Abstract
Background and Aims Trait-based plant ecology attempts to use small numbers of functional traits to predict plant ecological strategies. However, a major gap exists between our understanding of organ-level ecophysiological traits and our understanding of whole-plant fitness and environmental adaptation. In this gap lie whole-plant organizational traits, including those that describe how plant biomass is allocated among organs and the timing of plant reproduction. This study explores the role of whole-plant organizational traits in adaptation to diverse environments in the context of life history, growth form and leaf economic strategy in a well-studied herbaceous system.
Methods A phylogenetic comparative approach was used in conjunction with common garden phenotyping to assess the evolution of biomass allocation and reproductive timing across 83 populations of 27 species of the diverse genus Helianthus (the sunflowers).
Key Results Broad diversity exists among species in both relative biomass allocation and reproductive timing. Early reproduction is strongly associated with resource-acquisitive leaf economic strategy, while biomass allocation is less integrated with either reproductive timing or leaf economics. Both biomass allocation and reproductive timing are strongly related to source site environmental characteristics, including length of the growing season, temperature, precipitation and soil fertility.
Conclusions Herbaceous taxa can adapt to diverse environments in many ways, including modulation of phenology, plant architecture and organ-level ecophysiology. Although leaf economic strategy captures one key aspect of plant physiology, on their own leaf traits are not particularly predictive of ecological strategies in Helianthus outside of the context of growth form, life history and whole-plant organization. These results highlight the importance of including data on whole-plant organization alongside organ-level ecophysiological traits when attempting to bridge the gap between functional traits and plant fitness and environmental adaptation.
Keywords: Biomass, bud, climate, daylength, flower, growing season, Helianthus, soil fertility, life history, leaf, root, stem
INTRODUCTION
The past few decades have seen major strides in the development of ‘trait-based’ plant ecology. Such approaches focus on functional traits, often ecophysiological traits, individually and in small suites as proxies for whole-plant ecological strategies when attempting to understand adaptation (e.g. Westoby et al., 2002; Reich et al., 2003; Westoby and Wright, 2006; Ackerly and Cornwell, 2007). This approach is exemplified by the leaf economics spectrum (Wright et al., 2004), which summarized major aspects of ecophysiological variation among diverse species with a handful of key traits, and the broader plant economics spectrum (Reich et al., 2014), which attempts to unite functional trait axes from leaves, stems and roots into a coherent description of whole-plant ecological strategy. However, the link between easily measured functional traits and plant fitness is recognized to be rather tenuous (Shipley et al., 2016), as is the implicit assumption that ecophysiological traits contribute similarly to plant function and overall fitness across diverse species regardless of other aspects of whole-plant biology (Shipley et al., 2016). This is problematic given that the same classic literature that ties key ecophysiological traits to adaptation emphasizes almost equally the integration of such traits into whole-plant phenotypes, in particular the relative allocation of resources among the major plant organs (Mooney, 1972; Chapin, 1980; Bloom et al., 1985). Emerging empirical evidence has highlighted the importance of biomass allocation and whole-plant organization alongside key ecophysiological traits (Edwards et al., 2014; Freschet et al., 2015). Essentially, organ physiology (‘quality’) coupled with its relative abundance (‘quantity’) together determine the overall amount of organ function achieved by a plant. For example, from a carbon-capture perspective, whole-plant relative growth rate can be expressed as the product of three main factors – leaf construction cost, net photosynthetic return and leaf mass fraction (Poorter et al., 2012). Likewise, from a nutrient perspective, relative growth rate can be expressed as the product of net root uptake rate, average plant nutrient concentration and root mass fraction (Poorter et al., 2012). The relative allocation of biomass among organs is therefore theoretically on an equal footing with organ ecophysiology in driving plant resource acquisition and growth. Because of this, selection on organ ecophysiological traits is directly dependent on the larger context of biomass allocation, which determines the total whole-plant function achieved by a given set of ecophysiological traits. This interaction probably plays an important role in shaping plant adaptation to and persistence under disparate environmental conditions.
At a broad scale, the balance in allocation among organs is influenced by a variety of factors, including growth form and the relative availability of different key resources such as light, water and nutrients (Poorter et al., 2012). Modulation of allocation among organs is hypothesized to vary in response to resource supply, until the resources obtained by each organ limit growth equally (Chapin, 1980; Bloom et al., 1985). For instance, low water or nutrient availability should favour the evolution of increased allocation to roots, while insufficient carbon acquisition should favour increased allocation to leaves, or stems if the proximate cause is low light availability (Chapin, 1980; Bloom et al., 1985). Life history is also thought to influence biomass allocation, in particular the relative allocation to reproduction and storage functions between annuals and perennials (Bazzaz et al., 1987). While thorough meta-analyses have been conducted to examine a wide variety of factors affecting broad differences in biomass allocation among species (Poorter et al., 2012, 2015), there have been very few phylogenetically explicit studies of the role of biomass allocation in adaptation across diverse environments.
In addition to biomass allocation, reproductive timing is another whole-plant organizational trait that is key to determining plant fitness. Among habitats, a variety of factors may influence the optimal timing of reproduction, including growing season length and resource availability (Bazzaz et al., 1987; Guilbaud et al., 2015; Matthews and Mazer, 2016). If a plant begins reproduction too early, it sacrifices the potentially higher reproductive output that might be gained by delaying reproduction and growing to a larger vegetative size. If a plant begins reproduction too late, it may not complete seed production before the end of the growing season, whether terminated by frost, drought or other factors. Given the strong selection the environment places on optimal reproductive timing, this trait is known to be readily and rapidly differentiated among differing habitats (e.g. Hall and Willis, 2006; Franks et al., 2007; Kawakami et al., 2011; Brouillette et al., 2014; Matthews and Mazer, 2016). Plants possess multiple mechanisms for sensing environmental conditions, such that reproduction can be evolutionarily programmed to occur at the proper time, using cues in the form of temperature (Song et al., 2013; Kazan and Lyons, 2016), water availability (Fox, 1990; Kazan and Lyons, 2016), nutrient uptake (Guilbaud et al., 2015) and, perhaps most commonly, photoperiod (Blackman et al., 2011; Song et al., 2013). Reproductive timing is also expected to interact with biomass allocation, because allocation to productive vegetative structures has a compound interest effect on plant growth such that delaying allocation to reproduction in suitable environments can improve fitness (Bazzaz et al., 1987).
