Abstract
Background and Aims Berberidopsis beckleri is one of three species of the family Berberidopsidaceae. The flower of Berberidopsis is unusual for core eudicots in being spiral with an undifferentiated perianth. In a previous study of the sister species B. corallina, it was suggested that Berberidopsidaceae represent a prototype for the origin of the bipartite perianth and pentamery in core eudicots.
Methods The floral development of B. beckleri was investigated with a scanning electron microscope and compared with previous studies on B. corallina and Aextoxicon punctatum of Berberidopsidales.
Key Results Flowers are inserted at the end of short shoots, which are not distinguishable from a pedicel. The initiation of perianth parts is highly predictable and spiral with a divergence angle of 137·5°, in a progression of a variable number of bracts to weakly differentiated sepaloid and petaloid tepals. The androecium most often consists 11 stamens arising in a rapid sequence. Compared with B. corallina, the number of perianth parts and stamens is more variable and there is no evidence of an alternation of shorter and longer plastochrons leading to a whorled arrangement. However, the gynoecium is generally pentamerous and arises from five primordia. The carpels are laterally connected into massive intercarpellary ridges on which ovules are initiated.
Conclusions The position of Streptothamnus within Berberidopsidaceae is questioned. It is demonstrated that the floral development of Berberidopsis beckleri lies within a gradient from spiral flowers without perianth differentiation leading to flowers with differentiated sepals and petals. The arrangement of flowers in compact inflorescences in B. corallina and Aextoxicon leads to a more stabilized arrangement of organs in whorls. The inherent variability of the flower of Berberidopsis is well correlated with the limited canalization of flowers in taxa at the base of the core eudicots and could act as a prototype for the current eudicot floral Bauplan.
Keywords: Aextoxicon, Berberidopsis beckleri, B. corallina, Berberidopsidales, core eudicots, floral development, perianth evolution, pentamery, Streptothamnus
INTRODUCTION
The family Berberidopsidaceae belongs with the monotypic Aextoxicaceae to the order Berberidopsidales. The position of both families has been shifting for a long time (see the discussion in Ronse De Craene, 2004), but recent molecular evidence puts them in the same order close to the divergence of asterids, Caryophyllales and Santalales within core eudicots (Wang et al., 2009; Moore et al., 2010; Soltis et al., 2011; Angiosperm Phylogeny Group IV, 2016). However, the precise placement of Berberidopsidales remains problematic, with suggestions that Santalales is more basal in Superasteridae, although support is weak (Moore et al., 2010; Soltis et al., 2011; Maia et al., 2014). Berberidopsis beckleri (F. Muell.) Veldkamp is one of two species of the genus Berberidopsis. Berberidopsis corallina Hook. f. is found in Patagonian Chile and Argentina and is disjunct from B. beckleri, which occurs in Queensland and New South Wales, Australia. Together with the Australian monotypic genus Streptothamnus, Berberidopsis makes up the family Berberidopsidaceae (van Heel, 1984; Veldkamp, 1984; Kubitzki, 2007).
Berberidopsis beckleri was originally described by von Mueller (1862) as Streptothamnus beckleri F. Muell., with another species, Streptothamnus moorei F. Muell. However, Veldkamp (1984) moved the species to Berberidopsis on the basis of strong similarities in seed anatomy, pollen and wood, which are clearly different in Streptothamnus moorei (Baas, 1984; van Heel, 1984).
Ronse De Craene (2004) investigated the floral development of Berberidopsis corallina and suggested that Berberidopsis occupies a crucial position in the core eudicots regarding the origin of pentamery and the evolution of a bipartite perianth. Flowers of Berberidopsis have a spiral phyllotaxis without clear distinction between bracts, sepals and petals. Ronse De Craene (2004) suggested that the pentamerous flowers common to core eudicots have been derived from progenitors with flowers similar to those of Berberidopsis by an alternation of areas of shorter and longer plastochrons, leading to an arrangement of the perianth in alternating pentamerous whorls. This led to a consequent differentiation of the perianth into a calyx and corolla. Ronse De Craene (2007, 2008) also used the evidence of Berberidopsis to infer that the petals originated from an undifferentiated perianth in the majority of core eudicots and that they are homologous with the sepals. The sister group of the remaining core eudicots is Gunnerales, which are characterized by small dimerous flowers with a strong tendency for reduction linked to wind pollination (Wanntorp and Ronse De Craene, 2005; Ronse De Craene and Wanntorp, 2006; González and Bello, 2009; Ronse De Craene and Brockington, 2013; Ronse De Craene, 2016). Wanntorp and Ronse De Craene (2005) suggested that Gunnerales represent an evolutionary dead end as it is structurally difficult to derive the complex pentamerous flowers common to core eudicots from the strongly reduced flowers of Gunnera. However, Endress and Doyle (Endress and Doyle, 2009; Doyle and Endress, 2011; Doyle, 2013) argued that dimerous flowers could be ancestral to the pentamerous core eudicot flowers, because core eudicots are firmly nested within the dimerous grade. However, they did not discuss the physical transition from dimery to pentamery.
The derivation of pentamerous flowers from Berberidopsis-like ancestors is not parsimonious, especially because the family is nested in Superasteridae at the base of asterids and caryophyllids, possibly following the divergence of Santalales (Moore et al., 2010; Maia et al., 2014). This would imply that the ancestral floral Bauplan has been maintained within Berberidopsidaceae, while most other groups of eudicots became pentamerous and whorled. Kubitzki (2007) also pointed out that the family contains several derived characteristics, such as a parietal placentation, and the differentiation of a calyx and corolla correlated with polyandry in Streptothamnus. However, the floral Bauplan of Berberidopsis is unusual among the more derived core eudicots, and can be considered as a model for the evolution and establishment of a pentamerous merism, without inferring that it is ancestral, as the characters found in the family constitute a mixed bag of ancestral and derived features (see Ronse De Craene, 2004, 2010). In addition to Berberidopsidaceae, the monotypic family Aextoxicaceae belongs to Berberidopsidales. Ronse De Craene and Stuppy (2010) studied the floral development of Aextoxicon punctatum, and although flowers are pentamerous, they showed a similar lack of distinction between sepals and petals, at least in early developmental stages. They also considered Aextoxicon as a derived genus, because of the accumulation of several derived characters, such as a single carpel, the formation of calyptrate bracteoles, unisexual flowers, and the differentiation of the perianth into a calyx and corolla. The case of Aextoxicon demonstrates the possibility of an evolutionary transition of a spiral flower to a whorled pentamerous flower.
The availability of a second species of Berberidopsis provided the opportunity for a comparative study of the floral development of both species. This has the advantage that it enables a polarization of characters between ancestral and derived character states, in order to understand their evolution within the genus and with other genera of the Berberidopsidales.
MATERIALS AND METHODS
Flower buds and mature flowers were collected from potted plants at the Royal Botanic Garden Edinburgh (voucher number 19980223) grown from seed collected in Queensland (Australia). Material was fixed in FAA (formaldehyde 5 %, glacial acetic acid 5 %, 70 % ethanol 95 %) and transferred to 70 % ethanol. Buds were dissected under a Wild MZ8 dissecting microscope, critical-point-dried with CO2 and sputter-coated with platinum, before observations with a Cambridge Leo Supra scanning electron microscope (SEM). About ten mature buds were examined and compared with Berberidopsis corallina to understand the number of floral parts.
