Abstract
Background and Aims The geographical distributions of species are constrained by their ecological requirements. The aim of this work was to analyse the effects of environmental conditions, historical events and biogeographical constraints on the diversification of the three species of the western Mediterranean shrub genus Stauracanthus, which have a parapatric distribution in the Iberian Peninsula.
Methods Ecological niche factor analysis and generalized linear models were used to measure the response of all Stauracanthus species to the environmental gradients and map their potential distributions in the Iberian Peninsula. The bioclimatic niche overlap between the three species was determined by using Schoener's index. The genetic differentiation of the Iberian and northern African populations of Stauracanthus species was characterized with GenalEx. The effects on genetic distances of the most important environmental drivers were assessed through Mantel tests and non-metric multidimensional scaling.
Key Results The three Stauracanthus species show remarkably similar responses to climatic conditions. This supports the idea that all members of this recently diversified clade retain common adaptations to climate and consequently high levels of climatic niche overlap. This contrasts with the diverse edaphic requirements of Stauracanthus species. The populations of the S. genistoides–spectabilis clade grow on Miocene and Pliocene fine-textured sedimentary soils, whereas S. boivinii, the more genetically distant species, occurs on older and more coarse-textured sedimentary substrates. These patterns of diversification are largely consistent with a stochastic process of geographical range expansion and fragmentation coupled with niche evolution in the context of spatially complex environmental fluctuations.
Conclusions: The combined analysis of the distribution, realized environmental niche and phylogeographical relationships of parapatric species proposed in this work allows integration of the biogeographical, ecological and evolutionary processes driving the evolution of species adaptations and how they determine their current geographical ranges.
Keywords: Biogeography, diversification, ecological niche factor analysis (ENFA), Messinian salinity crisis, niche overlap, phylogeography, species distribution modelling, Stauracanthus
INTRODUCTION
The geographical distributions of species are constrained by their scenopoetic (i.e. abiotic) and bionomic requirements (Hutchinson, 1978; Soberón, 2007). Current understanding of the spatial and temporal dynamics of species ranges in space and time is tied to the Hutchinsonian niche concept (Colwell and Rangel, 2009). The duality between ‘niche’ and ‘biotope’ (Hutchinson, 1957, 1978) provides a conceptual framework that allows the joint analysis of environmental conditions, ecological interactions i.e. the so-called Grinnelian and Eltonian niches (Soberón, 2007), and geographical distributions (Colwell and Rangel, 2009). In addition to these factors, historical events and biogeographical constraints (such as barriers to dispersal) also determine species distributions (Soberón, 2007; Hortal et al., 2012), creating opportunities for diversification through isolation processes. The dynamic interaction between species’ requirements, environmental dynamics, ecological processes and biogeographical events determines the evolutionary history of a given group (Yesson and Culham, 2006; Wake et al., 2009).
To understand the origin and dynamics of species distributions it is necessary to study both their diversification and how their environmental requirements evolve through time. These analyses have been traditionally done by relating the position of the species in the bioclimatic space (as e.g. minimum temperature or average precipitation) to their positions in phylogeny through comparative analyses (e.g. Gouveia et al., 2014). However, analyses combining information on species distributions and niche-mediated bioclimatic responses with phylogeographical data may allow a more in-depth investigation of their joint effects and interactions on the diversification of a particular group of species. These analyses can help us to understand the evolution of new traits and adaptations (Knowles, 2003; Diniz-Filho et al., 2009), particularly in the study of speciation of sister species (Barraclough and Vogler, 2000). Georeferenced data on species occurrences gathered from atlases, museum collections and databases can nowadays be combined with high-resolution climate data by using statistical techniques (commonly referred to as ecological niche models or species distribution models; Guisan and Zimmermann, 2000; Chefaoui et al., 2005; Hortal et al., 2012; Peterson and Soberón, 2012) to predict the distributions of species and describe the climatic dimensions of their niches. In turn, species-level phylogenies estimated from DNA sequences (e.g. Pardo et al., 2008) allow the dating of speciation events via relaxed molecular clock methods (Lepage et al., 2007).
Stauracanthus (Genisteae) is a small legume genus comprising three thorny shrub species restricted to sandy and gravelly soils in the south-west of the Iberian Peninsula and north-western Africa (Morocco and Algeria; Paiva and Coutinho, 1999). Stauracanthus species have parapatric distributions (i.e. they occupy separate but contiguous areas; Bull, 1991) in the south-western Iberian Peninsula. This region is characterized by both high habitat heterogeneity and a complex palaeoclimatic history. Nonetheless, it can be safely assumed that the distribution of all Stauracanthus species within this region is largely the result of their responses to the environment rather than to biogeographical effects. Relatively little is known on the evolutionary patterns of this genus, besides the phylogeographical relationships among an array of Stauracanthus populations (Pardo et al., 2008). Therefore, the determinants of its diversification in space and time remain unclear.
In this work we studied the environmental responses of the three species of Stauracanthus, aiming to understand the effects of environmental conditions, ecological interactions, historical events and biogeographical constraints on the diversification of these closely related species. We hypothesized that edaphic and climate factors, rather than biogeographical processes, have been the main drivers of the distribution and diversification of Stauracanthus. Here, we believe that the effect of biogeographical processes will be comparatively less important because Stauracanthus species occupy most of the areas suitable for them in the Iberian Peninsula, and their dispersal abilities could have allowed all species to reach the areas occupied by their sister species. To evaluate this hypothesis, we first gathered and reviewed the available information on the distribution of Stauracanthus species in the Iberian Peninsula. Then, we: (1) measured their responses to the environmental gradients, (2) mapped their potential distribution, (3) determined the bioclimatic niche overlap between them, and (4) characterized their genetic differentiation using published genetic data. A mismatch between evolutionary relationships and environmental responses will imply that biogeographical processes different from mere responses to environment are behind the current distribution of Stauracanthus species, thereby making it possible to reject our hypothesis. Therefore, we finally discuss the geographical and environmental commonalities and differences between these species in the light of their known evolutionary patterns.
METHODS
Study system
Stauracanthus taxonomy is not totally consensual, but all authors agree upon the existence of three main taxa (Guinea and Webb, 1968; Díaz et al., 1990; Paiva and Coutinho, 1999). After analysing 141 specimens from the João de Carvalho e Vasconcellos herbarium (LISI) and from the Museu Nacional de História Natural e da Ciência of Lisbon (LISU), and using our taxonomic and ecological knowledge of the species (Supplementary Data Appendix 1), we decided to follow the more recent classification of Paiva and Coutinho (1999). This classification differentiates three species: Stauracanthus boivinii (Webb) Samp. – distributed throughout western Iberia and north-western Africa on either sandy or gravelly soils (Guinea and Webb, 1968), and S.genistoides (Brot) Samp. and S.spectabilis Webb – both growing on coastal sandy soils in south-western Iberia. An isolated population of S. spectabilis occurs on sandy soils on the Atlantic coast of Morocco (near Rabat). Although we restricted our environmental niche modelling and niche overlapping analyses to the parapatric Iberian populations, in order to minimize the effect of dispersal limitations on our assessment of the response of all three species to environmental gradients, we included Moroccan populations in the genetic analyses (see below) to fully characterize the evolutionary differentiation among all Stauracanthus populations.
