Abstract
Objective
Rheumatoid arthritis (RA) is a systemic, immune-mediated inflammatory disease that ultimately leads to bone erosions and joint destruction. Methotrexate (MTX) slows bone damage but the mechanism by which it acts is still unknown. In this study, we aimed to assess the effect of MTX and low-dose prednisolone (PDN) on circulating osteoclast (OC) precursors and OC differentiation in patients with RA.
Methods
Patients with RA before and at least 6 months after MTX therapy were analysed and compared with healthy donors. A blood sample was collected in order to assess receptor activator of NF-κβ (RANK) ligand surface expression on circulating leucocytes and frequency and phenotype of monocyte subpopulations. Quantification of serum levels of bone turnover markers and cytokines and OC differentiation assays were performed.
Results
Classical activation markers of monocytes and RANK increased in patients with RA at baseline, compared with control healthy donors, and after MTX and low-dose PDN (MTX+PDN) exposure they decreased to control levels. Although the number of OC was not different between groups, the percentage of resorbed area and the resorbed area per pit reduced after treatment. Serum soluble receptor activator of nuclear factor-kappa (RANKL) levels increased at baseline compared with healthy donors and normalised after therapy.
Conclusion
Our results suggest that MTX+PDN play an important role in downregulating OC function, which we believe occurs through the decrease in RANK surface expression in monocytes.
Keywords: Rheumatoid arthritis, Monocytes, DMARD, Osteoclast, Bone
Key messages.
What is already known about this subject?
Methotrexate slows bone damage but the mechanism by which it acts is still unknown.
What does this study add?
Methotrexate and low-dose corticosteroids downregulate osteoclast function through the decrease of receptor activator of NF-κB surface expression in monocytes.
How might this impact on clinical practice?
These observations reinforce the relevance of early treatment intervention in early RA with low-dose prednisolone and methotrexate.
Rheumatoid arthritis (RA) is a chronic, immune-mediated, inflammatory disease that mainly impacts joints. It affects around 1% of the world population and the majority of the affected patients are women.1 The main features of RA are synovial hyperplasia with pannus formation and bone erosions. On the other hand, systemic inflammation leads also to secondary osteoporosis and bone fragility.2 In RA, there is an imbalance between bone formation and bone resorption. In fact, the immune system contributes directly to bone resorption by producing receptor activator of NF-κβ (RANK) ligand and indirectly through the secretion of pro-inflammatory cytokines that act synergistically with the RANK-RANKL system, further enhancing osteoclast (OC) differentiation and bone resorption.3 Monocytes are OC precursors; these cells have phenotypical and functional heterogeneity and have a critical regulatory role in inflammation and innate immune response.4 5 Monocytes can be subdivided into three subpopulations based on their expression of CD14 and CD16 surface markers: the classical (CD14brightCD16−), intermediate (CD14brightCD16+) and non-classical (CD14dimCD16+) subsets.4 All of these precursors are able to differentiate into OC increasing bone resorption.6 7 Moreover, in RA, CD14+ monocytes continuously migrate into the injured joint tissues where they are activated and act as local and systemic amplifiers of the disease through a multitude of mediators.8–10
Methotrexate (MTX) combined with low-dose prednisolone (PDN) is the cornerstone of early RA treatment, reducing inflammation and slowing down bone damage.11–14 A recent report depicted that OC formation and OC gene expression, such as NFATc1 and DC-STAMP, which are induced by the cytokine RANKL, are significantly inhibited by MTX.15 In this study, we aimed to assess the effect of MTX and low-dose PDN (MTX+PDN) on the circulating OC precursors and OC differentiation in patients with RA.
Patients and methods
Patients
Patients with early untreated RA (<12 months of disease duration) fulfilling the American College of Rheumatology/European League Against Rheumatism criteria, 201016 were recruited from the Rheumatology and Bone Metabolic Disease Department, Hospital de Santa Maria, Lisbon Academic Medical Centre, Portugal. All patients included were naïve to disease-modifying antirheumatic drugs (DMARDs) and corticoids and were followed in the outpatient clinic during a minimum of 6 months after starting MTX+PDN treatment. Moderate-to-high disease activity was required for inclusion, defined as disease activity score 28 (DAS28) >3.2.17 Patients previously exposed or actively under corticoids and/or DMARD therapy were excluded from this study. The use of non-steroidal anti-inflammatory drugs was not an exclusion criterion. Information regarding patients' demographics, duration of symptoms, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), tender and swollen joints counts, presence of erosions, presence of rheumatoid factor (RF) and presence of anticitrullinated protein antibodies was collected. Both DAS28 and the Health Assessment Questionnaire18 were applied to patients. Patients with RA were managed by the standard-of-care approach, with no deviation induced by the participation in this study.
