Abstract
Background and Aims Independent evolution of derived complex characters provides a unique opportunity to assess whether and how similar genetic changes correlate with morphological convergence. Bilaterally symmetrical corollas have evolved multiple times independently from radially symmetrical ancestors and likely represent adaptations to attract specific pollinators. On the other hand, losses of bilateral corolla symmetry have occurred sporadically in various groups, due to either modification of bilaterally symmetrical corollas in late development or early establishment of radial symmetry.
Methods This study integrated phylogenetic, scanning electron microscopy (SEM)-based morphological, and gene expression approaches to assess the possible mechanisms underlying independent evolutionary losses of corolla bilateral symmetry.
Key Results This work compared three species of Lamiaceae having radially symmetrical mature corollas with a representative sister taxon having bilaterally symmetrical corollas and found that each reaches radial symmetry in a different way. Higher core Lamiales share a common duplication in the CYCLOIDEA (CYC) 2 gene lineage and show conserved and asymmetrical expression of CYC2 clade and RAD genes along the adaxial–abaxial floral axis in species having bilateral corolla symmetry. In Lycopus americanus, the development and expression pattern of La-CYC2A and La-CYC2B are similar to those of their bilaterally symmetrical relatives, whereas the loss of La-RAD expression correlates with a late switch to radial corolla symmetry. In Mentha longifolia, late radial symmetry may be explained by the loss of Ml-CYC2A, and by altered expression of two Ml-CYC2B and Ml-RAD genes. Finally, expanded expression of Cc-CYC2A and Cc-RAD strongly correlates with the early development of radially symmetrical corollas in Callicarpa cathayana.
Conclusions Repeated losses of mature corolla bilateral symmetry in Lamiaceae are not uncommon, and may be achieved by distinct mechanisms and various changes to symmetry genes, including the loss of a CYC2 clade gene from the genome, and/or contraction, expansion or alteration of CYC2 clade and RAD-like gene expression.
Keywords: Corolla symmetry, CYCLOIDEA, Lamiaceae, independent evolution, RADIALIS
INTRODUCTION
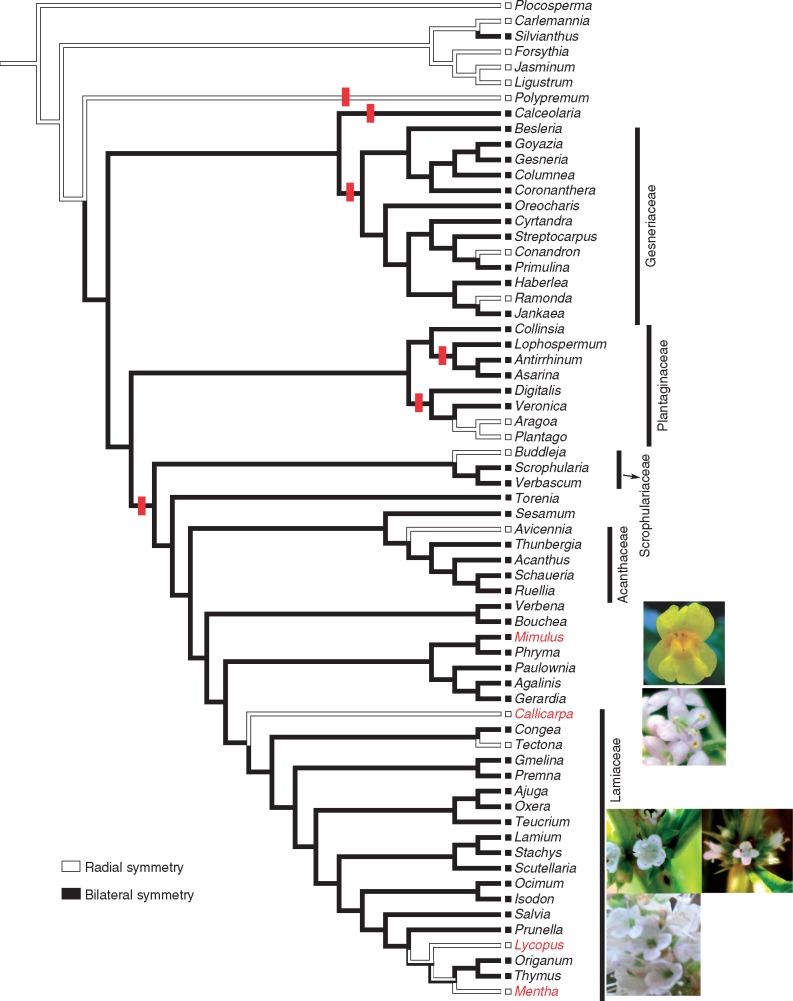
Within angiosperms, bilaterally symmetrical corollas have evolved from radially symmetrical corollas multiple times independently, and have long been considered evolutionary novelties that have increased conspecific pollination, thus promoting out-crossing and speciation (Neal et al., 1998; Ushimaru et al., 2009; Hileman, 2014a, b). In the core Lamiales, however, corollas in a number of distantly related taxa have reverted to near or complete radial symmetry (Donoghue et al., 1998; Endress, 2011) (Fig. 1). This raises the question of whether independent losses of Lamiales bilateral corolla symmetry have occurred through similar versus distinct developmental genetic mechanisms (Donoghue et al., 1998; Kadereit, 2004; Hileman, 2014a).
Fig. 1.
Evolution of corolla symmetry across the order Lamiales inferred by Mesquite 3.04 using parsimony ancestral state reconstruction (Maddison and Maddison, 2015). Phylogenetic tree and character states of corolla symmetry were compiled from previous studies (Donoghue et al., 1998; Endress, 1999; Stevens, 2001; Bello et al., 2004; Kadereit, 2004; Smith et al., 2004; Schäferhoff et al., 2010; Bendiksby et al., 2011; Drew and Sytsma, 2012; Zhong and Kellogg, 2015a). Black-filled lines represent corolla bilateral symmetry while white-filled lines show corolla radial symmetry. Vertical red bars show duplications of CYC2 clade genes (Zhong and Kellogg, 2015a and references therein). Families are assigned to lineages that have species with radially symmetrical corollas in the core Lamiales. The three focal species and reference outgroup are highlighted in red and bold.
The genetic basis of bilateral flower symmetry was first characterized in the Lamiales species Antirrhinum majus (snapdragon, Plantaginaceae), and focused initially on transcription factors in the TCP family, named for its founding members TEOSINTE BRANCHED1 from maize, CYCLOIDEA from snapdragon, and PROLIFERATING CELL FACTOR1 and 2 from rice (Cubas et al., 1999a). Two closely related TCP proteins from the CYCLOIDEA2 (CYC2) clade (sensuHowarth and Donoghue, 2006), CYCLOIDEA (CYC) and DICHOTOMA (DICH), induce the expression of the downstream MYB transcription factor RADIALIS (RAD), and together these protein products demarcate the adaxial part of the flower and affect the development of floral organs along the adaxial–abaxial axis (Luo et al., 1996, 1999; Corley et al., 2005; Raimundo et al., 2013).
CYC, DICH and RAD are expressed exclusively in the adaxial regions of snapdragon flowers, with cyc/dich double or rad single mutants showing complete and nearly radial floral symmetry, respectively (Luo et al., 1996, 1999; Corley et al., 2005). In mutant flowers, all petals acquire the morphology of wild-type abaxial petals, due to the ectopic expansion of the MYB protein DIVARICATA (DIV). DIV is normally restricted to the abaxial region through the antagonistic activity of RAD, which disrupts the formation of heterodimers of DIV and DIV-RAD interacting factors (DRIFs) (Raimundo et al., 2013). Unlike asymmetrical gene expression of CYC or RAD genes, DIV and DRIFs are widely transcribed in all petals (Almeida et al., 1997; Raimundo et al., 2013). Strikingly, comparative studies have demonstrated that CYC2 clade genes have played an essential role in floral symmetry patterning and have been recruited repeatedly and independently in a number of angiosperm clades that have bilaterally symmetrically flowers (e.g. Busch and Zachgo, 2007; Wang et al., 2008; Zhang et al., 2010; Howarth et al., 2011; Berger et al., 2016). Indeed, the CYC-RAD-DIV module may be conserved in the asterid core eudicot clade, although data suggest the evolution of distinct regulatory relationships between these three proteins in different clades (Luo et al., 1996, 1999; Feng et al., 2006; Yang et al., 2012; Garcês et al., 2016).