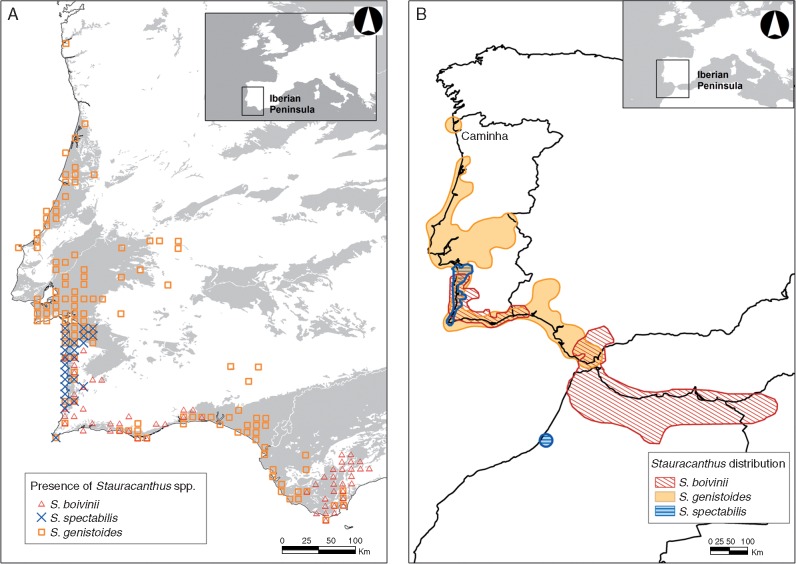
We referred environmental data and presence records of Stauracanthus species to the 6171 cells of 10 km × 10 km UTM grid squares that cover the 582 000 km2 of mainland Iberia. Records for the three Stauracanthus species were collected from the relevant herbaria [University of Coimbra (COI), LISI, LISU, Royal Botanic Garden of Madrid (MA), Complutense University of Madrid (MACB and MAF) and University of Seville (SEV)], specialized literature, the Anthos database (http://www.anthos.es/) and field work. In total, we compiled 740 presence records: 215 for S. boivinii, 394 for S. genistoides and 131 for S. spectabilis, corresponding to 60, 125 and 24 UTM grid cells (10 km × 10 km), respectively (Fig. 1A).
Fig. 1.
(A) Geographical range of Stauracanthus species in the Iberian Peninsula. (B) Total extent of occurrence of the genus. In (A) each symbol represents a 10×10 km UTM cell, according to the data compiled in this study; the shaded areas represent soils suitable for the genus, namely sedimentary soils.
Thirty-three topographical and climatic variables were extracted from the WorldClim interpolated map database (Hijmans et al., 2005; http://www.worldclim.org/) and from EDIT Geoplatform (http://edit.csic.es/GISdownloads.html), and reprocessed when necessary at 100 km2 resolution using a standard UTM grid (Supplementary Data Table S1). In addition, the shortest distance to the coast was calculated using ArcGIS Desktop 10 software (ESRI, 2011). As Stauracanthus species occur on sedimentary substrates, mainly sandy and gravelly soils, we reclassified the rock complexes described in the lithological maps of Portugal (APA, 1992) and Spain (IGME, 1994), homogenizing their information to create five consistent substrate classes particularly suited to the study of species growing in sedimentary soils: (1) Holocene and Pleistocene sedimentary substrates; (2) Miocene and Pliocene sedimentary substrates; (3) metamorphic and sedimentary substrates (older and more coarse-textured than classes 1 and 2); (4) plutonic substrates; and (5) volcanic substrates (Table S1). This classification was also applied to the lithological map of Morocco (MEM, 1985) in order to extract the edaphic requirements of Moroccan populations of Stauracanthus.
Environmental niche modelling
To model the environmental niche and estimate the potential distribution of the Stauracanthus species, we used two different methods: ecological niche factor analysis (ENFA; Hirzel et al., 2002a) and generalized linear models (GLMs; McCullagh and Nelder, 1989). Data for GLMs included the presence records for each species plus a stratified selection of pseudo-absences from the non-suitable habitats found by ENFA in the Iberian Peninsula. This contributes to obtaining a wider potential range of environmentally suitable habitats for the species (Chefaoui and Lobo, 2008). A total of 600 pseudo-absences for S. boivinii, 1250 for S. genistoides and 240 for S. spectabilis were selected. Although different species distribution modelling algorithms can provide different results, it is beyond the scope of this study to analyse that. We chose ENFA and GLM as both provide statistical knowledge of the most relevant variables and do not produce overfitted models.
Both presence and Box–Cox-transformed environmental data at 100 km2 resolution were used to perform ENFA analyses (Hirzel et al., 2002a). Briefly, ENFA is an ordination technique that describes the position of the niche in an ecological space composed of several orthogonal factors derived from the environmental descriptors and computes suitability functions for all points in the region considered. The first of these factors, called the ‘marginality axis’, is obtained from the direction of maximum difference between the species niche and the available conditions in the Iberian Peninsula, while the rest of the axes, called ‘specialization factors’, measure the ratio of ecological variance of the species in relation to the mean habitat (Hirzel et al., 2002a). From our initial set of 39 variables, we first removed those that were highly correlated (Pearson correlation coefficients ≥ |0·80|) or showed little contribution to the factors extracted by a preliminary ENFA for each species. Those with high correlations and/or without contribution to ENFA’s factors were excluded (Table S1). After this first selection, ENFA models were performed using two subsets of variables: (1) the complete set of variables remaining after selection; and (2) the coincident sets of relevant variables between the former ENFAs and GLMs for each species.
The ENFA models were validated by performing a 5-fold cross-validation and obtaining the absolute validation index (AVI), the contrast validation index (CVI) and the continuous Boyce index (BI). The AVI measures the proportion of presences above habitat suitability (HS) =0·5 and varies from 0 to 1. The CVI is the difference between AVI and a random model; it varies from 0 to 0·5. The continuous BI is a modification of the original BI (Boyce et al., 2002). This index varies from −1 to 1 and has shown a performance similar to AUC (Hirzel et al., 2006). The BI does not use HS classes, and is threshold-independent because it is computed using a moving window. We used a ‘small’ moving window of width 20 (i.e. BIcont(0·2)), as recommended by Hirzel et al. (2006) for giving the best results. ENFA computations were performed in Biomapper 4·0 (Hirzel et al., 2002b).