Heparinised blood and serum were collected from each participant at the starting of MTX and prednisolone and after 6 months of reaching the minimum effective dose of MTX. Blood was used for flow cytometry and peripheral blood mononuclear cell (PBMC) isolation. Healthy donors matched for age and gender were used as controls. The local ethics committee approved this study and all participants signed an informed consent. Patients were managed in accordance with the standard practice and the study was conducted in accordance with the Declaration of Helsinki as amended in Brazil (2013).
Antibodies and flow cytometry
Identification of B and T cells and neutrophils in peripheral blood and immunophenotyping of monocytes in the PBMC samples were performed using matched combinations of antihuman murine monoclonal antibodies. For peripheral blood staining, anti-CD19 PerCP-Cy5.5 (eBioscience, San Diego, California, USA), anti-CD3 PerCP (BD Biosciences, Franklin Lakes, New Jersey, USA), anti-CD66b FITC (Immunotools, Friesoythe, Germany) and anti-RANKL PE (Santa Cruz Biotechnology, Dallas, Texas, USA) were used. Monocyte subpopulations were identified with anti-CD14 FITC (BD Biosciences) or PerCPCy5.5 (Immunotools) and anti-CD16 APC (Immunotools) and stained with combinations of anti-CD11b PE-Cy7, CD105 PE, CD62L PE-Cy7, CD51/CD61 FITC (eBioscience), CCR2 PE (R&D Systems, Minneapolis, Minnesota, USA), human leucocyte antigen - antigen D related (HLA)-DR PerCP (BD Biosciences) and RANK PE (Santa Cruz Biotechnology). Cell death was assessed by staining with Annexin V Apoptosis Detection Kit APC (eBioscience). Acquisition was performed using a FACSCalibur (BD Biosciences).
Heparinised whole blood was used for staining. Erythrocytes were lysed with red blood cell lysis buffer and cells were incubated with IgG block solution 300 ng/mL (ChromPure Mouse IgG whole molecule, Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania, USA) before staining. Absolute cell counts were calculated from differential leucocyte count determined for all participants. PBMCs were isolated by density gradient centrifugation with Histopaque−1077 (Sigma-Aldrich, St. Louis, Missouri, USA). Monocyte subpopulations were identified as described before based on their CD14 and CD16 surface expression.4 Data were analysed using FlowJo software (TreeStar, Stanford University, Stanford, California, USA).
Osteoclast differentiation
PBMCs isolated by density gradient centrifugation were plated in 96-well culture plates as described before.19 PBMCs were left overnight for osteoclast precursors (OCPs) to adhere on bone slices (Immunodiagnostic Systems, Boldon, UK).20 On the following day (day 1 of culture), medium was changed to Dulbecco's modified Eagle's Medium (DMEM) supplemented with macrophage colony stimulating factors (M-CSF) 25 ng/mL (Peprotech, London, UK). Three days later, medium was again changed to DMEM with M-CSF (25 ng/mL), soluble RANKL (sRANKL) (50 ng/mL; Peprotech), dexamethasone (10 nM; Sigma-Aldrich) and transforming growth factor β (2.5 ng/mL; R&D Systems) in order to differentiate the OCPs into mature OC.21 The culture medium was then changed twice a week. Cells cultured on bone slices for 7, 14 and 21 days20 22 were used for functional assays and gene expression.
Functional assays
TRAP staining of OC was performed using the Acid Phosphate, Leukocyte Kit (tartrate resistant acid phosphatase (TRAP), Sigma-Aldrich) according to the manufacturer's instructions. This assay allows the identification of mature OC (TRAP-positive cells containing three or more nuclei). In the resorption assay, to measure the resorbed area, OC were removed from the bone slices using sodium hypochlorite and stained with 0.1% toluidine blue.
Bone slices from both TRAP staining and resorption functional assays were photographed in an area of 1.25 mm2 with a brightfield microscope (Leica DM2500, Leica, Wetzlar, Germany) under a 10x objective. The number of TRAP-stained OC was counted for each time-point per condition and the resorption pits were traced using ImageJ (NIH, Bethesda, Maryland, USA), a mean value of the resorbed area was calculated and expressed in percentage of total area.