Similar to the snapdragon and its close relatives, most core Lamiales have duplicated CYC2 clade genes (Hileman and Baum, 2003; Smith et al., 2004; Yang et al., 2012; Preston et al., 2014; Zhong and Kellogg, 2015a). Duplications in Gesneriaceae CYC2 clade genes correlate with their prolonged expression in adaxial petals during late developmental stages of organogenesis (Yang et al., 2012). Furthermore, duplicated CYC2 clade genes (CYC2A and CYC2B) across higher core Lamiales (core Lamiales excluding Gesneriaceae and Plantaginaceae) exhibit conserved expression patterns, with higher expression levels in the adaxial region of corollas compared with CYC2 clade genes in Gesneriaceae and Plantaginaceae (Yang et al., 2012; Zhong and Kellogg, 2015a). The need for RAD-like gene function for the patterning of bilateral floral symmetry in Lamiales species other than snapdragon has not been functionally tested (cf. S.H. Su and D. Luo, Sun Yat-sen University, Guangzhou, China, unpubl. res.). However, studies of gene expression in species of Plantaginaceae and Gesneriaceae and unpublished functional work in Torenia (Linderniaceae) suggest a conserved role of the CYC-RAD pathway in specifying adaxial identity across the core Lamiales (Zhou et al., 2008; Preston et al., 2009, 2011; Reardon et al., 2009, 2014; S.H. Su and D. Luo, unpubl. res.).
Losses of corolla bilateral symmetry are often linked with changes in expression of CYC2 clade genes, although changes in any other floral symmetry genes would likely have the same result (Cubas et al., 1999b; Citerne et al., 2006; Zhou et al., 2008; Reardon et al., 2009, 2014; Preston et al., 2011). Ontogenetic shifts in corolla symmetry appear to be common in radially symmetrical flowers of Plantaginaceae and Gesneriaceae in that flowers exhibit an early asymmetrical developmental pattern along the adaxial–abaxial floral axis (Zhou et al., 2008; Preston et al., 2009). However, it is unknown whether the establishment of early asymmetry can account for all mature radially symmetrical corollas within core Lamiales, and whether all losses of corolla bilateral symmetry are achieved by similar changes in CYC2 clade and/or RAD-like gene expression.
Recent studies have shown that shifts in corolla symmetry are not necessarily linked to loss of CYC2 clade genes from the genome. Indeed, despite having mature radially symmetrical corollas, Avicennia germinans, Buddleja davidii, Callicarpa cathayana and Tectona grandis all maintain both CYC2A and CYC2B genes, while Mentha longifolia has two CYC2B genes that appear to have duplicated recently following the loss of CYC2A (Zhong and Kellogg, 2015a). Thus, it remains unclear whether and how these shifts in corolla symmetry are linked with similar or distinct changes in the CYC-RAD corolla symmetry-patterning pathway. To determine the mechanisms underlying independent losses of bilateral symmetry, we reconstructed the ancestral character state of corolla symmetry across Lamiales, and focused on three Lamiaceae species (C. cathayana, Lycopus americanus and M. longifolia) that have tetramerous and near radially symmetrical corollas at mature stages for detailed developmental and gene expression assays. The phylogenetic position of Callicarpa in Lamiaceae remains uncertain, but recent studies have suggested that this genus may be sister to the remaining Lamiaceae (Schäferhoff et al., 2010; Bendiksby et al., 2011).
We sampled one additional species (Mimulus guttatus, Phrymaceae) that is closely related to Lamiaceae and has bilaterally symmetrical flowers, to understand the losses of corolla bilateral symmetry in the three focal Lamiaceae species (Stevens, 2001; Kadereit, 2004; Schäferhoff et al., 2010). Mimulus is more closely related to Lamiaceae than snapdragon, shares with Lamiaceae the same duplication of CYC2 clade genes, and both CYC2 genes are redundantly involved in specifying dorsal flower identity (Preston et al., 2014). Thus, we consider it a reasonable representative of the inferred ancestral state. We compared the trajectory of corolla development and expression of CYC2- and RAD-like genes in three focal species with that of M. guttatus. These results, in addition to previous findings, demonstrate various ontogenetic and CYC2/RAD-like gene expression patterns in Lamiales, suggesting diverse mechanisms underlying the independent losses of bilateral symmetry.
MATERIALS AND METHODS
Reconstructing the ancestral character state of corolla symmetry at maturity
To reconstruct the ancestral character state of corolla symmetry, we first compiled genus-level relationships of Lamiales from various recent phylogenetic studies using a manual subtree prune-and-regraft approach (Fig. 1) (Stevens, 2001; Kadereit, 2004; Smith et al., 2004; Schäferhoff et al., 2010; Bendiksby et al., 2011; Drew and Sytsma, 2012). Corolla symmetry at maturity was scored as a binary character, either bilateral or radial. We considered only post-anthesis symmetry because early floral development data are sparse across the Lamiales and symmetry may shift in some cases (Donoghue et al., 1998; Endress, 1999; Bello et al., 2004; Smith et al., 2004; Zhou et al., 2008; Zhong and Kellogg, 2015a). We then traced trait evolutionary histories of corolla symmetry using Mesquite 3.04 (Maddison and Maddison, 2015) with parsimony ancestral states.
Plant materials and focal species
Based on results of the ancestral character state reconstruction and the conserved expression patterns of CYC2 clade orthologues across higher core Lamiales, we chose to focus on three Lamiaceae species, each of which has tetramerous and near radially symmetrical corollas at maturity. We then compared our results with those for species from other Lamiales lineages (e.g. Gesneriaceae and Plantaginaceae) that have been described in the literature. Developing inflorescences and flowers of M. guttatus, L. americanus, M. longifolia and C. cathayana were harvested from greenhouses at the University of Kansas, Missouri Botanical Garden, or Shaw Nature Reserve in Missouri. Flower materials were collected in RNAlater (Ambion, USA) for RNA extraction or fixed in FAA (50 ml: 25 ml 95 % ethanol, 5 ml 37 % formaldehyde, 2·5 ml glacial acetic acid, 17·5 ml diethylpyrocarbonate-treated distilled H2O), and dehydrated in a graded series of ethanol. A subset of dehydrated material was put through a graded series of Histo-Clear (National Diagnostics, USA) and embedded in Paraplast (Fisher Scientific, USA) for in situ RNA hybridization.
Scanning electron microscopy
Inflorescence tissues fixed with FAA were dissected as necessary to reveal internal floral organs, and dried with a Tousimis Critical Point Dryer. Specimens were mounted on stubs, sputter-coated with gold using a Tousimis Sputter Coater, and examined using a scanning electron microscope (Hitachi S-2600H) at the University of Kansas and Washington University–St Louis. Micrographs were adjusted for brightness, contrast and colour balance using GIMP 2·8.
Amplification and sequence analyses of CYC2 clade and RAD-like genes
Total RNA from developing inflorescences was isolated using TRI Reagent followed by TURBO DNase treatment to remove contaminating DNA (Ambion, USA). Reverse transcriptase PCR (RT–PCR) was done using the SuperScript® III One-Step RT–PCR System with Platinum®Taq (Invitrogen, USA), and CYC2 clade and RAD-like genes were isolated using several sets of degenerate primer pairs (Supplementary Data Table S1; Zhong and Kellogg, 2015a, b). The coding regions of RAD genes are short (around 200 bp) and often include a rather divergent >1000-bp intron in the 3′ region (snapdragon and Mimulus), making it challenging to design primers and amplify genes from genomic DNA. Hence, we used RNA-derived cDNA as a template for amplification. Amplified products were subcloned into pGEM®-T vector (Promega, USA), and at least 12 white colonies were sequenced for each PCR reaction.