Through the dual lenses of biomass allocation and reproductive timing, this study examines the importance of whole-plant organization in the diversification of the herbaceous genus Helianthus. Wild sunflowers are an excellent system in which to examine trait evolution, as species vary widely in morphology and physiology, and occupy diverse habitats across North America, including deserts, wetlands, grasslands, forests, rock outcrops and coastal dunes (Heiser et al., 1969). Here we address two main questions using a phylogenetic comparative approach. First, how integrated are whole-plant organizational traits with one another and with organ-level ecophysiology? If biomass allocation and reproductive timing are tightly tied to one another and to leaf economic traits, then organ-level functional traits such as those of the leaf economics spectrum may be reasonable proxies for whole-plant ecological strategies. If not, then whole-plant organizational traits may be needed to describe plant function adequately. Secondly, what is the relative importance of whole-plant organizational traits to habitat differentiation as compared with organ-level functional traits? If biomass allocation or reproductive timing are more strongly tied to species diversification across environmental gradients than leaf economic traits, this calls into question the utility of using organ-level functional traits alone as proxies for whole-plant ecological strategies. Similarly, if the same leaf economic strategy allows for the occupancy of dramatically different habitats through interactions with plant architecture, phenology, growth form or life history, this undermines the assumption that organ-level functional traits contribute in similar ways to plant function and fitness across diverse species and environments (Shipley et al., 2016). By examining the relative predictive power of biomass allocation and reproductive timing in this system, we aim to gain an understanding of whether the current weak points in ‘trait-based’ ecology can be strengthened through the explicit consideration of plant architecture and phenology, and how these whole-plant organizational traits interact with organ-level ecophysiology.
MATERIALS AND METHODS
Study system
To assess the evolution of biomass allocation and reproductive timing across wild sunflowers, a diverse group of 27 diploid non-hybrid species of Helianthus (Asteraceae, Heliantheae) were selected for inclusion in this study, along with one of the two members of the sister genus Phoebanthus as an outgroup. This includes over half of the approx. 50 species described within Helianthus as well as four-fifths of diploid non-hybrid taxa (Heiser et al., 1969; Timme et al., 2007). Furthermore, these species were all included in the most recent and well-resolved phylogeny of the diploid backbone of Helianthus (Stephens et al., 2015), allowing for the use of robust phylogenetic comparative approaches. In order to incorporate natural intraspecific variation into assessments of trait evolution, multiple populations were included from across the geographic range of each species (2–4 each, 83 in total). Seed from these populations was either directly wild-collected or obtained from accessions established with the USDA National Genetic Resources Program (Supplementary Data Dataset S1).
Experimental design and plant growth
Because biomass allocation and reproductive timing are environmentally labile, plants were grown and traits assessed under controlled conditions in a high-resource greenhouse common garden experiment. By minimizing environmentally driven variation in this way, underlying genetic trait differentiation can be assessed for potential adaptive significance. Given the large number of populations under study, it was not feasible to grow all species simultaneously. Accordingly, the 28 species were divided into two common gardens grown in 2012 and 2013 (hereafter CG-1 and CG-2, respectively) at the University of Georgia Plant Biology greenhouses in Athens, GA, USA. To minimize differences between the two common gardens, the same experimental timing was used in both years, and environmental conditions were kept as similar as possible, including the use of identical pots, soil mixture, fertilization, watering regime and greenhouse temperature controls. To assess and correct for any remaining uncontrollable differences in conditions between years, three species reflecting a cross-section of growth form, life history and overall morphology (H. annuus, H. radula and H. silphioides) were replicated in both common gardens to serve as phytometers. Given the suitability of this design for addressing a variety of research questions, these common gardens were also used for the study of several other classes of physiological traits, including leaf economics (Mason and Donovan, 2015), leaf defences and secondary chemistry (Mason et al., 2016), and floral trait diversity (Mason et al., unpubl. res.). Experimental conditions are therefore described in detail in Mason and Donovan (2015). In short, seeds were scarified to induce uniform germination on moist filter paper in Petri dishes (Julian days 129–131), and transferred to seedling trays under a 12 h photoperiod once cotelydons had turned green (within 2–4 d). Eight replicate plants per population were then transplanted upon the emergence of true leaves (Julian days 142–154) into 6 L pots filled with a 3:1 mixture of sand and calcined clay. Pots were arranged in a randomized complete block design to account for any spatial environmental variation in the greenhouse. To provide high-nutrient conditions for plant growth, pots received 20 g of 9 month slow-release fertilizer with micronutrients (Osmocote Plus 15-9-12, Scotts, Marysville, OH, USA) mixed into the soil, as well as an initial liquid fertilization with supplemental Ca, Fe and Mg to promote seedling establishment. To ensure ample water availability, pots received daily drip irrigation to field capacity. Once transplanted into pots in the greenhouse, plants received ambient photoperiod for the remainder of the experiment, ranging from a maximum daylength of 14·4 h in mid-June (Julian days 168–174) to a minimum daylength of 9·9 h in mid-December (Julian days 350–360). Greenhouse temperatures were controlled by an automated system of heaters and evaporative coolers, set to 18 °C at night and 27 °C during the day (though daytime temperatures on especially hot summer days varied a few degrees above the daytime set point due to limitations of evaporative cooling).
Trait measurement
All plants were evaluated three times per week for the development of the first visible bud, and thereafter for the opening of the first flower (anthesis). These two dates were recorded for each plant. Almost all plants survived and successfully flowered (a mean of 7·52 ± 1·13 s.d. per population), and only a handful of individual plants remained vegetative and did not flower during the experiment. Once plants reached reproductive maturity, standardized harvests were performed. All members of a species (across all populations) were harvested together over a short period of time (Supplementary Data Table S1), with two criteria – reproduction and leaf senescence – used to define maturity. Timing harvests by the application of these two criteria captures both plant size and biomass allocation on reproductively mature plants at the end of a simulated growing season, in a way relevant to the biology of each species. First, to meet the reproduction criterion, all plants of a given species had to be past peak flowering, with the exception of any plants that remained vegetative and were not approaching reproduction (e.g. no buds). A small number of plants remained vegetative in a handful of species, mostly perennials. Secondly, to meet the leaf senescence criterion, all plants of a given species had to have senesced their most juvenile leaves. As these common gardens were also used to assess leaf economics traits, leaves were tagged on juvenile plants to estimate leaf life span (Mason and Donovan, 2015), and plants were not harvested until these leaves had senesced. In practice, the senescence of these juvenile leaves typically corresponded to the period between bud production and the onset of seed filling (and associated whole-plant senescence) in annual species, the onset of programmed end of growing season shoot senescence in the erect perennials (which are deciduous) and either the shift to winter dormancy or the production of new leaves after breaking dormancy in the basal rosette perennials. By combining these two criteria, we avoid issues that might arise from using either criterion in isolation – for instance, using only reproduction would not adequately capture the mature size or allocation of biomass in most basal rosette species, as these species often reproduce well before the end of their growing season and produce substantial additional vegetative growth before entering winter dormancy. Similarly, using only leaf senescence would result in barely capturing flowering in many annual species. Together, these two criteria allow for estimation of plant biomass traits across diverse taxa in a way that determines end of growing season maturity relative to the biology of each species.