RESULTS
Organography
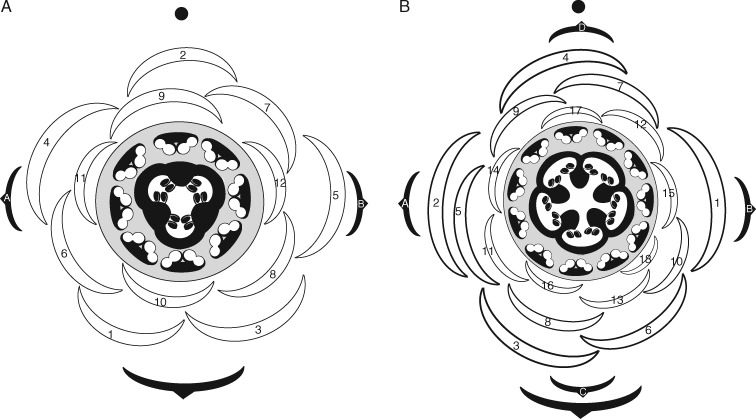
Berberidopsis beckleri is a vine growing along the margins of the rainforest. Each year new shoots are produced that develop flowers in the axils of leaves before turning vegetative and producing twining stems (Fig. 1A). Flowers appear solitary and pendent on long pedicels in the axil of an ovate leaf (Fig. 1A, B). The perianth is weakly differentiated. Bracts and tepals are carmine red, and the transition between outer and inner tepals is gradual, with a progressive loss of the red pigmentation towards the inner perianth parts (Fig. 1B, D). Flowers exhibit a spirally arranged perianth, with imbricately arranged parts increasing in size from bracts to inner tepals (Fig. 1D). The inner petaloid tepals exceed the outer tepals in size and form an urceolate structure with a narrowing mouth enclosing the sexual organs (Fig. 1B). The number of perianth parts including bracts fluctuates between 13 and 16; five can be described as petals, although they are morphologically similar to the intermediate sepals, except for their truncate apex. In all perianth parts six to ten vascular bundles are present, branching further in the upper half. The stamens are arranged in a single series with short filaments, introrse anthers and a prominent connective appendage (Fig. 1D). Stamen number fluctuates between (8–)11(–13). Stamens are surrounded by a crenelated disc nectary. The ovary is elliptical, with a massive style and green stigma lobes, reflecting the number of carpels, which is mostly five, rarely four or six. After anthesis the perianth and stamens drop off, leaving the ovary surrounded by the broad extrastaminal disc nectary and scars of fallen tissue (Fig. 1C). Fruits develop into berries with a persistent style and stigma.
Fig. 1.
Mature flowers of Berberidopsis beckleri (A–D) and comparison with Berberidopsis corallina (E). (A) Young flowering shoot bearing five flowers. (B) Detail of flower at anthesis. (C) Detail of young fruit. (D, E) Dissected flower with perianth parts spread out starting from the top left. (D) B. beckleri; (E) B. corallina.
Organogeny
Early flower development was investigated. The vegetative apex produces leaf initials that develop in broadly triangular structures bearing trichomes in the upper part (Fig. 2A). Flowers are produced acropetally on new shoots and the number of flowers produced on a stem is variable (mostly about five flowers) as the main axis resumes vegetative growth (Fig. 1A).
Fig. 2.
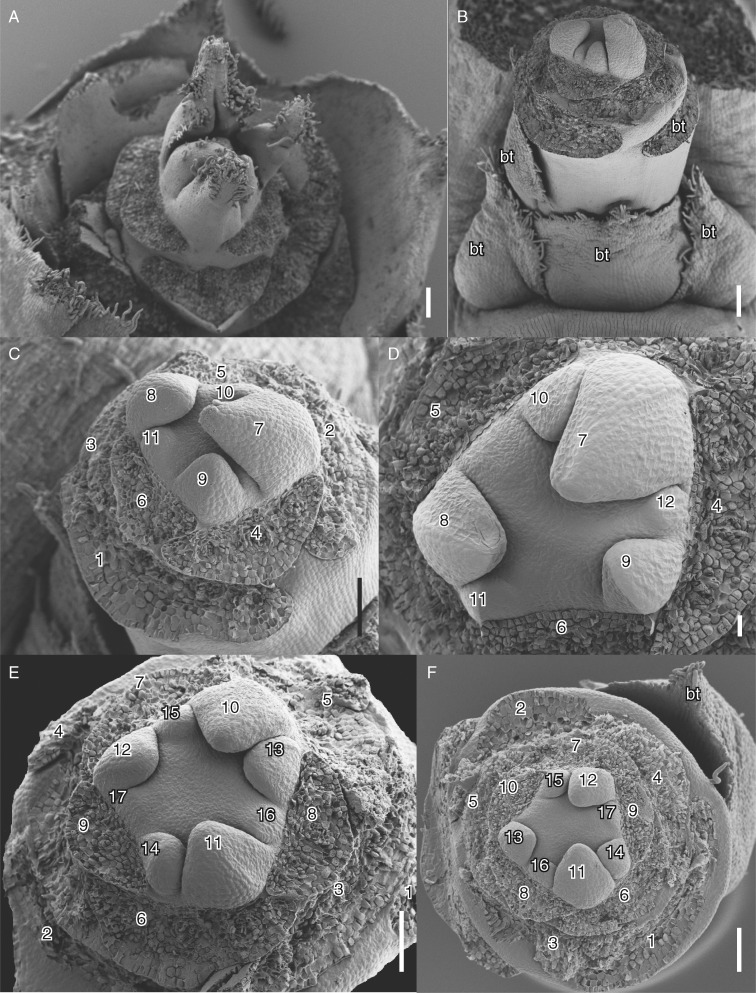
Vegetative and early floral development of Berberidopsis beckleri. (A) Vegetative shoot with spiral initiation of leaves. (B) Flower developing at the top of an expanding shoot. (C, D) Highly similar flowers at initiation of the outer tepals. (E) Older flower with initiation of inner (petaloid) tepals. (F) Slightly older flower with initiation of inner tepals complete. Numbers indicate the order of initiation of tepals and bracts; upper five tepals are shown with white numbers. bt, lower bracts of the shoot. Scale bars = 100 µm.
The flower-bearing shoots develop in the axil of a leaf and produce two lateral bracts followed by two median bracts (Fig. 2B). The shoot attains considerable length before the flower starts differentiating distally. The median adaxial bract remains at the level of the lateral bracts, while the median abaxial bract may be situated midway on the stem, together with a variable number of additional bracts (Fig. 2B). There is no clear distinction between stem and pedicel as there is a rapid sequence of spirally initiated phyllomes surrounding the periphery of the flower. Two lateral phyllomes inserted at different levels always precede the initiation of perianth parts (Fig. 2B, C). There is a conspicuous inflated zone of tissue immediately below these phyllomes, which allows the identification of the base of the flower (Fig. 2B, E, F). The position of the lower two phyllomes is clearly paired and these organs could be described as upper bracts as they remain smaller in the mature flower. The next phyllome (3 in Fig. 2C, E) is displaced between the two upper bracts and the following perianth parts undergo a change in phyllotaxis, with a divergence angle of 137·5° (starting from 4 in Figs 2C–F and 3B). The number of upper bracts plus tepals varies between 16 and 18, but sepaloid tepals are more or less seven in number, and petaloid tepals five (Fig. 1D). Growth of perianth parts is rapid and the outer organs rapidly cover the central area of the flower. Young perianth organs are triangular, with mainly basiplastic growth. While initially larger the bracteoles cease growth as the flower expands, they remain visible as small appendages below the flower (Figs 1D and 5A). The upper perianth parts appear much more crowded and the initiation of successive organs leads to an asymmetrical shape of the floral apex, which retains a flattened convex shape (Figs 2C–F and 3A). The diameter of the floral apex rapidly reaches its final size between 240 and 280 µm prior to the initiation of the androecium.
