Abstract
Background and Aims Roots have complex anatomical structures, and certain localized cell layers develop suberized apoplastic barriers. The size and tightness of these barriers depend on the growth conditions and on the age of the root. Such complex anatomical structures result in a composite water and solute transport in roots.
Methods Development of apoplastic barriers along barley seminal roots was detected using various staining methods, and the suberin amounts in the apical and basal zones were analysed using gas chromatography–mass spectometry (GC-MS). The hydraulic conductivity of roots (Lpr) and of cortical cells (Lpc) was measured using root and cell pressure probes.
Key Results When grown in hydroponics, barley roots did not form an exodermis, even at their basal zones. However, they developed an endodermis. Endodermal Casparian bands first appeared as ‘dots’ as early as at 20 mm from the apex, whereas a patchy suberin lamellae appeared at 60 mm. The endodermal suberin accounted for the total suberin of the roots. The absolute amount in the basal zone was significantly higher than in the apical zone, which was inversely proportional to the Lpr. Comparison of Lpr and Lpc suggested that cell to cell pathways dominate for water transport in roots. However, the calculation of Lpr from Lpc showed that at least 26 % of water transport occurs through the apoplast. Roots had different solute permeabilities (Psr) and reflection coefficients (σsr) for the solutes used. The σsr was below unity for the solutes, which have virtually zero permeability for semi-permeable membranes.
Conclusions Suberized endodermis significantly reduces Lpr of seminal roots. The water and solute transport across barley roots is composite in nature and they do not behave like ideal osmometers. The composite transport model should be extended by adding components arranged in series (cortex, endodermis) in addition to the currently included components arranged in parallel (apoplastic, cell to cell pathways).
Keywords: Apoplast, barley, composite transport, hydraulic conductivity, osmometer model, reflection coefficient, solute permeability
INTRODUCTION
In recent years, there has been an increasing amount of interest in modelling root hydraulics. This interest is due to the fact that within the soil–plant–air continuum (SPAC), the water taken up by plant roots either can be used for plant growth and development or can be lost by transpiration (Kramer, 1983). The discovery of aquaporins (AQPs) in the early 1990s suggested that they were a major regulatory component for water transport across cell membranes within the SPAC (Maurel, 1997; Tyerman et al., 1999; Maurel and Chrispeels, 2001). However, this picture has changed in light of further quantitative data from pressure probes concerning the hydraulic properties of individual root cells and the overall hydraulic conductivities of entire roots (Zhu and Steudle, 1991; Steudle and Peterson, 1998). In many of these studies, the results have indicated that the apoplastic path contributes to water transport, even across the endodermis. There are exceptions to these results, however, as reported in young roots of barley (Steudle and Jeschke, 1983), bean (Steudle and Brinckmann, 1989) and Arabidopsis thaliana (Ranathunge and Schreiber, 2011). In these plants, cell to cell water transport dominated in roots, suggesting that AQPs were the major influence on water transport. The existence of two pathways, along with composite transport, would provide some explanation for the observed variance in root hydraulic conductivity (Lpr) besides the action of AQPs (Brouwer, 1954; Weatherley, 1982; Kramer and Boyer, 1995). Regulation of Lpr has been discussed in terms of the variable contributions of different pathways to the overall water flow in response to stresses such as drought, which affect root anatomy as well as water channel activity (Vandeleur et al., 2009). This discussion is focused on the interaction between the two parallel pathways: the cell to cell and the apoplastic.
The simple composite transport model of the root, outlined in the previous paragraph, has been recently shown to be incomplete, because it does not include the root’s water storage capacity. This factor may be important when considering transient effects and thick roots (Meyer et al., 2011). More importantly, the model usually considers only parallel pathways. In reality, roots contain additional transport components, such as the cortex and the stele, which are arranged in series. The endodermis, in particular, is known to be a substantial barrier for both water and solutes. On the other hand, the axial hydraulic resistance is usually a component of minor importance (Frensch and Steudle, 1989).
Recently, Knipfer and Fricke (2010) used root pressure probes to repeat some of the osmotic experiments conducted by Steudle and Jeschke (1983), using NaCl as the osmotic solute. The authors concluded that the reflection coefficients of the roots (σsr) were very close to unity, suggesting that the roots behaved like ideal osmometers. These results were most probably due to an endodermis that was impermeable to the solute. This observation differed from the earlier results of Steudle and Jeschke (1983) for barley and for a number of other plant species (Steudle and Peterson, 1998, and references therein). Knipfer and Fricke (2010) concluded from their results that the cell to cell component of water transport, rather than the apoplastic component, was dominant in barley, confirming the previous data of Steudle and Jeschke (1983) that compared the cellular and overall root Lpr by pressure probe measurements.
In the present study, we critically investigate the proposed cell to cell transport model of barley roots (Steudle and Jeschke, 1983) by combining anatomical, biochemical and physiological studies at the cellular and root level. We also determine how suberized barriers in the cell walls affect water transport, in addition to the extended measurements of the permeability patterns of these roots using several electrolytes and non-electrolytes as test solutes. We also propose certain modifications/additions to the accepted composite transport model, in which certain parameters should be added. These modifications would explain the rather low values of σsr in the presence of the low permeability coefficients (Psr) found in the roots of some other plant species during the osmotic experiments.
MATERIALS AND METHODS
Plant material and growth conditions
Caryopses of barley (Hordeum vulgare L. cv. ‘Golf’, Scottish Crop Research Institute) were germinated in the dark at 23 °C on filter paper moistened with a 0·5 mm CaSO4 solution. Six days later, the seedlings were transferred into a hydroponic system containing modified half-strength Hoagland solution in a climatic chamber (Fricke and Peters, 2002). The plants used in the experiments were grown for 16–20 d, including the germination period. At this stage, the plants had 3–4 developed leaves and 6–7 seminal roots. The maximum length and average diameter of the seminal roots varied between 70 and 140 mm and 0·4 and 0·6 mm, respectively.
Histochemical detection of Casparian bands and suberin lamellae in roots
The seminal roots were cross-sectioned at distances of 10, 20, 30, 40, 50, 60 and 100 mm from the root apex. To detect the development of Casparian bands (CBs), the sections were stained with 0·1 % (w/v) berberine hemisulphate and 0·5 % (w/v) aniline blue (Brundrett et al., 1988). The suberin lamellae were stained with lipophilic 0·01 % (w/v) Fluorol Yellow 088 (Brundrett et al., 1991). The aliphatic suberin in cell walls was detected by yellow fluorescence under ultraviolet light (filter set: exciter filter, G 365; chromatic beam splitter, FT 395; barrier filter, LP 420). To detect the number of cell layers in the cortex and the cell dimensions, several cross-sections were stained with 0·05 % (w/v) Toludine blue O.
Chemical analysis of the root suberin
The seminal roots were divided into two zones. Zone-I was the younger part of the root, without laterals, which included the growing root tip. This zone was identical to the end segments/apical part of roots used in the root pressure probe measurements. The average length of Zone-I was 60 ± 15 mm (n = 15). Zone-II was the mature half of the root, towards the base, and included lateral roots. The length of the mature zone was 50 ± 12 mm (n = 15). Root segments were enzymatically digested to remove cellulose and pectins from the cell walls (Zeier and Schreiber, 1997), and the steles were isolated along with the suberized endodermis. The isolated cell wall samples were then purified, dried and subjected to transesterification to release suberin monomers according to the procedures of Kolattukudy and Agrawal (1974). Gas chromatographic analysis and mass spectrometric identification of the derivatized degradation products were performed as described by Zeier and Schreiber (1997, 1998). The amounts were calculated for the unit surface area of the roots. Four replicates were used for each root zone.
