Granulocyte macrophage colony-stimulating factor (GM-CSF), an immunomodulatory cytokine, is an emerging therapeutic target of rheumatoid arthritis (RA).1 Although GM-CSF is produced by various types of cells, including synovial fibroblasts, the importance of GM-CSF-producing CD4 T cells in the pathogenesis of RA was reported.2 Moreover, GM-CSF has been shown to be the critical effector cytokine produced by interleukin (IL)-23-stimulated Th17 cells in mice, leading to the prevailing notion that GM-CSF is a Th17-related cytokine.3 However, in humans, a distinct subset of CD4 T cells that produce only GM-CSF has recently been identified.4 The ‘GM-CSF-only’ T cells are characterised by the lack of lineage defining cytokines (interferon (IFN)-γ, IL-17 and IL-4) and are regulated independently of the master transcriptional regulators, T-bet, RORγt or GATA3. Furthermore, in contrast to mice, GM-CSF production by human CD4 T cells was promoted in Th1 conditions and was also detected in Th1 cells. At present, it is unclear which helper CD4 T cell subsets produce GM-CSF in human RA joints. Therefore, in this study, we performed multicolour flow cytometric analysis of cytokine production in CD4 T cells from patients with RA.
Lymphocytes in the peripheral blood (PB) and in joints from the same patients were analysed to compare the profiles of cytokine production. Synovial fluid (SF) samples were obtained from seven patients by arthrocentesis, while synovial membrane (SM) samples were obtained from seven other patients who underwent joint replacement surgery. The mean age and disease duration of the patients was 63.5±15.5 and 16.5±10.1 years, respectively. Twelve patients (86%) were positive for RF and/or anti-CCP2 antibody, and the mean C reactive protein level was 1.2±1.1 mg/dL. In total, 11 (79%), 10 (71%) and 4 (29%) patients received methotrexate, prednisolone and biologics, respectively. There was no difference in the characteristics between patients providing SF and SM samples.
Because GM-CSF was produced by CD45RA-negative CD4 T cells, whose frequency in CD4 T cells differs greatly between PB and the joints (data not shown), we compared the frequency of cytokine-expressing cells in the CD45RA-negative CD4 T cell compartment between PB and the joints (figure 1A, B). Of the cytokines examined, GM-CSF was the second most frequently produced one in PB. A comparable frequency of GM-CSF-producing cells was detected in the joints, in both SF and SM, although their frequency in overall CD4 T cells was higher in the joints than in PB (15.0% vs 6.1%, p<0.05), as most CD4 T cells in the joint were CD45RA-negative. In contrast, the frequency of IFN-γ-producing or IL-21-producing cells, even within the CD45RA-negative population, increased significantly in the joints, as reported previously.5 6 The IL-21-producing cells in RA joints are distinct from follicular helper T cells in the expression of CXCR5 and bcl6, although they also express programmed death-1 (PD-1) and provide B cell help.6
Figure 1.
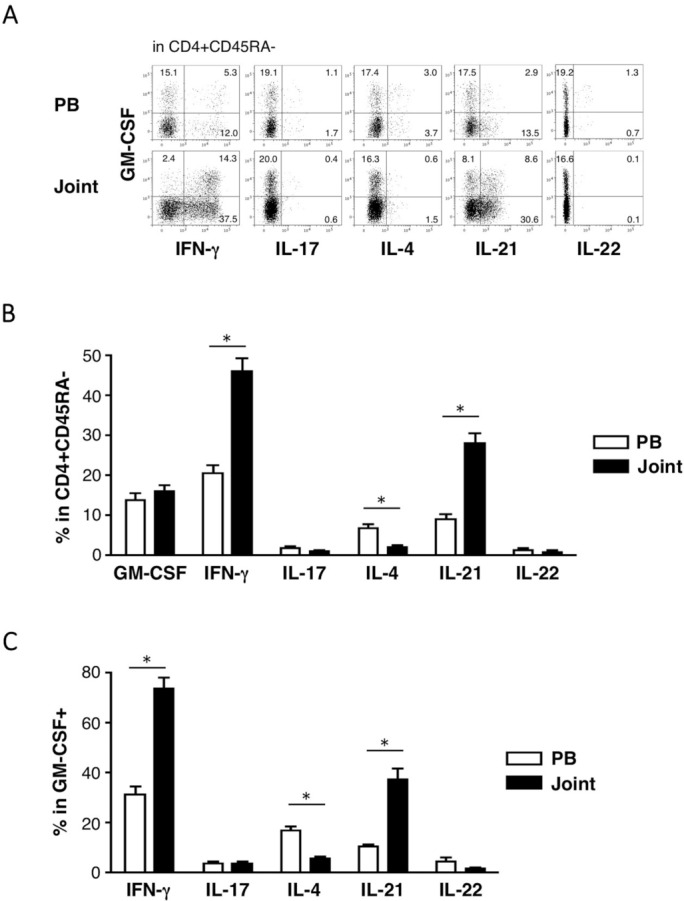
