 2003. Resource availability affects investment in carnivory in Drosera rotundifolia. New Phytologist 159: 507–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.