Abstract
Background and Aims Fossil plants are found as fragmentary remains and understanding them as natural species requires assembly of whole-organism concepts that integrate different plant parts. Such concepts are essential for incorporating fossils in hypotheses of plant evolution and phylogeny. Plants of the Early Devonian are crucial to reconstructing the initial radiation of tracheophytes, yet few are understood as whole organisms.
Methods This study assembles a whole-plant concept for the Early Devonian lycophyte Sengelia radicans gen. et sp. nov., based on morphometric data and taphonomic observations from >1000 specimens collected in the Beartooth Butte Formation (Wyoming, USA).
Key Results Sengelia radicans occupies a key position between stem-group and derived lycophyte lineages. Sengelia had a rooting system of downward-growing root-bearing stems, formed dense monotypic mats of prostrate shoots in areas that experienced periodic flooding, and was characterized by a life-history strategy adapted for survival after floods, dominated by clonality, and featuring infrequent sexual reproduction.
Conclusions Sengelia radicans is the oldest among the very few early tracheophytes for which a detailed, rigorous whole-plant concept integrates morphology, growth habit, life history and growth environment. This plant adds to the diversity of body plans documented among lycophytes and may help elucidate patterns of morphological evolution in the clade.
Keywords: Lycopsida, Devonian, evolution, morphology, fossil, Sengelia, Drepanophycus, organismal concept, natural history, microconchid
INTRODUCTION
The Devonian (419–359 million years ago) was a pivotal period in the evolution of plants. The evolutionary radiation of land plants during this relatively short interval explored morphospace, establishing innovations that led to tracheophyte success and generating all the major vascular plant lineages except the angiosperms. Among these innovations are stem–leaf–root organography of the sporophyte, rooting systems, secondary growth, arborescence and the seed. Lower Devonian strata preserve plants that record the early stages of this radiation. The Beartooth Butte Formation of Wyoming is one of the rare units hosting extensive Early Devonian plant assemblages in North America and the only one known from the western side of the continent. The plants of the Beartooth Butte Formation, documented as early as the 1930s (Dorf, 1933, 1934), were more extensively surveyed by Tanner (1982, 1983), but only a small fraction of the fossil diversity in the unit has seen formal publication. One of the Beartooth Butte Formation plants, an abundantly occurring lycophyte for which we recently described rooting structures (Matsunaga and Tomescu, 2016), provides excellent material for reconstruction as a whole plant.
Whole-plant concepts are organismal concepts of extinct taxa and are essential for inclusion of fossils in studies that address plant systematics, phylogeny and evolution (Kenrick and Crane, 1997; Rothwell, 1999; Bateman and Hilton, 2009). Because plant fossils are often fragmentary and single specimens rarely preserve fossil plants in their entirety, information from many specimens that preserve different parts of the same organism are integrated into whole-plant concepts, providing a holistic understanding of that organism as a natural species. When combined with stratigraphic and taphonomic information, whole-plant concepts also provide insights into the environments and ecology of the plants. More broadly, detailed knowledge of extinct species that sample the breadth and depth of plant phylogeny is key to uncovering patterns of morphological evolution, as well as to developing a framework for testing hypotheses of evolution derived from studies of extant plants (Klymiuk et al., 2011).
Although whole-plant concepts have been developed for numerous extinct species, few have been constructed for early vascular plants, particularly those of the Early Devonian. Early tracheophytes are the progenitors of modern vascular plant diversity and are central to understanding the evolution of fundamental aspects of plant structure and development, such as the complex sporophyte body plan, rooting structures and growth responses. Here we assemble a whole-plant concept for the Beartooth Butte Formation lycophyte Sengelia radicans gen. et sp. nov. This detailed picture of the morphology, growth habit, life history strategies and growth environment of Sengelia places it among the best understood Early Devonian plants to date, adding to our knowledge of the early colonizers of terrestrial ecosystems and our broader understanding of lycophyte body plans.
MATERIALS AND METHODS
Plant fossils were recovered from the Beartooth Butte Formation at the Cottonwood Canyon exposure in northern Wyoming (Big Horn Co., 44°51′51″ N, 108°02′46″ W). At Cottonwood Canyon the plant fossils occur in dense autochthonous and parautochthonous assemblages, and in situ preservation is frequent. Specimens of Sengelia radicans are preserved as coalified to oxidized compressions and impressions.