Bone turnover markers detection
Serum sRANKL and osteoprotegerin (OPG) were quantified by ELISA (Biomedica Grouppe, Vienna, Austria) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with SPSS Statistics V.17.0 (IBM, Portsmouth, UK). Categorical variables were expressed as frequencies and comparisons were tested using χ2 test. Continuous variables were expressed by median and IQR. Before and after treatment values within each sample were compared using Wilcoxon's matched-pairs signed-rank test. To compare patients with RA with healthy age-matched and sex-matched donors, Mann-Whitney U test was used. Clinical variables such as DAS28, CRP, ESR and tender and joint count were correlated with OC activation parameters (pit number and carboxy-terminal telopeptide of type I collagen (CTX-I)) using Spearman's correlation; p values <0.05 were considered to be statistically significant.
Results
Population characteristics
This study was conducted in 14 untreated patients with early RA, before and after initiation of combined MTX and PDN treatment, and in 18 age-matched and gender-matched healthy donors. All patients had <12 months disease duration and were corticosteroid and synthetic DMARD naïve at baseline. The clinical and demographic features of patients before and after treatment and of the healthy donors are described in table 1. All patients were under MTX (mean dose of 15 mg/week) and prednisolone (<10 mg/day). To three patients hydroxychloroquine (400 mg/day) was added later on and two patients received concomitant sulfasalazine (2 g/day), due to incomplete response to MTX. Patients' median MTX therapy duration was 13 months (minimum 6 and maximum 24 months).
Table 1.
Summary of patients and healthy controls' characteristics
| Patients with RA h | Healthy | p Value | ||
| Baseline | Treated | |||
| Sample size | 14 | 18 | ||
| Age (years) | 50 (32–60) | 44 (36–52) | 0.50853 | |
| Females (n, %) | 10, 71% | 11, 61% | 0.7120 | |
| Symptoms duration (months) | 9 (2–12) | NA | ||
| RF (n positive, % positive) | 8, 57% | NA | ||
| ACPA (n positive, % positive) | 5, 36% | NA | ||
| Erosive (n positive, % yes) | 3, 21% | NA | ||
| Treatment with NSAID (n, %) | 8, 57% | NA | ||
| NSAIDs duration (months) | 2.3 (0.3–5.5) | NA | ||
| Treatment with MTX (%) | 0% | 100%* | ||
| ESR (mm/hour) | 22 (11–44) | 13 (9–27) | NA | 0.1934 |
| CRP (mg/dL) | 0.3 (0.1–2.7) | 0.2 (0.1–0.3) | NA | 0.1289 |
| Tender joint count | 4 (0–7) | 0 (0–1) | NA | 0.3738 |
| Swollen joint count | 6 (2–10) | 1 (0–1) | NA | 0.0380* |
| DAS28 | 4.7 (4.2–5.4) | 2.4 (2.1–3.6) | NA | 0.0137* |
| HAQ | 1.4 (0.6–2.2) | 0.3 (0.0–0.7) | NA | 0.1250 |
*All patients were under MTX and prednisolone. To three patients hydroxychloroquine was added later on and two patients received concomitant sulfasalazine. *p<0.05 between patients before and after treatment with MTX.
Data are represented as median and IQR unless stated otherwise.
RA, rheumatoid arthritis; NA, not applicable; RF, rheumatoid factor; ACPA, anticitrullinated protein antibodies; NSAIDs, non-steroidal anti-inflammatory drugs; MTX, methotrexate; ESR, erythrocyte sedimentation rate; CRP, C reactive protein; DAS, disease activity score; HAQ, Health Assessment Questionnaire.