To identify RAD orthologues across Lamiales, RAD-like sequences of eudicots were obtained using BLAST searches from multiple public databases, including Phytozome (http://www.phytozome.net/), GenBank (http://www.ncbi.nlm.nih.gov/) and the 1KP database (http://218.188.108.77/Blast4OneKP/) (Matasci et al., 2014). We also used TRINITY 2.06 (Haas et al., 2013) to assemble de novo the leaf transcriptome of Mentha spicata (GenBank DRR016805) and the TransDecoder pipeline with default settings (Haas et al., 2013) to predict protein-coding regions. We then used BLASTN (e-value 1) and blastp (e-value 1e−5) to search for CYC2 clade and RAD-like genes in the M.longifolia genome (http://langelabtools.wsu.edu/mgr/blast) (Vining et al., 2016) and the M. spicata assembled and translated transcriptome, respectively. Genomic contigs were aligned to coding sequences from other Lamiales species to predict protein-coding regions. Nucleotide sequences were aligned using the translation alignment option with MAFFT (Katoh and Standley, 2013) in Geneious 6.1.8 (Biomatters, New Zealand). RAxML version 8 in CIPRES (Stamatakis, 2014) was used to infer the evolutionary histories of CYC2 clade and RAD-like nucleotide sequences with 1000 bootstrap replicates.
Expression of CYC- and RAD-like genes
Total RNA was extracted from different floral tissues at various developmental stages in M. guttatus to detect tissue- and stage-specific gene expression patterns. Mid-stage (3–5 mm long) flower buds were equivalent to the latest stages sectioned for in situ hybridization (see below), whereas late-stage (8–10 mm long) buds were in the final stages of organ growth prior to anthesis. cDNA was generated using the iScript cDNA synthesis kit (Bio-Rad, USA), and for quantitative PCR (qPCR) with gene-specific primers in a DNA Engine Opticon 2 real-time PCR machine (MJ Research, USA). Relative expression levels were assessed using SYBR Green I (Invitrogen, USA) and DyNAzyme II Hot Start DNA Polymerase (Finnzymes, USA). Each dissected tissue type was run in triplicate with three biological replicates, and Ct values were normalized with the geomean of Ct values for EF1alpha and UBQ5 (Scoville et al., 2011). The reference gene Actin was used for semi-quantitative RT–PCR gene expression assay.
For all four focal species, expression of CYC2 clade and RAD-like genes was also examined using in situ RNA hybridization following previous protocols (Malcomber and Kellogg, 2004). Briefly, locus-specific forward and reverse (or poly-T) primers (Table S1; Zhong and Kellogg, 2015a) were designed and used to amplify only the 3′-UTR and a small portion of the coding region. Sense and anti-sense probes were ∼ 200–400 bp in length and were generated by in vitro transcription with MEGAscript® T7/SP6 kits (Ambion, USA) and labelled by digoxigenin-UTP (Roche, USA) for visualization. Purified probes were used directly for in situ RNA hybridization without hydrolysis. In situ hybridization slides were photographed under the light microscope (Olympus BX40 or equivalent) at the Missouri Botanical Garden and University of Kansas, and images were adjusted for brightness, contrast and colour balance using GIMP 2.8.
RESULTS
Convergent ontogenetic origins of radially symmetrical corollas
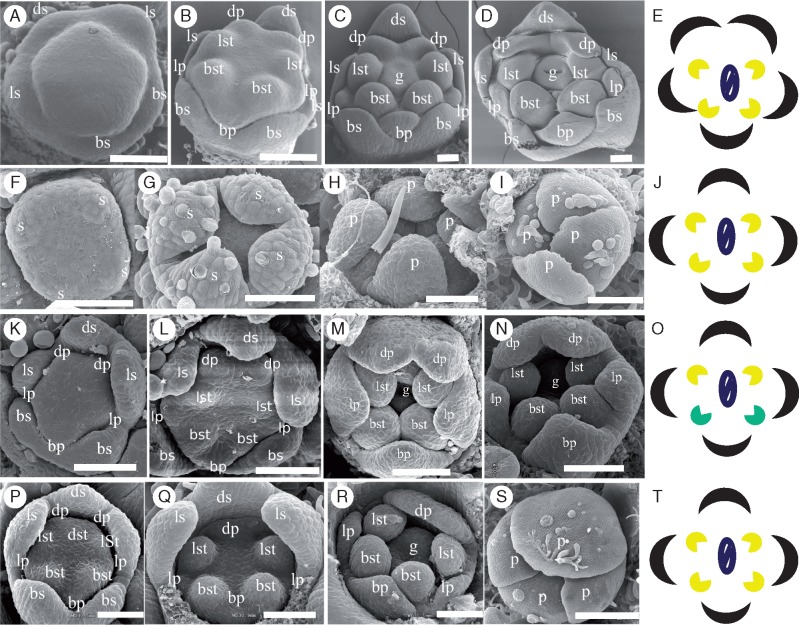
Ancestral character state reconstruction indicated a single origin of bilaterally symmetrical corollas early in core Lamiales with multiple independent shifts to corolla radial symmetry across the core Lamiales in many sub-lineages (Fig. 1; e.g. Gesneriaceae, Plantaginaceae, Scrophulariaceae, Lamiaceae and Acanthaceae). In order to decipher the ontogenetic trajectory that leads to independent origins of radial corolla symmetry within Lamiaceae, we focused on the Lamiaceae species C.cathayana, L.americanus and M.longifolia. Scanning electron micrographs were taken at different stages of development for the three focal species and compared with bilaterally symmetrical flower development in the outgroup M.guttatus. Unlike snapdragon or Bournea, each of our focal species failed to develop an adaxial stamen/staminode, and the sepals and petals generally developed in an adaxial (dorsal) to abaxial (ventral) direction (Vincent and Coen, 2004; Zhou et al., 2008). Specifically, M. guttatus flowers maintained bilateral symmetry from early to late corolla development, with the adaxial sepals and petals being much larger than their abaxial counterparts, and the lateral petals being smaller than both adaxial and abaxial perianth organs (Fig. 2A–E). In contrast, flowers of C.cathayana appeared to be tetramerous from the outset, with initiation and development of just four sepals, four petals and four stamens (Fig. 2F–J and Supplementary Data Fig. S4D). The calyx was slightly bilaterally symmetrical in very early development (Fig. 2F), but quickly shifted to radial symmetry before petal initiation (Fig. 2G), whereas the corolla was radially symmetrical from petal initiation onward (Fig. 2H–I).
Fig. 2.
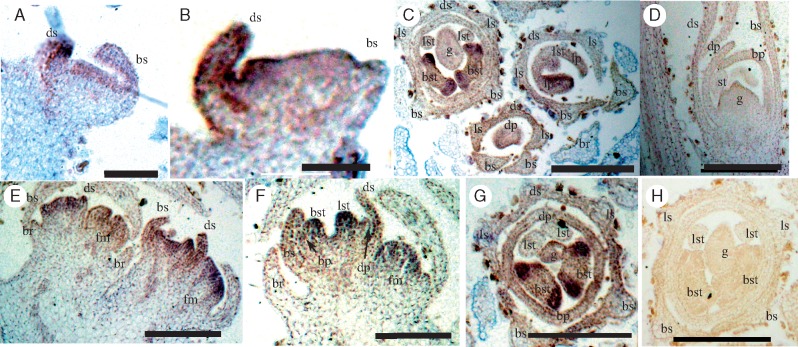
Ontogenetic origins of mature radially symmetrical corollas in (A–E) Mimulus guttatus, (F–J) Callicarpa cathayana, (K–O) Lycopus americanus and (P–T) Mentha longifolia. Cartoons (E), (J), (O) and (T) depict mature developmental stages for four focal species. Mimulus corollas are bilaterally symmetrical with five petals from early (B–D) to late (E) developmental stages; Callicarpa corollas are tetramerous and radially symmetrical from petal initiation to late development (G–I); corollas of Lycopus (L–N) and Mentha (P) are initially pentamerous and bilaterally symmetrical with two adaxial petals being fused during development (Lycopus, M, N; Mentha, Q, R), and the final corolla forms become tetramerous and radially symmetrical (Lycopus, O; Mentha, S, T). However, Lycopus has only two adaxial fertile stamens (yellow) with the other two abaxial (green) being sterile (O), while Mentha has four fertile stamens (yellow) (T); all species have two carpels (centre, blue). Scale bars = 50 μm except (I) = 100 μm and (S) = 200 μm. Sepal, s; petal, p; stamen, st; gynoecium, g; adaxial sepal, ds; abaxial sepal, bs; lateral sepal, ls; adaxial petal, dp; abaxial petal, bp; lateral petal, lp; lateral stamen, lst; abaxial stamen, bst.