Fig. 3.
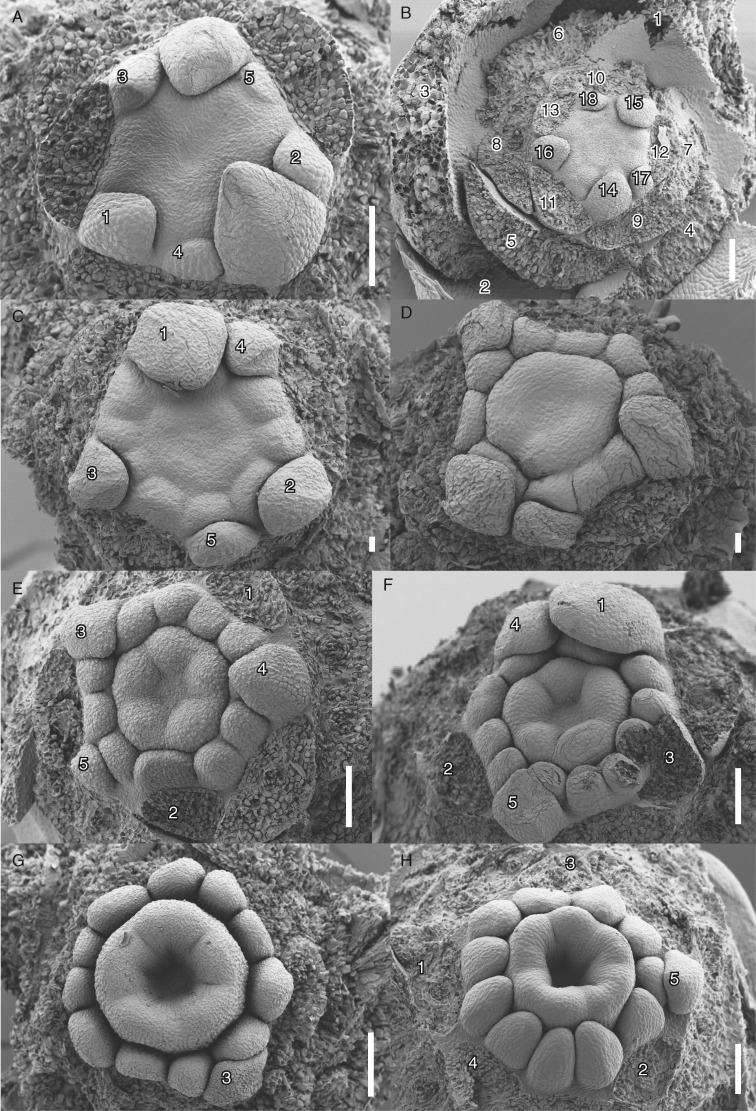
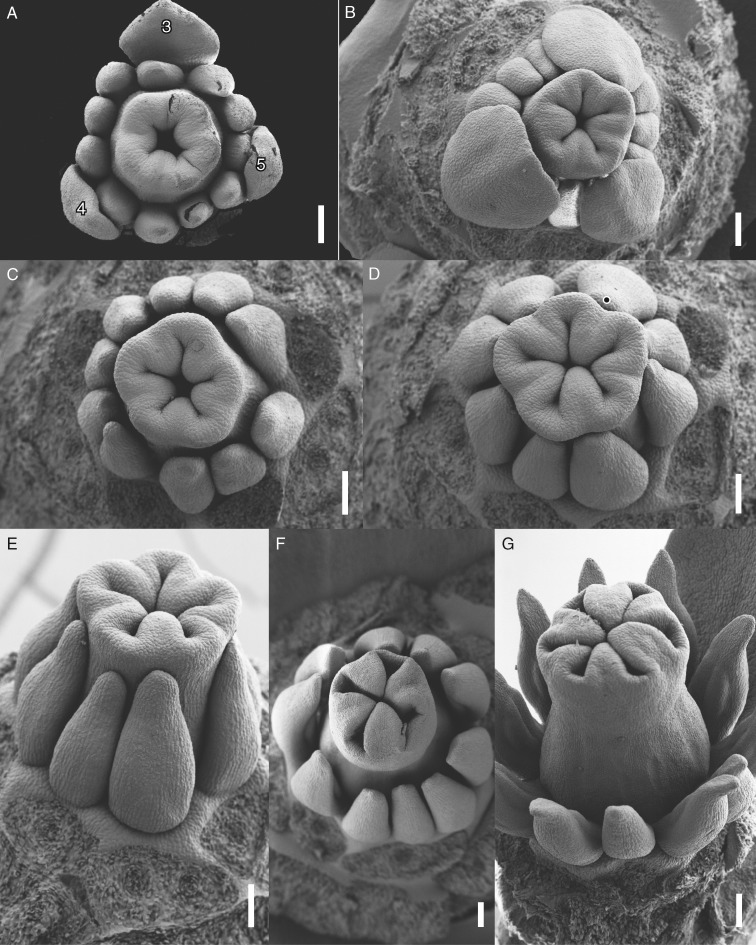
Floral development of Berberidopsis beckleri after stamen and carpel initiation. (A) Appearance of peripheral stamen protuberances and development of a central depression. (B) Similar stage with 18 organs, showing all bracts and tepals. (C) Differentiation of stamens at the floral periphery and appearance of carpels. (D) Upward growth of gynoecium and development of stamens. (E, F) Differentiation of five hemispherical carpels. (G) Apical view showing the development of a central gynoecial cavity. (H) Similar lateral view with progressive differentiation of the anthers. Numbers indicate order of initiation of tepals and bracts; the upper five petaloid tepals are numbered separately, except in (B), where they are shown as a full sequence of all tepals. Scale bars = 100 µm, except (C, D) = 20 µm.
Fig. 5.