At harvest, shoots were detached at the soil surface and divided into three categories: stems, leaves and reproductive parts (buds and flower heads). Pots were then emptied over a fine mesh screen, and below-ground biomass was gently washed to remove the soil mixture. This biomass consisted of coarse and fine roots, as well as rhizomes in many of the perennial species. Across Helianthus species, there is a wide continuum in the degree of rhizome formation, and in fact many perennial species lack rhizomes and instead root tissue or crown buds give rise to new shoots each year (Heiser et al., 1969). In many cases, rhizomes are not readily distinguishable from coarse roots, so rhizomes could not be consistently separated from roots with confidence and were thus included with roots as a single category of below-ground biomass. Separated biomass was dried at 60 °C in a forced-air drying oven for at least 72 h and then weighed for dry mass. Total plant biomass was calculated as the sum of below-ground, stem, leaf and reproductive biomass, and mass fractions were calculated as the most preferable presentation of biomass allocation patterns following the recommendations of Poorter and Sack (2012). Mass fractions were favoured over ratios (e.g. root:shoot) given the inherent problems with ratio-based metrics, including unboundedness, asymmetry, strong non-normality and loss of biological information (Poorter and Sack, 2012).
Data assessment and standardization
To test for differences between the two common gardens (CG-1 and CG-2) in total plant biomass and mass fractions, all replicates of the three phytometer species (nine populations) were assessed by a single analysis of variance (ANOVA; including year, population, year × population and block effects). Block was not found to have a significant effect for total biomass or any mass fractions (α = 0·05 was used as the significance threshold for all analyses unless otherwise stated). Total plant biomass was on average 36 % larger in CG-2 than in CG-1 across the phytometers, with no significant year × population interaction. While plant size differed substantially, mass fractions were found to vary negligibly between years (1–5 % of total dry mass). These phytometer results were used to inform standardization of data between the two common gardens. Population means were calculated over replicate plants without incorporating block effects. Population means for total biomass were then corrected upward by 36 % for all populations grown in CG-1, while mass fractions were not corrected between years. The summer of 2012 was hot and sunny, while the summer of 2013 was unusually rainy with milder peak temperatures. While precipitation itself could not affect plants in the greenhouse, differences in peak temperatures and cumulative insolation between years probably drove the differences seen in total plant size. It is important to point out that this phytometer-based correction is by no means a perfect solution to the problem of inter-year variation in total biomass, as the differences between years probably had differential impacts on different taxa and the phytometers cannot completely account for all variation. However, phytometer correction should put species grown in different years on a roughly equal footing, and in any case the shortcomings of phytometer correction for inter-year variation should only affect the trait of total biomass, not biomass allocation patterns (mass fractions).
With respect to reproductive timing, all but two plants of the KYL population of H. maximiliani did not flower in the greenhouse, either not producing buds or stalling after bud production, so this population was removed from analyses of reproductive timing. Additionally, plants of all three populations of H. radula grown in CG-2 behaved similarly, with most stalling after producing a few buds and only around a third of plants flowering after abnormally long delays. This slow-growing species does not always flower in the first year in the wild, and differences in conditions in CG-2 may have inhibited flowering in favour of vegetative growth. As such, populations of H. radula grown in CG-2 were excluded from further consideration for reproductive analyses and not used as a phytometer species for assessment of reproductive timing. The other two phytometer species were used to test for differences in the Julian dates of first bud and first flower between years in the same manner as for biomass traits. Block was not found to have a significant effect for either first bud or first flower. Date of first bud was on average 5·0 % later in CG-2, with a significant year × population interaction (varying between 1·4 and 7·4 % later among populations). Date of first flower was on average 9·7 % later in CG-2, also with a significant year × population interaction (varying between 4·4 and 13·9 % among populations). It seems that the same difference in conditions that resulted in overall larger plants in CG-2 also delayed reproduction. As such, population means were calculated over replicate plants without incorporating block effects, and first bud and first flower dates were corrected upward by 5·0 and 9·7 %, respectively, for all populations grown in CG-1. Again, this correction by phytometer is far from a perfect solution, but should ameliorate some of the effect of inter-year variation and put species on a roughly equal footing.
After correction, population means of first bud and first flower dates were used to calculate the length of time between bud initiation and anthesis (hereafter referred to as ‘interim period’). Daylengths were also calculated for each observed first bud and first flower date using the US Naval Observatory sunrise and sunset tables for Athens, GA.
Environmental data
Climate and soil characteristics of each population source site were obtained as reported previously in Mason and Donovan (2015). In brief, climate data were aggregated from a variety of sources. Altitude, mean annual temperature (MAT), mean diurnal temperature range, temperature seasonality, mean annual precipitation (MAP) and precipitation seasonality were obtained from the WorldClim database (Hijmans et al., 2005). Potential evapotranspiration (PET) and aridity index (temperature-adjusted water availability, the ratio of MAP to PET) were taken from the CGIAR Global Aridity and PET database (Zomer et al., 2008). Additionally, length of the frost-free period was derived from the USDA NRCS Soil Survey Geographic Database (Soil Survey Staff, NRCS), and moisture-based growing season length was retrieved from the UN FAO Global Agro-Ecological Zones version 3·0 Module I (IIFAS/FAO, 2012).
To characterize soil characteristics, five approx. 20 cm deep soil cores were taken spread throughout the population at each source site, dried at 60 °C and homogenized prior to analysis. Soil carbon content, nitrogen content and C:N ratio were assessed with Micro-Dumas combustion (NA1500, Carlo Erba Strumentazione, Milan, Italy) at the University of Georgia Analytical Chemistry Laboratory. Soil samples were also submitted for standard bulk soil analysis by A&L Eastern Laboratories (Richmond, VA, USA). This analysis provided estimates of soil pH, organic matter content, cation exchange capacity (CEC), available phosphorus and exchangeable calcium, potassium and magnesium. All soil characteristics were averaged across the five soil cores to yield population site means (Dataset 1). Covariation among environmental characteristics is given in Supplementary Data Table S3.
Phylogenetic analysis
All phylogenetic analyses were performed using the phylogeny of Stephens et al. (2015), the most robust and well-resolved phylogeny of the diploid backbone of the genus Helianthus. Phylogenetic signal was assessed as Pagel’s λ for all plant traits using the ‘phylosig’ function in the R package ‘phytools’ (Pagel, 1999; Revell, 2012). All but one trait was found to exhibit significant phylogenetic signal (Supplementary Data Table S2), confirming the need for phylogenetically explicit analyses. Macroevolutionary correlations among plant traits and between plant traits and environmental characteristics were assessed using phylogenetic mixed models on population means (Housworth et al., 2004; Felsenstein, 2008). These models were implemented using the ‘phylopars’ function in the R package ‘Rphylopars’ to estimate evolutionary covariance while accounting for within-species variation and missing data (Bruggeman et al., 2009; Goolsby et al., 2016). Additionally, macroevolutionary correlations were assessed between the plant traits assessed in this study and leaf economic traits assessed on the common gardens in a previous study (Mason and Donovan, 2015). Furthermore, to test for trait differences between annuals, erect perennials and basal rosette perennials, species means were calculated on population means, and phylogenetic ANOVA was performed using the ‘phylANOVA’ function in the R package ‘phytools’ (Garland et al., 1993; Revell, 2012), which tests for differences among groups while accounting for expected residual autocorrelation due to evolutionary relatedness.