Corollas of L. americanus shifted from early bilateral to late radial symmetry (Fig. 2K–O). During early development, L. americanus sepals developed from the adaxial to the abaxial side (Fig. 2K), with five petal and four stamen primordia developing almost synchronously (Fig. 2K–M). The two adaxial petals became partly and increasingly fused at later developmental stages (Fig. 2L–N) and appeared as a single petal at anthesis (Fig. 2O). In addition, the paired abaxial stamens were well developed during early stages (Fig. 2M, N), but this development was arrested after anthesis, resulting in mature flowers with two functional lateral stamens and two abaxial staminodes (Fig. 2O).
Flowers of M.longifolia also shifted from bilateral to radial symmetry from early to late stages of development (Fig. 2P–T and Supplementary Data Fig. S4A–C). At early stages, adaxial sepals developed faster than the abaxial ones (Fig. S4A, B), with the adaxial stamen failing to grow out (Fig. 2P–R and Fig. S4B). In contrast to sepals and stamens, petal development was synchronous throughout the flower, with the lateral petals being smaller than the adaxial and abaxial ones, as found in Mimulus (Fig. 2Q, R). This synchronous growth, combined with fusion of the two distinct adaxial petals (Fig. 2P–R), resulted in near radial symmetry by mid-stages (Fig. 2S), which was maintained until anthesis (Fig. 2T). Thus, despite their similarity in corolla symmetry at maturity, the four-petalled, radially symmetrical corollas of Callicarpa, Lycopus and Mentha differ structurally and developmentally.
Lineage-specific duplications of CYC2 clade and RAD-like genes
Copy number and phylogenetic analyses suggest CYC2 clade and RAD-like genes have experienced extensive duplication events in Lamiales (Supplementary Data Figs S1 and S2). Confirming our previous analysis (Zhong and Kellogg, 2015a), Lamiaceae and Phrymaceae species share a common ancient CYC2 clade gene duplication before the radiation of higher core Lamiales. Specifically, M.guttatus, C.cathayana and L.americanus have preserved both CYC2A and CYC2B paralogues, whereas Mentha has maintained only CYC2B clade paralogues (Ml-CYC2B1 and Ml-CYC2B2; genomic contigs Contig3580 and Contig64753) and lost Ml-CYC2A from its genome (Fig. S2).
The majority of sampled Lamiales species have preserved two RAD-like genes that likely resulted from multiple independent duplication events (Fig. S1). However, unlike the timing of CYC2 duplication events, that of extensive RAD duplication events is hard to determine using phylogenetic analysis alone as only about 200 bp of the RAD-like sequences could be confidently aligned, resulting in little support for relationships among paralogues (Zhong and Kellogg, 2015a, b). This makes it difficult to determine orthologous relationships among duplicates between species with functional data and our focal species. In the case of M. guttatus, three RAD-like genes were isolated, two of which fall into the same clade as snapdragon RAD, and one of which (Mg-RAD2) is a distantly related paralogue. It is unclear whether Mg-RAD1 and Mg-RAD3 are derived from a duplication event that occurred before or after the split of Phrymaceae and Plantaginaceae, but snapdragon RAD is more similar to Mg-RAD1 than Mg-RAD3 at the nucleotide (77 versus 72 %), amino acid and primary structure (e.g. hydrophobicity and polarity) levels (Supplementary Data Fig. S3) and in terms of the asymmetrical expression pattern along the adaxial–abaxial axis (see below). It is thus most likely that Mg-RAD1 is functionally analogous to snapdragon RAD in specifying flower adaxial identity.
The single RAD gene from C. cathayana (Cc-RAD) shares higher nucleotide sequence identity with Mg-RAD1 (73 %) than with Mg-RAD3 (71 %). No RAD-like genes were isolated from developing inflorescences of L. americanus or M. longifolia despite using multiple different degenerate primers derived from various parts of the conserved MYB domain of known RAD sequences from GenBank. Additionally, only a single RAD-like gene was found in the M. spicata leaf transcriptome and M. longifolia genome (Contig27170), respectively, and each is more similar (66·3 versus 62·0 % identity) and more closely related to Mg-RAD3 (Fig. S1; bootstrap support 90 %) than Mg-RAD1. Other closely related Lamiaceae species that have an Mg-RAD3 clade orthologue also possess one additional RAD-like paralogue (Fig. S1; e.g. Salvia, Thymus and Micromeria). We thus assume that Mentha and Lycopus have likely lost expression of the RAD genes in developing inflorescence and/or leaves.
Asymmetrical expression of CYC2 clade and RAD-like genes correlates with late bilateral flower symmetry in Mimulus
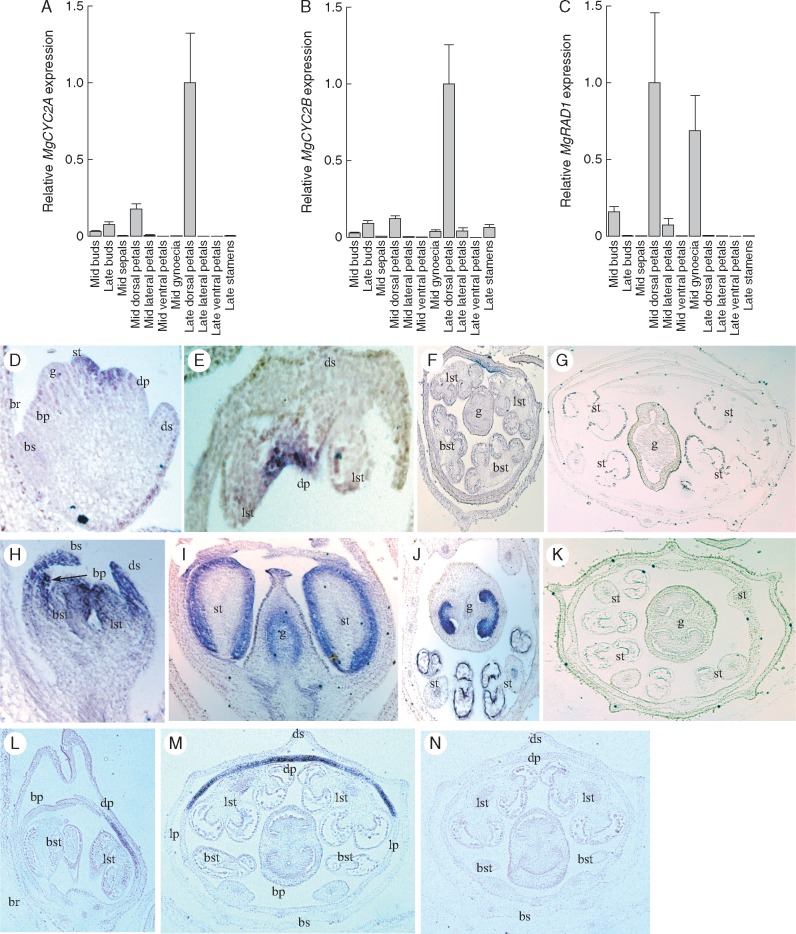
To assess whether changes in expression patterns of CYC2 clade and RAD-like genes correlate with switches from bilateral to radial symmetry, we first generated expression data for the bilaterally symmetrical M. guttatus, which has the presumed ancestral morphology (Fig. 1). Quantitative PCR analyses of dissected floral tissues at different stages of development showed that the putative flower symmetry genes Mg-CYC2A, Mg-CYC2B and Mg-RAD1 are all expressed most strongly in petals relative to other floral organs, with much higher expression in adaxial versus lateral and abaxial petals (Fig. 3A–C). Mg-CYC2A and Mg-CYC2B expression increased from mid-stage to late-stage buds. However, although Mg-RAD1 was detectable only in dorsal petals of late-stage petals (small column in Fig. 3C), expression was much higher in mid-stage versus late-stage buds.
Fig. 3.
Expression of floral symmetry genes in Mimulus guttatus (Phrymaceae). (A–C) Quantitative reverse transcriptase PCR (qRT–PCR) for Mg-CYC2A, Mg-CYC2B and Mg-RAD1, respectively. (D–F) Mg-CYC2A expression using anti-sense probes with (G) as sense control. (H–J) Mg-CYC2B expression using anti-sense probes using (K) as sense control. (L, M) Expression detected by anti-sense probes of Mg-RAD1 and (N) sense probes of Mg-RAD1. Scale bars = 50 μm. Bracts, br; stamen, st; gynoecium, g; adaxial sepal, ds; abaxial sepal, bs; lateral sepal, ls; adaxial petal, dp; abaxial petal, bp; lateral petal, lp; lateral stamen, lst; abaxial stamen, bst.