Measurement of hydraulic conductivity of roots (Lpr) and root cortical cells (Lpc) using pressure probes
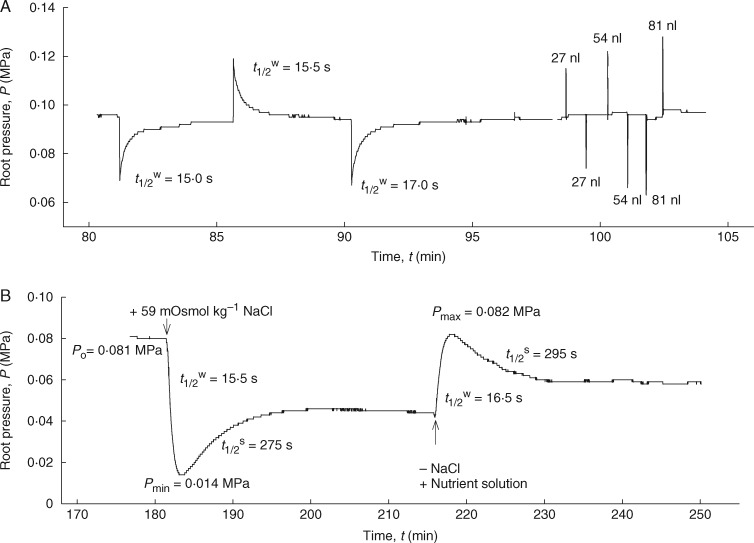
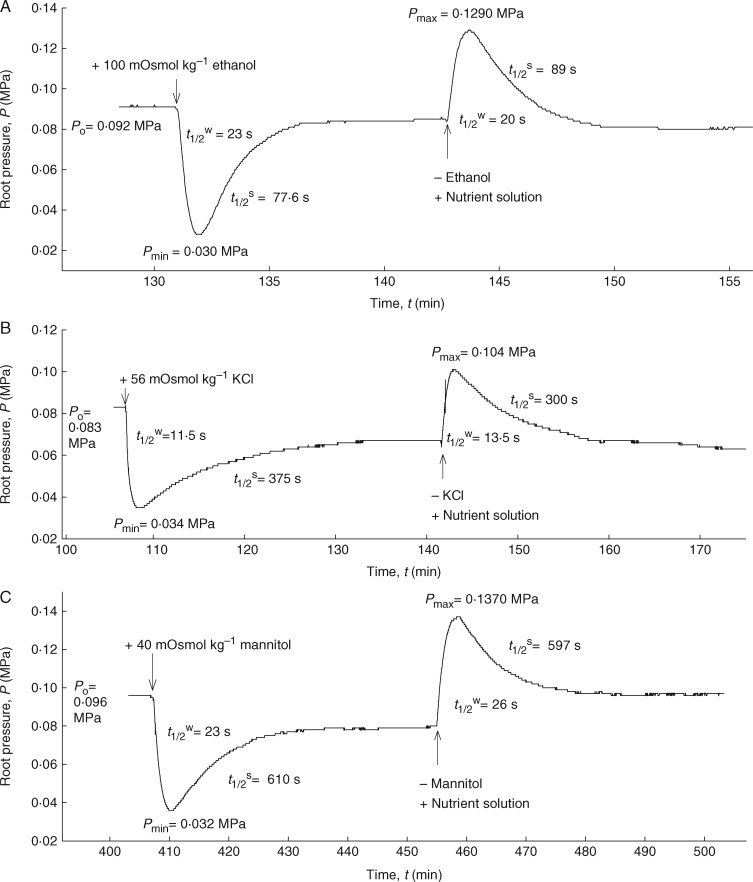
The Lpr of the end segments/apical part of the seminal roots (Zone-I; length: 50–75 mm) and total seminal roots (length: approx. 200 mm) was measured separately using a root pressure probe as earlier described by Steudle et al. (1987). Stable root pressure (Pr) developed within 2–4 h after fixing the roots to the pressure probe. In the hydrostatic experiments, water flows were induced by moving the meniscus either forward to induce exosmotic water flow or backward to induce endosmotic water flow. The resulting hydrostatic relaxation curves were composed of two exponential phases brought about by different rates of changes of Pr over time. The initial rapid phase covered about 85 % of the entire pressure (volume) curve; followed by a slow reversible phase related to the concentration polarization effects at the endodermis (Steudle and Frensch, 1989; Hose et al., 2000). The initial phase (see Fig. 3A) was used to measure the half-time of water exchange, t1/2w; and the hydrostatic hydraulic conductivity (Lpr) was determined from the rate constant of water exchange (kwr) (Steudle et al., 1987, Steudle, 1989):
| (1) |
Fig. 3.
Time course vs. pressure change during a typical experiment with the root pressure probe. A hydroponically grown, 20-day-old barley seminal root (without laterals) was attached to the root pressure probe. (A) Measurements of the hydrostatic pressure relaxations and elastic coefficient (β) by moving the metal rod forwards and backwards. (B) Responses of root pressure (Pr) in relation to the change in osmotic pressure in the external medium (either by adding 59 mOsmol kg–1 NaCl to the medium or by removing it from the medium). Biphasic responses consist of rapid water efflux (or influx) followed by slow solute influx (or efflux). The addition of NaCl to the medium resulted in a drop of Pr but it failed to reach the original pressure due to the inhibition of other electrogenic pumps in the plasma membrane.
Here β (=ΔPr/ΔVs) is the elastic coefficient of the measuring system, ln(2) is the natural logarithm of 2 or 0·693, and Ar is the surface area of the conductive part of the root, which was approx. 85 % of the total surface area. This part had dead and functional (conductive) xylem vessels with lignified cell walls. The non-conductive root tip part (hydraulically isolated zone), which was approx. 15 mm long, was not taken into account for surface area calculations. This part did not have mature and functional xylem vessels, which were filled with the cytoplasm. Ten replicates were used for each measurement.
In the osmotic experiments, the nutrient solution in the external medium was rapidly exchanged by 30 mm (approx. 59 mOsmol kg–1) NaCl, a permeating solute (Fig. 3B). The osmotic Lpr was calculated from the rate constant of the water phase of the biphasic osmotic root pressure relaxations (half-time of the first phase; Fig. 3B). Both experiments were conducted in two ways. In the first, the root medium was rapidly stirred to minimize the external unstirred layers, while in the second, the medium was kept stagnant without stirring to determine the effect of external unstirred layers on water permeability. Stirring was achieved by rapidly bubbling the medium with air (Ye and Steudle, 2006). During stagnant (unstirred) conditions, the bubbling was stopped.
The hydraulic conductivity of root cortical cells (Lpc) was measured by a cell pressure probe, as has been done for several different types of higher plant tissues, including roots (e.g. Azaizeh et al., 1992; Steudle, 1993; Wan et al., 2004; Kim and Steudle, 2007). Excised roots were mounted on a metal sledge carefully holding them by magnetic bars. The nutrient solution, used to grow plants in the hydroponic culture, ran along the roots using a recirculation system. Mid cortical cells of roots (third or fourth cell layer from the outside) were punctured at approx, 50 mm from the apex using an oil-filled microcapillary (average tip diameter: approx, 6 μm) to measure the cell Lpc. Once the cell was punctured, a meniscus formed between the cell sap and oil. Hydrostatic pressure relaxations were performed by gently moving the meniscus forward and backward to inject water into and out of the cells, respectively. Successful cell pressure probe experiments resulted in measuring cell turgor (P), the volumetric elastic modulus (ε) and the half-time of water exchange of individual cortical cells (tc1/2w). The ε was determined by the change in pressure (dP) according to the relative volume change (dV/V) by:
| (2) |
The hydraulic conductivity of the cell membrane (Lpc) was calculated using measured tc1/2w, ε and calculated mean values of cell volume (V) and cell surface area (A) from the following equation:
| (3) |
The osmotic pressure of the cells (πi) was approximated by the stationary turgor pressure (P) of the cortical cells in the nutrient solution. Cell volume (V) and cell surface area (A) was calculated from the length and the diameter of cortical cells, assuming they are cylindrical in shape.
Measurement of solute permeabilities (Psr) and reflection coefficients (σsr) of roots
Several solutes which tend to have very different membrane permeabilities were used to measure the permeabilities (Psr) and reflection coefficients (σsr) of the roots. Ethanol (100 mm = 100 mOsmol kg–1) was used as a solute that rapidly permeated the plasma membrane (Steudle and Tyerman, 1983; Tyerman and Steudle, 1984) and the root cylinder. NaCl (30 mm = 59 mOsmol kg–1), KCl (30 mm = 56 mOsmol kg–1) and mannitol (40 mm = 40 mOsmol kg–1) were used as less permeating solutes, while sucrose (60 mm = 60 mOsmol kg–1) and the 1:4 monovalent cation salt, K4[Fe(CN)6] (8 mm = 26 mOsmol kg–1) were used as virtually non-permeating solutes through the membranes. The osmotic concentration of solutes in the nutrient solution had an osmolality of 16 mOsmol kg–1. The Psr of each solute was determined separately by the rate constant of solute exchange [ksr = ln(2)/ts1/2] using the second phases in biphasic relaxations of osmotic experiments as given by Steudle et al. (1987):
| (4) |
In this equation, t1/2s is the half-time of solute exchange and Vx is the volume of functional xylem in the root system, which is approx. 1 % of the total root volume, as measured from the cross-sections. The total root volume was calculated using the conductive length and the diameter of the root. The osmotic concentrations of the tested solutes were measured using a freezing point depression osmometer (Osmomat 030; Gonotec, Berlin, Germany).
The σsr values of the test solutes used in the Psr measurements were calculated from the following equation:
| (5) |
where ΔPr is the maximum change in root pressure caused by changes in the osmotic pressure of the medium (Δπs° = RT × Cs; R = universal gas constant, T = absolute temperature, Cs = concentration of solute ‘s’ in the medium) and tmin is the time required to reach the minimum root pressure. The exponential term on the right side of eqn (2) is larger than unity and corrects for the solute flow during the time interval of tmin. However, for non-permeating solutes, the curve was monophasic and the second phase or solute phase was missing. Hence, it was not possible to determine the exponential component of ksr = ln(2)/ts1/2. For such solutes, σsr was calculated from the ΔPr, the maximum change in root pressure, caused by changes in the osmotic pressure of the medium (Kedem and Katchalsky, 1963a, b). For a semi-permeable membrane, which holds that σsr = 1, the addition of 40 mOsmol kg–1 would result in a pressure drop of 0·1 MP (1 bar). Since the osmotic pressure of the solutes in the medium was known, it was possible to calculate σsr for non-permeating solutes.
After each measurement, the proper function of the seal was checked by cutting off the root at the seal. When the xylem of the root remained open, there was a drastic decrease in the half-times (by at least one order of magnitude) and an increase in hydraulic conductance after the cut. If these results were not observed, the experiment was discarded.