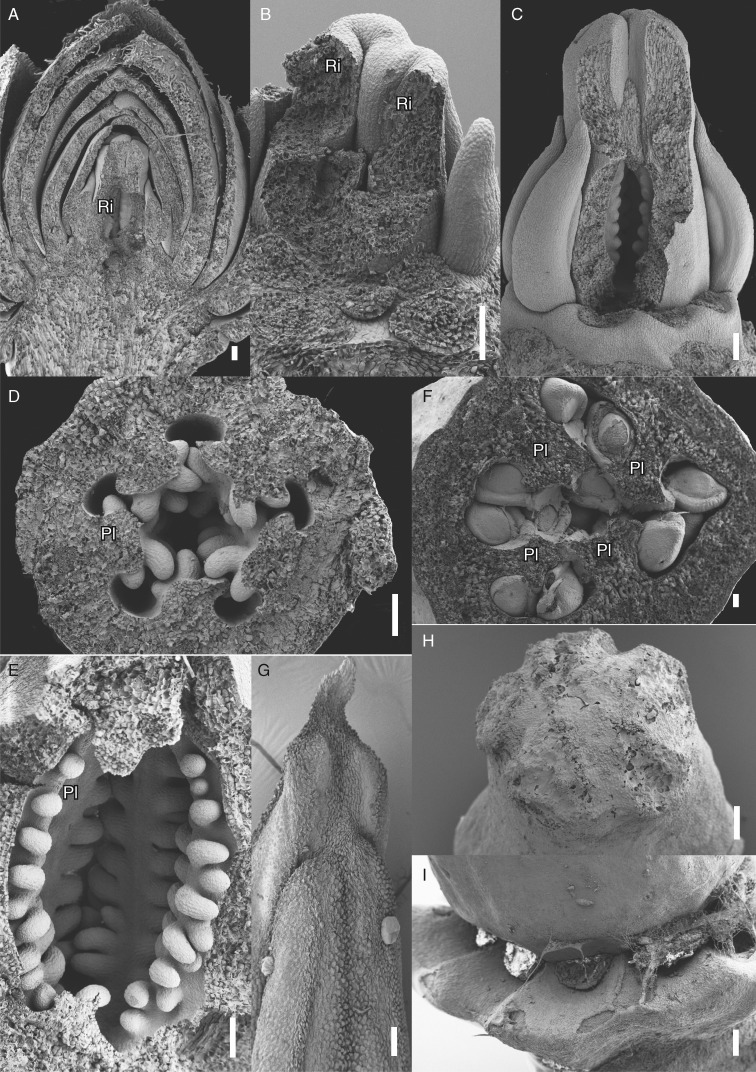
Late floral and gynoecial differentiation of Berberidopsis beckleri. (A) Longitudinal section of young flower. Note the progressive change in size of bracts and tepals. (B) Partially sectioned flower showing the massive protruding intercarpellary ridges in the ovary. (C) Flower with partly dissected ovary showing early ovule differentiation on the intercarpellary protrusions. (D) Transverse section of young ovary showing five parietal placental ridges with two series of ovules each. (E) Longitudinal section of ovary showing three of the five placentas with ovules. (F) Transverse section of nearly mature ovary with four placental ridges and anatropous ovules. (G) Adaxial view of the upper part of immature anther with long apical connective. (H) View of five-lobed stigma at anthesis completely covered with exudate. (I) Lateral view of lobed, extrastaminal disc nectary; stamens removed. Ri, intercarpellary protuberance; Pl, placenta. Scale bars = 100 µm, except (G–I) = 200 µm.
The position of the five upper perianth parts is highly predictable (numbered 1–5 in Fig. 3C, E, F, H). At the time the last perianth part has been initiated the floral apex takes a flattened shape. Stamens are initiated in a very rapid sequence on the periphery of the apex, with stamens slightly displaced relative to the inner perianth parts, forming indistinct parastichies (Fig. 3A–C). Young stamens have different sizes and shapes and it was difficult to discern a clear sequence of initiation, although their position corresponds to the expected phyllotactic pattern (Fig. 3C–F). The number of stamens fluctuates between eight (Fig. 4D; with an indication of a larger stamen), ten (Figs 3D and 4C, F) and 12, while 11 stamens is the most common number (Figs 3E, F and 4A). Stamens grow upwards as elliptical–triangular structures (Fig. 4C–G); the apical part becomes slender and differentiates as a connective appendage , while anther tissue becomes visible as a longitudinal abaxial furrow separating two thecae (Fig. 5C, G). At this stage receptacular tissue expands between the stamens and tepals and will eventually form the nectary (Figs 4E, F and 5C). Filaments are short and develop very late in development (Fig. 5C). The three inner tepals remain smaller than the other tepals for some time and give a triangular impression to the flower while the gynoecium expands (Fig. 4A, B).
Fig. 4.
Floral development of Berberidopsis beckleri at stamen and carpel differentiation. (A) Initiation of intercarpellary protuberances closing the gynoecial cavity. (B) Apical view with expansion of the ovary above the three inner tepals and further development of the intercarpellary ridges. (C) Similar stage with the development of a stigmatic plate above the developing stamens. (D, E) Apical and lateral view of the closure of the cavity by the ridges. Flower with eight stamens and possibly a double stamen (black dot in D). (F) Apical view of flower with tetramerous gynoecium. (G) Lateral view of the development of the style and differentiation of thecal tissue. Note the sixth smaller intercarpellary protuberance. Scale bars = 100 µm.
Contrary to the variable androecium, the number of carpels is generally five, rarely four (Figs 4F and 5F) or six (Fig. 4G). The gynoecium emerges rapidly around a central depression at the time of androecium initiation (Fig. 3B, C). Five hemispherical carpels appear in a rapid sequence (Fig. 3B–E) with three being generally larger, at the time of stamen growth. The five carpels expand by basal zonal growth (Fig. 3F–H). Upward growth of the basal part of the gynoecium leads to the development of a globular elongated ovary (Figs 4E–G and 5A–C). In alternation with the original lobes, five protuberances grow inwards beyond the initial contact zones of the individual carpels and develop into short intercarpellary ridges (Fig. 4B–D). These protuberances as well as the original lobes are visible on top of the gynoecium and will eventually form the stigmatic zone, while the lower part of the ovary expands upwards. The intercarpellary ridges do not fuse and leave a five-armed narrow slit in the middle of the ovary (Fig. 4B–G). In the case of a tetramerous ovary, the slit takes the shape of a Maltese cross (Fig. 4F). Ridges and slit are unequal because of the difference in size of the carpels. The original carpel lobes initially expand slightly above the gynoecium, but cease further growth as the intercarpellary ridges extend in size and fuse postgenitally by abundant secretion (Fig. 5C, H). Ovules arise within the ovary on the intercarpellary ridges in two irregular rows (Fig. 5A–E). Placentation appears as parietal (Fig. 5D), but the inner locular space becomes progressively compressed by the irregular inward growth of the intercarpellary ridges (Fig. 5F). Ovules develop laterally and bend horizontally towards the ovary wall before curving completely to become anatropous and bitegmic (Fig. 5F).
At maturity the nectary expands as a crenelated ring between the inner tepals and stamens (Fig. 5I).