RESULTS
Variation in biomass allocation and reproductive timing
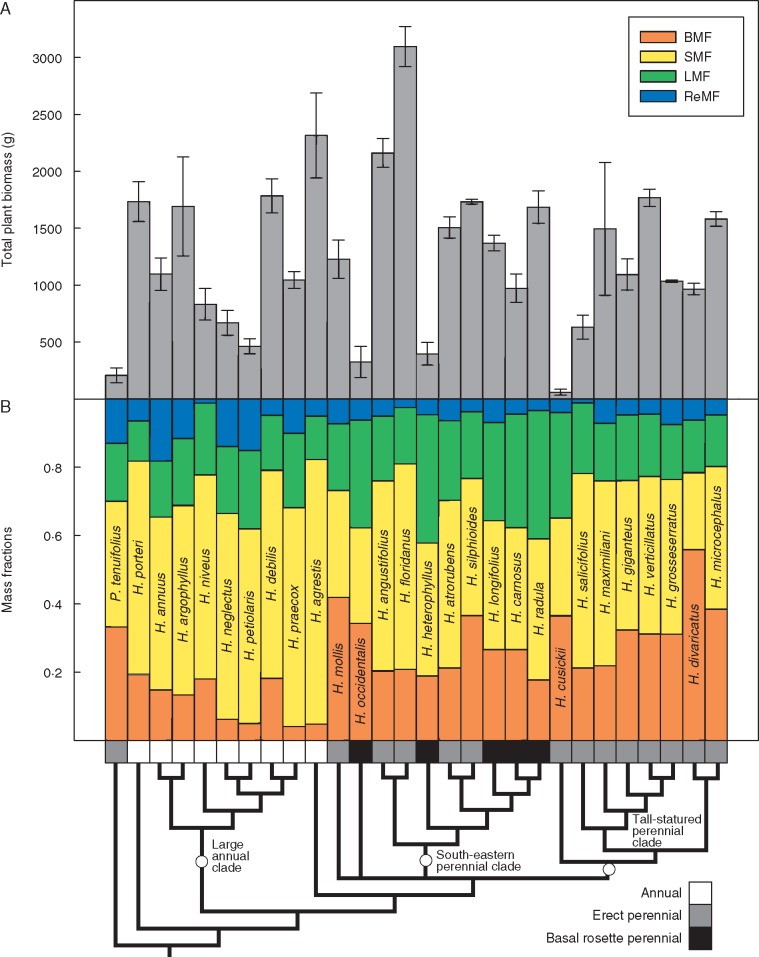
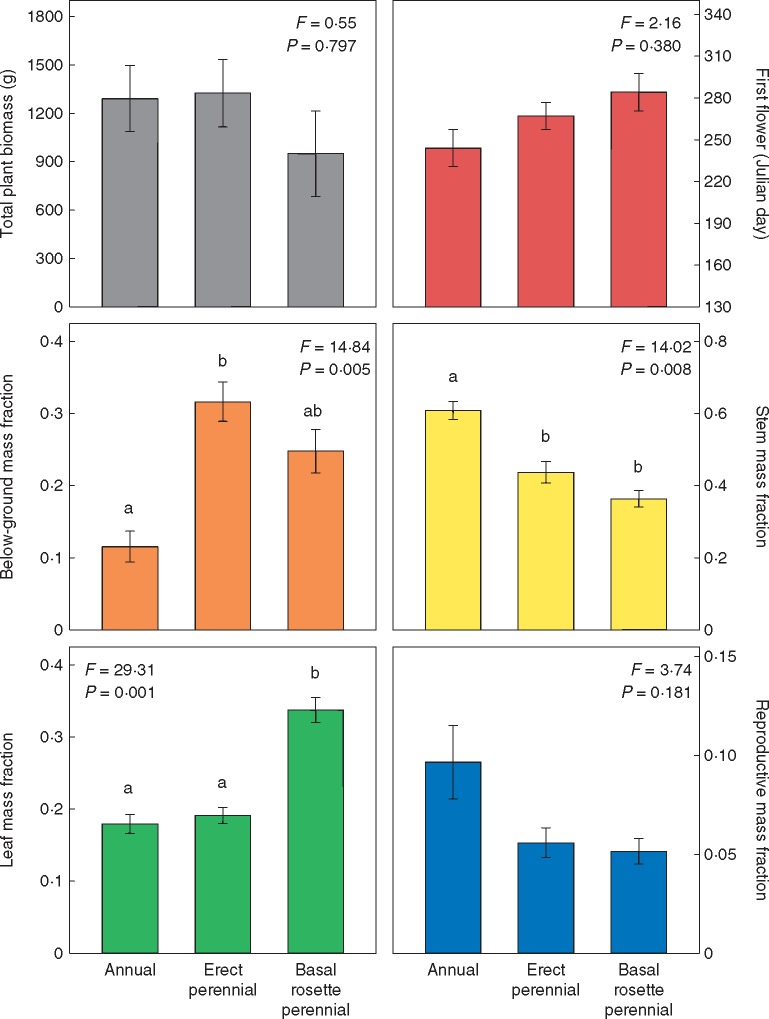
Across the genus, there is broad diversity in plant size and organization (Fig. 1). Total plant biomass varies approx. 50-fold, from the very small H. cusickii to the very large H. floridanus. Below-ground mass fraction varies from <5 % of total plant biomass in several annual species to > 55 % in H. divaricatus. Stem mass fraction is similarly variable, ranging from 28 % in H. occidentalis ssp. occidentalis to nearly 78 % in H. agrestis. Leaf mass fraction varies somewhat less, from <12 % in H. porteri to > 37 % in both H. heterophyllus and H. radula. Reproductive mass fraction varies the least though still substantially, from about 1 % in H. salicifolius and H. niveus ssp. tephrodes to > 18 % in H. annuus. Among species, the evolution of larger overall plant size at maturity is associated with the evolution of higher stem mass fraction and lower leaf mass fraction (Table 1). Stem mass fraction trades off strongly with below-ground mass fraction, and to a lesser extent with leaf mass fraction, though no other significant pairwise trade-offs exist among mass fractions (Table 1). With respect to growth form and life history, there are no significant differences in overall plant size, though there are large differences in vegetative mass fractions (Fig. 2). Annual species have significantly higher allocation to stems than perennial species, while basal rosette species have significantly higher allocation to leaves than erect species (Fig. 2). Erect perennials also have significantly higher allocation to roots and rhizomes than annual species (Fig. 2). Reproductive allocation, while on average higher in annuals than in perennials, does not vary significantly among groups (Fig. 2).
Fig. 1.
Variation in biomass allocation and total plant size across Helianthus. (A) Species mean total plant biomass at harvest. Error bars reflect standard errors of species means calculated from population means. (B) Relative biomass allocation among plant organs represented as species mean below-ground, stem, leaf and reproductive mass fractions. Note that infraspecific epithets are omitted for space.
Table 1.