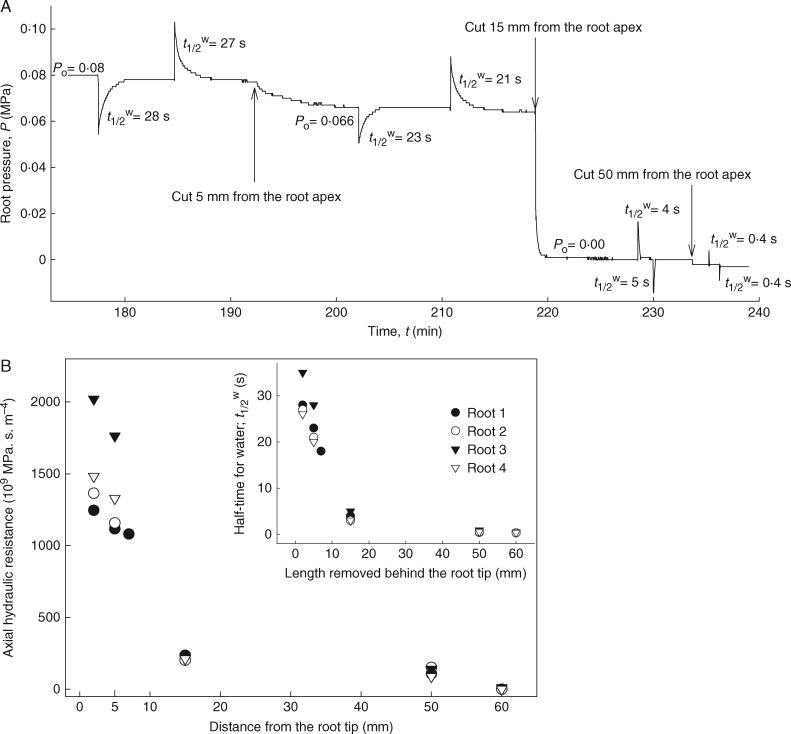
Measurement of root axial resistance by cutting experiments
To determine the maturity and functionality of xylem vessels and the development of axial resistance along the root axis, excised roots were attached to the root pressure probe (as described above). Once roots attained steady-state root pressures, successive cuts were made from the apex (at 5, 15 and 50 mm) using a sharp double-edged razor blade (Fig. 5). Once mature xylem vessels were cut, the root pressure (Pr) quickly dropped to zero. Hydrostatic pressure relaxations were also performed between cuts to determine changes in the axial hydraulic resistance. Since the hydraulic capacitance (ΔVs/ΔPr) of the system remained constant, the recorded changes in t1/2w directly reflected changes in the axial hydraulic resistance of roots (R; MPa s m–4).
| (6) |
where Lpr is the root hydraulic conductance (m3 MPa–1 s–1) and lrl (m) is the remaining length of the root after each cut.
Fig. 5.
Cutting experiment on an excised maize root attached to the root pressure probe. When the root was successively cut with a razor blade, starting at the apex, the root pressure dropped immediately when developed xylem was cut. Between cuts, hydrostatic pressure relaxations were performed in order to measure changes in the hydraulic resistance of the root. The cutting experiments also allowed the longitudinal hydraulic resistance in the root to be estimated and provided information about the tightness of the silicone seal. In the pressure probe measurement, it is assumed that the radial resistance is significantly greater than the axial resistance to overall water flow in roots. The cutting experiment demonstrated that this is true. When the root apical end of a 5 mm long segment (including the root tip) was removed, the root pressure (Pr) and the t1/2w dropped by 20 % (A), indicating that some early metaxylem vessels are partially matured and semi-conductive at this distance from the tip. However, as soon as the fully matured and functional early metaxylem vessels (starts at approx. 15 mm from the apex) were cut, the Pr immediately dropped to zero and the t1/2w decreased by 6- to 7-fold due to leaking out of the open xylem vessels. A further cut at 50 mm from the apex, where the central late metaxylem vessel (central duct) is conductive, resulted in decreasing the t1/2w to zero. There is a direct and positive correlation between the t1/2w and the axial hydraulic resistance of the root (xylem vessels) (B). The experimental data proved that the measured t1/2w and Lpr refer to the radial transport of water into the roots (from the external medium to the root xylem) but not to the axial transport along the root xylem vessels.
Statistical analysis
Data were normally distributed and have been presented in figures and tables as means ± s.d. The Student’s t-test was employed to compare the means of the water permeabilities, while an analysis of variance (ANOVA) with the least significant difference (LSD) test was used to compare the means of Psr and σsr for the different solutes used in the experiments. All statistical analyses were conducted with a confidence level of 95 %.
RESULTS
Root anatomy: development of Casparian bands and of suberin lamellae
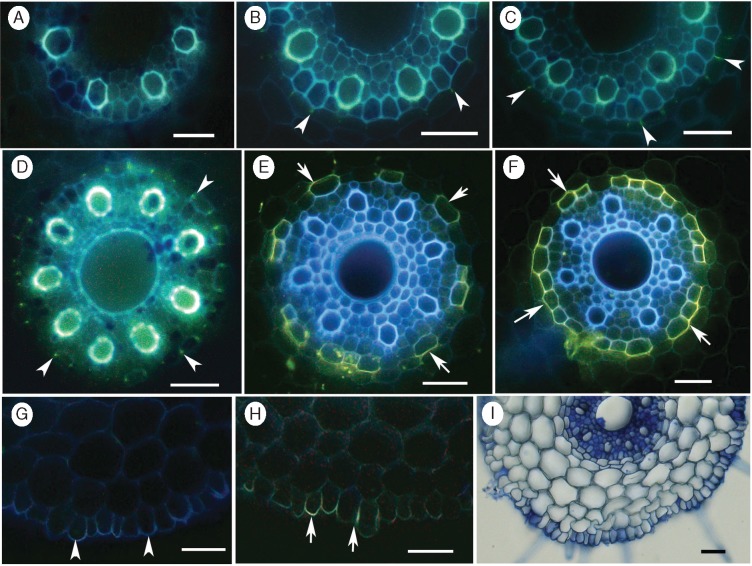
Casparian bands in the endodermis were detected by a yellowish green fluorescence in the radial cell walls after staining with berberine–aniline blue (Fig. 1A–D). No CBs were detected at 10 mm from the apex (Fig. 1A). The bands first appeared as ‘dots‘, indicated by a faint green fluorescence, at 20 mm from the apex (Fig. 1B; arrowheads). At 30 and 40 mm, continuous bands with intense yellowish green fluorescence were observed in the radial walls of the endodermis (Fig. 1C, D; arrowheads).
Fig. 1.
Appearance of Casparian bands (CBs) and suberin lamellae (SL) in the endodermis, and presence of suberin in the rhizodermis of barley roots. Freehand cross-sections of seminal roots of 16- to 20-day-old barley plants stained with either berberine–aniline blue (A-D) or lipophilic fluorochrome, Fluorol yellow 088 (E, F and H). At 10 mm from the apex, no CBs appeared in the endodermis (A), whereas, ‘dot-like’ bands, indicated by a faint green fluorescence, appeared at 20 mm from the apex (B; arrowheads). Continuous bands with intense yellowish green fluorescence in the radial walls at 30 (C) and 40 mm (D) from the tip, respectively. Stained SL appeared as a yellow, patchy ring in the endodermis (arrows) at 60 mm (E), but as a complete lamellae ring with bright yellow fluorescence (arrows) at 100 mm from the apex (F). Autofluorescence (G) and greenish yellow fluorescence (H) in the radial and outer tangential walls of the rhizodermis at 100 mm from the apex. Section stained with Toluidine blue O, showing only four cortical cell layers in the cortex (I). Scale bars = 50 µm.
The suberin lamellae (SL) were detected by an intense, bright yellow fluorescence in the cell walls after staining cross-sections with fluorol yellow 088 (Fig. 1E, F). At 60 mm from the apex, the stained SL was visible as a yellow, patchy ring in the endodermis (Fig. 1E; arrows). About 50 % of the endodermal cells were passage cells without lamellae. At 100 mm, well-developed SL with bright yellow fluorescence were apparent, and the passage cells accounted for <10 % (Fig. 1F). No exodermis was detected in the hydroponically grown barley roots. Even at 100 mm from the apex, neither CBs (Fig. 1G) nor SL (Fig. 1H) developed in the hypodermis (see Supplementary data, Fig. S1). However, clear autofluorescence (Fig. 1G) and yellow green fluorescence (Fig. 1H) in the outer tangential walls of the rhizodermis suggested the presence of ‘diffuse suberin’ (see Peterson et al., 1978). The cross-section, stained with Toludine blue O, confirmed that there were four cortical cell layers present in the cortex (Fig. 1I).
Aliphatic and aromatic suberin in the barley endodermis
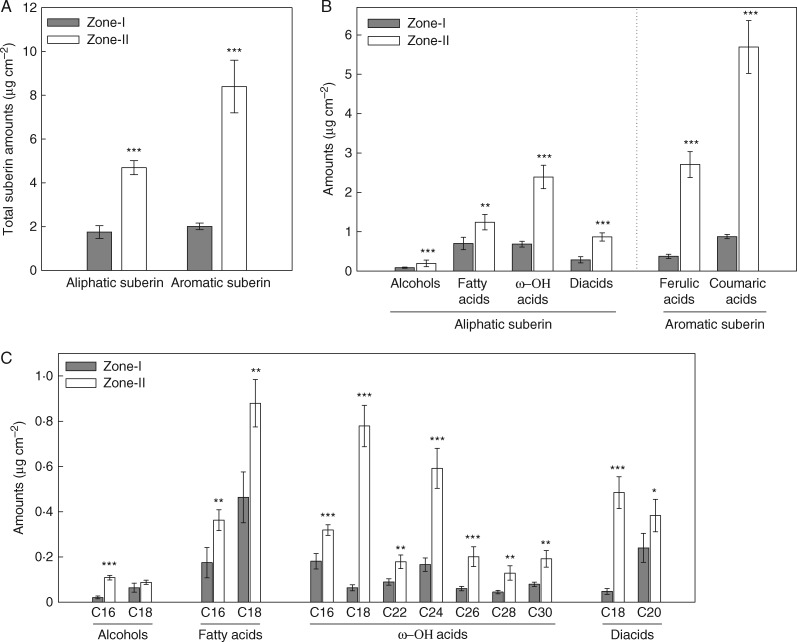
The whole stele, which included the endodermis, was resistant to enzymatic digestion. The suberin in all of the endodermal samples was composed of two main classes: aliphatic and aromatic suberin (Fig. 2A). The total aliphatic and aromatic suberin contents in the stele of Zone-I (the apical part of the root) were significantly lower than in Zone-II (the basal part of the root) (P < 0·001; Fig. 2A). On average, Zone-II had 2·6-fold greater amounts of aliphatic suberin and 4·2-fold greater amounts of aromatic suberin compared with Zone-I. These data were well correlated with the anatomical studies, in which Zone-II had brighter suberin staining than Zone-I (see Fig. 1E, F).