In situ hybridization of antisense Mg-CYC2A (Fig. 3D–F) and Mg-RAD1 (Fig. 3L–N) RNA probes confirmed asymmetrical gene expression at early to mid-stages of flower development. Whereas Mg-CYC2A was expressed only on the inside of the adaxial petals (Fig. 3D–F), Mg-RAD1 expression extended across the dorsal petals into the adaxial side of the lateral petals (Fig. 3L, M). In contrast to Mg-CYC2A, Mg-CYC2B was expressed broadly across petals in early development, and within developing stamens and the gynoecium at later stages (Fig. 3H–J). Additionally, the other RAD-like gene, Mg-RAD3, was expressed at high levels in the abaxial region of flower meristems, broadly in all petals, and in stamens during late developmental stages (Supplementary Data Fig. S5), whereas the more distantly related Mg-RAD2 was ubiquitously expressed across floral organs (Fig. S5A). No obvious staining was found in control floral sections probed with sense RNA probes (Fig. 3G, K, N).
Differential expression of CYC2 clade and RAD-like genes in radially symmetrical flowers
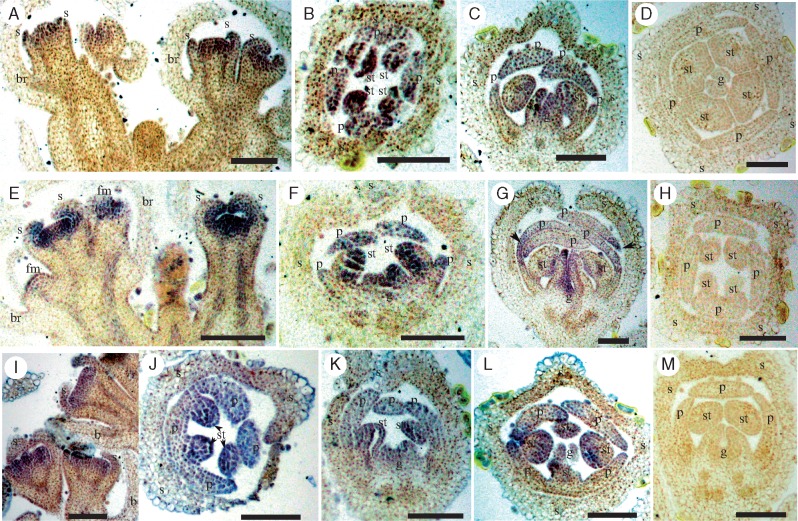
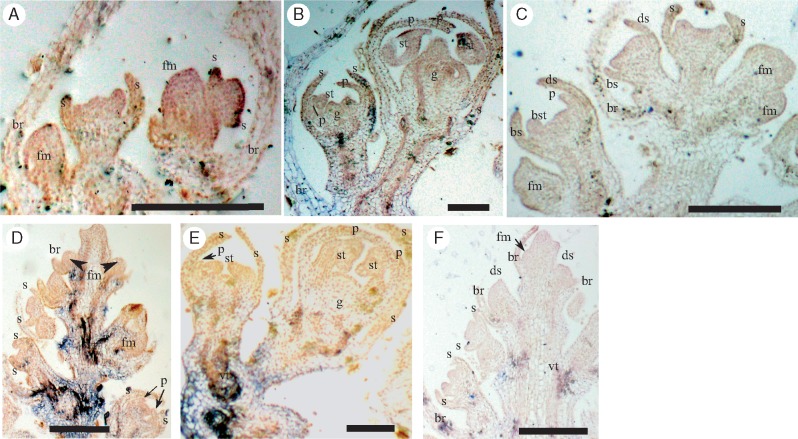
In C. cathayana, where flowers establish radial symmetry early in development, both CYC2 clade genes were strongly expressed across floral meristems, and in all developing sepal primordia (Fig. 4A, E). At later stages of development, Cc-CYC2A (Fig. 4B, C) and Cc-CYC2B (Fig. 4F, G) transcript levels decreased in sepals. However, expression was strong in developing petals, stamens and gynoecia, in the adaxial, lateral and abaxial regions of the flower.
Fig. 4.
RNA in situ hybridization expression of CYC2 clade (A–H) and RAD-like (I–M) genes during Callicarpa cathayana (Lamiaceae) flower development. (A–C) Cc-CYC2A is widely expressed using anti-sense probes. (D) Sense probes of Cc-CYC2A. (E–G) Cc-CYC2B is detected broadly using anti-sense probes of (H) sense probes of Cc-CYC2B; Cc-RAD is widely expressed using anti-sense probes (I–L) with minimal staining of sense probes (M). Scale bars = 50 μm. Bracts, br; floral meristem, fm; sepal, s; petal, p; stamen, st; gynoecium, g.
Similar to M. guttatus CYC2A, L. americanus CYC2A (La-CYC2A) was asymmetrically expressed in developing adaxial sepals and petals (Fig. 5A, B) and late-stage abaxial stamens (Fig. 5D). However, the sections were too ambiguous to determine the late expression patterns of La-CYC2A (Fig. 5D and Supplementary Data Fig. S6, notched adaxial corolla). In contrast, La-CYC2B was expressed widely throughout the floral meristem at very early developmental stages, but was absent from subtending leafy bracts (Fig. 5F, G). During later developmental stages, La-CYC2B was expressed strongly in abaxial stamens and gynoecia, but weakly across sepals and petals (Fig. 5H, I). In M. longifolia, despite having early bilateral flower symmetry, no asymmetrical expression of CYC2 clade genes was found. Instead, Ml-CYC2B1 was only detected early during sepal initiation (Fig. 6A) with no apparent staining during later developmental stages (Fig. 6B), while minimal staining was found for Ml-CYC2B2 (Fig. 6D, E). Apparent vascular staining of the Ml-CYC2B2 antisense probe was probably non-specific (Fig. 6D, E), since similar staining was observed with the sense control probe (Fig. 6F). All other sense probes for L. americanus and M. longifolia genes showed little or no staining (Figs 5E, J and 6C).
Fig. 5.
RNA in situ hybridization gene expression using anti-sense probes for La-CYC2A (A–C) and La-CYC2B (E–G) during Lycopus americanus flower development. (D, H) Sense probe of La-CYC2A and La-CYC2B, respectively. (A–C) La-CYC2A is restricted to adaxial regions of the flowers during early developmental stages, and detected in abaxial stamens later. (E–G) La-CYC2B is found widely in the floral tissues but not in subtending leaves, and is later expressed in abaxial stamens and gynecia. Scale bars = 50 μm. Bracts, br; stamen, st; gynoecium, g; adaxial sepal, ds; abaxial sepal, bs; floral meristem, fm; lateral sepal, ls; adaxial petal, dp; abaxial petal, bp; lateral petal, lp; lateral stamen, lst; abaxial stamen, bst.
Fig. 6.
RNA in situ hybridization expression of CYC2 clade genes during Mentha longifolia (Lamiaceae) flower development. (A, B) Early broad expression of Ml-CYC2B1 using anti-sense probes. (C) Ml-CYC2B1 sense probes. (D, E) Minimal staining using anti-sense probes of Ml-CYC2B2; staining in vascular tissues may be non-specific staining, which is also observed using sense probes (F). Scale bars = 50 μm. Bracts, br; sepal, s; petal, p; stamen, st; gynoecium, g; adaxial sepal, ds; abaxial sepal, bs; lateral sepal, ls; abaxial stamen, bst; vascular tissues, vt.
No RAD-like gene transcripts were isolated from total RNA from the developing inflorescences of L. americanus or M. longifolia despite using multiple different degenerate primers derived from various parts of the conserved MYB domain. However, the newly identified RAD-like gene Cc-RAD in C. cathayana exhibited a similar expression pattern to Cc-CYC2A and Cc-CYC2B, being expressed widely throughout the floral meristem, early sepals, petals, stamens and gynoecia (Fig. 4I–L). Staining was minimal in tissue sections hybridized with sense control probes (Fig. 4D, H, M), supporting specificity of the antisense probes.