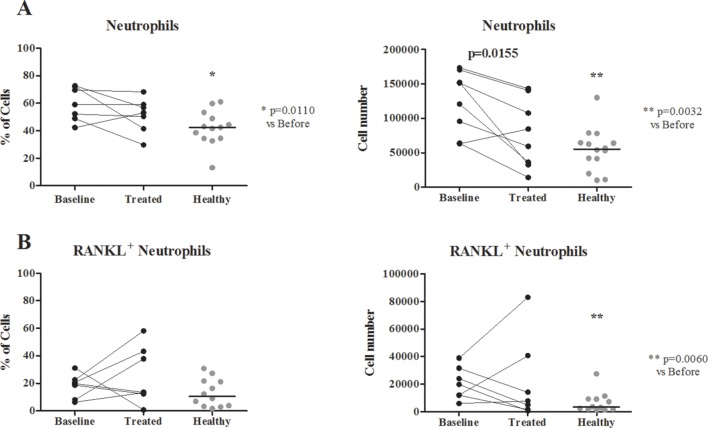
Patients with RA had a higher number of neutrophils expressing RANKL than controls, which regressed to control levels after treatment
The number of neutrophils was significantly higher in patients with RA at baseline when compared with healthy donors (both frequency p=0.0110 and absolute number p=0.0032; figure 1). Moreover, the absolute number of neutrophils expressing RANKL on their surface was also higher than in controls (p=0.0060). After treatment, the absolute counts of neutrophils decreased (p=0.0155). No difference was found in the total number of circulating T or B cells before and after therapy or when compared with healthy donors (data not shown). Moreover, no differences were found regarding RANKL surface expression on neutrophils, T and B cells.
Figure 1.
Frequency of neutrophils and RANKL neutrophils. (A) Neutrophil frequency is significantly higher in patients with RA at baseline when compared with healthy donors (both frequency p=0.0110 and absolute number p=0.0032). (B) RANKL+neutrophil count is also higher at baseline when compared with healthy donors (p=0.0060). Neutrophil number is decreased after MTX and low-dose prednisolone treatment in patients with RA (absolute number only, p=0.0155). Each dot represents a sample and the line in healthy represents median. RA, rheumatoid arthritis; MTX, methotrexate; RANKL, receptor activator of nuclear factor-κβ ligand.
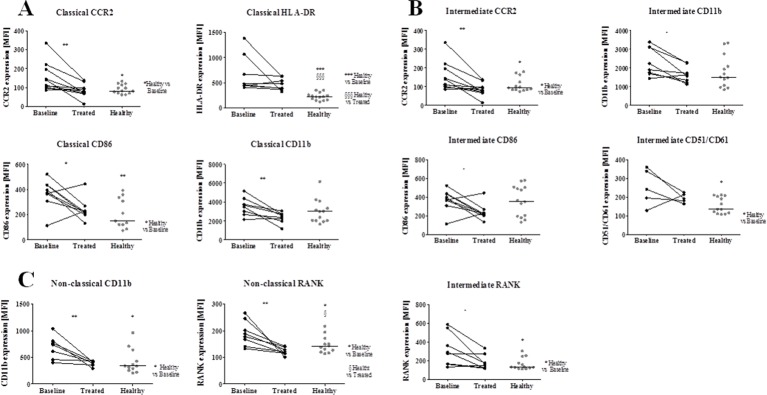
Treatment reduced RANK expression in monocyte subpopulations in patients with RA
Regarding the monocyte subpopulations (classical CD14brightCD16−, intermediate CD14brightCD16+ and non-classical CD14dimCD16+), no differences in frequency or cell death among the three subpopulations or between groups were found (data not shown).
In patients with RA at baseline, CCR2 expression was higher both in the classical and intermediate subpopulations (p=0.0120 and p=0.0471 when compared with controls; figure 2) and decreased after therapy (p=0.0039 for classical and p=0.0237). HLA-DR and CD86 expression were also higher in the classical subpopulation of patients with RA at baseline (p=0.0002 and p=0.0068) as compared with controls. However, while HLA-DR expression remained elevated after treatment (p=0.0006 compared with controls), CD86 expression decreased (p=0.0237). RANK expression was higher than in controls, both in the intermediate and non-classical subpopulations (p=0.0112 and p=0.0475, respectively), while CD51/CD61 (αVβ3 integrin) was higher in the intermediate subpopulation (p=0.0487) and CD11b in the non-classical subpopulation (p=0.0160) as compared with controls. Treatment with MTX+PDN decreased RANK and CD11b in the intermediate (p=0.0234 and p=0.0125) and non-classical subpopulations (p=0.0084 and p=0.0132) and CD11b in the classical subpopulation (p=0.0091).
Figure 2.
Phenotype of monocyte subpopulations. (A) Classical subpopulation phenotype, (B) intermediate subpopulation, (C) non-classical subpopulation phenotype. Before treatment there was higher expression of CCR2 in classical and intermediate subpopulations, HLA-DR and CD86 in classical subpopulation, CD51/CD61 in the intermediate subpopulation, CD11b in non-classical subpopulation and RANK both in intermediate and non-classical subpopulations. HLA-DR expression remained higher in patients after treatment when compared with controls. CCR2, CD86, CD11b and RANK were decreased in the referred populations after treatment. *p<0.05, **p<0.01. Each dot represents a sample and the line in healthy represents median. MFI, median fluorescence intensity; CCR2, C-C chemokine receptor type 2; HLA, human leucocyte antigen; RANK, receptor activator of nuclear factor-κB; MTX, methotrexate.