Fig. 2.
Total amounts (A), substance classes (B) and monomer compositions (C) of aliphatic and aromatic suberin in the stele of 16- to 20-day-old barley seminal roots. Analyses were done for two root zones: Zone-I was the younger zone that included the root apex, and Zone-II was the mature zone, including laterals, towards the base. Enzymatically digested and solvent-extracted root cell walls were subjected to BF3/MeOH transesterification and analysed using gas chromatography and mass spectrometry. Absolute amounts are given as means in µg cm–2 ± s.d. (n = 4 roots). The significant levels of P ≤ 0·05, P ≤ 0·01 and P ≤ 0·001 are indicated by *, ** and ***, respectively (two sample t-test).
Alcohols, fatty acids, ω-hydroxy acids (ω-OH acids) and diacids were the major substance classes of aliphatic suberin (Fig. 2B). The total amounts of these substances were significantly higher in Zone-II than in Zone-I (P < 0·01; Fig. 2B). The largest differences between the zones were observed for ω-hydroxy acids and diacids. Aromatic suberin was mainly composed of ferulic and coumaric acids (Fig. 2B). The ferulic and coumaric acid contents were 6·5-fold greater in Zone-II than in Zone-I.
The chain length distribution of the aliphatic monomers varied from C16 to C30 (Fig. 2C). Very short chains, such as C16 and C18, were prominent in all substance classes. The ω-hydroxy acids showed the greatest diversity; carbon chain lengths for these compounds varied from C16 to C30 (Fig. 2C). Overall, all monomer contents were markedly greater in the mature part of the root (Zone-II) than in the younger part of the root (Zone-I) (P < 0·05; Fig. 2C).
Hydraulic conductivity of the roots
When connected to the root pressure probe, the seminal roots took 2–3 h to generate steady-state root pressures. Stable pressures varied according to the individual roots, and the mean values ranged between 0·1 and 0·2 MPa. When measured using hydrostatic pressure gradients (Fig. 3A), the hydrostatic Lpr of end-segments of seminal roots (Zone-I) was 9·4 × 10–8 m s–1 MPa–1 under well-stirred external root medium conditions (Table 1). The hydrostatic Lpr measured with unstirred root medium was 9·7 × 10–8 m s–1 MPa–1. The ratio of well-stirred to unstirred Lpr was not significantly different from unity, suggesting that the unstirred layers around the root did not affect the hydrostatic Lpr. When measured using an osmotic pressure gradient (replacing the nutrient solution with 30 mm NaCl or 59 mOsmol kg–1; Fig. 3B), the average osmotic Lpr values were 9·5 and 4·2 × 10–8 m s–1 MPa–1 for well-stirred and unstirred conditions, respectively. Although unstirred layers in the external medium had no effect on the hydrostatic Lpr, they decreased the osmotic Lpr by 2·4-fold. When measured under well-stirred conditions, the estimated hydrostatic/osmotic ratios of Lpr were approximately at unity, indicating a relatively greater cell to cell water flow in barley roots (Table 1). However, when measured under unstirred conditions, this ratio was increased by 2·6-fold, indicating that the unstirred layers primarily affect osmotic Lpr rather than hydrostatic Lpr.
Table 1.
Hydraulic conductivity (Lpr) of end-segments of seminal roots of barley grown in aerated hydroponics for 14–20 d, measured with a root pressure probe
| Root medium | Hydraulic conductivity, Lpr (10–8 m s–1 MPa–1) |
HY:OS ratio | |
|---|---|---|---|
| Hydrostatic (HY) | Osmotic (OS) | ||
| Well-stirred (WS) | 9·4 ± 3·1 (n = 15)a | 9·5 ± 3·7 (n = 15)a | 1·1 ± 0·3 |
| Unstirred (US) | 9·7 ± 4·2 (n = 6)a | 4·2 ± 2·6 (n = 6)b | 2·6 ± 0·8 |
| WS/US ratio | 1·0 ± 0·1 | 2·4 ± 0·9 | |
Hydrostatic and osmotic Lpr were measured either rapidly stirring (well-stirred) or without stirring (unstirred) the root medium. Osmotic Lpr was measured by replacing the external nutrient solution by 59 mOsmol kg–1 NaCl in the nutrient solution.
Values given are means ± s.d. and the numbers of measured roots are in parentheses. Different letters indicate significant differences at the P < 0·05 level.
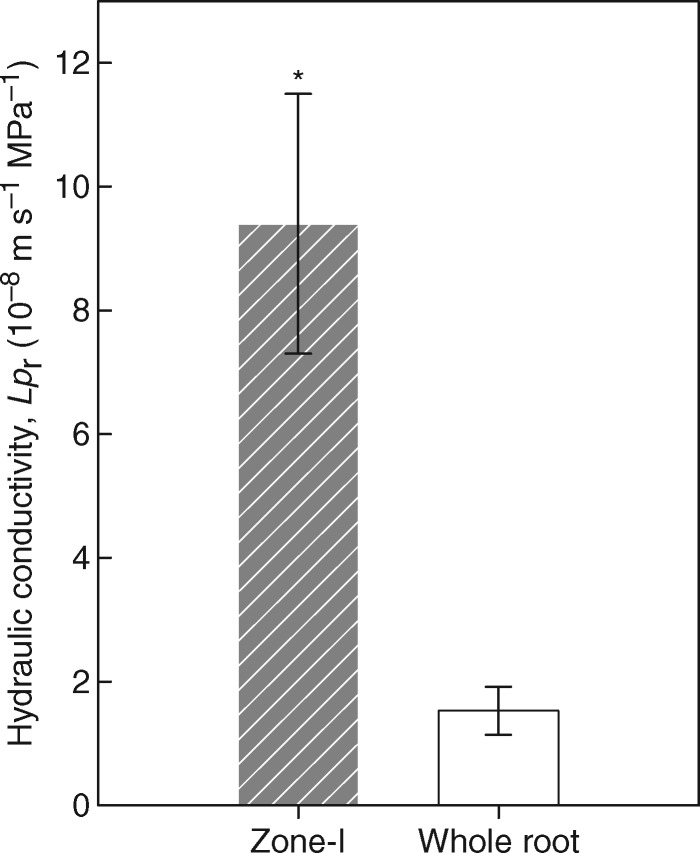
The comparison of Lpr of whole seminal roots and the apical zone (Zone-I) revealed that the younger zone had approx. 6-fold greater water permeability than the whole root (9·4 vs. 1·5 × 10–8 m s–1 MPa–1), which included mature, well-suberized Zone-II (Fig. 4). This indicated that the deposition of SL in the endodermis of the mature/basal zone (Zone-II) significantly decreased the overall radial water permeability of the whole root.
Fig. 4.
The Lpr of the apical zone (Zone-I) and whole roots of 16- to 20-day-old barley seminal roots, grown in aerated hydroponics, and measured with a root pressure probe under well-stirred conditions. The Lpr of the whole root is approx. 6-fold smaller than that of the apical zone (Zone-I). Values are the means ± s.d. of ten replicates. A significant difference of P ≤ 0·05 is denoted by * (two sample t-test).
Water permeability of cell membranes of cortical cells measured by a cell pressure probe
The root cortical cells (unmodified cells in the mid cortex) had turgor pressure values ranging from 0·36 to 0·69 MPa (Table 2). The elastic modulus values of the cells (ε) varied from 0·4 to 14 MPa, and the half-times of water exchange (tc1/2w) ranged from 1 to 80 s. The relatively large range of ε values is probably due to the variation in cell volume. The actual volume calculations were not possible for the exactly measured cells and instead average cell diameter and length obtained from the root cross-sections of the same place were used for the calculations. Assuming cylindrical cell geometry, the average volume of a cortex cell was 1·3 × 10–13 m3 (130 pL). There was also a rather large range of tc1/2w in single cell measurements. At the beginning of the experiment (just after puncturing the cell), seven out of nine cells had a short tc1/2w ranging from 1 to 6 s, while two cells had tc1/2w >10 s. Continuous measurements showed that the tc1/2w increased up to 80 s then recovered to short tc1/2w within 40–160 min (recovery to the initial values in four cells). A similar phenomenon was observed in corn root cortical cells (Wan et al., 2004). Their results were interpreted as being caused by the tremendous water flux during the puncturing procedure, which acted as a mechanical stress and resulted in the closure of AQPs. Wan et al. (2004) demonstrated that about 1 h was needed to recover to short tc1/2w, which are probably the original values. The calculated Lpc values were 1·9 × 10–7 and 1·5 × 10–6 m s–1 MPa–1 for the minimum and maximum, respectively. When calculating the minimum Lpc, one cell was excluded, as it did not show any increase in the tc1/2w during a 15 min long measurement. During calculations of the maximum Lpc, two cells that showed long tc1/2w after puncturing were excluded.