Flow cytometric analysis of cytokine production by lymphocytes in the peripheral blood (PB) and in joints. Paired samples of lymphocytes prepared from the PB and joints were stimulated briefly (4 hours) with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of brefeldin A for intracellular staining of the cytokines. The following monoclonal antibodies were used: Alexa fluor 488 anti-IL-17A (clone N49-653; BD Biosciences, San Diego, California, USA), PE anti-IL-21 (clone 3A3-N2; eBioscience, San Diego, California, USA), PerCP-eFluor710 anti-IL22 (clone 22URTI, eBioscience), allophycocyanin (APC) anti-GM-CSF (clone BVD2-21C11; BioLegend, San Diego, California, USA), anti-IFN-γ-PE/Cy7 (clone 4S.B3, BioLegend), anti-IL-4-brilliant violet 421 (clone MP4-25D2, BioLegend), anti-CD45RA-brilliant violet 510 (clone HI100, BioLegend) and anti-CD4-Biotin (clone RPA-T4, eBioscience) with APC/Cy7 sreptavidin (BD Biosciences). Stained cells were run on a FACSVerse flow cytometer (BD Biosciences), and the data were analysed by FlowLogic software (Inivai Technologies, Mentone, Victoria, Australia). (A) Representative dot plots of cytokine-producing cells in the CD4+ CD45RA– gate are shown. Quadrant gates were set according to the staining levels of isotype-matched control mAbs. (B, C) Mean percentages of the indicated cell populations within the analysis gates are shown (PB, open bars; joints, closed bars). *p<0.05, determined by a paired t-test. IFN, interferon; IL, interleukin.
We next analysed the overlaps between different cytokine-producing T cell subsets. The majority of GM-CSF-producing CD4 T cells in PB did not produce other cytokines (figure 1A, C). They could thus be the recently identified ‘GM-CSF-only’ T cells.4 In contrast, nearly 80% of GM-CSF-producing CD4 T cells in the joint produced IFN-γ. The frequency of GM-CSF-producing cells within IFN-γ-producing cells was also higher in the joint than PB (26.8% vs 20.5%, p<0.05). IL-21 was produced by some GM-CSF-producing CD4 T cells in the joint, but the frequency of GM-CSF-producing cells within IL-21-producing cells was not increased in the joints. Only a few GM-CSF-producing CD4 T cells produced IL-17, IL-4 and IL-22 even in the joint. IL-9 production was not detected in any samples, while tumour necrosis factor (TNF)-α was produced by virtually all GM-CSF-producing CD4 T cells in both PB and the joints (data not shown).
Since we found that GM-CSF, IFN-γ and IL-21 were the major effector cytokines preferentially produced by joint-infiltrating CD4 T cells, we further analysed the relationship between those cytokines. As depicted in figure 2, the three cytokines were mostly produced by distinct T cell populations in PB. On the other hand, there was a large overlap of cell populations producing those cytokines in the joints, and, notably, about 60% of CD4 T cells in the joint produced at least one of the three major cytokines. Thus, in RA joints, GM-CSF is mainly produced by IFN-γ-producing Th1-like cells, a part of which also produce IL-21.
Figure 2.