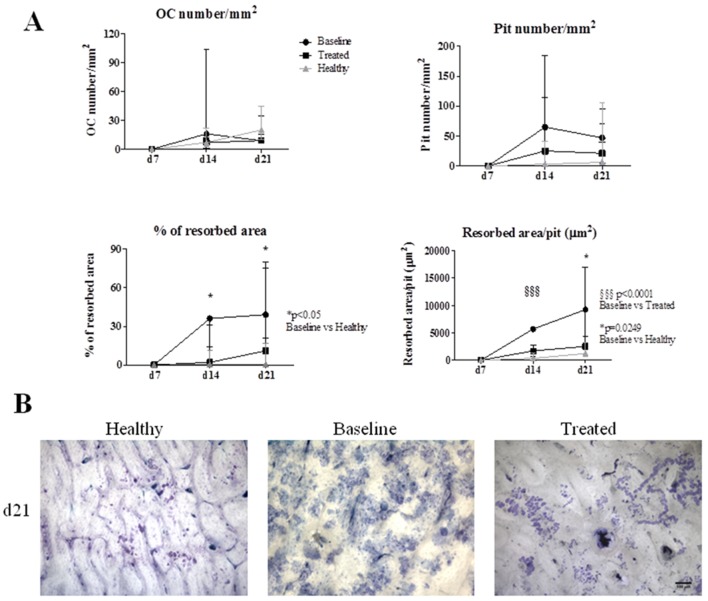
In vitro assays showed that OCs from patients with RA at baseline are more active than the ones from treated patients and controls
The number of OC increased in all groups throughout culture time. No significant differences in OC number/mm2 or pit number were found (figure 3). However, the percentage of resorbed area was higher in patients with RA at baseline at culture days 14 and 21 when compared with controls (p=0.0333 and p=0.0436). Resorbed area per pit was higher at culture day 21 before treatment when compared with controls (p=0.0249) and decreased after therapy, reaching statistical significance at day 14 of culture (p<0.0001). Pit number at day 21 was positively correlated with tender joint count when all patients were analysed (r=0.499, p=0.042).
Figure 3.
Functional assays in differentiated OC. (A) No differences in OC number/mm2 or in pit number were found. Percentage of resorbed area was higher in patients at baseline at culture day 14 and 21 when compared with controls. Resorbed area per pit was lower at culture day 14 when compared with patients at baseline. At culture day 21, patients with RA at baseline had higher resorbed area per pit than controls. Dots and lines represent median and IQR. (B) Representative images of resorption pit assay in all groups at culture day 21; scale bar represents 100 μm. d, day; OC, osteoclast; RA, rheumatoid arthritis.
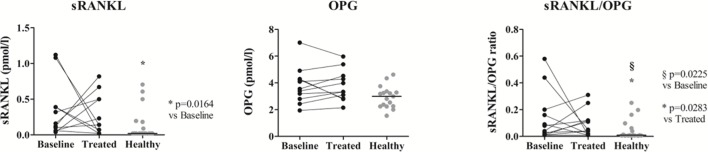
Bone turnover markers increased in patients with RA at baseline and normalised after treatment
Serum level of sRANKL was higher in patiets with RA at baseline than in controls (p=0.0164; figure 4) and normalised after treatment. OPG levels did not change with treatment and was not different from controls. The sRANKL/OPG ratio was higher in patients when compared with controls and it was not affected by treatment (p=0.0228 and p=0.0283, respectively). The ratio of CTX-I/P1NP positively correlated with DAS28 in patients before therapy (r=0.786, p=0.036). After treatment with MTX and prednisolone, both CTX-I and P1NP negatively correlated with swollen joint count (r=−0.913, p=0.002 and r=−0.756, p=0.030, respectively).
Figure 4.
Serum sRANKL and OPG levels in healthy donors and patients with RA at baseline and after MTX and low-dose prednisolone treatment. Both sRANKL and the sRANKL/OPG ratio increased in patients at baseline when compared with healthy donors. There were no differences in circulating OPG level before and after treatment or when compared with controls. The sRANKL/OPG ratio remained significantly higher after treatment. Each dot represents a sample and the line in healthy represents median. sRANKL, soluble receptor activator of nuclear factor-κB ligand; OPG, osteoprotegerin; RA, rheumatoid arthritis.