Presence and pseudo-absence data for each species were used to accomplish GLMs with binomial distribution and the logit link function. We selected 10-fold more pseudo-absences than presences randomly from the non-suitable area found in the Iberian Peninsula (i.e. with HS = 0) predefined by ENFA (Chefaoui and Lobo, 2008) to be used in GLMs. After including all linear, quadratic and cubic terms of each variable, we performed stepwise model selection using the Akaike information criterion (AIC) in both directions using the MASS package (Venables and Ripley, 2002). To test whether the model terms were significant, we performed a χ2 ANOVA. We transformed the continuous predictions into a binary output, adjusting the threshold value to the prevalence of our data (= 0·1), as suggested by Lobo et al. (2008) to map predicted distributions. Models were 5-fold cross-validated, partitioning both presence and pseudo-absence data sets, and not using a higher fold number due to the small data size. We calculated the area under the receiver operating characteristic (ROC) curve (AUC; Fielding and Bell, 1997), sensitivity (presences correctly predicted), specificity (absences correctly predicted) and κ statistics, using the maximum of the sum of the sensitivity and specificity as threshold value. All analyses were conducted in R (R Core Team, 2015).
Niche overlap
We measured niche overlap among Stauracanthus species using two subsets of variables to account for differences in the interpretation of the niches: (1) all variables selected for at least one species; and (2) the best subset of variables found in ENFAs and GLMs for all species. We calculated environmental niche overlap using Schoener's index (D metric; Schoener, 1970; Broennimann et al., 2012; Silva et al., 2016), which compares the occupancy of the environment between pairs of species in a given environmental space. It allows an intuitive interpretation because it varies between 0 (no overlap) and 1 (identical niches). Calculations of D were performed in the space derived from a PCA-env ordination, namely a principal component analysis calibrated on the entire environmental space. This method delivers the most accurate outputs among the ordination techniques implemented in R by Broennimann et al. (2012). We also explored the spatial niche overlap among the species by overlaying the habitat suitability maps obtained from ENFA. Values of HS were previously reclassified according to the same BI as that used for validation, to discriminate among optimal, suitable, marginal and unsuitable HS areas (Hirzel et al., 2006).
Genetic distance
We used the data provided by Pardo et al. (2008) for different populations of Stauracanthus spp. in the Iberian Peninsula and north-western Africa to assess the genetic distance between Stauracanthus populations. Such genetic data provide solid evidence on the evolutionary differentiation between populations in a small clade in which species definition is, on occasion, complicated. Such differentiation can arise by genetic drift associated with either adaptations of spatially interconnected populations to different environmental conditions or non-adaptive phenotypic variations associated with differences in performance on several substrates. Regardless of the adaptive or neutral nature of these genetic differences, their study makes it possible to relate environmental variations to a continuous variable describing the evolutionary differences between populations (i.e. genetic distance), rather than associating these responses to the environment with doubtful discrete categories (in this case, species) within groups without taxonomical consensus.
Pardo et al. (2008) provided a table showing the different haplotypes obtained in the analyses of seven polymorphic chloroplast microsatellites (SSRs) and their frequency in each sampled population. Pooling all microsatellites, there were a total of 38 distinct alleles, which, combined, constituted 44 different haplotypes. The pairwise distance between all individuals was calculated using the module available at GenalEx for haploid SSRs (Peakall and Smouse, 2006, 2012). Briefly, for each locus we calculated the sum of the squared size difference between the two alleles in the comparisons: (S1 – S2)2, where S1 is the size (base pairs) of the allele of individual 1 and S2 the size of the allele of individual 2. Distances were then summed across loci. The pairwise genetic distances between populations were obtained by averaging the genetic distances between all the individuals of each population with respect to the rest.
To separate truly causal processes acting on genetic distance among Stauracanthus species from those that merely reflect spatial covariation (Legendre and Legendre, 1998) we applied simple and partial Mantel tests (SMTs and PMTs, respectively) to the matrix of pairwise genetic distances between populations. Firstly, SMTs between geographical and genetic distances indicated whether species distributions were spatially structured (Borcard et al., 2004). When significant relationships between genetic distances and location were found, PMTs were used to examine the relationship between genetic distance and the environmental variables selected by the previous analyses once geographical distance was taken into account (Goslee and Urban, 2007). These analyses were conducted in the ecodist software package (Goslee and Urban, 2007).
Then, a non-metric multidimensional scaling (NMS) ordination of Stauracanthus populations based on pairwise genetic distances was used to analyse the relationship between genetic distance and the environmental variables identified by the previous analyses using the function metaMDS of the R package vegan. The response of the genetic distance to NMS axes was assessed (Oksanen et al. 2013) through a graphic illustration of these relationships, obtained by including vectors representing the environmental variables onto the population ordination using the envit function of the vegan R software package.
RESULTS
Environmental niche modelling
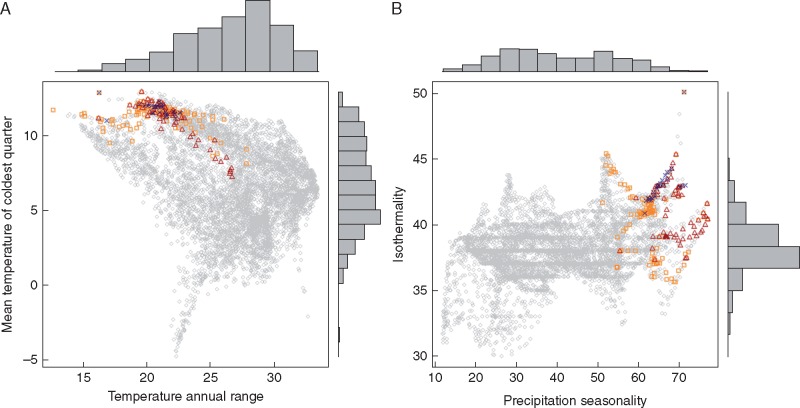
The number of variables considered after the initial selection procedure was reduced to less than half of the starting set: nine for S. boivinii, 12 for S. genistoides and 12 for S.spectabilis (Table 1). The most important variables estimated by ENFA and GLMs were coincident for the three species. GLMs identified four statistically significant (P <0·001) variables that were able to differentiate all Stauracanthus species distributions with respect to their less suitable climates: mean temperature of the coldest quarter; annual range of temperature; isothermality (i.e. mean diurnal temperature range/annual temperature range); and seasonality of precipitation. The same four climatic variables contributed the most to the ENFA marginality factors, being precipitation seasonality and the mean temperature of the coldest quarter the most relevant of them (Table 1). Marginality scores were positive for isothermality, seasonality of precipitation and the mean temperature of the coldest quarter, indicating that these species are present in locations with higher values of each of these variables than the overall mean conditions of the study area. However, Stauracanthus populations are found in habitats with a lower annual range of temperature in comparison with the rest of the Iberian Peninsula (Table 1, Fig. 2). Specialization scores were in general higher for S.spectabilis, indicating a more restricted range of the species on most of the variables within the Iberian Peninsula.