Table 2.
Turgor pressure (P), volumetric elastic modulus (ε), half times (tC1/2w) and hydraulic conductivity (Lpc) of cortical cells at a distance of 40–50 mm from the root tip
| Water relation parameters | Values | |
|---|---|---|
| Stationary turgor pressure (P; MPa) | Range (min – max) | 0·36–0·69 |
| Mean ± s.d. | 0·56 ± 0·11 (n = 9) | |
| Volumetric elastic modulus (ε; MPa) | Range (min – max) | 0·4–14 |
| Mean ± s.d. | 3·4 ± 4·8 (n = 9) | |
| Half-time of water exchange (tC1/2w; s) | Range (min – max) | 1–80 |
| Minimum, which is < 10 s | Mean ± s.d. | 3·6 ± 1·7 (n = 7) |
| Maximum | Mean ± s.d. | 32 ± 22 (n = 8) |
| Hydraulic conductivity (Lpc; 10–6 m s–1MPa–1) | Range (min – max) | 0·013–5·7 |
| Minimum | Mean ± s.d. | 0·19 ± 0·16 (n = 8) |
| Maximum | Mean ± s.d. | 1·5 ± 2·0 (n = 7) |
Values given are ranges (minimum and maximum), and means ± s.d.
Volumes of cells were calculated assuming that they were cylindrical.
A mean value of 1·3 × 10–13 m3 (130 pL) was taken to calculate ε and Lpc.
Numbers of measured cells are in parentheses.
Root hydraulic conductivity as calculated from the hydraulic conductivity of cortical cells
According to the earlier work of Steudle and Jeschke (1983) and Steudle and Brinckmann (1989), average values of cell Lpc were used to estimate the root Lpr values of corn. This estimation assumed that the radial flow of water across the root cylinder was due solely to cell to cell movement through the plasma membrane, excluding apoplastic passage. The root was assumed to consist of parallel concentric rings of membranes. In this model, the overall root Lpr relates to the Lpc values of individual cells by the following equation:
| (7) |
In this equation, ro refers to the outer radius of roots (300 μm), and ri refers to the radii of the n membrane cylinders to be crossed (six layers including the endodermis, which equates to n = 12 concentric rings of plasma membranes). The equation assumes that the hydraulic resistances of individual cell layers arranged in series are additive and that cell membranes must be crossed twice per layer. For geometric reasons, the inner rings contribute more to the overall resistance than the outer rings. Both the rhizodermis and cortex, including the endodermis, were considered in these calculations. For the endodermal resistance, it is believed that only the passage cells (around 80 % of the total endodermal cells in Zone-I) contribute to the water flow, which is a realistic assumption. However, in this calculation for barley, it was assumed that all the endodermal cells contributed to the water flow through membranes. For the roots used here, the average Σro/ri was 21, and the average Lpc (max) = 1·5 × 10–6 m s–1 MPa–1, which resulted in Lpr = 7·1 × 10–8 m s–1 MPa–1. However, the calculated Lpr value should be considered the upper limit due to the exclusion of the endodermal resistance.
Root axial resistance for water transport
In the pressure probe measurement, it is assumed that the radial resistance is significantly greater than the axial resistance to overall water flow in roots. The cutting experiment demonstrated that this is true. When the root apical end of a 5 mm long segment (including the root tip) was removed, the root pressure (Pr) and the t1/2w dropped by 20 % (Fig. 5A), indicating that early metaxylem vessels (the number can vary between seven and nine), which locate around the central duct/late metaxylem, are partially matured and semi-conductive at this distance from the tip. It is likely that these vessels still contain debris of cell content which made a detectable resistance to the axial water flow. However, as soon as the mature and fully functional early metaxylem vessels (starts at approx. 15 mm from the apex) were cut, the Pr immediately dropped to zero and the t1/2w decreased by 6- to 7-fold due to leaking out of the open xylem vessels. Nevertheless, even at this stage, the t1/2w was not zero. A further cut at 50 mm from the apex, where the fully developed central late metaxylem appeared (Fig. 1D, E), resulted in decreasing the t1/2w close to zero due to complete leak out of xylem vessels (negligible axial resistance). There is a direct and positive correlation between the t1/2w and the axial hydraulic resistance of the root (xylem vessels) (Fig. 5B). The experimental data proved that the measured t1/2w and Lpr refer to the radial transport of water into the roots (from the external medium to the root xylem) but not to the axial transport along the root xylem vessels.
Solute permeability and reflection coefficient of roots
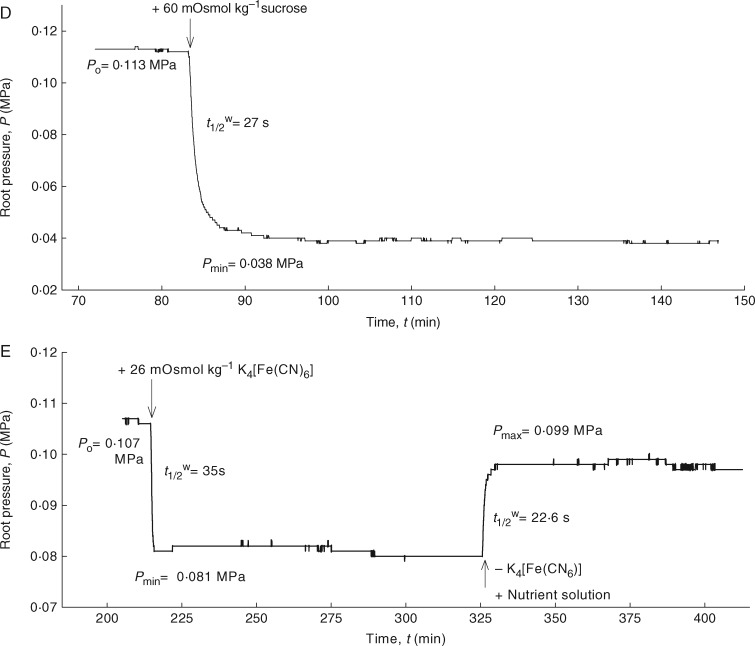
Once the roots had achieved steady-state pressures, the osmotic pressure of the medium was changed by adding different osmotic solutes, which were rapidly permeating (ethanol, NaCl and KCl), less rapidly perneating (mannitol) or virtually non-permeating (sucrose and K4[Fe(CN)6]) the membranes. Hypertonic conditions caused an efflux of water, whereas hypotonic conditions caused an influx. However, for the permeating solutes, there was a reverse influx (efflux) of solutes into (and out of) the root, which was denoted as a ‘solute phase’ (second phase of the biphasic relaxations; Figs 3B and 6A–C). Following a minimum (or a maximum) in pressure, the root pressure was expected to return to the baseline value. With the roots used here, however, the original root pressure was nearly but not fully recovered in the solute phase with ethanol, NaCl and KCl. This observation suggests a possible inhibition of the plasma membrane ion pumps at high solute concentrations, as has previously been found in corn (Steudle et al., 1987; see the Discussion). For non-permeating solutes, such as sucrose and K4[Fe(CN)6], the pressure relaxations were monophasic (Fig. 6D, E). The solute phase was either lacking or very long due to the long half-times caused by low Psr [eqn (4)]. In contrast to previous results in corn (Steudle et al., 1987), osmotic experiments with mannitol showed biphasic relaxations in the young barley roots, indicating a measurable uptake of this solute (Fig. 6C). Determination of the Psr and σsr values for the different solutes using eqn (4) or eqn (5) required the estimation of the volume of the functioning xylem, which was obtained from the root cross-sections.
Fig. 6.
Measurement of solute permeabilities (Psr) and reflection coefficients (σsr) of seminal roots of barley for different test solutes using the root pressure probe in well-stirred conditions. Responses of root pressure (Pr) in relation to the change in osmotic pressure in the medium either by adding 100 mOsmol kg–1 ethanol (A), 56 mOsmol kg–1 KCl (B), 40 mOsmol kg–1 mannitol (C), 60 mOsmol kg–1 sucrose (D) and 26 mOsmol kg–1 K4[Fe(CN)6] (E) or by removing them from the medium. The addition of ethanol, KCl and mannitol to the medium resulted in biphasic responses due to rapid efflux of water, followed by slow influx of solutes. In contrast, addition of sucrose and K4[Fe(CN)6] to the external medium gave monophasic responses in which the second phase or solute influx (solute permeation into the root) is missing.
The results indicated a marked difference between the Psr values of barley roots for different solutes (Table 3). Most interesting was the fact that K4[Fe(CN)6] did not permeate at all, similar to previous observations by Ranathunge et al. (2005) in rice. The Psr values for NaCl and KCl were 4·5-fold lower than that of ethanol, which is lipophilic and rapidly permeated through the plasmalemma (Steudle and Peterson, 1998; Miyamoto et al., 2001; Meyer et al., 2011). However, values of Psr for NaCl and KCl were significantly higher than that of mannitol (F3,40 = 159·89; P < 0·0001). The Psr values for sucrose (which has a larger molecular weight than mannitol) and K4[Fe(CN)6] were not measurable because the solute phase was missing for these compounds (second phase in the osmotic experiments; Fig. 6D, E).