DISCUSSION
Floral symmetry is regulated by asymmetrical activities of CYC2 clade and RAD genes along the adaxial–abaxial axis in snapdragon (Plantaginaceae) (Luo et al., 1996, 1999; Corley et al., 2005). We found similar asymmetrical expression patterns of CYC2 clade and RAD genes in M.guttatus (Fig. 3) and other higher core Lamiales species (CYC2 clade; Zhong and Kellogg, 2015a), similar to the asymmetrical expression that was previously described in the higher core Lamiales species Torenia (S. H. Su et al., Sun Yat-sen University, Guangzhou, China, pers. comm.). Possible differences exist in the exact interactions between CYC, RAD and DIV genes, as exemplified by the regulation of Sv-RAD in Senecio vulgaris (Asteraceae) by Sv-DIV1B (Garcês et al., 2016), and the temporal mismatch between levels of Mg-CYC and Mg-RAD1 expression in M. guttatus, both indicative of additional non-CYC regulators of RAD. However, the CYC-RAD-DIV genetic module appears to be conserved in influencing petal growth, and thus the patterning of floral symmetry, in asterids (Luo et al., 1996, 1999; Almeida et al., 1997; Corley et al., 2005; Garcês et al., 2016). Together, these results strongly suggest conserved CYC-RAD genetic regulation of bilateral corolla symmetry across the core Lamiales (Luo et al., 1996, 1999; Corley et al., 2005; Yang et al., 2012; Preston et al., 2014; S. H. Su and D. Luo, Sun Yat-sen University, Guangzhou, China, unpubl. res.). We thus infer that the asymmetrical expression of CYC2 clade and RAD-like genes is ancestral to, and responsible for, the early establishment of bilateral symmetry in Lamiaceae and Phrymaceae.
In our three focal species, we find three distinct patterns of radial corolla development. The first is exhibited by C.cathayana, in which the flower is radially symmetrical from inception. CYC2 clade and RAD-like genes are broadly expressed, implying that an abaxial–adaxial gradient in CYC2 gene expression is never set up. Because the inferred ancestral function of CYC2 is to upregulate RAD, it is not surprising that RAD expression follows the CYC2 pattern. This suggests that radial symmetry was caused not by mutations in CYC2 clade coding sequences themselves but rather by changes in their regulation.
The pattern seen in C. cathayana is also seen in in Aragoa and Plantago (Plantaginaceae) (Endress, 1999; Bello et al., 2004). Callicarpacathayana and Plantago are both tetramerous from inception, initiating only four sepals, petals and stamens, while Aragoa develops five sepals but only four petals and four stamens (Endress, 1999; Bello et al., 2004). Similar to Aragoa (Plantaginaceae) (Preston et al., 2011), C. cathayana has preserved both CYC2 clade paralogues. Expanded expression of CYC2 clade genes also correlates with shifts to corolla radial symmetry in some rosid lineages (e.g. Citerne et al., 2006; Zhang et al., 2013). For example, in radially symmetrical flowers of Cadia purpurea (Fabaceae), CYC2 clade genes are expressed symmetrically throughout all floral organs (Citerne et al., 2006). Collectively, expanded expression of CYC-RAD genes may result in early development of radially symmetrical corollas (Bello et al., 2004; Preston et al., 2011).
A second pattern of radial corolla development is observed in L.americanus, in which early development is similar to that of strongly bilaterally symmetrical corollas in having two adaxial petals, two lateral petals and one abaxial petal. During development, symmetry shifts such that flowers are radially symmetrical by the time of anthesis. Likewise, L. americanus CYC2 clade genes show early expression patterns similar to those of their orthologues in M. guttatus, although it remains relatively unclear whether there is also similar late expression of CYC2 clade genes. Nevertheless, early asymmetrical expression of CYC2 clade genes in L. americanus appears to correlate with the developmental patterns of typical bilaterally symmetrical corollas before anthesis.
The mature corolla’s radial symmetry may be produced by changes in expression of the downstream target of CYC2 clade proteins, i.e. RAD. Despite the ancestral bilateral pattern of early development and CYC2 expression, RAD transcripts are undetectable in L. americanus flowers. In snapdragon, rad mutants, unlike cyc/dich double mutants, are still slightly bilaterally symmetrical (Corley et al., 2005). Thus, the apparent loss of RAD-like gene expression in floral tissues of L. americanus suggests a possible mechanism for early-to-mid corolla bilateral symmetry similar to that in rad mutants. This change in expression could be explained by protein coding changes in upstream CYC2 clade proteins, cis-regulatory changes in RAD-like genes, or complete loss of RAD-like genes from the genome. A non-mutually exclusive alternative possibility could be heterochronic changes in the expression of CYC2 clade genes during development that are responsible for late radial symmetry, as shown in some species of Brassicaceae and Gesneriaceae, in that CYC2 clade genes show early asymmetrical expression (Busch and Zachgo, 2007; Zhou et al., 2008). Further sequencing, expression and protein binding assays would be required to distinguish among these alternative hypotheses.
The third pattern of corolla development is that seen in M.longifolia, where early flowers are bilaterally symmetrical, but in which CYC2 clade genes appear not to be involved. In this case, Ml-CYC2A is lost from the M. longifolia genome and only transient broad expression of Ml-CYC2B1 was detected, with no detectable expression of Ml-CYC2B2 (Fig. 6). Early transient broad expression of Ml-CYC2B1 alone might not be sufficient to establish early asymmetry along the adaxial–abaxial axis. Rather, it is more likely that mechanisms other than CYC2 are involved in early asymmetrical growth. In addition, it is possible that genetic pathways that regulate organ fusion play a critical role, followed by loss of CYC2 clade and RAD-like gene function (Fig. 2Q, R).
No RAD-like transcript was found in floral tissues of M. longifolia. Two RAD-like genes are present in many higher core Lamiales leaf transcriptomes (Fig. S1; e.g. Salvia, Thymus and Micromeria), but only one is found in the M.spicata and M. longifolia genomes (Fig. S1). In particular, newly identified RAD-like genes from the Mentha leaf transcriptome and genome are strongly supported as orthologous to Mimulus Mg-RAD3 (Ml-RAD3), which is broadly expressed in all petals. We thus infer that Mentha species have lost an Mg-RAD1-like gene (Ml-RAD1), but preserved a broadly expressed Mg-RAD3-like gene (Fig. S1). Taking these findings together, late radial symmetry in M. longifolia corollas is likely linked with losses of Ml-CYC2A and Ml-RAD1 (an Mg-RAD1 orthologue) genes.
Perhaps surprisingly, each of the three distinct mechanisms for producing radially symmetrical flowers correlates with the shift from pentamerous to tetramerous flowers. This pattern is found in other radially symmetrical flowers in Lamiales, such as Aegiphila, Aragoa, Avicennia and Buddleja (Endress, 1999; Bello et al., 2004; Kadereit, 2004). However, this correlation is not absolute since there are cases of pentamerous/polymerous radially symmetrical corollas (Figs 1 and 7) (e.g. Ramonda and Tectona) and tetramerous but strongly bilaterally symmetrical corollas (Fig. 7) (e.g. Veronica) (Smith et al., 2004; Kadereit, 2004). Whether the CYC-RAD genes are involved in this shift in merosity is unknown. The four-lobed corollas in both M. longifolia and L. americanus are caused by fusion of the two adaxial petals, although the timing of fusion is early in M. longifolia and late in L. americanus. Exactly whether and how the floral symmetry and sympetaly patterning pathways interplay and influence each other is unclear (Zhong and Preston, 2015).
Fig. 7.