Table 1.
Results of ENFA using all the variables, showing the contributions of each variable to the marginality/specificity factors of each species. Contributions to the marginality factor higher than 0·2 are in bold. The four variables with higher scores for the three species, which were also identified by GLMs and used in subsequent analyses, are indicated with an asterisk*
| ENFA results | S. boivinii | S. genistoides | S. spectabilis |
|---|---|---|---|
| Marginality | 1·587 | 1·757 | 1·936 |
| Specialization | 7·822 | 3·991 | 9·257 |
| Marginality factor (% explained) | 40 | 32 | 69 |
| First specialization factor (% explained) | 50 | 33 | 15 |
| Environmental variables | |||
| Mean temperature of coldest quarter* | 0·47/175·8 | 0·47/55·34 | 0·48/452·28 |
| Annual temperature range* | −0·36/98·52 | −0·40/73·59 | −0·36/330·34 |
| Isothermality* | 0·37/85·34 | 0·34/28·19 | 0·41/302·94 |
| Seasonality of precipitation* | 0·59/255·03 | 0·43/83·18 | 0·44/443·95 |
| Mean temperature of warmest quarter | – | 0·14/72·17 | – |
| Mean temperature of wettest quarter | 0·13/77·10 | 0·16/29·26 | 0·19/245·62 |
| Hydric balance | −0·14/204·6 | – | −0·22/189·7 |
| Altitude range | – | −0·32/36·82 | −0·33/276·66 |
| Average monthly radiation | – | 0·05/54·27 | 0·04/186·33 |
| Precipitation in coldest quarter | 0·29/286·6 | 0·23/21·52 | – |
| Holocene and Pleistocene sedimentary substrates | – | 0·18/16·33 | 0·11/89·80 |
| Metamorphic and sedimentary substrates | – | −0·22/18·70 | −0·21/166·47 |
| Miocene and Pliocene sedimentary substrates | 0·15/36·72 | 0·11/12·59 | 0·11/93·92 |
| Plutonic substrates | −0·11/30·12 | – | −0·13/110·55 |
Fig. 2.
Responses of the three Stauracanthus species to the most significant bioclimatic variables determining their geographical distribution, according to ecological niche factor analysis (ENFA) and generalized linear model (GLM) analysis. Circles, cells without presences; triangles, S. boivinii; squares, S. genistoides; crosses, S. spectabilis.
The GLM results allowed discriminating of the importance of these variables: seasonality of precipitation was the most powerful explanatory variable for all species, which occur in habitats with a high difference in precipitation among seasons (Supplementary Data Fig. S1). Besides this common variable, GLMs identified differences in the climatic conditions inhabited by each of the species. While S. boivinii is restricted to areas with lower annual ranges of temperature, the most favourable areas for S. genistoides are found where the mean temperature of the coldest quarter is >7·5 °C, and S. spectabilis prefers isothermal regions (Fig. S1).
Sedimentary soils seem to exert a secondary role as correlates of Stauracanthus distributions. The presence of metamorphic and sedimentary substrates was identified by ENFAs as slightly relevant for S. genistoides and S. spectabilis (marginality > 0·2), whereas other types of soil were less relevant (Table 1). Such low predictive power of the substrate compared with climate may be due to the widespread distribution of sedimentary soils throughout the Iberian Peninsula. However, as Stauracanthus species have been observed exclusively on sedimentary substrates, we used soil conditions afterwards to filter all predictive maps.
After using the two subsets of variables [(1) all the variables selected and (2) only the four most relevant variables mentioned above] to perform ENFA models, the worst evaluation results were obtained for S. spectabilis using all the variables (BI = −0·074; Supplementary Data Table S2). This result means that the model is not different from random (Hirzel et al., 2006) and can be explained by the smaller number of presences available for this species. On the other hand, GLMs were quite parsimonious, showing similarly low AIC values for the three species (AIC ranging from 6 to 8) and a very high percentage of total deviance explained (D2 = 100 %). Cross-validation results were also optimal (Supplementary Data Table S3), but these overoptimistic results often occur when using pseudo-absences from non-suitable areas (Chefaoui and Lobo, 2008), as this method creates a complete separation between presences and absences regarding good predictors. Thus, to prevent this statistical phenomenon from affecting our models, we decided to discard GLM outputs and not use them to create maps or for the niche overlap analyses. Rather, we preferred ENFAs performed with the concurrent set of four variables to predict suitable habitats, as we considered that the match between ENFAs and GLMs is a sign of the robustness of our results.