Macroevolutionary correlations among plant traits as assessed by a phylogenetic mixed model incorporating intraspecific variation using population means (Housworth et al., 2004; Felsenstein, 2008)
| BMF | SMF | LMF | ReMF | First bud | First flower | Interim period | |
|---|---|---|---|---|---|---|---|
| Total biomass | – | (+) 0·32 | (–) 0·34 | – | (+) 0·38 | (+) 0·44 | (+) 0·42 |
| BMF | (–) 0·66 | – | – | – | – | – | |
| SMF | (–) 0·20 | – | (+) 0·20 | (+) 0·18 | – | ||
| LMF | – | – | – | – | |||
| ReMF | (–) 0·33 | (–) 0·28 | – | ||||
| First bud | (+) 0·93 | (+) 0·56 | |||||
| First flower | (+) 0·80 |
R2 and directionality of significant correlations are presented.
Abbreviations: BMF, below-ground mass fraction; SMF, stem mass fraction; LMF, leaf mass fraction; ReMF, reproductive mass fraction.
First bud and first flower dates are considered in Julian days.
Fig. 2.
Differences in plant traits among species based on growth form and life history using phylogenetic ANOVA (Garland et al., 1993) in the R package ‘phytools’ (Revell, 2012). Bars that do not share letters are significantly different by the Holm post-hoc test.
Helianthus species vary substantially in reproductive timing, here with first bud varying from as little as under 2 months post-germination in several annual species to well over 4 months in multiple south-eastern species. Date of first flower varies even more, from a little over 2 months to well over 6 months post-germination. This corresponds to variation in the interim period between bud and flower from just over 2 weeks to over 2 months. Dates of first bud and first flower are strongly positively correlated among species, as expected, and later reproducing species also have longer interim periods (Table 1). The evolution of later reproduction is positively correlated with the evolution of both higher total plant biomass and higher stem mass fraction at maturity, but later reproduction is also associated with lower reproductive mass fraction (Table 1). Dates of first bud and first flower do not appear to be significantly associated with life history or growth form (Fig. 2).
Reproductive timing, biomass allocation among vegetative organs, and plant size at maturity are all evolutionarily correlated with previously reported leaf economic traits. The evolution of more resource-acquisitive leaf traits (higher photosynthetic rate and leaf nutrient contents) is correlated with the evolution of earlier reproduction (Table 2). The evolution of higher leaf mass per area (LMA), indicating higher leaf construction cost, is correlated with the evolution of higher leaf mass fraction, while the evolution of higher leaf phosphorus and longer leaf life span is correlated with increased allocation to roots and rhizomes over stems (Table 2). Overall, the evolution of higher total plant biomass at maturity is associated with more resource-acquisitive traits such as lower LMA and higher leaf phosphorus (Table 2). Allocation to reproductive organs, however, appears unrelated to leaf economic traits (Table 2).
Table 2.
Macroevolutionary correlations between biomass allocation and reproductive timing traits in this study vs. leaf economic traits reported previously (Mason and Donovan, 2015)
| Amass | Rmass | Nmass | Pmass | LMA | LL | |
|---|---|---|---|---|---|---|
| Total biomass | – | – | – | (–) 0·21 | (–) 0·22 | – |
| BMF | – | – | – | (+) 0·16 | – | (+) 0·21 |
| SMF | – | – | – | (–) 0·16 | – | (–) 0·21 |
| LMF | – | – | – | – | (+) 0·17 | – |
| ReMF | – | – | – | – | – | – |
| First bud | (–) 0·26 | – | (–) 0·23 | – | – | – |
| First flower | (–) 0·36 | – | (–) 0·26 | (–) 0·18 | – | – |
| Interim period | (–) 0·43 | – | (–) 0·23 | (–) 0·19 | – | (+) 0·19 |
Correlations were assessed by a phylogenetic mixed model.
R2 and directionality of significant correlations (P < 0·05) are presented.
Abbreviations: Amass, leaf photosynthetic rate; BMF, below-ground mass fraction; LL, leaf life span; LMA, leaf mass per area; LMF, leaf mass fraction; Nmass, leaf nitrogen concentration; Pmass, leaf phosphorus concentration; Rmass, leaf respiration rate; ReMF, reproductive mass fraction; SMF, stem mass fraction.
First bud and first flower dates are considered in Julian days.
Diversification across environmental gradients
Both total plant biomass at maturity and relative mass fractions are significantly evolutionarily correlated with a wide variety of environmental factors. Higher total plant biomass at maturity is correlated with evolutionary shifts into habitats with longer frost-free periods and moisture-based growing periods as well as higher MAT and MAP and lower temperature seasonality, all of which predominate at lower latitudes and altitudes (Table 3). Soil characteristics appear unrelated to the evolution of total plant biomass at maturity (Table 3).
Table 3.
Macroevolutionary correlations between plant traits and source site environmental characteristics as assessed by a phylogenetic mixed model incorporating intraspecific variation using population means (Housworth et al., 2004; Felsenstein, 2008)
| Total biomass | BMF | SMF | LMF | ReMF | First bud | First flower | Interim period | |
|---|---|---|---|---|---|---|---|---|
| Latitude | (–) 0·25 | (+) 0·25 | (–) 0·31 | – | – | (–) 0·26 | (–) 0·29 | (–) 0·24 |
| Altitude | (–) 0·28 | – | – | – | (+) 0·30 | (–) 0·42 | (–) 0·44 | (–) 0·37 |
| Frost-free period | (+) 0·23 | (–) 0·18 | (+) 0·31 | – | – | (+) 0·28 | (+) 0·32 | (+) 0·28 |
| Moisture-based growing period | (+) 0·19 | – | – | – | – | (+) 0·34 | (+) 0·38 | (+) 0·35 |
| Mean annual temperature | (+) 0·21 | (–) 0·20 | (+) 0·31 | – | – | (+) 0·27 | (+) 0·30 | (+) 0·25 |
| Mean diurnal range | – | – | – | – | – | – | – | – |
| Temperature seasonality | (–) 0·18 | (+) 0·15 | (–) 0·16 | – | – | (–) 0·19 | (–) 0·22 | (–) 0·18 |
| Mean annual precipitation | (+) 0·21 | – | – | – | – | (+) 0·34 | (+) 0·38 | (+) 0·33 |
| Precipitation seasonality | – | (–) 0·39 | (+) 0·19 | – | – | – | – | – |
| Potential evapotranspiration | – | (–) 0·14 | (+) 0·24 | – | – | (+) 0·25 | (+) 0·27 | (+) 0·22 |
| Aridity index | – | – | – | – | – | (+) 0·19 | (+) 0·21 | (+) 0·20 |
| Soil N | – | – | (+) 0·19 | (–) 0·19 | – | – | – | – |
| Soil C | – | – | (+) 0·20 | (–) 0·20 | – | – | – | – |
| Soil C:N | – | (–) 0·29 | (+) 0·38 | – | – | – | – | – |
| Soil P | – | – | – | – | (+) 0·23 | (–) 0·15 | (–) 0·22 | (–) 0·29 |
| Soil K | – | – | – | – | – | (–) 0·22 | (–) 0·20 | – |
| Soil Mg | – | – | – | – | – | (–) 0·27 | (–) 0·28 | (–) 0·21 |
| Soil Ca | – | (–) 0·18 | (+) 0·23 | – | – | – | – | – |
| Soil pH | – | – | – | – | – | (–) 0·14 | (–) 0·17 | (–) 0·17 |
| Soil organic matter content | – | – | (+) 0·15 | (–) 0·18 | – | – | – | – |
| Soil cation exchange capacity | – | (–) 0·15 | (+) 0·17 | – | – | – | – | – |
R2 and directionality of significant correlations (P < 0·05) are presented, with correlations significant after implementation of a false discovery rate correction at q = 0·05 presented in bold (Benjamini and Hochberg, 1995).