DISCUSSION
Circumscription of Berberidopsidaceae
Despite the fact that the Berberidopsidales contain only four species in three genera, the high morphological divergence and disjunct distribution reflect an ancient, relictual nature of the group. It remains questionable whether Streptothamnus moorei belongs to Berberidopsidaceae. Carlquist (2003) did not include the species in his wood anatomical comparison with Aextoxicaceae. The seed anatomical evidence presented by van Heel (1984) is not conclusive, and the author admits that more material needs to be studied. Baas (1984) mentioned the cyclocytic and bicyclic stomata as common characters in the family, although most shared characters are of a more generalized nature. Additionally, pollen grains of Streptothamnus differ from those of Berberidopsis in being small with a perforated tectum, and the ovary is tetramerous (Keating, 1975; Baas, 1984; van Heel, 1984). Keating reported that the pollen of Streptothamnus is indistinguishable from that of Erythrospermum (Achariaceae). The studies of van Heel (1984) and Baas (1984) were centred on finding differences with Flacourtiaceae, a family that has since then been split into Salicaceae and Achariaceae by molecular evidence (Angiosperm Phylogeny Group IV, 2016), and which remains anatomically understudied. Descriptions in local floras (e.g. Sleumer, 1963; Jessup, 1982; Stanley and Ross, 1986; Harden, 1990) and a single illustration of flowers (a photograph taken by Hugh Nicholson, obtainable from www.rainforestpublishing.com.au) show important differences from the other Berberidopsidales, such as pentamery with a differentiated perianth, a persistent calyx and deciduous, reflexed corolla, many stamens described as having long filaments and apiculate anthers, and absence of an extrastaminal disc nectary. The ovary and placentation is discussed nowhere. Generic descriptions in older floras (including B. beckleri) appear to be based on a superficial description of S. moorei and are identical in content (Sleumer, 1963; Jessup, 1982; Stanley and Ross, 1986). The real affinities of the genus might be in Malpighiales. This possibility can only be answered through a molecular study of the genus, which is currently unavailable.
Comparison of Berberidopsis beckleri with Berberidopsis corallina
Berberidopsis beckleri resembles B. corallina to a great extent (Figs 1D, E and 6A, B). Both species are vines with flowers arising at the end of lateral shoots developing on a main axis. The number of flowers is higher in B. corallina and they appear to be grouped in terminal racemes because of shorter internodes. In B. beckleri flowers are fewer with longer internodes, and the main stem reverts to a vegetative state after producing a few flowers (occasionally in B. corallina). One could argue that flowers are solitary in the axil of a leaf or bract in both cases, which demonstrates the difficulty of describing inflorescences because of a shift between vegetative systems and flower systems (see Classen-Bockhoff and Bull-Hereñu, 2013). Pedicels in B. beckleri can be interpreted as the terminal extension of short branches. There are always two pairs of decussate bracts at the base of the branch and a variable number of bracts higher up the stem (Fig. 2B, 6B). Two small bracts are always present below the flower at a level where the axis is swollen, and this might correspond to a stunted pedicel (Fig. 2B, C, F).
Fig. 6.
Floral diagram of Berberidopsis corallina (A) with floral formula B1 Bt2 ★PC12 A8 psV1:1 G(3) lo1 styA1stiCA3 ovP∞ and of Berberidopsis beckleri (B) with floral formula B1 Bt2 + 2 ★PC17 A11 psV1:1 G(5) lo1 styA1stiCA5 ovP∞, based on Ronse De Craene et al. (2014).
The mature floral parts of B. beckleri were separated and laid out, and compared with those of B. corallina (Fig. 1D, E). Bracts cannot be distinguished from tepals and they become progressively larger at the base of the flower in both species (Figs 1D, E and 6A, B). Harden (1990) reports 15–18 spirally inserted perianth parts, corresponding to the findings of this study. While in B. corallina outer sepals and upper bracts may be persistent, they are always deciduous in B. beckleri (Fig. 1C). Berberidopsis corallina has a similar but more constant arrangement of perianth parts (fluctuating between 13 and 14, including smaller outer bracts), but the distinction between outer sepaloid tepals and inner petaloid tepals is less clear compared with B. beckleri (Fig. 1E). In B. corallina all perianth parts and bracts are bright red, while in B. beckleri there is a progressive change towards the inner tepals. In both species the inner tepals are longer and better developed than the intermediate and outer tepals (Fig. 1B, D, E).
The inner parts of the flower are similar, as both species possess an extrastaminal lobed disc. The stamens are arranged in a single series with comparable short filaments, introrse anthers and a prominent connective. However, stamen number in B. beckleri [fluctuating between (8–)10–11(–13)] is more variable than B. corallina, where eight stamens are the norm. Australian regional floras (e.g. Sleumer, 1963; Jessup, 1982; Stanley and Ross, 1986; Harden, 2000) unanimously report the stamens to be 12 or 13 and it is not clear whether this is based on genuine observations. In both species the ovary is globular with a tapering style. The ovary and style are more slender in B. beckleri (Fig. 1C, D). The stigma lobes reflect the number of carpels, which are constantly three in B. corallina and (4–)5(–6) in B. beckleri.
The plastochrons of bracts and tepals appear to be equal in B. beckleri, while in B. corallina the arrangement of tepals shows a tendency to a quincuncial imbricate (2/5) pattern by an interruption in the sequence of equal plastochrons. However, the divergence angles appear to be highly similar between the two species, and some stages of development are almost similar. A possible reason for this difference in plastochron is that in B. corallina flowers arise on much shorter branches and they develop close to the main axis; this strongly influences the position and initiation sequence of bracts and sepals, especially in early stages of development. In B. beckleri the development of a strong main shoot below the pedicel removes the physical constraint of main axis and subtending bracts and this maintains the equal plastochron and a more constant differentiation of organs along the axis, reducing the eventuality probability for organs to be arranged in pentamerous whorls. The androecium and gynoecium show a very rapid initiation in both species, with a variable number of stamens due to stamens arising in double positions. Ovary development shows strong similarities in the two species, although the number of ovules is higher in B. beckleri. The ovary develops strong inward-growing intercarpellary protuberances and the style is formed by extension of the apical parts of carpels and ridges.
Comparison of Berberidopsis with Aextoxicon
Flowers of Aextoxicon punctatum appear morphologically highly different from those of Berberidopsis, although they share a number of features (Ronse De Craene and Stuppy, 2010). In all species flowers are preceded by two transverse upper bracts, although more bracts are variably present in Berberidopsis. While in Berberidopsis the upper bracts are soon arrested in their growth and remain small at maturity, they are persistent and surround the flower as a calyptra in Aextoxicon. Floral organ appearance is also sequential and helical, although it often appears unidirectional in Aextoxicon, with occasional variation in the number of tepals, stamens and carpels. Aextoxicon is mostly unicarpellate, but the orientation of the single carpel depends on the number and initiation sequence of preceding organs. Paired stamens are a frequent occurrence in all species, and are more frequent in Berberidopsis (Ronse De Craene, 2004; B. corallina), but as in both species of Berberidopsis they are unlikely the result of dédoublement in Aextoxicon, as they may differ in size (Ronse De Craene and Stuppy, 2010). A massive nectary occurs in the three species, but in Aextoxicon interstaminal sickle-shaped scales are formed, while in Berberidopsis an extrastaminal crenelated disc is formed on the receptacle. All three species of Berberidopsidales possess anatropous ovules with two integuments. In Aextoxicon there is a single carpel with two marginal ovules, but in Berberidopsis the ovary develops strong intercarpellary ridges with two rows of ovules. The absence of clearly developed septa could indicate that the parietal placentation is the result of a concrescence of ancestrally free carpels and is not derived from an ancestral axile placentation (see also Fig. 7). The presence of five globular carpel primordia clearly resemble originally free carpels that become postgenitally connected. Interestingly, van Heel (1977) mentioned that the ovules of B. corallina arise directly on the carpel wall and not on a placenta, emphasizing the difference from a more common parietal placentation. He also reports that the stele is reconstructed after separation of the pistil traces and that a floral axis protrudes for a very short distance into the base of the locule. There is no evidence of pseudomonomery in Aextoxicon or a suggestion that the single carpel has been derived from a syncarpous gynoecium (Ronse De Craene and Stuppy, 2010).