Throughout northern Wyoming and southern Montana, the Beartooth Butte Formation displays geometries reminiscent of channel-fill deposits – long narrow volumes of sediment that are lenticular in cross-section. The deposits consist primarily of dolomitized siltstone and shale with dolomitized sandstone interbeds (Sandberg, 1961, 1967; Caruso and Tomescu, 2012). The unit contains fish, eurypterid and terrestrial plant fossils, as well as encrusting microconchid lophophorates (Dorf, 1933, 1934; Schultes and Dorf, 1938; Denison, 1956; Hueber, 1972; Tanner, 1982, 1983; Elliot and Johnson, 1997; Tetlie, 2007; Lamsdell and Legg, 2010; Lamsdell and Selden, 2013; Caruso and Tomescu, 2012; Steenbock and Tomescu, 2013). Based on geometry and fossil content, the unit has been interpreted as fresh- and brackish-water deposits of estuarine to fluvial origin (Dorf, 1934; Denison, 1956; Sandberg, 1961), an interpretation corroborated by limited isotope data (Fiorillo, 2000). Palynological analyses by D. C. McGregor (reported by Tanner, 1983) suggest a late Lochkovian to early Pragian (∼411 Ma) age for the plant fossil layers at Cottonwood Canyon. This age has been independently confirmed by fish biostratigraphy (Elliot and Johnson, 1997).
This study used over 400 rock specimens held in the collections curated at several institutions: the National Museum of Natural History – Smithsonian Institution (USNM), the Denver Museum of Nature and Science (DMNH), the Field Museum of Natural History (FMNH), the University of Kansas Biodiversity Institute (KU) and Humboldt State University (HPH). The specimens originate from a 1·5- to 1·8-m thick section and, within it, from locations along the outcrop no more than 10 m apart. Despite collection by several independent researchers, all specimens originate broadly from the same stratigraphic level because accessibility issues in the field, at Cottonwood Canyon, preclude collection of specimens from other levels and locations.
High-resolution images obtained in parallel with direct examination of collection specimens were used for morphological and morphometric analyses. When needed for resolution of morphological detail, selected specimens were obtained on loan from KU and NMNH. Leaf length was measured from the centre of the base to the tip. Leaf density was measured as the distance between successive leaves along the side of compressed stems, measured between the centres of leaf bases. Only specimens with well-preserved, intact leaves exhibiting regular arrangement were used to determine leaf density. A total of 1131 leafy stem- and root-bearing axis segments were measured, including 606 leafy stems, 380 horizontal root-bearing axes, and 145 vertical root-bearing axes; 615 roots were observed on 310 root-bearing axes. Stems were separated into six size (width) classes, which provide a framework for exploring the distribution of architectural features, such as branching, across the gradient in stem size (Fig. 4A–C). Size classes have 4-mm ranges, except the largest one, which includes a broader size range (23–34 mm) because specimens >28 mm wide were rare and uninformative when placed in a separate size class.
Fig. 4.
Summary of morphometric data on Sengelia radicans. (A) Number of individual stem specimens in each stem width class. All stems larger than 23 mm are lumped into a single size class, as stems wider than 30 mm are relatively rare and uninformative when separated into their own size class. Note that stems of intermediate size are most abundant in the sample. (B) Percentage of stems within each width class that exhibit branching (= branching frequency). The absolute number of specimens is indicated above histogram bars. Note that branching frequency is similar among stems of all sizes, except those in the largest size class. (C) Developmental stages of K-branching documented in the Sengelia stems shown in (B). Dark grey bars correspond to complete K-branches that exhibit full development of both branches. Black bars represent incomplete K-branches, where one branch is fully developed and the other remains dormant as a bud. Light grey bars indicate K-branch buds, where both apices are dormant. The final category, unresolved K-branches (white), indicate specimens for which preservation precludes identification of developmental stage (see Fig. 5B for example). (D) Relationship between leaf density, measured as the distance between leaves compressed in profile along the stem, and stem size. Leaf density is highest in smaller stems, and declines and becomes more variable as stem size increases. P < 0·001; r2 = 0·43. (E) Relationship between leaf length and stem size. Note that leaf length is variable relative to stem size, but that the smallest leaves are concentrated on the smallest stems. P < 0 ·001; r2 = 0·57. (F) Comparison of main stem size with the size of a subtending lateral leafy stem produced by K-branching. Although there are exceptions, lateral branches are generally smaller than subtending main stems, indicating a tendency towards subordination of lateral branch size in Sengelia.
For details of cuticular anatomy, fragments of cuticle were removed from plant compressions using cellulose acetate sheets. Peels were placed in 0·5 % hydrochloric acid to dissolve residual minerals before mounting on slides using Eukitt (O. Kindler, Freiburg, Germany). Oxidative clearing of cuticles was not necessary.