Abbreviations: BMF, below-ground mass fraction; SMF, stem mass fraction; LMF, leaf mass fraction; ReMF, reproductive mass fraction.
First bud and first flower dates are considered in Julian days.
Given their strong trade-off among species, below-ground mass fraction and stem mass fraction are inversely related to many of the same climate factors. Habitats with shorter frost-free periods, lower MAT, higher temperature seasonality and lower precipitation seasonality (all conditions typical of higher latitudes) favour the evolution of increased allocation to roots and rhizomes at the expense of stems (Table 3). Unlike below-ground and stem allocation, climate factors do not appear to influence the evolution of leaf mass fraction or reproductive mass fraction, with the single exception that a higher reproductive mass fraction evolves repeatedly at higher altitudes (Table 2). Underground, some soil fertility metrics appear to mediate relative allocation between below-ground biomass and stems, while others appear to drive relative allocation between stems and leaves. Shifts onto soils with higher soil calcium, CEC and C:N ratio are evolutionarily correlated with lower below-ground allocation and higher stem allocation (Table 2). Shifts onto soils with higher nitrogen, carbon and organic matter content are all evolutionarily correlated with lower leaf allocation and higher stem allocation (Table 2). In all cases shifts into habitats with less fertile soils appear to favour decreased allocation to stems. Additionally, the occupation of soils with higher phosphorus content is evolutionarily correlated with increased allocation to reproduction.
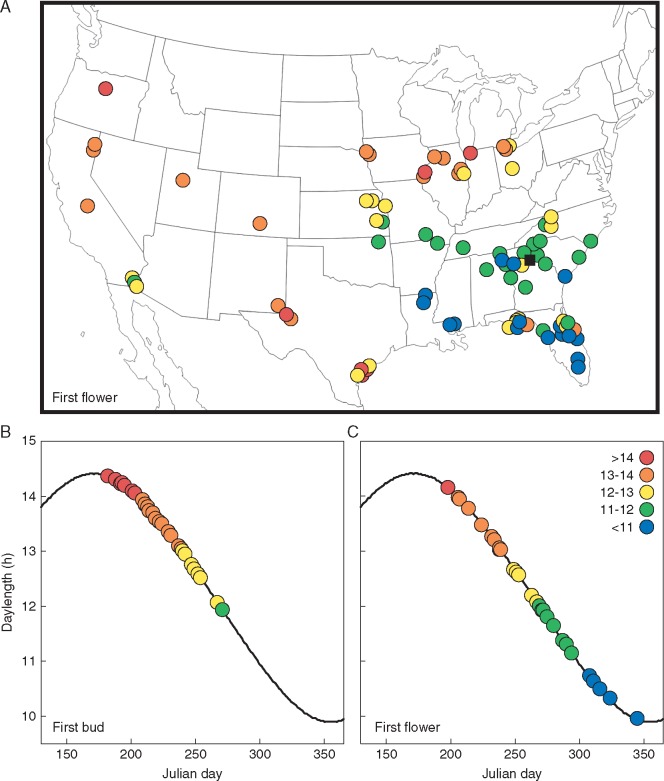
Much like total plant biomass at maturity, the evolution of later reproductive timing is strongly correlated with evolutionary shifts into habitats with longer growing seasons and associated environmental factors (Table 2). In addition to those factors correlated with the evolution of total plant biomass at maturity, a high potential evapotranspiration and aridity index also favour the evolution of later reproduction (Table 2). Geographically, it is easy to see that earlier reproduction is associated with populations distributed in the cooler northern and more arid western USA, while later reproduction is largely found in the warm and mesic south-east (Fig. 3). With respect to soil fertility, earlier reproduction appears to be favoured by shifts onto more acidic soils, as well as those with higher levels of phosphorus, potassium and magnesium (Table 2).
Fig. 3.
Variation in reproductive timing across Helianthus. (A) Population mean daylength at first flower in Athens, Georgia common gardens (black square) plotted on population source sites across North America. (B) Species mean daylength at first bud and (C) first flower in Athens, Georgia common gardens.
DISCUSSION
Integration of traits into whole-plant ecological strategies
The most current sunflower phylogeny (Stephens et al., 2015) indicates that the diploid backbone of Helianthus is comprised of three main clades – a large annual clade, a clade of tall-statured perennials and a south-eastern perennial clade made up of both erect and basal rosette perennials. With respect to whole-plant organization, members of the large annual clade typically have low below-ground allocation (not built for multiyear persistence) coupled with high stem allocation allowing for the shading of competitors in the open habitats they typically occupy. This architecture is accompanied by a resource-acquisitive leaf economic strategy supporting rapid growth (Mason and Donovan, 2015), as well as a generally high reproductive allocation. These species also have fairly early reproductive timing regardless of geography, a characteristic that is probably favoured in the habitats this clade occupies: more arid environments such as deserts and grasslands, or edaphically stressful habitats such as coastal dunes and sand prairies in less arid regions (Heiser et al., 1969). Members of the tall-statured perennial clade typically have high allocation to roots and rhizomes, supporting their multiyear persistence, as well as a typically resource-acquisitive leaf economic strategy capable of generating large deciduous shoots each growing season (Mason and Donovan, 2015). Reproductive timing in this clade is highly variable by geographic region – quite early in northern latitudes vs. later in southern Appalachia and the middle Great Plains. The south-eastern perennial clade contains an even mix of basal rosette and erect perennial species, united by a more resource-conservative leaf economic strategy supporting slower growth and late reproduction over the long growing seasons characteristic of the warm and moist south-eastern USA (Mason and Donovan, 2015). The growth forms, however, differ in allocation, with the erect perennials having similar allocation patterns to those in the large-statured perennial clade, while the basal rosette species maintain above-ground rosettes year-round and have high leaf allocation. Across the genus as a whole, biomass allocation is not particularly strongly integrated with reproductive timing. Later reproducing species do have higher average stem allocation and lower reproductive allocation, but both early and late reproducing species possess a diversity of allocation patterns. Surprisingly, life history is only associated with significant differences in vegetative allocation, and not with significant differences in reproductive timing, overall plant size or reproductive mass fraction. Annual plants are typically expected to invest more in reproduction than perennial close relatives, especially for annuals that occupy ruderal environments (Gaines et al., 1974; Bazzaz et al., 1987). While some annual Helianthus species often occupy human-disturbed habitats (e.g. Helianthus annuus and Helianthus petiolaris) and several more are native to habitats with natural forms of disturbance (e.g. unstable sand dunes), others are found in habitats with little disturbance (e.g. H. porteri and H. agrestis) which might obscure a general pattern. For rhizomatous perennials, estimates of below-ground mass fraction may also contain some clonal reproductive function, especially in the handful of members of the tall-statured perennial clade known to achieve substantial vegetative spread and senesce connections to parent plants (Heiser et al., 1969). However, given that this study only assessed a single year of growth from seed, the rhizomatous allocation that could be considered ‘reproductive’ rather than simply storage for resprouting is negligible.