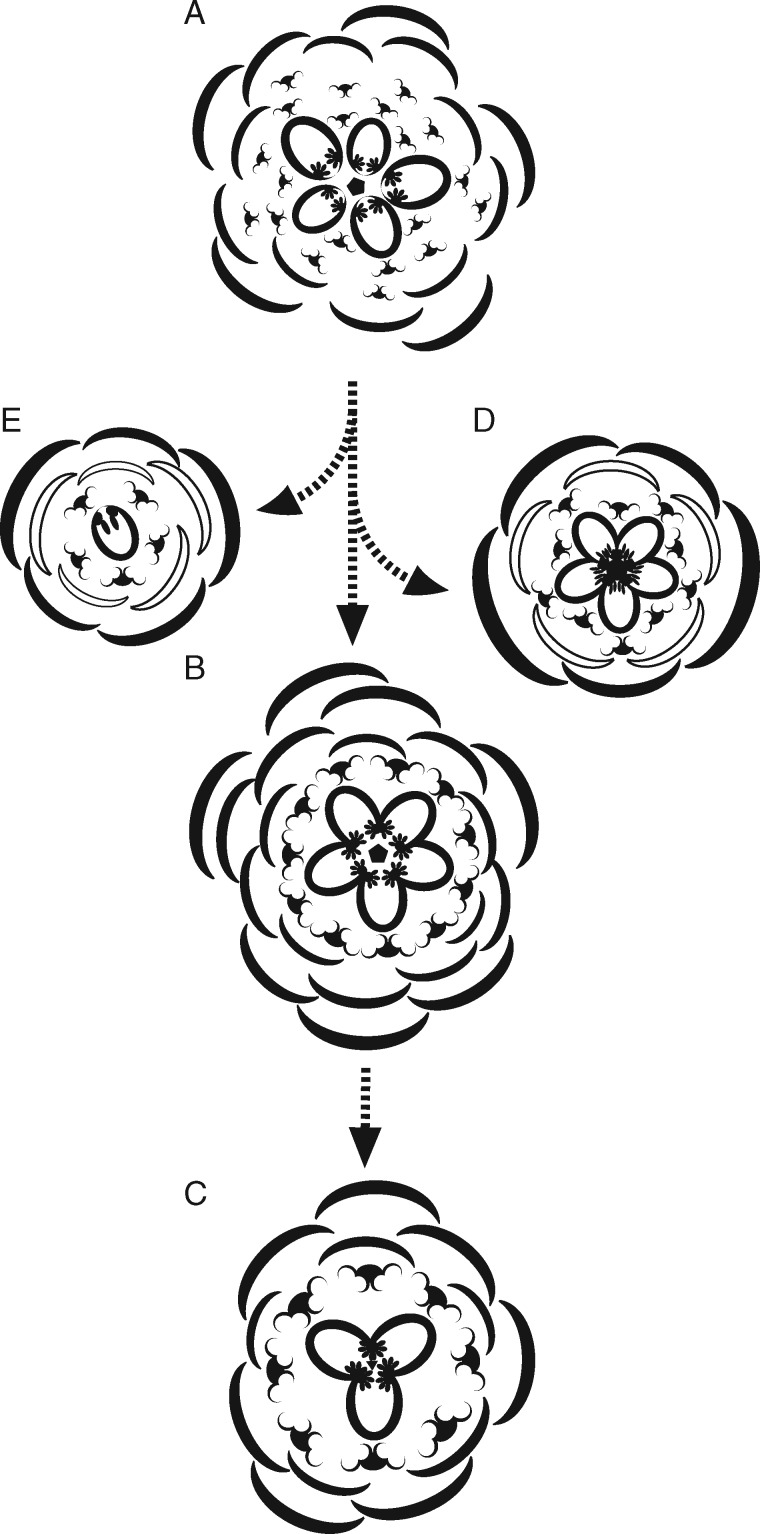
Fig. 7.
Diagrams showing putative floral evolution of Berberidopsidales and early core eudicots from a putative ancestor (A); (B) Berberidopsis beckleri; (C) Berberidopsis corallina; (D) basic core eudicot flower; (E) Aextoxicon punctatum.
Character evolution in Berberidopsidales
Comparing B. beckleri with B. corallina reveals a number of differences that can be polarized to reflect floral evolution. Berberidopsis corallina appears more stable than B. beckleri in the incipient whorled arrangement of floral parts, the lower number of bracts and more condensed arrangement of flowers, the greater regularity in organ number, and the lower carpel number. In B. beckleri organ number is less constant, without indication of a whorled arrangement and with a continuous plastochron. On the other hand, the differentiation of the inner petaloid tepals appears more pronounced in B. beckleri, in both coloration and morphology. However, this may be linked to a different adaptation to pollination, but nothing is known about the pollination strategy of the two species. Both species represent unique floral morphologies within the core eudicots, with a probably far-reaching ancestry (Fig. 7). A transition from B. beckleri to B. corallina and in parallel to A. punctatum can be visualized as an increased regularity linked with a stabilization of a lower number of floral organs and a clearer differentiation of perianth parts. The lack of more immediate relatives of Berberidopsis makes it difficult to use the morphological information in a clear phylogenetic context. It is likely that Berberidopsidales represent an offshoot from an ancestor with an arrangement of organs in a tight spiral, with B. beckleri morphologically closer to the ancestral state than B. corallina. Typical core eudicot flowers are probably derived in a similar way by stabilization of a pentamerous whorled pattern (Fig. 7).
Perianth differentiation and petal evolution in core eudicots
Most core eudicots have a well differentiated perianth of sepals and petals (Ronse De Craene, 2008; Ronse De Craene and Brockington, 2013). However, in a few clades, including Berberidopsidaceae, the perianth is weakly or not differentiated, with a gradual spiral transition between bracts, sepals and petals that are morphologically difficult to distinguish. This condition is more widespread in early-diverging eudicots, such as Buxaceae and Trochodendraceae (Von Balthazar and Endress, 2002), or Gunneraceae, sister to other core eudicots (Wanntorp and Ronse De Craene, 2005). In general the number of perianth parts is rarely fixed. Flowers with several weakly differentiated perianth parts are generally large and associated with numerous stamens and carpels (e.g. Achariaceae, Actinidiaceae, Cactaceae, Clusiaceae, Dilleniaceae, Paeoniaceae, Theaceae). Ronse De Craene (2007, 2008, 2016) hypothesized that a secondary increase in stamens and possibly carpels disrupted the whorled arrangement of organs, leading to a break-up of the strict genetic boundaries between perianth parts, and a diffusion and mixing-up of different organ identity genes in the perianth. This would indicate that these massive flowers with a variable perianth are secondarily complex. Taking the phylogenetic position of Berberidopsidales into account (see Introduction), the possibility of a similar reversal to a spiral flower in Berberidopsis cannot be excluded, but flowers are smaller with fewer parts and there is no correlation with a secondary stamen and carpel increase. Moreover, a comparison between the two species of Berberidopsis and Aextoxicon points to a clear unidirectional evolution from a spiral flower to a whorled pentamerous flower (see above).