Niche overlap
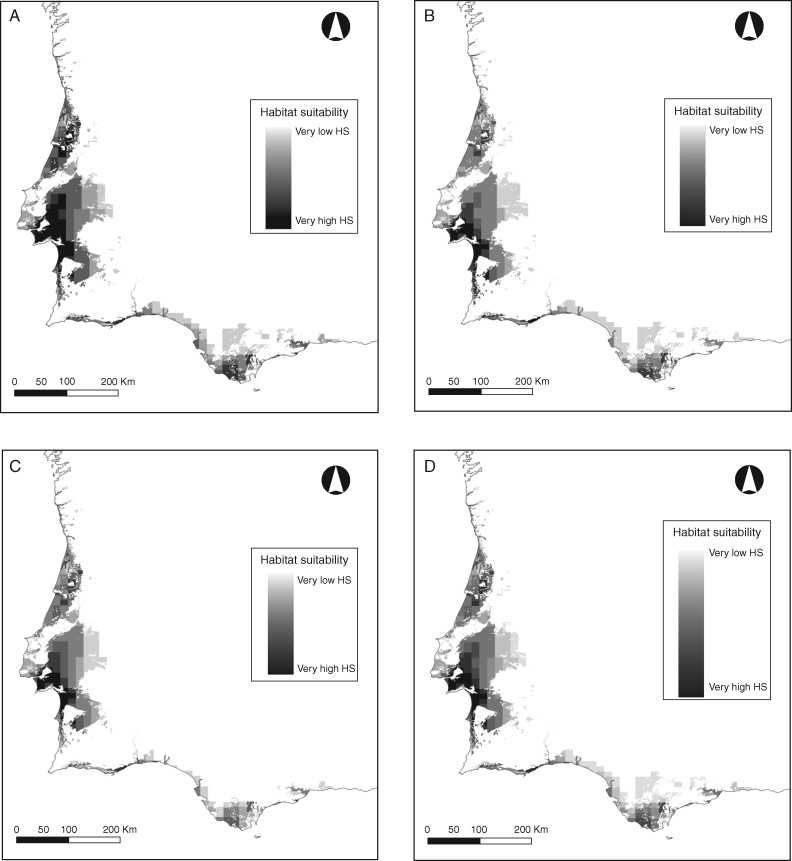
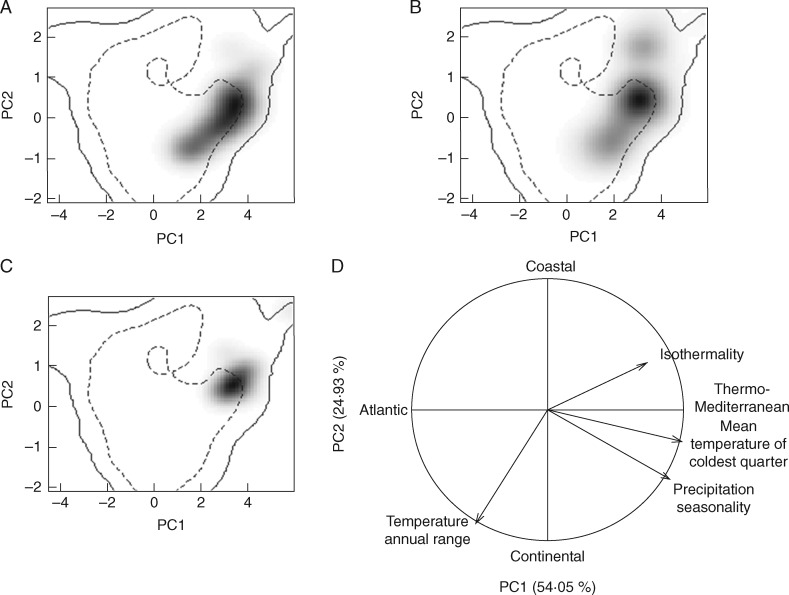
After filtering with lithological data, predicted suitable habitat was reduced by 54 % on average (Fig. 3). The assessment of environmental niche overlap between pairs of species using the two subsets of variables is shown in Table 2. The three Stauracanthus species showed remarkably similar responses to the climatic conditions in the Iberian Peninsula. The total value of D was similar between the two subsets of variables (total Dallvariables = 1·538; total Dfourvariables = 1·517), and the niche overlap obtained among all species was on average 0·51. Considering the two subsets, environmental niches of S. boivinii and S. genistoides were the most coincident, overlapping 67 % on average. The ordination of the four most relevant variables in the environmental background (Fig. 4) indicated the existence of a climatic maximal variance direction in which temperature and precipitation fitted firstly Thermo-Mediterranean versus Atlantic vegetation zones (PC1) and secondly coastal versus continental conditions (PC2).
Fig. 3.
Overlap of habitat suitability (HS) maps obtained with ecological niche factor analysis (ENFA) using the set of four variables identified as the most relevant for the distribution of the three species (see text). (A–D) Overlap of HS maps between S. boivinii and S. genistoides (A), S. boivinii and S. spectabilis (B), S. genistoides and S. spectabilis (C) and the three species (D), after reclassification using the continuous Boyce index.
Table 2.
Niche overlap between pairs of species of Stauracanthus found using all variables selected and only the four most relevant (see text). Schoener’s measure of niche overlap (D) was computed over the whole environmental space of the study area
| All variables |
Four variables |
|||
|---|---|---|---|---|
| D | HS area (km2) | D | HS area (km2) | |
| SBO–SGE | 0·726 | 1239 | 0·612 | 3894 |
| SBO–SSP | 0·395 | 1947 | 0·468 | 1593 |
| SGE–SSP | 0·417 | 1770 | 0·437 | 1593 |
HS area, area in which both species were found to have an optimum HS value; SBO, S. boivinii; SGE, S. genistoides; SSP, S. spectabilis.
Fig. 4.
Realized niches of the three Stauracanthus species in the climatic space available in the Iberian Peninsula. (A–C) Niches along the two first axes of the PCA in the Iberian Peninsula. (A) S. boivinii. (B) S. genistoides. (C) S. spectabilis. Grey shading shows the density of occurrences of each species by grid cell. Solid and dashed contour lines illustrate, respectively, 100 and 50 % of the available (background) environment. (D) Contribution of the climatic variables on the two axes of the PCA. PC1 (x axis) reflects a gradient between Mediterranean and Atlantic conditions in the Iberian Peninsula; PC2 (y axis) reproduces a gradient of continentality.
Using the four-variables subset, we found a correspondence between D and the geographical extent of the areas with HS defined as optimal for each pair of species. For example, S. boivinii and S. genistoides, the species that showed greater environmental overlap, also revealed a greater area using this subset (Table 2). However, there was no such correspondence using all the variables.
Interestingly, the marginality factors of the three species were highly correlated. There was a significant positive Pearson correlation among them using both the four-variable subset (0·995 ≤ r ≤ 0·998) and all variables (0·882 ≤ r ≤ 0·965). The first specialization factors using all the variables were correlated only for S. spectabilis and S. genistoides (r = −0·943).
Genetic distances
Stauracanthus populations and environmental variations showed a strong effect of spatial distance on genetic differentiation both in the Iberian Peninsula and throughout the whole distribution range of the genus (Table 3). This effect disappeared when only the S. genistoides–spectabilis clade was considered. After accounting for the effects of spatial distance, lithology (i.e. substrate) clearly separated S. boivinii from the other two species (Table 3, Fig. 5), an effect that remained significant when all Stauracanthus populations were considered at both spatial scales (Table 3). However, no relationship between genetic differentiation and lithology was found when considering only populations from the S. genistoides–spectabilis clade. Strikingly, climate bore no relationship with genetic distance in any case.
Table 3.
Mantel correlations between genetic distances and climatic and lithological differences among Iberian Stauracanthus populations. Results are shown for tests including populations of either all the species in the genus or only S. genistoides sensu lato, both over the total range of the genus’s distribution or just in the Iberian Peninsula. For climate and lithology, two correlation values are provided; the second is the correlation factor after accounting for the effect of inter-population spatial distance with a partial Mantel test (see text)
| Space |
Climate |
Lithology |
||||
|---|---|---|---|---|---|---|
| Genetic distance | Total range | Iberian peninsula | Total range | Iberian peninsula | Total range | Iberian peninsula |
| Stauracanthus | 0·24* | NS | 0·28*/ns | NS | 0·38**/0·40*** | 0·27*/0·27* |
| S. genistoides–spectabilis clade | NS | NS | NS | NS | NS | NS |
NS, not significant.