Whole-plant traits and environmental differentiation
Across North America, biomass allocation in Helianthus varies strongly across environmental gradients. Meta-analysis of biomass allocation in herbs suggests that several of the macroevolutionary patterns seen here mirror typical plastic plant responses to environmental conditions (Poorter et al., 2012). For instance, lower nutrient availability favours increased root allocation, lower temperatures favour increased root and decreased stem allocation, and larger herbs on average have lower leaf mass fraction and higher stem mass fraction (Poorter et al., 2012). Water availability is an apparent exception, as plastically herbs typically increase root allocation in response to drought (Poorter et al., 2012), though among Helianthus there are no significant relationships between below-ground mass fraction and mean annual precipitation or aridity index. Additionally, a lower below-ground mass fraction is associated with higher precipitation seasonality and higher potential evapotranspiration, indicating if anything lower root allocation in environments more prone to low water availability. This is probably due to contrasting architectural mechanisms for achieving drought escape in annuals vs. deciduous perennials – in annuals, growing quickly and completing reproduction during favourable periods, in deciduous perennials, investing heavily in below-ground storage and becoming dormant during droughts (Kigel et al., 2011; Brouillette et al., 2014). Unlike water availability, macroevolutionary differentiation with respect to soil fertility appears to be unified across Helianthus, with low soil fertility favouring higher allocation to roots to increase plant acquisition of growth-limiting nutrients (Chapin, 1980; Bloom et al., 1985). Additionally, high soil fertility favours reduced allocation to leaves, probably because more resource-acquisitive leaf economic strategies are found on more fertile soils, and more productive leaves allow for the maintenance of total plant carbon capture with lower relative leaf allocation (Mason and Donovan, 2015).
Reproductive timing across Helianthus is even more strongly correlated with environmental gradients than biomass allocation. Reproduction across Helianthus is known to be strongly photoperiod controlled, with most species native to lower latitudes possessing an obligate short-day requirement for flowering (Henry et al., 2014). Previous work in three species shows adaptation for earlier flowering in populations at higher latitudes as well as in drier habitats (Blackman et al., 2011; Kawakami et al., 2011; Brouillette et al., 2014), identical to the patterns seen here among species. Overall Helianthus species appear to tailor reproduction strongly to growing season length, whether defined by temperature or water availability. Additionally, the relationship between soil nutrient availability and reproductive timing is consistent with the ‘peak-nutrient’ hypothesis of flowering time, where selection favours the trigger for flowering occurring just before the whole-plant nutrient uptake rate is maximized, a point that is reached earlier in faster-growing species and in more fertile soils (Guilbaud et al., 2015). This phenomenon is probably folded on top of selection in response to growing season length across the genus.
On the relative predictive power of leaf economic traits
In general, biomass allocation and reproductive timing are as or more strongly linked to differentiation across environments as leaf economics are in Helianthus (Mason and Donovan, 2015). Leaf economic strategy and reproductive timing appear tightly integrated across the genus, but both are less strongly associated with biomass allocation. On the whole, it seems that all three classes of traits are shaped by selection from the environment in the context of plant growth form and life history, with biomass allocation and leaf economic strategy evolving together to support the rate of whole plant growth necessary given optimal reproductive timing. Our results underscore the importance of whole-plant strategies in plant adaptation and the interaction of multiple classes of traits, in agreement with emerging evidence in other systems, both herbaceous and woody (Edwards et al., 2014; Freschet et al., 2015). While different growth forms may differ strongly in typical allocation across organs (e.g. forbs vs. trees), species within these forms (and often even within closely related groups such as genera) vary so much in size, architecture, longevity and seasonal phenology that they very probably experience similar interactions between organ functional traits and whole-plant allocation and phenology. Single classes of traits such as those that are usually considered to define the leaf economic spectrum are often poor proxies for whole-plant strategies without the context of whole-plant ecology and economics, as seen in Helianthus where species can have similar leaf economic traits despite differing dramatically in habitat occupancy, flowering time and whole-plant architecture. For example, using the first principal component of the standard six mass-based leaf economic traits, leaf economic strategy is nearly identical between the desert annual H. neglectus and the tallgrass prairie perennial H. grosseserratus, between the granite outcrop annual H. porteri and the forest gap perennial H. verticillatus, and between the wetland annual H. agrestis and the pine savanna basal rosette perennial H. radula (Mason and Donovan, 2015). Clearly more information than leaf functional traits alone is needed to understand the role of leaf functional traits in species diversification, especially predicting how trait values determine individual fitness and how trait values are shaped by environment (Shipley et al., 2016). Both of these current weak points in ‘trait-based’ ecology described by Shipley et al. (2016) seem largely unaddressable outside of the context of whole-plant organization. Furthermore, the idea of building broad generalizations about functional traits in plant ecology without at least a rough understanding of how general plant architecture and phenology interact with key ecophysiological traits in roots, stems and leaves seems insurmountable (Godoy and Levine, 2014; Klimešová and Herben, 2016). The current lack of data on whole-plant organization in functional trait databases would therefore appear to be a major impediment to progress in ‘trait-based’ ecology, and expanded consideration of these key aspects of plant physiology in light of growth form and life history is needed to achieve meaningful synthesis.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: harvest dates for all species in both common garden years. Table S2: phylogenetic signal in plant traits across Helianthus. Table S3: pairwise correlations among source site environmental characteristics. Dataset S1: source information, trait data and environmental metrics used in this study.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank S. McGaughey, K. Schofield, C. Woody, J. Vaughn, X. French and B. Hudson for assistance with plant trait data collection, A. Bowsher for assistance with soil trait characterization, and the handling editor and two anonymous reviewers for helpful comments on the manuscript. This work was supported by the National Science Foundation [grant IOS-1122842], as well as a 2011 Rosemary Grant Award to C.M.M. from the Society for the Study of Evolution.
Author contributions: C.M.M. and L.A.D. designed the study. C.M.M., K.E.D. and D.V.B. collected data. C.M.M., E.W.G. and K.E.D. designed and implemented analyses. All authors contributed to the writing of the manuscript.
Data accessibility: Data used in this study are available in the Supplementary Data, and also available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.v3824.