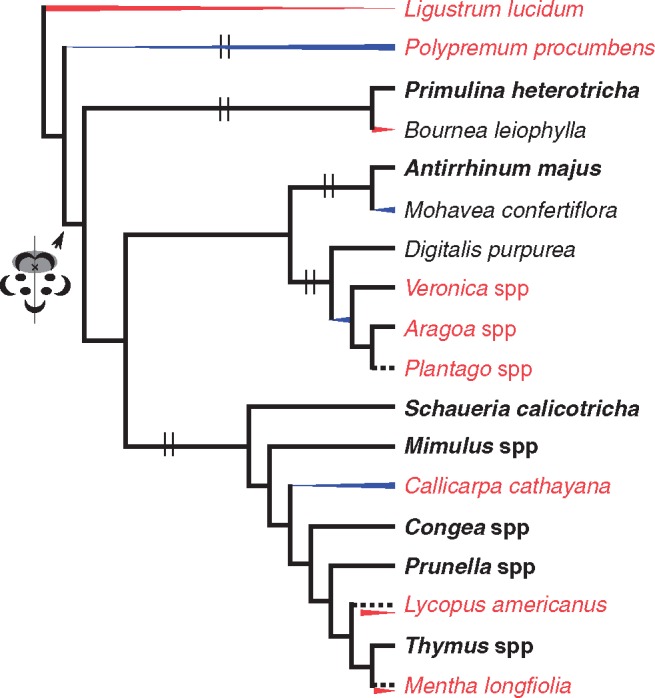
Summary of evolutionary losses of corolla bilateral symmetry in Lamiales. Red-coded species have radially symmetrical corollas and were examined for expression of floral symmetry genes. Species in bold have typical bilaterally symmetrical corollas. A red triangle decreasing in height indicates transient expression of CYC2 clade genes early in flower development in corresponding species. A blue triangle increasing in height shows expanded expression of CYC2 clade and/or RAD genes. Dashed lines indicate possible losses of one CYC2 clade gene and/or RAD expression. Double vertical lines depict lineage-specific duplications of CYC2 clade gene. Flower cartoon with shaded circles points to the origin of asymmetrical expression of corolla symmetry CYC2 clade and RAD genes. Results were compiled from previous studies (Luo et al., 1996, 1999; Corley et al., 2005; Zhou et al., 2008; Preston et al., 2009, 2011; Reardon et al., 2009, 2014; Yang et al., 2012; Zhong and Kellogg, 2015a, b).
The connection between corolla symmetry and CYC2 clade and/or RAD-like gene copy number is neither simple nor direct, as has been shown previously (Reardon et al., 2009, 2014; Preston et al., 2011; Zhong and Kellogg, 2015a). Some radially symmetrical flowers, such as those of C.cathayana, have orthologues of the CYC2 clade and RAD-like genes present in bilaterally symmetrical flowers. On the other hand, Plantago species (Plantaginaceae) have only a single CYC2 clade gene, which is expressed broadly in all petals (Reardon et al., 2009, 2014; Preston et al., 2011). Similarly, M. longifolia has lost one CYC2 clade gene (CYC2A), although a recent duplication of CYC2B restored copy number of CYC2 clade genes (two CYC2Bs). Rather, temporal and spatial changes in the expression of CYC-RAD genetic programme may be better predictors of the ontogenetic shifts in corolla symmetry and mature corolla symmetry.
In summary, losses of mature corolla bilateral symmetry in Lamiales and other core eudicots (e.g. Fabaceae, Malpighiales) are not uncommon, and may be achieved by diverse developmental mechanisms and by various changes to symmetry genes, including the loss of a CYC2 clade and/or RAD-like genes from the genome, and/or contraction or expansion of CYC2 clade and/or RAD-like gene expression across the corolla (Fig. 7) (Smith et al., 2004; Citerne et al., 2006; Zhou et al., 2008; Reardon et al., 2009, 2014; Preston et al., 2009, 2011; Zhang et al., 2013). Importantly, close examination of developmental trajectories and symmetry gene expression assays appear to be informative in distinguishing convergence (i.e. divergent developmental and genetic changes) and parallelism (i.e. the same developmental and genetic changes) of shifts to corolla radial symmetry. Specifically, independent shifts to radial symmetry may be linked with similar changes in symmetry genes, such as the expanded expression of CYC2 clade genes in Aragoa (Plantaginaceae) and Callicarpa (Lamiaceae) (Preston et al., 2011) (Fig. 7). Similar expanded expression of a CYC2 gene, along with further loss of the other CYC2 and RAD genes, is associated with a shift in floral symmetry in Plantago (Reardon et al., 2009, 2014; Preston et al., 2011) (Fig. 7). Indeed, broad expression of CYC2 clade and RAD-like genes is also found in the early diverging Lamiales Polypremum, which is sister to the core Lamiales with predominantly bilaterally symmetrically corollas (Zhong and Kellogg, 2015b) (Fig. 7). In addition, heterochronic changes during development and disrupted interactions of symmetry genes may also produce radially symmetrical corollas. Development and early expression patterns of CYC2 clade genes in Bournea and Lycopus are similar to those of its relatives with bilaterally symmetrical corollas (Zhou et al., 2008). However, alteration of La-CYC2A expression during late developmental stages and/or lack of La-RAD expression might have led to a shift to late corolla radial symmetry, similar to altered CYC2 but not RAD expression in Bournea (Zhou et al., 2008) (Fig. 7). Finally, loss of a gene copy and expression may lead to shifts in floral symmetry. Loss of Ml-CYC2A and Ml-RAD1 and altered/transient expression of Ml-CYC2Bs might account for mature radial symmetry in Mentha (Fig. 7). We have also shown in this study that petal fusion may affect final floral forms and thereby the interaction between pollinators and plants, although the exact mechanism for petal fusion is not clear. Future work on whether and how organ fusion and symmetry patterning pathways interact would be interesting and helpful in understanding floral evolution.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: primers used for the preparation of locus-specific probes in this study. Figure S1: phylogeny of RAD-like genes in the order Lamiales. Figure S2: phylogeny of Lamiaceae CYC2 clade genes; sequences of focal species are highlighted in red. Figure S3: alignment of amino acids of RAD-like proteins colour-coded for distinct hydrophobicity. Figure S4: flower development in Mentha and Callicarpa. Figure S5: expression of Mimulus guttatus RAD-like genes. Figure S6: expression of La-CYC2A.
Author contributions
J.Z., J.C.P., L.C.H. and E.A.K. conceived and designed the research. J.Z. and J.C.P. performed the research and analysed the data. J.Z., J.C.P., L.C.H. and E.A.K. wrote the manuscript.
Supplementary Material
ACKNOWLEDGEMENTS
This work was partly supported by a Doctoral Dissertation Improvement Grant from the National Science Foundation (grant number DEB 1210540 to E.A.K. and J.Z.), NSF IOS-0616025 to L.C.H., the Jane Harris Scholarship in Tropical Botany from Whitney R. Harris World Ecology Center at the University of Missouri–St Louis, a Graduate Student Research Award from the Botanical Society of America, and an Alumni Fellowship from the Missouri Botanical Garden. We are grateful for sampling assistance from the Missouri Botanical Garden, Shaw Nature Reserve and the 1000 Plant (1KP) project. We also thank S. H. Su (Sun Yat-sen University, Guangzhou, China) for sharing his unpublished work. The transcriptome assembly and post-analyses were done using iPlant Collaborative Cyberinfrastructure and the Texas Advanced Computing Center (TACC) at the University of Texas at Austin.