P< 0·05;
P< 0·01;
P< 0·001.
Fig. 5.
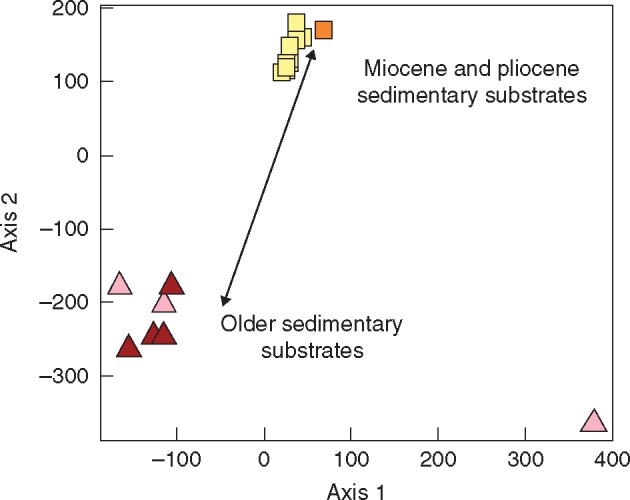
Axes 1 and 2 of the two-dimensional non-metric multidimensional scaling ordinations of populations based on their pairwise genetic distances. The final stress value for the two-dimensional configuration was 0·08. Vectors fitted represent the substrate classes dominating the Stauracanthus populations: Miocene and Pliocene substrates (r2 = 0·51, P< 0·01) and older and coarse-textured sedimentary substrates (r2 = 0·66, P< 0·001). Triangles, S. boivinii; squares, S. genistoides and S. spectabilis; dark colours, Moroccan populations; pale colours, Iberian populations.
DISCUSSION
Environmental niche of Stauracanthus species
Our analysis of the realized niche of the Stauracanthus species has shown that the distributions of these three species are largely constrained by local edaphic conditions, as well as by yearly and daily climatic variability and winter temperatures in the Iberian Peninsula. Strikingly, Stauracanthus species present high degrees of climatic niche overlap, and all of them are discriminated by the same climatic variables despite the differences in their geographical distributions. Such similarity evidences that many common adaptations are still shared by the members of a recently diversified clade (Burns and Strauss, 2011). Stauracanthus species occur on sandy and gravelly soils of mainly coastal areas and on some mountains with a large difference in precipitation among seasons, relatively mild winters and moderately low variations between both daily and annual temperatures. These conditions characterize the Thermo-Mediterranean bioclimate with Atlantic influence (Barbero and Quezel, 1982). However, each species shows particular requirements that determine its distribution within these bioclimatic conditions. Currently, S. boivinii occurs on coarse-textured sandy and gravelly soils in both coastal areas and mountains of the south-western Iberian Peninsula, but always with oceanic influence. In contrast, both S. genistoides and S. spectabilis mainly occur on Miocene to Pliocene fine-textured sandy soils. While the distribution of S. spectabilis mostly coincides with the area of warm summer Mediterranean climate in the south-western Iberian Peninsula (AEMET-IM, 2011), S.genistoides mostly occupies the hot summer Mediterranean climate coastal areas in this region, extending its distribution also to the coastal area of warm summer Mediterranean climate in north-western Iberian Peninsula.
Our results indicate that the spatial segregation of Stauracanthus species is first determined by edaphic conditions. The type of substrate determines the parapatry between the S. boivinii and S. genistoides–spectabilis clades, pointing to it as a potentially key factor for their diversification. Additionally, the responses of S. genistoides and S. spectabilis to climate are clearly behind their parapatry in the Iberian Peninsula. This points to the existence of a, perhaps ongoing, process of speciation within this clade involving some new physioclimatic adaptations, at least for one of the taxa. Since our results show that S. genistoides and S. boivinii largely share their main climate adaptations, it can be argued that the former would have retained the ancestral climatic responses of the genus. These findings are consistent with those of Anacker and Strauss (2014) for Californian plants, underlining the importance of shifts in soil types and/or climate used by sister species.
Genetic diversification of Stauracanthus populations
Inter-population genetic differentiation in Stauracanthus was largely explained by geographical distance. This may in part be due to the fact that the genetically most differentiated species, S. boivinii, is also the only one present in north-western Africa, with the exception of an isolated population of S. spectabilis found near Rabat. In any case, the strong effect of geographical distance suggests that historical factors (see below) and long-term isolation could have promoted diversification within the genus. However, our results show that, irrespective of distance, ecological adaptation has also promoted inter-specific segregation. Differences in substrate lithology explained a significant proportion of genetic variability and separated S.boivinii from the populations of the genistoides–spectabilis clade when either all the genus’s distribution range or just the Iberian Peninsula was considered. In contrast, climate was not correlated with genetic variability at any level, probably due to the reduced distribution of the populations used in the genetic analyses.
The low correlation of genetic and climatic differences among populations indicates that climate acts as a common determinant for the whole genus, constraining its distribution to the south-west of the Iberian Peninsula, whereas soil requirements may have played a significant role in the diversification within Stauracanthus. Such adaptation to different substrates may have taken place in allopatry, favouring species segregation after further expansion of their distribution ranges. This is consistent with a process of ecological speciation, which occurs when sister species occupy ecological niches that do not overlap in space, thus favouring reproductive isolation (for a review see Rundle and Nosil, 2005). In this sense, adaptation to different substrates can provoke a sharper spatial segregation of Stauracanthus species compared with the effect of climatic gradients. This may be the reason why the former explains a higher proportion of the overall genetic variability. In fact, the diversification of Stauracanthus is largely consistent with a stochastic process of geographical range expansion and fragmentation coupled with niche evolution in the context of spatially complex environmental fluctuations (Rangel et al., 2007; Colwell and Rangel, 2009).
A probable evolutionary scenario for Stauracanthus
The current distribution of the Stauracanthus genus (Fig. 1B) is thought to be largely conditioned by the Messinian Salinity Crisis (MSC; Late Miocene) and the opening of the Strait of Gibraltar in the Early Pliocene (Pardo et al., 2008). The genus may have appeared before the end of the Palaeogene (Pardo et al., 2008), so Stauracanthus could have been distributed along the Betic–Rif mountain belt formed during the African–Iberian collision (Early Miocene), as has been hypothesized for its sister genus Ulex (Cubas et al., 2005). Both the formation of the Alboran Sea by the Middle Miocene and the drier climate conditions during the MSC reduced and fragmented the geographical ranges of Stauracanthus species (probably at that moment S. boivinii and S. spectabilis–genistoides), although the former event reduced the gene flow while the latter also allowed the recolonization of new suitable areas.