LITERATURE CITED
- Ackerly DD, Cornwell WK.. 2007. A trait-based approach to community assembly: partitioning of species trait values into within- and among-community components. Ecology Letters 10: 135–145. [DOI] [PubMed] [Google Scholar]
- Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF.. 1987. Allocating resources to reproduction and defense. Bioscience 37: 58–67. [Google Scholar]
- Blackman BK, Michaels SD, Rieseberg LH.. 2011. Connecting the sun to flowering in sunflower adaptation. Molecular Ecology 20: 3503–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS III, Mooney HA.. 1985. Resource limitation in plants – an economic analogy. Annual Review of Ecology and Systematics 16: 363–392. [Google Scholar]
- Brouillette LC, Mason CM, Shirk RY, Donovan LA.. 2014. Adaptive differentiation of traits related to resource use in a desert annual along a resource gradient. New Phytologist 201: 1316–1327. [DOI] [PubMed] [Google Scholar]
- Bruggeman J, Heringa J, Brandt BW.. 2009. PhyloPars: estimation of missing parameter values using phylogeny. Nucleic Acids Research 37: W179–W184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS. 1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233–260. [Google Scholar]
- Edwards EJ, Chatelet DS, Sack L, Donoghue MJ.. 2014. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. Journal of Ecology 102: 328–336. [Google Scholar]
- Felsenstein J. 2008. Comparative methods with sampling error and within-species variation: contrasts revisited and revised. American Naturalist 171: 713–725. [DOI] [PubMed] [Google Scholar]
- Fox GA. 1990. Drought and the evolution of flowering time in desert annuals. American Journal of Botany 77: 1508–1518. [Google Scholar]
- Franks SJ, Sim S, Weis AE.. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences, USA 104: 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet GT, Swart EM, Cornelissen JHC.. 2015. Integrated plant phenotypic responses to contrasting above- and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytologist 206: 1247–1260. [DOI] [PubMed] [Google Scholar]
- Gaines MS, Vogt KJ, Hamrick JL, Caldwell J.. 1974. Reproductive strategies and growth patterns in sunflowers (Helianthus). American Naturalist 108: 889–894. [Google Scholar]
- Garland T, Dickerman AW, Janis CM, Jones JA.. 1993. Phylogenetic analysis of covariance by computer simulation. Systematic Biology 42: 265–292. [Google Scholar]
- Godoy O, Levine JM.. 2014. Phenology effects on invasion success: insights from coupling field experiments to coexistence theory. Ecology 95: 726–736. [DOI] [PubMed] [Google Scholar]
- Goolsby EW, Bruggeman J, Ané C.. 2016. Rphylopars: fast multivariate phylogenetic comparative methods for missing data and within-species variation. Methods in Ecology and Evolution (in press). [Google Scholar]
- Guilbaud CSE, Dalchau N, Purves DW, Turnbull LA.. 2015. Is ‘peak N’ key to understanding the timing of flowering in annual plants? New Phytologist 205: 918–927. [DOI] [PubMed] [Google Scholar]
- Hall MC, Willis JH.. 2006. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60: 2466–2477. [PubMed] [Google Scholar]
- Heiser CBJ, Smith DM, Clevenger SB, Martin WCJ.. 1969. The North American sunflowers: Helianthus. Memoirs of the Torrey Botanical Club 22: 1–218. [Google Scholar]
- Henry LP, Watson RHB, Blackman BK.. 2014. Transitions in photoperiodic flowering are common and involve few loci in wild sunflowers (Helianthus; Asteraceae). American Journal of Botany 101: 1748–1758. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A.. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Housworth EA, Martins EP, Lynch M.. 2004. The phylogenetic mixed model. American Naturalist 163: 84–96. [DOI] [PubMed] [Google Scholar]
- IIASA/FAO. 2012. Global agro-ecological zones (GAEZ v 3.0). Laxenburg, Austria: IIASA, and Rome, Italy: FAO. [Google Scholar]
- Kawakami T, Morgan TJ, Nippert JB, et al. 2011. Natural selection drives clinal life history patterns in the perennial sunflower species, Helianthus maximiliani. Molecular Ecology 20: 2318–2328. [DOI] [PubMed] [Google Scholar]
- Kazan K, Lyons R.. 2016. The link between flowering time and stress tolerance. Journal of Experimental Botany 67: 47–60. [DOI] [PubMed] [Google Scholar]
- Kigel J, Konsens I, Rosen N, Rotem G, Kon A, Fragman-Sapir O.. 2011. Relationships between flowering time and rainfall gradients across Mediterranean–desert transects. Israel Journal of Ecology and Evolution 57: 91–109. [Google Scholar]
- Klimešová J, Tackenberg O, Herben T.. 2016. Herbs are different: clonal and bud bank traits can matter more than leaf–height–seed traits. New Phytologist 210: 13–17. [DOI] [PubMed] [Google Scholar]
- Mason CM, Donovan LA.. 2015. Evolution of the leaf economics spectrum in herbs: evidence from environmental divergences in leaf physiology across Helianthus (Asteraceae). Evolution 69: 2705–2720. [DOI] [PubMed] [Google Scholar]
- Mason CM, Bowsher AW, Crowell BL, Celoy RM, Tsai CJ, Donovan LA.. 2016. Macroevolution of leaf defenses and secondary metabolites across the genus Helianthus. New Phytologist 209: 1720–1733. [DOI] [PubMed] [Google Scholar]
- Matthews ER, Mazer SJ.. 2016. Historical changes in flowering phenology are governed by temperature × precipitation interactions in a widespread perennial herb in western North America. New Phytologist 210: 157–167. [DOI] [PubMed] [Google Scholar]
- Mooney HA. 1972. The carbon balance of plants. Annual Review of Ecology and Systematics 3: 315–346. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Poorter H, Sack L.. 2012. Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Frontiers in Plant Science 3: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L.. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193: 30–50. [DOI] [PubMed] [Google Scholar]
- Poorter H, Jagodzinski AM, Ruiz-Peinado R, et al. 2015. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytologist 208: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Shipley B, Bello F, Cornelissen JHC, Laliberté E, Laughlin DC, Reich PB.. 2016. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 180: 923–931. [DOI] [PubMed] [Google Scholar]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture Web Soil Survey Available online at http://websoilsurvey.nrcs.usda.gov/.
- Song YH, Ito S, Imaizumi T.. 2013. Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends in Plant Science 18: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JD, Rogers WL, Mason CM, Donovan LA, Malmberg RL.. 2015. Species tree estimation of diploid Helianthus (Asteraceae) using target enrichment. American Journal of Botany 102: 910–920. [DOI] [PubMed] [Google Scholar]
- Timme RE, Simpson BB, Linder CR.. 2007. High-resolution phylogeny for Helianthus (Asteraceae) using the 18s–26s ribosomal DNA external transcribed spacer. American Journal of Botany 94: 1837–1852. [DOI] [PubMed] [Google Scholar]
- Westoby M, Wright IJ.. 2006. Land-plant ecology on the basis of functional traits. Trends in Ecology and Evolution 21: 261–268. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ.. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Zomer RJ, Trabucco A, Bossio DA, Verchot LV.. 2008. Climate change mitigation: a spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agriculture, Ecosystems and Environment 126: 67–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.