In most core eudicot flowers there is a transition from a decussate arrangement of vegetative parts or bracteoles to a spiral in the calyx and further to whorls in the rest of the flower (Endress, 1987; Ronse De Craene, 2010). However, the transition of the decussate phyllotaxis to a spiral phyllotaxis occurs at the level of the bracts in Berberidopsidales, contrary to most core eudicots, where it happens at the level of the two bracteoles. The pattern of phyllotactic transition of 180° to 137·5°, as well as the progressive increase in the size of perianth parts, is reminiscent of basal angiosperms (see also Endress, 2008; Endress and Doyle, 2007; e.g. Amborellaceae: Buzgo et al., 2004; Austrobaileyaceae: Endress, 1980b, 2008; Calycanthaceae: Staedler et al., 2007; Monimiaceae: Endress, 1980a; Staedler and Endress, 2009; Schisandraceae: Dong et al., 2012; Trimeniaceae: Endress and Sampson, 1983).
Ronse De Craene (2004) suggested that B. corallina represents a prototype for the origin of the typical core eudicot pentamerous flowers with a well differentiated perianth. The arguments presented are the quincuncial arrangement of perianth parts (2/5 initiation sequence) in the flower of B. corallina with evidence of an alternation of whorls of five. Other core eudicots often share a spiral initiation sequence of petals, although the plastochron for the petals is much shorter than in Berberidopsis. Berberidopsis beckleri resembles B. corallina, although a 2/5 sequence is not visible, and there is no evidence of a whorled arrangement. The high-level phylogenetic position of Berberidopsidales (at the base of asterids, caryophyllids and Santalales) and the advanced characters of some parts of the flower (e.g. a syncarpous ovary with apparently parietal placentation, extrastaminal disc nectary) could be used as arguments against this interpretation. However, there is no indication that the homogenous perianth morphology of Berberidopsis is derived by the loss of strict boundaries between floral organs. Genetic studies of the expression of ABCE genes might clarify this. The development of a pentamerous syncarpous ovary, as in Berberidopsis beckleri, might be a first step in a progressive arrangement of organs in pentamerous whorls, extending to other organs in a centrifugal direction. This would be different from the centripetal transition of pentamery as found in Ranunculaceae and Sabiaceae. In the early diverging eudicots, there is evidence for derivation of pentamery from trimerous whorled ancestors through a reduction of organs in each whorl or from dimerous ancestors through the increase in the diameter of the flower meristem (see discussion in Ronse De Craene et al., 2003, 2015; Wanntorp and Ronse De Craene, 2007). It is tempting to derive the bipartite pentamerous eudicot flower from a dimerous ancestry, as it is the most common condition in the basal eudicot grade preceding the core eudicots and including Gunneraceae as sister of Pentapetalae (Doyle and Endress, 2011). There are known cases of pentamerous flowers with irregularly whorled phyllotaxis, when one of the inner perianth parts appears in double position (e.g. Clematis: Ren et al., 2010), or the perianth is pentamerous by an increase in the diameter of the apex (e.g. Thalictrum: Ren et al., 2011). However, to physically understand the development of the most common core eudicot flower with floral formula K5C5A5 + 5G5, one would need a dimerous precursor with several whorls (K2 + 2C2 + 2A2 + 2 + 2 + 2G2 + 2) and an irregular increase in each alternating whorl, leading to amalgamations of compound whorls with a 2/5 sequence of initiation. The closest resemblance of this flower structure is in Berberidaceae (e.g. Epimedium), but there are difficulties, as dimery is closely linked to trimery in the family, the number of stamens and carpels is lower, and petals are probably staminodial structures. The closest sister group of core eudicots is Gunneraceae, where the few-parted flowers are clearly apomorphic (Wanntorp and Ronse De Craene, 2005; Ronse De Craene and Wanntorp, 2006).
Conclusions
This investigation throws light on a little-known distinctive genus, clarifying possible pathways in the early differentiation of the flower of core eudicots. Berberidopsis beckleri is more variable than B. corallina and A. punctatum, and stands at one end of a sequence leading to the more regular flowers of the latter (Fig. 7). Aextoxicon (Ronse De Craene and Stuppy, 2010; Ronse De Craene, 2010) is mostly pentamerous, although all floral parts, including sepals and petals, develop sequentially. The reduction in the internode length of the flower-bearing axis together with a reduction in the number of bracts and carpels places B. corallina in an intermediate position.
The almost ubiquitous Bauplan of pentamerous pentacyclic flowers evolved extremely rapidly in all major clades of core eudicots, but the question how this structure originated from early diverging eudicots remains currently unanswered. Berberidopsis combines several primitive and derived features with a floral Bauplan that could likely be close to the ancestral flower of core eudicots.
ACKNOWLEDGEMENTS
I thank the horticultural staff of the Royal Botanic Garden Edinburgh for expertly growing Berberidopsis beckleri in their greenhouse and for respecting my request not to prune plants for my studies. I thank Lynsey Wilson for the photographs of living material. I also thank Frieda Christie for technical support with the SEM and the Systematics Association for financial support in the earlier stages of this project. Two anonymous reviewers are thanked for helpful suggestions to improve the manuscript.