Table 3.
Solute permeability (Psr) and reflection coefficient (σsr) of end-segments of seminal roots of barley, grown in aerated hydroponics for 14–20 d, measured with a root pressure probe
| Type of solute | Solute permeability (Psr; 10–9 m s–1) | Reflection coefficient (σsr) |
|
|---|---|---|---|
| Measured | Corrected | ||
| Ethanol | 12·5 ± 2·4a | 0·23 ± 0·11a | 0·35 ± 0·19a |
| NaCl | 2·8 ± 0·5b | 0·51 ± 0·09b | 0·69 ± 0·12b |
| KCl | 2·5 ± 0·3b | 0·52 ± 0·06b | 0·68 ± 0·10b |
| Mannitol | 1·7 ± 0·4c | 0·71 ± 0·09c | 0·90 ± 0·16c |
| Sucrose | n.m. | 0·45 ± 0·10b | – |
| K4[Fe(CN)6] | n.m. | 0·61 ± 0·20bc | – |
Values given are means ± s.d. for tenroots.
Different letters in the same column indicate significant differences at the P < 0·05 level.
n.m., not measurable.
The measured values of σsr for barley roots ranged from 0·2 to 0·7, depending on the solute used (Table 3). The lowest σsr was observed for ethanol, which was the solute that permeated most rapidly through the root cylinder. This value was 2-fold smaller than the σsr values of NaCl and KCl, and >3-fold smaller than that of mannitol (F5,41 = 20·24; P < 0·0001). However, non-permeating solutes, such as sucrose and K4[Fe(CN)6], had unexpectedly lower σsr values, which are normally at unity for the plasmalemma (an ideally semi-permeable membrane). The σsr value for sucrose was in the same range as those of NaCl and KCl, while the σsr value for K4[Fe(CN)6] was located between those of mannitol and sucrose (Table 3). The σsr values, when corrected for solute flow, were approx. 20–35 % greater than the ‘raw’ data of uncorrected ratios of ΔPr/Δπ°s (Table 3). These corrected values showed that mannitol had the greatest σsr value, while ethanol had the lowest. The σsr values for NaCl and KCl were greater than that of ethanol but smaller than that of mannitol {the σsr values for sucrose and K4[Fe(CN)6] could not be corrected). Although the σsr values increased as Psr decreased, they were below unity for those solutes that had virtually zero permeability for semi-permeable membranes.
DISCUSSION
For the first time, we have investigated the role of suberized barriers, a part of the apoplast, for water and solute transport of barley seminal roots. The findings showed that these barriers negatively affect the overall radial water and solute transport of barley roots. The basal zone with a greater content of suberin exhibited markedly lower permeability for water than the apical zone with a lower content of suberin. The contribution of the root apical zone for overall water transport is significantly greater than the contribution of the basal zone. In the former, the relative contribution of the cell to cell path is greater for water transport than its associated apoplast, which is similar to the case for A. thaliana (Ranathunge and Schreiber, 2011). However, the water transport through the apoplast is not negligible.
In roots, suberized cell walls in the endo- and exodermis form transport barriers to water and solutes (Peterson and Cholewa, 1998). In barley, when grown in hydroponics, roots develop no exodermis, even in their basal zones, which is in agreement with the previous finding of Robards et al. (1973). This anatomical feature is different from other monocot plants such as rice, onion and Iris germanica (Zimmermann and Steudle, 1998; Ranathunge et al., 2005; Meyer et al., 2011). Hydroponically grown corn plants developed a patchy exodermis which resulted in a greater suberin content of the root periphery (Barrowclough et al., 2000; Zimmermann et al., 2000; Schreiber et al., 2005). Barley roots developed an endodermis, where early deposition of CBs appeared as ‘dots’ 20 mm from the root apex (Fig. 1B), which is similar to soybean (Ranathunge et al., 2008) and other angiosperm plants (Ma and Peterson, 2003, and references therein). With maturity, these bands extended in the anticlinal walls (Fig. 1D) which probably added a greater resistance to the apoplastic permeability of the endodermis. SL deposited further back from the apex (60 mm) and initially exhibited as a patchy structure. At the base of Zone-I, SL were not deposited in the complete ring of the endodermis (Fig. 1E). Only further back at the base of the root (100 mm) did SL develop as a complete ring which had approx. 10 % of passage cells without lamellae, (Fig. 1F). The passage cells without SL facilitated the water and solute transport through the transcellular and symplastic components of the cell to cell pathway, but still present a barrier for apoplastic transport due to the presence of CBs.
The absence of an exodermis suggests that the endodermal suberin mostly accounted for the total suberin of barley roots, whereas, faintly stained, diffuse suberin in the rhizodermis accounted for the rest. The total root suberin is made up from aliphatic and aromatic components (Fig. 2A, B), similar to other monocot plants such as rice (Kotula et al., 2009; Ranathunge et al., 2011) and corn (Zimmermann et al., 2000). Even though, the basal zone (Zone-II) had markedly greater amounts of both aliphatic and aromatic suberin than the apical zone (Zone-I), the difference was more pronounced for the aromatic suberin (Fig. 2A). This might be due to some associated aromatics/polyphenolics in the strongly lignified stele of the basal zone of barley roots. The most abundant aliphatic monomers were ω-hydroxy fatty acids, α,ω-dicarboxylic acids and primary carboxylic acids, whereas aromatics were composed of ferulic and coumaric acids, of which the latter is prominent (Fig. 2B). These monomers are also known to be typical components of suberin lamellae in other plant species (Matzke and Riederer 1991; Schreiber et al., 1999; Kolattukudy, 2001; De Simone et al., 2003; Franke et al., 2005; Kotula et al., 2009). However, barley endodermis contained markedly greater amounts of aliphatic suberin than corn endodermis, depending on the root zone (for comparison, see Fig. 3A and Schreiber et al., 2005). On average, differences were approx. 9-fold for Zone-I (apical part) and 4-fold for Zone-II (basal part). For the first time, this study also shows the whole spectrum of monomer chain length distribution in the endodermis of barley roots. Short chain lengths, such as C16 and C18, dominate in the endodermis (Fig. 2C). More lipophilic very long chains (C26, C28 and C30) were only found in the ω-hydroxy fatty acids (Fig. 2C). In comparison with rice and corn, the aliphatic suberin in barley has a less diverse monomer composition. Such interspecies differences are likely to play a major role in the differences of water and solute transport of roots.
For barley roots, the ratio of measured Lpr using a hydrostatic pressure gradient (water flow through the apoplast as well as cell to cell path) and an osmotic gradient (water flow through the cell to cell path) was a little over unity. This suggests a dominant cell to cell water flow rather than a porous apoplastic bypass flow (Steudle and Peterson, 1998). This result can be interpreted using the composite transport model (Steudle and Peterson, 1998), i.e. in the absence of a hydrostatic pressure gradient, the apoplastic path is inefficient due to its low reflection coefficient. As such, water will flow predominantly via the protoplastic (cell to cell) path in response to an osmotic driving force. Further, comparisons between the cell Lpc and root Lpr of Zone-I also supported the view that the cell to cell pathway provides a greater contribution to the overall radial water flow than the apoplastic pathway. Although this finding was in agreement with the earlier results of Steudle and Jeschke (1983), concerns about the importance of each pathway for overall water flow still remained. On average, 20 % of endodermal cells contained the SL in the root segments (Zone-I) used for the measurements. The deposition of lamellae between the primary cell walls and the plasmalemma is known to reduce the water flow through the plasma membrane (Steudle and Peterson, 1998). Therefore, only those remaining cells without lamellae (passage cells) allowed water to move freely through the endodermis. Assuming that no water moved through the apoplast, the theoretically calculated overall root Lpr of Zone-I from the cell Lpc (not including the SL resistance) was 26 % lower than the measured root Lpr value. This result suggests that there are some apoplastic bypasses in the cortex, which may represent the difference between the measured and calculated values of Lpr. However, the theoretically calculated Lpr should be the upper limit and it should even be smaller if the resistance of suberized endodermal cells (20 % of the total endodermis) accounted for the calculations. This hypothesis of Steudle and Jeschke (1983) was further supported by a recent study by Knipfer et al. (2011), in which the closure of AQPs by HgCl2 resulted in a reduction of root Lpr values by 53 %. Nevertheless, there is still a substantial cell to cell component similar to the results of Steudle and Jeschke (1983). The results of this study conclude that the cell to cell water transport is more pronounced in barley roots, as found for A. thaliana (Ranathunge and Schreiber, 2011).
The rate of water transport into the shoot is usually determined by (1) the radial water transport across roots from the soil solution to the root xylem crossing different complex cell layers, including the endodermis, and (2) axial water transport inside the root xylem. It is known that the major hydraulic resistance or rate-limiting step for water transport into the shoot is the former but not the axial/longitudinal resistance in the xylem (Steudle and Peterson, 2000). Usually, the mature and dead xylem vessels/ducts provide exceedingly low resistance to the axial water flow (Steudle and Peterson, 2000). Using the pressure probe technique, we have demonstrated that this is true for the conductive part of Zone-I of barley roots in which the early metaxylem vessels and central late metaxylem vessel are fully matured (Fig. 5). In these roots, even though the column of central metaxylem cells can be identified much earlier, closer to the root apex, these cells fully mature much later, and the distance from the apex depends on the growth conditions (Heimsch, 1951; Lux, 1981). The undifferentiated and immature xylem vessels in the very apical region of Zone-I (at least up to 15 mm) provide some resistance to the axial water flow in the xylem. Hence, the axial hydraulic resistance in the very apical part of Zone-I cannot be neglected. However, in our Lpr measurements, this very apical part or ‘hydraulically isolated zone’ was not accounted for in the calculation. This experiment demonstrates that the measured Lpr data refer to the radial transport of water in barley roots but not to the axial transport in the xylem vessels.