Discussion
In this study, we have shown a decrease in the expression of surface markers on the different monocyte subpopulations, as well as a decrease in OC activity, in patients with early RA treated with MTX and low-dose PDN. The characteristics of these patients were similar to other very early RA cohorts.23
Neutrophils are involved in RA pathology since the early phase of the disease and they express surface RANKL when activated.23–26 We showed that the number of neutrophils and RANKL+ neutrophils increased in untreated patients with RA when compared with controls and that, after treatment with MTX+PDN, the number of neutrophils decreased, reaching control values. Despite also being a source of RANKL,27–29 no differences were found in T or B lymphocyte RANKL surface expression before and after therapy.
Patients with RA have increased expression of monocyte surface markers of adhesion, activation and proteins involved in OC differentiation, like RANK and CD51/CD61 (αVβ3 integrin).30–32 In our study, after exposure to MTX+PDN, CCR2, CD86, CD11b and RANK expression reduced in several monocyte subpopulations, reaching normal expression levels. Dendritic cells, also present in circulation, express CD14, CD16 and CD8633 34 and can also differentiate into OC.35 36 These cells were also represented in the CD14/CD16 subpopulations of monocytes since we did not exclude them. It was previously shown that CCR2 is upregulated in monocytes and T cells in patients with RA. In vivo studies have shown that this surface marker is involved in recruitment and activation of monocytes in response to monocyte chemoattractant protein-1 (MCP-1).37 38 CD86 is a costimulatory molecule involved in the antigen-presenting process and it has been shown to have an important role in T cells activation in RA synovia.33 CD11b expression is increased in RA monocytes, promoting their binding and migrating properties and it has been shown that corticosteroid therapy decreases CD11b expression.39–41 These surface molecules (CCR2, CD86 and CD11b) are important in migration, activation and promotion of inflammation contributing to the continuous inflammatory infiltrate in the synovia. After therapy with MTX and corticoids, these surface markers were reduced in line with a decrease of swollen and tender joints.
We verified that the serum sRANKL/OPG ratio increased in RA, as previously described.42 After treatment with MTX and low-dose PDN, the sRANKL/OPG ratio decreased. MTX has been recently associated with both reduction of the RANKL/OPG ratio and of dickkopf-1 levels43 and it has been previously shown that MTX directly inhibits osteoclastogenesis in vitro in synovial fibroblasts and PBMC cocultures by downregulating RANKL expression on synovial fibroblasts, but no effect on RANK expression was found.44 45
Finally, RANK is the essential receptor for monocyte differentiation in OC, it is upregulated in patients with RA and has been implicated in RA bone loss, both systemic and locally.42 We propose that the decrease in monocyte migration/adhesion molecules and downregulation of RANK expression after therapy decrease the osteoclastogenic potential of these cells. In fact, MTX-treated patients with RA reached the maximum OC number later than untreated patients and also than controls and the resorbed area per pit decreased significantly after treatment. This suggests that MTX+PDN have an important role in downregulating OC function, which we believe is at least partially through the decrease in RANK surface expression by monocytes.
In conclusion, our results suggest that the European League Against Rheumatism recommended treatment approach for patients with early RA (MTX combined with low-dose corticosteroids14) downregulates OC function, which we propose to occur through the decrease in RANK surface expression by monocytes.
Acknowledgments
The authors would also like to thank Biobanco-IMM, Lisbon Academic Medical Center, Lisbon, Portugal for sample collection and storage.
Footnotes
Contributors: Conceived and designed the experiments: IPP, JC-L, JEF, HC, MA. Performed the experiments: IPP, JC-L. Analysed the data: IPP, JC-L, CP, RC-M, AMR. Contributed reagents/materials/analysis tools:
IPP, RC-M, CP, AMR, HC. Wrote the paper: IPP, JC-L, RC-M, CP, AMR, HC, MA, JEF.
Funding: This work was supported by Fundação para a Ciência e Tecnologia (SFRH/BD/70533/2010 to IPP) and by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp (Merck_P08574 to JEF). The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. The funding agencies had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Patient consent: There is no identifiable individual in this paper. All patients that were enrolled signed the institutional Biobank consent form and it complies with Data Protection Laws from Portugal as approved by our local ethics committee and by the Portuguese National Data Protection Authority .