According to Pardo et al. (2008), some S. spectabilis populations (or related ones) located in or near its current distribution area diversified into the more resilient S. genistoides, undergoing niche evolution processes, and enlarging its distribution area to more northern and southern locations. However, the observed patterns of niche and genetic differentiation do not exclude the possibility of the existence of an ancestral S. genistoides taxon already separated from S. spectabilis before the MSC. In fact, our analyses of the realized responses of Stauracanthus to climate point to this second hypothesis as the most likely, for it provides the more parsimonious process of niche evolution, given the ancestral character of S. genistoides. Although we agree that S. genistoides expansion after the Miocene may be related to its wider response to climate, including larger variations in daily temperatures, it is unlikely that these adaptations correspond to an enlargement of its niche compared with that of S. spectabilis. Rather, its considerable similarity to the niche of S. boivinii would point to both species retaining in large part the ancestral adaptations of the Stauracanthus lineage.
According to this scenario, we hypothesize that the populations that gave rise to the current S. spectabilis – genetically more basal according to Pardo et al. (2008) – underwent a process of adaptation to the climatic conditions of the fragmented landscape of the west Mediterranean during the MSC. These particular conditions have been less prevalent during the Pleistocene, so the distribution of S. spectabilis has been progressively reduced to a few pockets of fixed dunes under oceanic influence in the Iberian Peninsula and north-western Morocco. In contrast, the populations of S. genistoides show a wider response to climate. This could be partly explained by the greater genetic heterogeneity of the taxon (Fig. 3 in Pardo et al., 2008), so it could be formed by a complex of monophyletic but distantly related populations that show diverging responses to climate, and therefore a wider overall bioclimatic niche, close to that of S. boivinii. That said, the large overlap between the realized bioclimatic niches of S. genistoides and S. boivinii supports the ancestral character of their current response to climate, particularly because high degrees of niche overlap are not necessarily present among sister species (Warren et al., 2008). In any case, the expansion of the genistoides lineage in the Iberian Peninsula since MSC (Pardo et al., 2008) would have been facilitated by the increase in sandy coastal areas created during the lowering of sea level associated with Pleistocene glaciations (Zazo and Goy, 1989). These sea-level changes could explain the existence of isolated populations such as those located in Caminha, on the border between Portugal and Spain (Fig. 1B).
Conclusions
The evolution and current distribution of Stauracanthus species has been strongly conditioned by their adaptation to soil and climate conditions during the complex history of the western Mediterranean region during the Miocene and the Pleistocene. Therefore, the current distributions of the three species that can now be recognized are shaped by edaphic and climatic requirements, as well as by historical events and biogeographical constraints. Our results are consistent with other studies on the diversification of sister species (Anacker and Strauss, 2014) and taxa sharing a similar distribution and evolutionary history (for a review see Rodríguez-Sánchez et al., 2008). Further analyses of the evolutionary relationships between the Iberian populations of S. spectabilis and S. genistoides are required to ascertain whether the genetic heterogeneity of this latter species corresponds to a rapid expansion from a restricted number of populations, or to a series of relatively less connected populations that are occupying different parts of the bioclimatic niche space.
In sum, the approach of combining analyses of the distribution, realized environmental niche and phylogeographical relationships of parapatric species provides a deeper knowledge of Stauracanthus diversification. The kind of methodological approximation proposed here allows integration of the complex array of biogeographical, ecological and evolutionary processes that determine the evolution of species adaptations in a geographical and environmentally dynamic region through time. It also enhances our understanding of how these adaptations determine the current geographical distributions of species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: climatic, topographic and lithological variables initially tested. Table S2: validation of ENFA analyses obtained after a 5-fold cross-validation using only presence measures: Boyce index, the absolute validation index and the contrast validation index. SBO, S. boivinii; SGE, S. genistoides; SSP, S. spectabilis. Table S3: results of GLMs. Five-fold cross-validation for GLMs results summarized as mean ± s.d. scores. Figure S1: environmental response of Stauracanthus species in relation to the variables found significant by GLMs. Appendix S1: notes on the taxonomy of Stauracanthus Link.
Supplementary Material
ACKNOWLEDGEMENTS
We are particularly thankful to Juan Carlos Moreno, who pointed us towards the study of the distribution of Stauracanthus species. We are also grateful to Alex Fajardo, Miguel Berdugo and an anonymous referee for their useful comments on an earlier version of the manuscript. We also acknowledge FCT funding by “UID/Multi/04326/2013” for CCMAR. This work was partly funded by the Portuguese FCT project COMDUNES (EXPL/BIA-BIC/2311/2013). S.C. was supported by the FCT PhD grant SFRH/BD/65659/2009 and an FCT BI grant funded by the project COMDUNES, R.M.C. by the FCT postdoctoral fellowship SFRH/BPD/85040/2012 and R.B. by a contract of the Atracción de Talento Investigador Programme (Gobierno de Extremadura TA13032).