In addition to water uptake, roots serve as the primary site for the uptake of nutrients and other solutes by plants. In barley roots, there are significant differences between the permeability (Psr) of electrolytes, such as nutrient salts and non-electrolytes, depending on their size and molecular structure. Small, lipophilic molecules (i.e. ethanol) move faster crossing the plasma membranes of the root than larger molecules with or without charges. These flows may also differ among different plant species, according to the composite transport model of roots (Steudle et al., 1987; Frensch and Steudle, 1989; Steudle and Peterson, 1998; Martinka et al., 2014). Differences between species may be caused by differences in root morphological and anatomical structures. For example, corn roots contained 12–14 cortical cell layers in series in the cylinder (Ye and Steudle, 2006), while barley contained 4–5 layers. The stele of barley was completely lignified with thick walls and densely packed cells containing no apparent air spaces. Only those cells surrounding the xylem were lignified in corn. As expected, barley roots had very low or no permeability for K4[Fe(CN)6] (Fig. 6E). It has been previously demonstrated that this salt with four negative charges moves slowly in the apoplast of corn and rice roots. The ferrocyanide anion is known to be repelled by the fixed negative charges of the cell walls (Ranathunge et al., 2005). There were also differences between barley and corn for sucrose, the non-electrolyte with the largest molar weight in the experiment. Sucrose permeated slowly but significantly across the root cylinder of corn during a very long treatment time of 4–5 h (Steudle et al., 1987). In barley, however, sucrose did not move into the stele for a period of 1·5–2 h (Fig. 6D). Longer experiments with sucrose (>2 h) resulted in a gradual decline of the root pressure, most probably due to some degree of membrane leakage. Conversely, mannitol slowly permeated into the roots of barley (Fig. 6C) but did not move across the roots of corn (Steudle et al., 1987). However, in corn, the authors conducted the experiment only for a short period of time (approx. 40 min) and they probably did not wait long enough to observe the permeation of mannitol into the root. The permeability of the roots for ethanol was similar for both species (Fig. 6A). This result would be expected because this lipophilic solute rapidly crosses the plasmalemma (Steudle and Henzler, 1995; Henzler et al., 2004). In summary, if barley roots behaved as perfect osmometers and were impermeable for all the solutes, biphasic responses would not have been seen for the permeable solutes, such as ethanol, KCl, NaCl and mannitol. In contrast, larger molecules with or without charges, such as sucrose and K4[Fe(CN)6], failed to permeate across the root. These results indicate that Psr of barley roots depends on the solute used, and the roots are not perfect osmometers.
Reflection coefficients (σsr) smaller than unity have been found for many roots using different techniques (Steudle and Peterson, 1998). It has been stressed many times in the literature that an σsr value is not a direct measure of the Psr of a root, although these values sometimes correlate such that a low σsr may correspond to a high Psr (Steudle and Peterson, 1998). The correlation may be simple and straightforward for uniform, homogeneous membranes (see figs 3–12 in Nobel, 1999) but may be complicated in composite membrane systems (Kedem and Katchalsky, 1963a, b). According to Kedem and Katchalsky (1958), the definition of semi-permeability holds if σsr = 1 and Psr = 0 at the same time. Further, by definition, Psr = 0 and Lpr > 0 should also hold for a semi-permeable barrier. However, in barley, the measured values of root Psr > 0 and σsr < 1 revealed that they do not behave like an ideal osmometer for the tested solutes.
Similarly to root Lpr and Psr values, the σsr value may be subjected to errors due to unstirred layers (USLs) both inside and outside of the root (Ye and Steudle, 2006; Knipfer et al., 2007; Knipfer and Steudle, 2008). Under well-stirred conditions, the estimated effect of internal USLs on the σsr values of young corn roots was as small as 7 % (Steudle and Frensch, 1989). For the thinner roots of barley, this effect should be even smaller. The impact of USLs was minimized by vigorously stirring the medium surrounding the root, but stirring cannot affect the USLs within the root. When measured in the stagnant solution, the effects of USLs were substantial in the osmotic experiments (Table 1). External USLs around the root reduced osmotic Lpr values by 2·4-fold compared with the well-stirred conditions. In contrast, USLs failed to reduce hydrostatic Lpr values below those of well-stirred conditions. This result is understandable because water flow driven by a hydrostatic pressure gradient should not be affected, as has been previously described by Steudle and Frensch (1989). In agreement with the present study, Knipfer and Fricke (2010) also found that root σsr values for solutes were significantly smaller when measured in a stagnant medium.
In young barley roots, the cortical apoplast allows solutes to diffuse up to the endodermis. Therefore, the cortical apoplast forms an internal USL. The cortex should have a rather low σsr, which may be even close to zero in the presence of a rather high Psr. For a composite structure consisting of an endodermis (the innermost modified cortical cell layer with rather low Psr and high σsr) and other cortical cells arranged in series, the basic principles of irreversible thermodynamics propose that the cortex should contribute to the overall σsr according to its permeability for the solute, which should be high (Kedem and Katchalsky, 1963a, b). Denoting the cortex with the superscript ‘cor’ and the endodermis with ‘en’, the overall σsr is described by the following equation:
| (8) |
Therefore, roots may exhibit a low σsr in the presence of a low Psr. For tree roots, σsr values were as low as 0·2–0·4. At the same time, the root Psr value was too small to be measured by the pressure probe (Steudle and Peterson, 1998). Similarly, in barley, the σsr values were substantially smaller than unity for K4[Fe(CN)6] and sucrose (which were virtually non-permeating). Alternatively, as suggested by Knipfer and Fricke (2010), if roots have a value of Psren = 0 (and a value of Psr = 0), the contribution of a USL (due either to the cortex or to an adjacent layer in the medium) should be small. Under these conditions, it holds that Psr/Psrcor ≅ 0 and Psr/Psren ≅ 1. However, in barley, it was not the case and Psren was not negligible. If both the apoplastic and the cell to cell permeability of ions in the endodermis are considered, the apoplastic component may be small but not negligible. Kronzucker and Britto (2011) demonstrated the importance of apoplastic bypasses of solutes in the endodermis compared with the plasma membrane permeability for sodium.
In conclusion, the data show that suberized cell walls make strong barriers for water transport in barley roots, especially in the basal zones. Barley roots did not form an exodermis, and the endodermal suberin accounted for the total root suberin. The absolute suberin amount in the basal zone was significantly higher than in the apical zone, which was inversely proportional to the Lpr. Comparison of the Lpc and Lpr of the apical zone indicated that the results were comparable and suggested a dominant cell to cell transport of water. It is likely that the passage cells without SL facilitate a significantly greater water flow through the endodermis, at least in the younger zone. At the root level, there was also some apoplastic bypass of water in the cortex. The xylem resistance for axial water flow depends on the xylem maturation. When the xylem is fully matured and functional, the axial hydraulic resistance dropped to virtually zero (Fig. 5). The permeability of barley roots for solutes depends on their size, molecular structure and root anatomy, and relates to the proposed composite transport model. The data support the hypothesis that water and solute transport across barley roots is composite in nature. The composite transport model should be extended to include serial arrays of components (cortex, endodermis) alongside the parallel components (apoplast, cell to cell).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: schematic diagram showing the development of Casparian bands (CBs) and suberin lamellae (SL) of apical (Zone-I) and basal (Zone-II) zones of barley seminal roots, grown in aerated hydroponics for 16–20 d.
Supplementary Material
ACKNOWLEDGEMENTS
This work is dedicated to inspiring and pioneering scientist Professor Dr Ernst Steudle who passed away far too early even before finishing this article. His enthusiasm, encouragement and enormous brainstorming ideas made this research possible. The authors gratefully acknowledge the Alexander-von-Humboldt foundation for awarding postdoctoral fellowships to K.R. and Y.X.K. The financial support by the Deutsche Forschungsgemeinschaft (GRK 2064) and Deutscher Akademischer Austauschdienst (DAAD) to L.S. is also highly appreciated.