Ethics approval: Ethics committee from Hospital de Santa Maria, Lisbon Academic Medical Centre, Lisboa, Portugal.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 2015;11 10.1038/nrrheum.2015.8 [DOI] [PubMed] [Google Scholar]
- 2.van Vollenhoven RF. Rheumatoid arthritis in 2012: progress in RA genetics, pathology and therapy. Nat Rev Rheumatol 2013;9:70–2. 10.1038/nrrheum.2012.232 [DOI] [PubMed] [Google Scholar]
- 3.Danks L, Takayanagi H. Immunology and bone. J Biochem 2013;154 10.1093/jb/mvt049 [DOI] [PubMed] [Google Scholar]
- 4.Wong KL, Yeap WH, Tai JJ, et al. The three human monocyte subsets: implications for health and disease. Immunol Res 2012;53:41–57. 10.1007/s12026-012-8297-3 [DOI] [PubMed] [Google Scholar]
- 5.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–70. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- 6.Costa-Rodrigues J, Fernandes A, Fernandes MH. Spontaneous and induced osteoclastogenic behaviour of human peripheral blood mononuclear cells and their CD14(+) and CD14(-) cell fractions. Cell Prolif 2011;44:410–9. 10.1111/j.1365-2184.2011.00768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprangers S, Schoenmaker T, Cao Y, et al. Different Blood-Borne human osteoclast precursors respond in distinct Ways to IL-17A. J Cell Physiol 2016;231 10.1002/jcp.25220 [DOI] [PubMed] [Google Scholar]
- 8.Thurlings RM, Wijbrandts CA, Bennink RJ, et al. Monocyte scintigraphy in rheumatoid arthritis: the dynamics of monocyte migration in immune-mediated inflammatory disease. PLoS One 2009;4:e7865 10.1371/journal.pone.0007865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandooren B, Noordenbos T, Ambarus C, et al. Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum 2009;60:966–75. 10.1002/art.24406 [DOI] [PubMed] [Google Scholar]
- 10.Kinne RW, Stuhlmüller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther 2007;9:224. 10.1186/ar2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 2005;57:163–72. 10.1124/pr.57.2.3 [DOI] [PubMed] [Google Scholar]
- 12.de Jong PH, Hazes JM, Han HK, et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis 2014;73:1331–9. 10.1136/annrheumdis-2013-204788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortendahl M, Holmes T, Schettler JD, et al. The methotrexate therapeutic response in rheumatoid arthritis. J Rheumatol 2002;29:2084–91. [PubMed] [Google Scholar]
- 14.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanagawa H, Masuyama R, Morita M, et al. Methotrexate inhibits osteoclastogenesis by decreasing RANKL-induced calcium influx into osteoclast progenitors. J Bone Miner Metab 2016;34 10.1007/s00774-015-0702-2 [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 17.Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 2005;52:2625–36. 10.1002/art.21235 [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992;35:498–502. 10.1002/art.1780350502 [DOI] [PubMed] [Google Scholar]
- 19.Perpétuo IP, Raposeiro R, Caetano-Lopes J, et al. Effect of tumor necrosis factor inhibitor therapy on Osteoclasts precursors in Ankylosing Spondylitis. PLoS One 2015;10:e0144655 10.1371/journal.pone.0144655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holloway WR, Collier FM, Aitken CJ, et al. Leptin inhibits osteoclast generation. J Bone Miner Res 2002;17:200–9. 10.1359/jbmr.2002.17.2.200 [DOI] [PubMed] [Google Scholar]
- 21.Takuma A, Kaneda T, Sato T, et al. Dexamethasone enhances osteoclast formation synergistically with transforming growth factor-beta by stimulating the priming of osteoclast progenitors for differentiation into osteoclasts. J Biol Chem 2003;278:44667–74. 10.1074/jbc.M300213200 [DOI] [PubMed] [Google Scholar]
- 22.Husheem M, Nyman JK, Vääräniemi J, et al. Characterization of circulating human osteoclast progenitors: development of in vitro resorption assay. Calcif Tissue Int 2005;76:222–30. 10.1007/s00223-004-0123-z [DOI] [PubMed] [Google Scholar]
- 23.Cascão R, Moura RA, Perpétuo I, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther 2010;12:R196. 10.1186/ar3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cascão R, Rosário HS, Souto-Carneiro MM, et al. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 2010;9:531–5. 10.1016/j.autrev.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 25.Chakravarti A, Raquil MA, Tessier P, et al. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood 2009;114:1633–44. 10.1182/blood-2008-09-178301 [DOI] [PubMed] [Google Scholar]