Images were obtained using a Nikon Coolpix E8800 digital camera (mounted on a Nikon Eclipse E400 microscope), a Canon EOS 5D Mark II digital camera with a Canon macro lens (EF 100 mm 1:2·8 USM) and a Nikon D70 digital camera with a bellows adapter. Measurements were obtained using ImageJ (National Institutes of Health, Bethesda, MD, USA). Figures were assembled in Photoshop CC (Adobe Systems Inc.) and the whole-plant reconstruction was rendered using Pixelmator 3·1 (Pixelmator Team, London, UK). Linear regressions for leaf size and density relative to stem size were performed using StatPlus:mac 5.8.2 statistical analysis software for Microsoft Excel (AnalystSoft Inc., Alexandria, VA, USA).
RESULTS
Systematics
The classification system follows that proposed by Li et al. (2000).
Division: Tracheophyta
Subdivision: Lycophytina
Class: Lycopsida
Order: Drepanophycales (Plesion Drepanophycales of Kenrick and Crane, 1997)
Family: Drepanophycaceae Kräusel and Weyland
Genus:Sengelia Matsunaga et Tomescu gen. nov.
Generic diagnosis: Stems branched by K-branching; simple dichotomous branching sometimes present. K-branching produces stems and root-bearing axes. Stems with lobed protostele and helical phyllotaxis; leaves vascularized by one vein. Root-bearing axes leafless or with reduced leaves only on axis base. Roots borne laterally on root-bearing axes, dichotomously branched. Sporangia cauline, round to reniform.
Etymology:Sengelia (anagram of Genselia) is named in honour of Patricia G. Gensel, University of North Carolina at Chapel Hill, in recognition of the significant contributions her research has made to our understanding of Early Devonian floras.
Type species:Sengelia radicans Matsunaga et Tomescu sp. nov.
Specific diagnosis: As for genus. Stems prostrate, up to 35 mm wide; stele up to 2 mm thick; cortex parenchymatous. K-branches with short base, each producing a subordinate stem and a root-bearing axis. Some K-branches latent on main stem as dormant buds consisting of two apical meristems; some K-branches with only one arm developed while the other forms a dormant meristem. Leaves short-triangular, with tangentially widened base and thick cuticle at tips, up to 7 mm long, helically arranged, eight to ten per gyre; gyres spaced up to 26 mm apart. Stem epidermis with numerous evenly distributed anomocytic stomata aligned with long axis parallel to stem length; epidermal cells isodiametric to slightly oblong, up to 96 × 66 µm. Leaf epidermal cells in basal two-thirds of leaf elongated towards tip, up to 150 × 60 µm, and becoming smaller and isodiametric at leaf tip. Leaf stomata rare or absent. Root-bearing axes 2–8 mm wide, unbranched, growing horizontally or downward; leafless except for small, sparse triangular leaves at base. Root-bearing axis epidermis with rare anomocytic stomata and epidermal cells longitudinally elongated, up to 155 × 31 µm. Roots lateral on root-bearing axes, slender (0·4–0·7 mm), branching apically and dichotomously up to five times. Sporangia round to slightly ovoid (2·3–3·7 mm), cauline and sessile, with no consistent position relative to leaves, and randomly distributed in loose fertile zones; dehiscence probably around distal margin.
Holotype hic designatus: PP15959 (FMNH) (Fig. 5B).
Paratypes: KU D1812 (Fig. 2B); USNM 598156 (Fig. 5A); USNM 598158 (Fig. 6A); KU D1471 (Fig. 5C); KU D1618b (Matsunaga and Tomescu, 2016, Fig. 2b); KU D1804 (Matsunaga and Tomescu, 2016, Fig. 2C); KU D1806 (Fig. 1A); KU D1810b; KU D1813 (Matsunaga and Tomescu, 2016, Fig. 2A); KU CC79-110 (Fig. 5D); DMNH 29594 (Fig. 7C); HPH 210 (Matsunaga and Tomescu, 2016, Fig. 8A–C); HPH 356 part and counterpart (Matsunaga and Tomescu, 2016, Fig. 3A); slide HPH 76-1 (Fig. 8C); slide HPH 330-1 (Fig. 8B).
Locality: South wall of Cottonwood Canyon (Big Horn County, Wyoming), 44°51′51″ N, 108°02′46″ W.
Stratigraphic position and age: Beartooth Butte Formation, late Lochkovian to early Pragian.
Etymology: The specific epithet emphasizes the strong development of rooting structures and the presence of roots.