LITERATURE CITED
- Almeida J, Rocheta M, Galego L.. 1997. Genetic control of flower shape in Antirrhinum majus. Development 124: 1387–1392. [DOI] [PubMed] [Google Scholar]
- Bello MA, Rudall PJ, Gonzalez F, Fernandez-Alonso JL.. 2004. Floral morphology and development in Aragoa (Plantaginaceae) and related members of the order Lamiales. International Journal of Plant Sciences 165: 723–738. [Google Scholar]
- Bendiksby M, Thorbek L, Scheen A-C, Lindqvist C, Ryding O.. 2011. An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. Taxon 60: 471–484. [Google Scholar]
- Berger BA, Thompson V, Lim A, Ricigliano V, Howarth DG.. 2016. Elaboration of bilateral symmetry across Knautia macedonica capitula related to changes in ventral petal expression of CYCLOIDEA-like genes. EvoDevo 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A, Zachgo S.. 2007. Control of corolla monosymmetry in the Brassicaceae Iberis amara. Proceedings of the National Academy of Sciences of the USA 104: 16714–16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citerne HL, Pennington RT, Cronk QCB.. 2006. An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. Proceedings of the National Academy of Sciences of the USA 103: 12017–12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley SB, Carpenter R, Copsey L, Coen E.. 2005. Floral asymmetry involves an interplay between TCP and MYB transcription factors in Antirrhinum. Proceedings of the National Academy of Sciences of the USA 102: 5068–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas P, Lauter N, Doebley J, Coen E.. 1999a. The TCP domain: a motif found in proteins regulating plant growth and development. The Plant Journal 18: 215–222. [DOI] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E.. 1999b. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401: 157–161. [DOI] [PubMed] [Google Scholar]
- Donoghue MJ, Ree RH, Baum DA.. 1998. Phylogeny and the evolution of flower symmetry in the Asteridae. Trends in Plant Science 3: 311–317. [Google Scholar]
- Drew BT, Sytsma KJ.. 2012. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). American Journal of Botany 99: 933–953. [DOI] [PubMed] [Google Scholar]
- Endress PK. 1999. Symmetry in flowers: diversity and evolution. International Journal of Plant Sciences 160: S3–S23. [DOI] [PubMed] [Google Scholar]
- Endress PK. 2011. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany 98: 370–396. [DOI] [PubMed] [Google Scholar]
- Feng X, Zhao Z, Tian Z, et al. 2006. Control of petal shape and floral zygomorphy in Lotus japonicus. Proceedings of the National Academy of Sciences of the USA 103: 4970–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcês HMP, Spencer VMR, Kim M.. 2016. Control of floret symmetry by RAY3, SvDIV1B, and SvRAD in the capitulum of Senecio vulgaris. Plant Physiology 171: 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, et al. 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protocols 8: 1494–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman LC. 2014a. Bilateral flower symmetry – how, when and why? Current Opinion in Plant Biology 17: 146–152. [DOI] [PubMed] [Google Scholar]
- Hileman LC. 2014b. Trends in flower symmetry evolution revealed through phylogenetic and developmental genetic advances. Philosophical Transactions of the Royal Society of London B: Biological Sciences 369: 20130348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman LC, Baum DA.. 2003. Why do paralogs persist? Molecular evolution of CYCLOIDEA and related floral symmetry genes in Antirrhineae (Veronicaceae). Molecular Biology and Evolution 20: 591–600. [DOI] [PubMed] [Google Scholar]
- Howarth DG, Donoghue MJ.. 2006. Phylogenetic analysis of the “ECE” (CYC/TB1) clade reveals duplications predating the core eudicots. Proceedings of the National Academy of Sciences of the USA 103: 9101–9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth DG, Martins T, Chimney E, Donoghue MJ.. 2011. Diversification of CYCLOIDEA expression in the evolution of bilateral flower symmetry in Caprifoliaceae and Lonicera (Dipsacales). Annals of Botany 107: 1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit JW. 2004. The families and genera of vascular plants. VII, Flowering plants, dicotyledons, Lamiales (except Acanthaceae including Avicenniaceae). Berlin: Springer. [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Carpenter R, Vincent C, Copsey L, Coen E.. 1996. Origin of floral asymmetry in Antirrhinum. Nature 383: 794–799. [DOI] [PubMed] [Google Scholar]
- Luo D, Carpenter R, Copsey L, Vincent C, Clark J, Coen E.. 1999. Control of organ asymmetry in flowers of Antirrhinum. Cell 99: 367–376. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR.. 2015. Mesquite: a modular system for evolutionary analysis. Version 3.04. http://mesquiteproject.org. (last accessed 15 September 2015).
- Malcomber ST, Kellogg EA.. 2004. Heterogeneous expression patterns and separate roles of the SEPALLATA gene LEAFY HULL STERILE1 in grasses. The Plant Cell 16: 1692–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matasci N, Hung L-H, Yan Z, et al. 2014. Data access for the 1,000 Plants (1KP) project. GigaScience 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal PR, Dafni A, Giurfa M.. 1998. Floral symmetry and its role in plant-pollinator systems: terminology, distribution, and hypotheses. Annual Review of Ecology and Systematics 29: 345–373. [Google Scholar]
- Preston JC, Kost MA, Hileman LC.. 2009. Conservation and diversification of the symmetry developmental program among close relatives of snapdragon with divergent floral morphologies. New Phytologist 182: 751–762. [DOI] [PubMed] [Google Scholar]
- Preston JC, Martinez CC, Hileman LC.. 2011. Gradual disintegration of the floral symmetry gene network is implicated in the evolution of a wind-pollination syndrome. Proceedings of the National Academy of Sciences of the USA 108: 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Barnett LL, Kost MA, Oborny NJ, Hileman LC.. 2014. Optimization of virus-induced gene silencing to facilitate evo-devo studies in the emerging model species Mimulus guttatus (Phrymaceae). Annals of the Missouri Botanical Garden 99: 301–312. [Google Scholar]
- Raimundo J, Sobral R, Bailey P, et al. 2013. A subcellular tug of war involving three MYB-like proteins underlies a molecular antagonism in Antirrhinum flower asymmetry. The Plant Journal 75: 527–538. [DOI] [PubMed] [Google Scholar]
- Reardon W, Fitzpatrick DA, Fares MA, Nugent JM.. 2009. Evolution of flower shape in Plantago lanceolata. Plant Molecular Biology 71: 241–250. [DOI] [PubMed] [Google Scholar]
- Reardon W, Gallagher P, Nolan KM, et al. 2014. Different outcomes for the MYB floral symmetry genes DIVARICATA and RADIALIS during the evolution of derived actinomorphy in Plantago. New Phytologist 202: 716–725. [DOI] [PubMed] [Google Scholar]
- Schäferhoff B, Fleischmann A, Fischer E, et al. 2010. Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences. BMC Evolutionary Biology 10: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville AG, Barnett LL, Bodbyl-Roels S, Kelly JK, Hileman LC.. 2011. Differential regulation of a MYB transcription factor is correlated with transgenerational epigenetic inheritance of trichome density in Mimulus guttatus. New Phytologist 191: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Hileman LC, Powell MP, Baum DA.. 2004. Evolution of GCYC, a Gesneriaceae homolog of CYCLOIDEA, within Gesnerioideae (Gesneriaceae). Molecular Phylogenetics and Evolution 31: 765–779. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens PF. 2001. Angiosperm phylogeny website.http://www.mobot.org/mobot/research/apweb/. (last accessed 17 September 2015).
- Ushimaru A, Dohzono I, Takami Y, Hyodo F.. 2009. Flower orientation enhances pollen transfer in bilaterally symmetrical flowers. Oecologia 160: 667–674. [DOI] [PubMed] [Google Scholar]
- Vincent CA, Coen ES.. 2004. A temporal and morphological framework for flower development in Antirrhinum majus. Canadian Journal of Botany 82: 681–690. [Google Scholar]
- Vining KJ, Johnson SR, Ahkami A, et al. 2016. Draft genome sequence of Mentha longifolia and development of resources for mint cultivar improvement. Molecular Plant http://dx.doi.org/10.1016/j.molp.2016.10.018. [DOI] [PubMed]
- Wang Z, Luo Y, Li X, et al. 2008. Genetic control of floral zygomorphy in pea (Pisum sativum L.). Proceedings of the National Academy of Sciences of the USA 105: 10414–10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Pang H-B, Liu B-L, et al. 2012. Evolution of double positive autoregulatory feedback loops in CYCLOIDEA2 clade genes is associated with the origin of floral zygomorphy. The Plant Cell 24: 1834–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kramer EM, Davis CC.. 2010. Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proceedings of the National Academy of Sciences of the USA 107: 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Steinmann VW, Nikolov L, Kramer EM, Davis CC.. 2013. Divergent genetic mechanisms underlie reversals to radial floral symmetry from diverse zygomorphic flowered ancestors. Frontiers in Plant Science 4: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Kellogg EA.. 2015a. Duplication and expression of CYC2-like genes in the origin and maintenance of corolla zygomorphy in Lamiales. New Phytologist 205: 852–868. [DOI] [PubMed] [Google Scholar]
- Zhong J, Kellogg EA.. 2015b. Stepwise evolution of corolla symmetry in CYCLOIDEA2-like and RADIALIS-like gene expression patterns in Lamiales. American Journal of Botany 102: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Zhong J, Preston JC.. 2015. Bridging the gaps: evolution and development of perianth fusion. New Phytologist 208: 330–335. [DOI] [PubMed] [Google Scholar]
- Zhou X-R, Wang Y-Z, Smith JF, Chen R.. 2008. Altered expression patterns of TCP and MYB genes relating to the floral developmental transition from initial zygomorphy to actinomorphy in Bournea (Gesneriaceae). New Phytologist 178: 532–543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.