- 26.Poubelle PE, Chakravarti A, Fernandes MJ, et al. Differential expression of RANK, RANK-L, and osteoprotegerin by synovial fluid neutrophils from patients with rheumatoid arthritis and by healthy human blood neutrophils. Arthritis Res Ther 2007;9:R25. 10.1186/ar2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwood NJ, Kartsogiannis V, Quinn JM, et al. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun 1999;265:144–50. 10.1006/bbrc.1999.1623 [DOI] [PubMed] [Google Scholar]
- 28.Miranda-Carús ME, Benito-Miguel M, Balsa A, et al. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum 2006;54:1151–64. 10.1002/art.21731 [DOI] [PubMed] [Google Scholar]
- 29.Manilay JO, Zouali M. Tight relationships between B lymphocytes and the skeletal system. Trends Mol Med 2014;20:405–12. 10.1016/j.molmed.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Davignon JL, Hayder M, Baron M, et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology 2013;52:590–8. 10.1093/rheumatology/kes304 [DOI] [PubMed] [Google Scholar]
- 31.Kawanaka N, Yamamura M, Aita T, et al. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum 2002;46:2578–86. 10.1002/art.10545 [DOI] [PubMed] [Google Scholar]
- 32.Rossol M, Kraus S, Pierer M, et al. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 2012;64:671–7. 10.1002/art.33418 [DOI] [PubMed] [Google Scholar]
- 33.Thomas R, Quinn C. Functional differentiation of dendritic cells in rheumatoid arthritis: role of CD86 in the synovium. J Immunol 1996;156:3074–86. [PubMed] [Google Scholar]
- 34.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology 2013;140:22–30. 10.1111/imm.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maitra R, Follenzi A, Yaghoobian A, et al. Dendritic cell-mediated in vivo bone resorption. J Immunol 2010;185:1485–91. 10.4049/jimmunol.0903560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivollier A, Mazzorana M, Tebib J, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 2004;104:4029–37. 10.1182/blood-2004-01-0041 [DOI] [PubMed] [Google Scholar]
- 37.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem 2005;280:16163–9. 10.1074/jbc.M412713200 [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Magno CL, Day CJ, et al. Induction of chemokines and chemokine receptors CCR2b and CCR4 in authentic human osteoclasts differentiated with RANKL and osteoclast like cells differentiated by MCP-1 and RANTES. J Cell Biochem 2006;97:512–8. 10.1002/jcb.20649 [DOI] [PubMed] [Google Scholar]
- 39.Higaki M, Miyasaka N, Sato K. Increased expression of CD11b (Mo1) on peripheral blood monocytes of patients with rheumatoid arthritis. J Rheumatol 1992;19:825–6. [PubMed] [Google Scholar]
- 40.Mazure G, Jayawardene SA, Perry JD, et al. Abnormal binding properties of blood monocytes in rheumatoid arthritis. Agents Actions 1993;38 Spec No:C41–C43. 10.1007/BF01991131 [DOI] [PubMed] [Google Scholar]
- 41.Torsteinsdóttir I, Arvidson NG, Hällgren R, et al. Monocyte activation in rheumatoid arthritis (RA): increased integrin, fc gamma and complement receptor expression and the effect of glucocorticoids. Clin Exp Immunol 1999;115:554–60. 10.1046/j.1365-2249.1999.00817.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geusens P. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2012;4:225–33. 10.1177/1759720X12438080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Świerkot J, Gruszecka K, Matuszewska A, et al. Assessment of the effect of Methotrexate therapy on bone metabolism in patients with Rheumatoid Arthritis. Arch Immunol Ther Exp 2015;63 10.1007/s00005-015-0338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schett G, Stach C, Zwerina J, et al. How antirheumatic drugs protect joints from damage in rheumatoid arthritis. Arthritis Rheum 2008;58:2936–48. 10.1002/art.23951 [DOI] [PubMed] [Google Scholar]
- 45.Lee CK, Lee EY, Chung SM, et al. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor kappaB, osteoprotegerin, and receptor activator of nuclear factor kappaB ligand. Arthritis Rheum 2004;50:3831–43. 10.1002/art.20637 [DOI] [PubMed] [Google Scholar]