Remarks: Two previously described species are transferred to the genus Sengelia (see discussion below).
Species: Sengelia devonica (Schweitzer et Giesen) Matsunaga et Tomescu comb. nov. The specific diagnosis, type material and material source are as stated by Schweitzer and Giesen (1980) for Drepanophycus devonicus. Basionym: Protolycopodites devonicus Weyland et Berendt (1968). (SM.B. 10 720 in Palaeontographica Abt. B 122: p. 177, Figs 18–21, Table 29).
Species: Sengelia minor (Xu, Feng, Jiang et Wang) Matsunaga et Tomescu comb. nov. The specific diagnosis, type material and material source are as stated by Xu et al. (2013) for Drepanophycus minor. Basionym: Drepanophycus minor Xu, Feng, Jiang et Wang (Xu et al., 2013). (PB21656 in Journal of Systematics and Evolution 61: p. 769, Fig. 3B, 3D, p. 768).
Fig. 5.
Shoot apex morphology and branching of Sengelia radicans. (A) Shoot apex with three successive, closely spaced K-branches illustrating different developmental stages and a dormant bud (black arrow). The two K-branches closest to the apex appear to show development of both K-branch arms (complete K-branches), with one of the branches (rooting axes) overlapped by the leafy stem of the adjacent K-branch. The third K-branch, furthest from the apex, is an incomplete K-branch with a bud at the base (white arrow). Scale bar = 10 mm. USNM 598156. (B) Complete K-branch producing a leafy stem (left) and narrower root-bearing axis (right), cut off by the right edge of the rock specimen. Note the reduced leaves at the base of the root-bearing axis (arrowheads). The diverging leafy stem branches ∼3 cm from its point of divergence from the K-branch (boxed area and inset). However, the resulting branches are not preserved on the bedding plane shown and the primary evidence of branching is a vascular trace of the K-branch in the cortex of the subtending stem (white arrowhead); this is an example of an unresolved K-branch. Scale bar = 20 mm. FMNH PP15959. (C) Leafy stem with large lateral bud (arrow). Scale bar = 20 mm. KU D1471b. (D) Leafy stem with an incomplete K-branch, consisting of a fully developed arm and a dormant bud (arrow). Scale bar = 20 mm. KU CC79-110.
Fig. 2.
Sengelia radicans phyllotaxis and leaf venation. (A) Stem with leaves (arrows) compressed on stem surface, showing loose helical phyllotaxis. Note four leaves per gyre on side of stem shown, corresponding to a full helix of eight or nine leaves. Scale bar = 10 mm. KU D1499. (B–D) Leaves with a single robust leaf trace (arrows). Note thick strands possibly representing leaf traces in the stem cortex (white arrowheads) in (C). Scale bars = 5 mm. KU D1812a (B), FMNH PP16098 (C), HPH 248 (D). (E, F) Leaves in which two strands (black arrows) converge to form a single, thicker trace. Thin lines indicated by white arrowheads in the stem cortex (E) most likely represent artefacts of compression and not leaf traces. Scale bars = 3 mm. DMNH 6370 (E), DMNH 6362 (F). (G) Leaf with a thin central strand, possibly representing an artefact of compression. Scale bar = 5 mm. HPH 227.
Fig. 6.
Sporangia of Sengelia radicans. (A) Stem with at least six sporangia preserved. Sporangia indicated by arrows are shown in (B) and (C). Scale bar = 10 mm. USNM 598158. (B, C) Details of sporangia indicated by arrows in (A). Scale bars = 3 mm. (D, E) Sporangia from specimen DMNH 6362. Scale bars = 3 mm. (F) Sporangium (arrow) from oxidized stem. Note numerous encrusting microconchids on the stem, and the presence of strands in the cortex possibly representing leaf traces. Scale bar = 10 mm. KU D1217b. (G) Sporangium with marginal dehiscence line (arrow). Scale bar = 3 mm. USNM 598159. (H) Sporangium from specimen KU D1265a. Scale bar = 3 mm.