LITERATURE CITED
- Angiosperm Phylogeny Group IV. 2016. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Baas P. 1984. Vegetative anatomy and taxonomy of Berberidopsis and Streptothamnus (Flacourtiaceae). Blumea 30: 39–44. [Google Scholar]
- Von Balthazar M, Endress PK.. 2002. Development of inflorescences and flow/ers in Buxaceae and the problem of perianth interpretation. International Journal of Plant Sciences 163: 847–876. [Google Scholar]
- Buzgo M, Soltis PS, Soltis DE.. 2004. Floral developmental morphology of Amborella trichopoda (Amborellaceae). International Journal of Plant Sciences 165: 925–947. [Google Scholar]
- Carlquist S. 2003. Wood anatomy of Aextoxicaceae and Berberidopsidaceae is compatible with their inclusion in Berberidopsidales. Systematic Botany 28: 317–325. [Google Scholar]
- Classen-Bockhoff R, Bull-Hereñu K.. 2013. Towards an ontogenetic understanding of inflorescence diversity. Annals of Botany 112: 1523–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X-Y, Zhong L, Saunders RMK, Chen Z-D.. 2012. Floral ontogeny of Schisandra chinensis (Schisandraceae): implications for androecial evolution within Schisandra and Kadsura. Plant Systematics and Evolution 298: 713–722. [Google Scholar]
- Doyle JA. 2013. Phylogenetic analyses and morphological innovations in land plants. Annual Plant Reviews 45: 1–50. [Google Scholar]
- Doyle JA, Endress PK.. 2011. Tracing the early evolutionary diversification of the angiosperm flower In: Ronse De Craene LP, Wanntorp L, eds. Flowers on the tree of life. Cambridge: Cambridge University Press, 88–119. [Google Scholar]
- Endress PK. 1980a. Floral structure and relationships of Hortonia (Monimiaceae). Plant Systematics and Evolution 133: 199–221. [Google Scholar]
- Endress PK. 1980b. The reproductive structures and systematic position of the Austrobaileyaceae. Botanische Jahrbücher für Systematik 101: 393–433. [Google Scholar]
- Endress PK. 1987. Floral phyllotaxis and floral evolution. Botanische Jahrbücher fur Systematik 108: 417–438. [Google Scholar]
- Endress PK. 2008. Perianth biology in the basal grade of extant angiosperms. International Journal of Plant Sciences 16: 844–862. [Google Scholar]
- Endress PK, Doyle JA.. 2007. Floral phyllotaxis in basal angiosperms: development and evolution. Current Opinion in Plant Biology 10: 52–57. [DOI] [PubMed] [Google Scholar]
- Endress PK, Doyle JA.. 2009. Reconstructing the ancestral angiosperm flower and its initial specializations. American Journal of Botany 96: 22–66. [DOI] [PubMed] [Google Scholar]
- Endress PK, Sampson FB.. 1983. Floral structure and relationships of the Trimeniaceae (Laurales). Journal of the Arnold Arboretum 64: 447–473. [Google Scholar]
- González F, Bello MA.. 2009. Intra-individual variation of flowers in Gunnera subgenus Panke (Gunneraceae) and proposed apomorphies for Gunnerales. Botanical Journal of the Linnean Society 160: 262–283. [Google Scholar]
- Harden GJ. 1990. Flacourtiaceae In: Harden GJ, ed. Flora of New South Wales, Vol. 1 Sydney: UNSW Press, 430–432. [Google Scholar]
- van Heel WA. 1977. Flowers and fruits in Flacourtiaceae III. Some Oncobeae. Blumea 23: 349–369. [Google Scholar]
- van Heel WA. 1984. Flowers and fruits in Flacourtiaceae V. The seed anatomy and pollen morphology of Berberidopsis and Streptothamnus. Blumea 30: 31–37. [Google Scholar]
- Jessup LW. 1982. Flacourtiaceae In: George AS, ed. Flora of Australia, Vol. 8. Lecythidales to Batales. Canberra: Australian Government Publishing Service, 66–84. [Google Scholar]
- Keating RC. 1975. Trends of specialization in pollen of Flacourtiaceae with comparative observations of Cochlospermaceae and Bixaceae. Grana 15: 29–49. [Google Scholar]
- Kubitzki K. 2007. Berberidopsidaceae In: Kubitzki K, ed. The families and genera of vascular plants, Vol. IX Berlin: Springer, 33–35. [Google Scholar]
- Maia VH, Gitzendanner MA, Soltis PS, Wong GK-S, Soltis DE.. 2014. Angiosperm phylogeny based on 18S/26S rDNA sequence data: constructing a large data set using next-generation sequence data. International Journal of Plant Sciences 175: 613–650. [Google Scholar]
- Moore MJ, Soltis PS, Bell CD, Burleigh G, Soltis DE.. 2010. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proceedings of the National Academy of Sciences of the USA 107: 4623–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mueller F. 1862. Fragmenta phytographiae Australiae 3: 27–28. [Google Scholar]
- Ren Y, Chang H-L, Endress PK.. 2010. Floral development in Anemoneae (Ranunculaceae). Botanical Journal of the Linnean Society 162: 77–100. [Google Scholar]
- Ren Y, Gu T-q, Chang H-l.. 2011. Floral development of Dichocarpum, Thalictrum, and Aquilegia (Thalictroideae, Ranunculaceae). Plant Systematics and Evolution 292: 203–213. [Google Scholar]
- Ronse De Craene LP. 2004. Floral development of Berberidopsis corallina: a crucial link in the evolution of flowers in the core eudicots. Annals of Botany 94: 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse De Craene LP. 2007. Are petals sterile stamens or bracts? The origin and evolution of petals in the core eudicots. Annals of Botany 100: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse De Craene LP. 2008. Homology and evolution of petals in the core eudicots. Systematic Botany 33: 301–325. [Google Scholar]
- Ronse De Craene LP. 2010. Floral diagrams. An aid to understanding floral morphology and evolution. Cambridge: Cambridge University Press. [Google Scholar]
- Ronse De Craene LP. 2016. Meristic changes in flowering plants. How flowers play with numbers. Flora 221: 22–37. [Google Scholar]
- Ronse De Craene LP, Brockington S.. 2013. Origin and evolution of petals in the angiosperms. Plant Ecology and Evolution 146: 5–25. [Google Scholar]
- Ronse De Craene LP, Stuppy W.. 2010. Floral development and anatomy of Aextoxicon punctatum (Aextoxicaceae – Berberidopsidales) – an enigmatic tree at the base of core eudicots. International Journal of Plant Sciences 171: 244–257. [Google Scholar]
- Ronse De Craene LP, Wanntorp L.. 2006. Evolution of floral characters in Gunnera (Gunneraceae). Systematic Botany 31: 671–688. [Google Scholar]
- Ronse De Craene LP, Soltis PS, Soltis DE.. 2003. Evolution of floral structures in basal angiosperms. International Journal of Plant Sciences 164 (5 Suppl): S329–S363. [Google Scholar]
- Ronse De Craene LP, Iwamoto A, Bull-Hereñu K, Dos Santos P, Luna-Castro J, Farrar J.. 2014. Understanding the structure of flowers – the wonderful tool of floral formulae: a response to Prenner & al. Taxon 63: 1103–1111. [Google Scholar]
- Ronse De Craene LP, Quandt D, Wanntorp L.. 2015. Floral development of Sabia (Sabiaceae): evidence for the derivation of pentamery from a trimerous ancestry. American Journal of Botany 102: 336–349. [DOI] [PubMed] [Google Scholar]
- Sleumer H. 1963. Flacourtiaceae In: Anderson RH, ed. Contributions from the New South Wales National Herbarium. Flora Series, No. 136. Sydney: NSW Department of Agriculture, 1–4. [Google Scholar]
- Soltis DE, Smith SA, Cellinese N, et al. 2011. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98: 704–730. [DOI] [PubMed] [Google Scholar]
- Staedler YM, Endress PK.. 2009. Diversity and lability of floral phyllotaxis in the pluricarpellate families of core Laurales (Gomortegaceae, Atherospermataceae, Siparunaceae, Monimiaceae). International Journal of Plant Sciences 170: 522–550. [Google Scholar]
- Staedler YM, Weston PH, Endress PK.. 2007. Floral phyllotaxis and floral architecture in Calycanthaceae (Laurales). International Journal of Plant Sciences 168: 285–306. [Google Scholar]
- Stanley TD, Ross EM.. 1986. Flacourtiaceae Flora of south-eastern Queensland , Vol.2 Brisbane: Queensland Department of Primary Industries, 97–99. [Google Scholar]
- Veldkamp JP. 1984. Berberidopsis (Flacourtiaceae) in Australia. Blumea 30: 21–29. [Google Scholar]
- Wang H, Moore MJ, Soltis PS, et al. 2009. Rosid radiation and the rapid rise of angiosperm-dominated forests. Proceedings of the National Academy of Sciences 106: 3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanntorp L, Ronse De Craene LP.. 2005. The Gunnera flower: key to eudicot diversification or response to pollination mode? International Journal of Plant Sciences 166: 945–953. [Google Scholar]
- Wanntorp L, Ronse De Craene LP.. 2007. Flower development of Meliosma (Sabiaceae) – evidence for multiple origins of pentamery in the eudicots. American Journal of Botany 94: 1828–1836. [DOI] [PubMed] [Google Scholar]