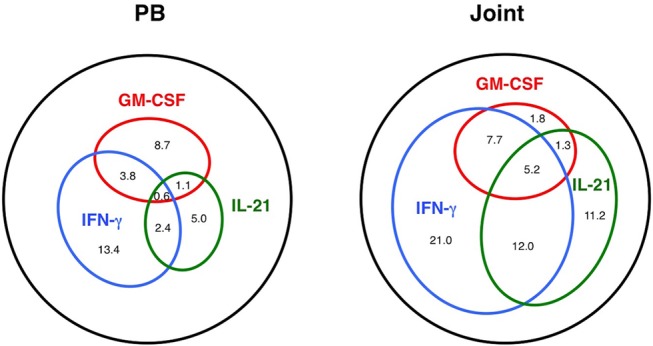
The landscape of cytokine-producing CD4 T cell populations in the peripheral blood (PB) and in the joints. Venn diagrams show the frequency of overlapping and non-overlapping population of cells producing GM-CSF, interferon (IFN)-γ and interleukin (IL)-21 in CD4+CD45RA– cells.
Efforts have been made to identify the CD4 T cell subset critically involved in the pathogenesis of RA. Studies in animal models suggested that Th17 cells are the candidates, but this claim was not supported by the results of clinical trials targeting IL-17 in RA. On the other hand, favourable outcomes of GM-SCF-targeting therapy have been reported.1 These are in line with our results showing a much higher prevalence of GM-CSF-producing T cells than IL-17-producing T cells in human RA joints. Notably, the GM-CSF-producing T cells also produce TNF-α, an established target of RA. We also found that GM-CSF is mainly produced by Th1-like cells in RA joints, further highlighting the difference in immune pathogenesis between humans and mice. In the latter, GM-SCF is associated with Th17 cells.
Despite possible influence of various treatment and relatively long disease duration in our cohort, we think our data are valuable in understanding the features of T cells infiltrating into inflamed joints. Interestingly, we observed a higher frequency of IFN-γ-producing cells, but not other subsets, in SF than SM (52.8% vs 40.8%, p<0.05), which may be because SF samples were obtained only from patients with joint effusion.
Our comprehensive analysis of cytokine production demonstrates the landscape of helper T cell populations in RA joints: partially overlapping populations of CD4 T cells producing IFN-γ, IL-21 and GM-CSF predominate in RA joints. Interestingly, it has been shown that not only IFN-γ, but GM-CSF and IL-21 are also induced by IL-12 in human CD4 T cells in vitro.4 7 8 Our results add in vivo relevance to these observations and suggest the presence of common developmental pathway for these T cells. Furthermore, such Th1-like cells with multiple effector functions might be involved in the pathogenesis of RA and thus be a potential therapeutic target.
Footnotes
Contributors: Study design: HY. Data collection: HY, AH, KS, KO, J-IF, HM-U, YA, YE, SK, KF, MK and HM. Data analysis: HY and AH. Manuscript preparation: HY, YN and YY.
Funding: This work was supported by Grants-in-aid for Scientific Research, The Japan Society for the Promotion of Science and by Grants for Excellent Graduate Schools, the Japanese Ministry of Education, Culture, Sports, Science and Technology, Japan.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Regional Committee of Ethics for Human Research at the Faculty of Medicine of Kyushu University, Fukuoka, Japan.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Avci AB, Feist E, Burmester GR. Targeting GM-CSF in rheumatoid arthritis. Clin Exp Rheumatol 2016;34(4 Suppl 98):39–44. [PubMed] [Google Scholar]
- 2. Reynolds G, Gibbon JR, Pratt AG, et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis 2016;75:899–907. 10.1136/annrheumdis-2014-206578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becher B, Segal BM. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol 2011;23:707–12. 10.1016/j.coi.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noster R, Riedel R, Mashreghi MF, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med 2014;6:241ra80. 10.1126/scitranslmed.3008706 [DOI] [PubMed] [Google Scholar]
- 5. Yamada H, Nakashima Y, Okazaki K, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis 2008;67:1299–304. 10.1136/ard.2007.080341 [DOI] [PubMed] [Google Scholar]
- 6. Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017;542:110–4. 10.1038/nature20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma CS, Suryani S, Avery DT, et al. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol 2009;87:590–600. 10.1038/icb.2009.64 [DOI] [PubMed] [Google Scholar]
- 8. Schmitt N, Morita R, Bourdery L, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009;31:158–69. 10.1016/j.immuni.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