REFERENCES
- AEMET-IM. 2011. Iberian climate atlas. Madrid: AEMET-IM. [Google Scholar]
- Anacker BL, Strauss SY.. 2014. The geography and ecology of plant speciation: range overlap and niche divergence in sister species. Proceedings of the Royal Society of London B: Biological Sciences 281: 20132980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. 1992. Atlas do ambiente. Lisbon: Agência Portuguesa do Ambiente. [Google Scholar]
- Barbero M, Quezel P.. 1982. Classifying Mediterranean ecosystems in the Mediterranean rim countries and in southwestern U.S.A. General Technical Report PSW-58. Berkeley, USA: Pacific Southwest Forest and Range Experiment Station, USDA Forest Service. [Google Scholar]
- Barraclough Vogler. 2000. Detecting the geographical pattern of speciation from species-level phylogenies. American Naturalist 155: 419–434. [DOI] [PubMed] [Google Scholar]
- Boyce MS, Vernier PR, Nielson SE, Schmiegelow FKA.. 2002. Evaluating resource selection functions. Ecological Modelling 157: 281–300. [Google Scholar]
- Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H. 2004. Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832. [Google Scholar]
- Broennimann O, Fitzpatrick MC, Pearman PB, et al. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography 21: 481–497. [Google Scholar]
- Bull CM. 1991. Ecology of parapatric distributions. Annual Review of Ecology and Systematics 22: 9–36. [Google Scholar]
- Burns JH, Strauss SY.. 2011. More closely related species are more ecologically similar in an experimental test. Proceedings of the National Academy of Sciences of the USA 108: 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefaoui RM, Lobo JM.. 2008. Assessing the effects of pseudo-absences on predictive distribution model performance. Ecological Modelling 210: 478–486. [Google Scholar]
- Chefaoui RM, Hortal J, Lobo JM.. 2005. Potential distribution modelling, niche characterization and conservation status assessment using GIS tools: a case study of Iberian Copris species. Biological Conservation 122: 327–338. [Google Scholar]
- Colwell RK, Rangel TF.. 2009. Hutchinson’ s duality: the once and future niche. Proceedings of the National Academy of Sciences of the USA 106: 19651–19658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas P, Pardo C, Tahiri H.. 2005. Genetic variation and relationships among Ulex (Fabaceae) species in southern Spain and northern Morocco assessed by chloroplast microsatellite (cpSSR) markers. American Journal of Botany 92: 2031–2043. [DOI] [PubMed] [Google Scholar]
- Díaz TE, Rivas Martínez S, Fernández González F.. 1990. Stauracanthus Link (Leguminosae) en la Península Ibérica. Itinera Geobotanica 3: 131–135. [Google Scholar]
- Diniz-Filho JAF, Nabout JC, Bini LM, et al. 2009. Niche modelling and landscape genetics of Caryocar brasiliense (‘Pequi’ tree: Caryocaraceae) in Brazilian Cerrado: an integrative approach for evaluating central–peripheral population patterns. Tree Genetics & Genomes 5: 617–627. [Google Scholar]
- ESRI. 2011. ArcGIS Desktop: release 10. Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- Fielding AH, Bell JF.. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation 24: 38–49. [Google Scholar]
- Goslee SC, Urban DL.. 2007. The ecodist package for dissimilarity-based analysis of ecological data. Journal of Statistical Software 22: 1–19. [Google Scholar]
- Gouveia SF, Hortal J, Tejedo M, et al. 2014. Climatic niche at physiological and macroecological scales: the thermal tolerance-geographical range interface and niche dimensionality. Global Ecology and Biogeography 23: 446–456. [Google Scholar]
- Guinea E, Webb DA.. 1968. Stauracanthus Link In: Tutin TG, Heywood VH, Burges NA, et al. , eds. Flora Europaea, Vol. 2 Cambridge: Cambridge University Press, 103–104. [Google Scholar]
- Guisan A, Zimmermann NE.. 2000. Predictive habitat distribution models in ecology. Ecological Modelling 135: 147–186. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A.. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hirzel AH, Hausser J, Chessel D, Perrin N.. 2002a. Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology 83: 2027–2036. [Google Scholar]
- Hirzel AH, Hausser J, Perrin N.. 2002b. Biomapper 4.0 Lausanne: Laboratory for Conservation Biology. http://www.unil.ch/biomapper.
- Hirzel AH, Le Lay G, Helfer V, Randin C, Guisan A.. 2006. Evaluating the ability of habitat suitability models to predict species presences. Ecological Modelling 199: 142–152. [Google Scholar]
- Hortal J, De Marco P Jr, Santos AMC, Diniz-Filho JAF.. 2012. Integrating biogeographical processes and local community assembly. Journal of Biogeography 39: 627–628. [Google Scholar]
- Hutchinson GE. 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427. [Google Scholar]
- Hutchinson GE. 1978. An introduction to population ecology. New Haven: Yale University Press. [Google Scholar]
- IGME. 1994. Mapa geológico de España. Madrid: Instituto Geológico y Minero de España. [Google Scholar]
- Knowles LL. 2003. The burgeoning field of statistical phylogeography. Journal of Evolutionary Biology 17: 1–10. [DOI] [PubMed] [Google Scholar]
- Legendre P, Legendre L. 1998. Numerical ecology. Amsterdam: Elsevier Science BV. [Google Scholar]
- Lepage T, Bryant D, Philippe H, Lartillot N.. 2007. A general comparison of relaxed molecular clock models. Molecular Biology and Evolution 24: 2669–2680. [DOI] [PubMed] [Google Scholar]
- Lobo JM, Jiménez-Valverde A, Real R.. 2008. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography 17: 145–151. [Google Scholar]
- McCullagh P, Nelder JA. 1989. Generalized linear models, Second Edition. London: Chapman & Hall. [Google Scholar]
- MEM. 1985. Carte géologique du Maroc Èchelle 1:1000,000. Rabat: Ministére de l’Energie et des Mines, Direction de la Géologie.
- Oksanen J, Guillaume Blanchet F, Roeland Kindt, et al. 2013. vegan: Community Ecology Package. R package version 2.0-7. [Google Scholar]
- Paiva J, Coutinho AXP.. 1999. Stauracanthus Link In: Talavera S, Aedo C, Castroviejo S, et al. , eds. Flora Ibérica 7(II). Madrid: Real Jardín Botánico, CSIC, 240–245. [Google Scholar]
- Pardo C, Cubas P, Tahiri H.. 2008. Genetic variation and phylogeography of Stauracanthus (Fabaceae, Genisteae) from the Iberian Peninsula and northern Morocco assessed by chloroplast microsatellite (cpSSR) markers. American Journal of Botany 95: 98–109. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE.. 2006. GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse PE.. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics 28: 2537–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AT, Soberón J.. 2012. Species distribution modeling and ecological niche modeling: getting the concepts right. Natureza & Conservação 10: 102–107. [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Rangel TFLVB, Diniz-Filho JAF, Colwell RK.. 2007. Species richness and evolutionary niche dynamics: a spatial pattern-oriented simulation experiment. American Naturalist 170: 602–616. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Sánchez F, Pérez-Barrales R, Ojeda F, Vargas P, Arroyo J.. 2008. The Strait of Gibraltar as a melting pot for plant biodiversity. Quaternary Science Reviews 27: 2100–2117. [Google Scholar]
- Rundle HD, Nosil P.. 2005. Ecological speciation. Ecology Letters 8: 336–352. [Google Scholar]
- Schoener TW. 1970. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 51: 408–418. [Google Scholar]
- Silva DP, Vilela B, Buzatto BA, Moczek AP, Hortal J.. 2016. Contextualized niche shifts upon independent invasions by the dung beetle Onthophagus taurus. Biological Invasions 18: 3137–3148. [Google Scholar]
- Soberón J. 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters 10: 1115–1123. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD.. 2002. Modern applied statistics with S. New York: Springer. [Google Scholar]
- Wake DB, Hadly EA, Ackerly DD.. 2009. Biogeography, changing climates, and niche evolution. Proceedings of the National Academy of Sciences of the USA 106: 19631–19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DL, Glor RE, Turelli M.. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62: 2868–2883. [DOI] [PubMed] [Google Scholar]
- Yesson C, Culham A.. 2006. Phyloclimatic modeling: combining phylogenetics and bioclimatic modeling. Systematic Biology 55: 785–802. [DOI] [PubMed] [Google Scholar]
- Zazo C, Goy JL.. 1989. Sea-level changes in the Iberian Peninsula during the last 200,000 years In: Scott D, Pirazzoli P, Honing G, ed. Late Quaternary Sea-level correlation and applications. Dordrecht: Kluwer Academic, 27–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.