LITERATURE CITED
- Azaizeh H, Gunse B, Steudle E.. 1992. Effects of NaCl and CaCl2 on water transport across cells of maize (Zea mays L.) seedlings. Plant Physiology 99: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowclough DE, Peterson CA, Steudle E.. 2000. Radial hydraulic conductivity of developing onion roots. Journal of Experimental Botany 51: 547–557. [DOI] [PubMed] [Google Scholar]
- Brouwer R. 1954. The regulating influence of transpiration and suction tension on the water and salt uptake by roots of intact Vicia faba plants. Acta Botanica Neerlanica 3: 264–312. [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA.. 1988. A berberine–aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissue. Protoplasma 146: 133–142. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA.. 1991. Efficient lipid staining in plant material with Sudan red 7B or Fluorol yellow 088 in polyethylene glycol–glycerol. Biotechnic and Histochemistry 66: 111–116. [DOI] [PubMed] [Google Scholar]
- De Simone O, Haase K, Muller E, et al. 2003. Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four Amazonian tree species. Plant Physiology 132: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, et al. 2005. Apoplastic polyesters in Arabidopsis surface tissues – a typical suberin and a particular cutin. Phytochemistry 66: 2643–2658. [DOI] [PubMed] [Google Scholar]
- Frensch J, Steudle E.. 1989. Axial and radial hydraulic resistance to roots of maize (Zea mays L.). Plant Physiology 91: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, Peters WS.. 2002. The biophysics of leaf growth in salt-stressed barley: a study at the cell level. Plant Physiology 129: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimsch Ch. 1951. Development of vascular tissues in barley root. American Journal of Botany 38: 523–537. [Google Scholar]
- Henzler T, Ye Q, Steudle E.. 2004. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant, Cell & Environment 27: 1184–1195. [DOI] [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W.. 2000. Abscisic acid and hydraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta 211: 874–882. [DOI] [PubMed] [Google Scholar]
- Kedem O, Katchalsky A.. 1958. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochimica et Biophysica Acta 27: 229–246. [DOI] [PubMed] [Google Scholar]
- Kedem O, Katchalsky A.. 1963a. Permeability of composite membranes. Part 2. Parallel arrays of elements. Transactions of the Faraday Society (London) 59: 1931–1940. [Google Scholar]
- Kedem O, Katchalsky A.. 1963b. Permeability of composite membranes. Part 3. Series arrays of elements. Transactions of the Faraday Society (London) 59: 1941–1953. [Google Scholar]
- Kim YX, Steudle E.. 2007. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. Journal of Experimental Botany 58: 4119–4129. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W.. 2010. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytologist 187: 159–170. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Steudle E.. 2008. Root hydraulic conductivity measured by pressure clam is substantially affected by internal unstirred layers. Journal of Experimental Botany 59: 2071–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer T, Das D, Steudle E.. 2007. During measurements of root hydraulics with pressure probes, the contribution of unstirred layers is minimized in the pressure relaxation mode: comparison with pressure clamp and high-pressure flowmeter. Plant, Cell & Environment 30: 845–860. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Besse M, Verdeil JL, Fricke W.. 2011. Aquaporin-facilitated water transport in barley (Hordeum vulgare) roots. Journal of Experimental Botany 62: 4115–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE. 2001. Polyesters in higher plants In: Babel W, Steinbuchel A, eds. Advances in biochemical engineering/biotechnology: biopolyester 1. Berlin: Springer, 1–49. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Agrawal VP.. 1974. Structure and the composition of the aliphatic components of potato tuber skin. Lipids 9: 682–691. [Google Scholar]
- Kotula L, Ranathunge K, Schreiber L, Steudle E.. 2009. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany 60: 2155–2167. [DOI] [PubMed] [Google Scholar]
- Kramer PJ. 1983. Water relations of plants. New York: Academic Press. [Google Scholar]
- Kramer PJ, Boyer JS.. 1995. Water relations of plants and soil. Orlando: Academic Press. [Google Scholar]
- Kronzucker HJ, Britto DT.. 2011. Sodium transport in plants: a critical review. New Phytologist 189: 54–81. [DOI] [PubMed] [Google Scholar]
- Lux A. 1981. A light microscopic study of the central metaxylem ontogenesis in the root of barley (Hordeum distichum L.) In: Bouwer R, Gasparikova O, Kolek J, Loughman BC, eds. Structure and function of plant roots. The Hague: Martin Nijhoff/W. Junk Publishers, 71–76. [Google Scholar]
- Ma F, Peterson CA.. 2005. Current insights into the development, structure and chemistry of the endodermis and exodermis of roots. Canadian Journal of Botany 80: 405 – 421. [Google Scholar]
- Martinka M, Vaculík M, Lux A.. 2014. Plant cell responses to cadmium and zinc In: Nick P, Opatrný Z, eds. Applied plant cell biology. The Plant Cell Monographs, Vol. 22 Berlin: Springer, 209–246. [Google Scholar]
- Matzke K, Riederer M.. 1991. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst., Quercus robur L., and Fagus silvatica L. Planta 185: 233–245. [DOI] [PubMed] [Google Scholar]
- Maurel C. 1997. Aquaporins and water permeability of plant membranes. Annual Review of Plant Physiology and Plant Molecular Biology 48: 399–429. [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ.. 2001. Aquaporins. A molecular entry into plant water relations. Plant Physiology 125: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CJ, Peterson CA, Steudle E.. 2011. Permeability of Iris germanica’s multiseriate exodermis to water, NaCl, and ethanol. Journal of Experimental Botany 62: 1911–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R.. 2001. Hydraulic conductivity of rice roots. Journal of Experimental Botany 52: 1835–1846. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1999. Physicochemical and environmental plant physiology. San Diego: Academic Press Inc. [Google Scholar]
- Peterson CA, Cholewa E.. 1998. Structural modifications of the apoplast and their potential impact on ion uptake. (Proceeding of the German Society of Plant Nutrition, Kiel, Germany). Zeitschrift Pflanzenernährung und Bodenkunde 161: 521–531. [Google Scholar]
- Peterson CA, Peterson RL, Robards AW.. 1978. A correlated histochemical and ultrastructural study of the epidermis and hypodermis of the onion roots. Protoplasma 96: 1–21. [Google Scholar]
- Ranathunge K, Schreiber L.. 2011. Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. Journal of Experimental Botany 62: 1961–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R.. 2005. A new precipitation technique provides evidence for the permeability of Casparian bands to ions in young roots of corn (Zea mays L.) and rice (Oryza sativa L.). Plant, Cell & Environment 28: 1450–1462. [Google Scholar]
- Ranathunge K, Thomas RH, Fang X, Peterson CA, Gijzen M, Bernards MA.. 2008. Soybean root suberin and partial resistance to root rot caused by Phytophthora sojae. Phytopathology 98: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L, Franke R.. 2011. Suberin research in the genomics era – new interest for an old polymer. Plant Sciences 180: 399–413. [DOI] [PubMed] [Google Scholar]
- Robards AW, Jackson SM, Clarkson DT, Sanderson J.. 1973. The structure of barley roots in relation to the transport of ions into the stele. Protoplasma 77: 291–311. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J.. 1999. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany 50: 1267–1280. [Google Scholar]
- Schreiber L, Franke R, Hartmann KD, Ranathunge K, Steudle E.. 2005. The chemical composition of suberin in apoplastic barriers affects radial hydraulic conductivity differently in roots of rice (Oryza sativa L. cv. IR64) and corn (Zea mays L. cv. Helix). Journal of Experimental Botany 56: 1427–1436. [DOI] [PubMed] [Google Scholar]
- Steudle E. 1989. Water flow in plants and its coupling to other processes: an overview. Methods in Enzymology 174: 183–225. [Google Scholar]
- Steudle E. 1993. Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level In: Smith JAC, Griffiths H, eds. Water deficits: plant responses from cell to community. Oxford: BIOS Scientific Publishers, 5–36. [Google Scholar]
- Steudle E, Brinckmann E.. 1989. The osmometer model of the root: water and solute relations of Phaseolus coccineus. Botanica Acta 102: 5–95. [Google Scholar]
- Steudle E, Frensch J.. 1989. Osmotic responses of maize roots. Planta 177: 281–295. [DOI] [PubMed] [Google Scholar]
- Steudle E, Henzler T.. 1995. Water channels in plants: do basic concepts of water transport change? Journal of Experimental Botany 46: 1067–1076. [Google Scholar]
- Steudle E, Jeschke WD.. 1983. Water transport in barley roots. Planta 158: 237–248. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA.. 1998. How does water get through roots? Journal of Experimental Botany 49: 775–788. [Google Scholar]
- Steudle E, Tyerman SD.. 1983. Determination of permeability coefficients, reflection coefficients, and hydraulic conductivity of Chara corallina using the pressure probe: effects of solute concentrations. Journal of Membrane Biology 75: 85–96. [Google Scholar]
- Steudle E, Oren R, Schulze ED.. 1987. Water transport in maize roots. Plant Physiology 84: 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Steudle E.. 1984. Determination of solute permeability in Chara internodes by a turgor minimum method. Effects of external pH. Plant Physiology 74: 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA.. 1999. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. Journal of Experimental Botany 50: 1055–1071. [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD.. 2009. The role of PIP aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology 149: 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XC, Steudle E, Hartung W.. 2004. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. Journal of Experimental Botany 55: 411–422. [DOI] [PubMed] [Google Scholar]
- Weatherley PE. 1982. Water uptake and flow into roots In: Lange OL, Nobel PS, Osmond CB, Zeigler H, eds. Encyclopedia of plant physiology, Vol. 12B, Berlin: Springer Verlag, 79–109 [Google Scholar]
- Ye Q, Steudle E.. 2006. Oxidative gating of water channels (aquaporins) in corn roots. Plant, Cell & Environment 29: 459–470. [DOI] [PubMed] [Google Scholar]
- Zeier J, Schreiber L.. 1997. Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata. Plant Physiology 113: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Schreiber L.. 1998. Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledonous species: chemical composition in relation to fine structure. Planta 206: 349–361. [Google Scholar]
- Zhu GL, Steudle E.. 1991. Water transport across maize roots: simultaneous measurement of flows at the cell and root level by double pressure probe technique. Plant Physiology 95: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HM, Steudle E.. 1998. Apoplastic transport across young maize roots: effect of the exodermis. Planta 206: 7–19. [Google Scholar]
- Zimmermann HM, Hartmann KD, Schreiber L, Steudle E.. 2000. Chemical composition of apoplastic transport barriers in relation to root hydraulic conductivity of maize (Zea mays L.) roots. Planta 210: 302–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.