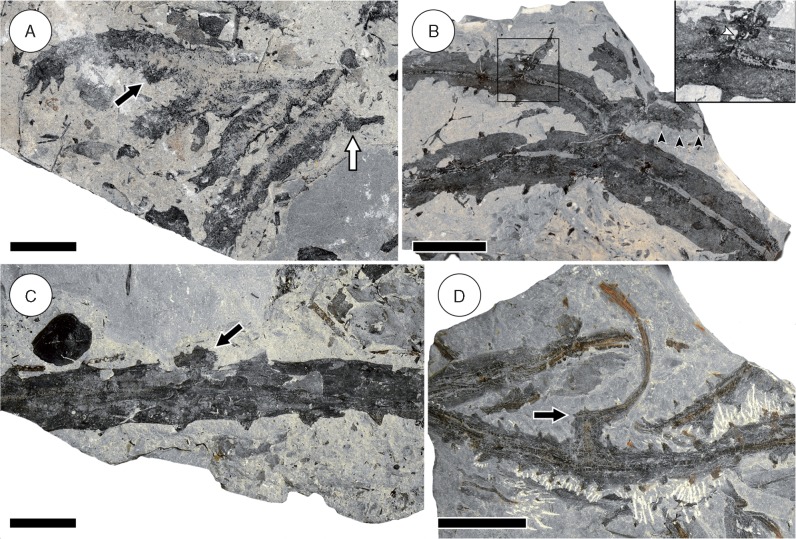
Fig. 1.
Organography of Sengelia radicans. (A) Intertwined leafy stems from a stem mat layer. Note thin central vascular strand and small triangular leaves. Scale bar = 20 mm. KU D1806. (B) Horizontal root-bearing axes with fine lateral roots. Note parallel orientation of the axes. Scale bar = 5 mm. HPH 28. (C) Dichotomously branching root showing three orders of branching. Note fine central vascular strand. Subtending root-bearing axis not visible on the bedding plane shown. Scale bar = 5 mm. KU D1616b.
Fig. 7.
in situ preservation and stratigraphy of fossiliferous layers at Cottonwood Canyon. (A) Rock specimen from dense stem mat layer consisting of finely laminated shales. Arrows indicate several separate bedding planes containing Sengelia stems over <1 cm of rock thickness. Scale bar = 40 mm. HPH 77. (B) Outcrop at Cottonwood Canyon showing heterolithic deposits of finely laminated shale (black arrows) containing in situ Sengelia stem mats [e.g. panel (A)] and siltstone layers (white arrows) containing in situ vertical root-bearing axes [e.g. panel (C)]. The sequence corresponds to cycles of colonization and burial of Sengelia mats by flood events. Hammer head = 17 cm across. (C) Vertical root-bearing axes crossing bedding planes in a siltstone layer. Lateral roots diverging horizontally indicated by arrows. Scale bar = 20 mm. DMNH 29594. (D) Three successive layers of Sengelia stems (arrows) separated by ∼5 mm of sediment, indicative of smaller-scale flood episodes. Vertical axes crossing bedding planes (arrowheads) most likely represent root-bearing axes. Scale bar = 20 mm. HPH 231. (E) Sengelia stem mat layer (arrow) underlain by siltstone that exhibits a zone of mottling (black bar), which corresponds to the amplitude of water table oscillations in the sediment. Hammer head = 17 cm across.
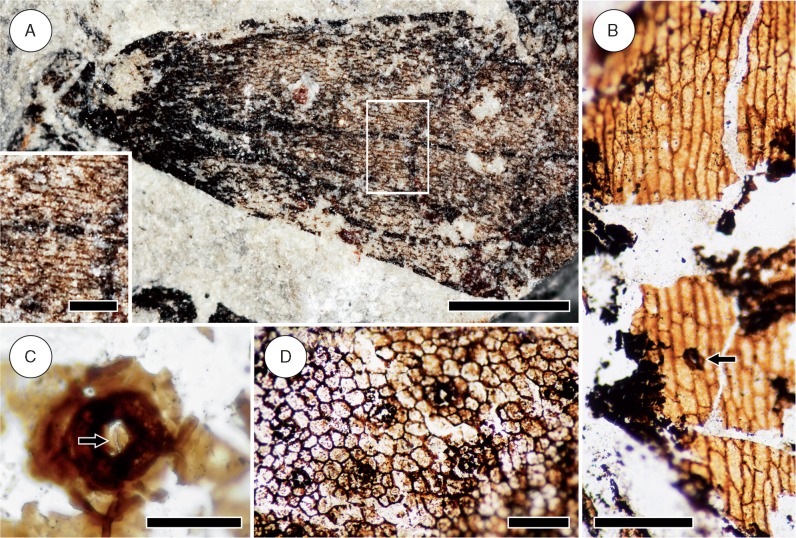
Fig. 8.
Epidermal anatomy of Sengelia radicans. (A) Leaf cuticle showing epidermal anatomy. Epidermal cells are elongate near the leaf base and medially (boxed area and inset; scale bar = 250 µm) and become more isodiametric towards the leaf tip. No stomata are visible. Note darkened leaf tip and the dark lines in the leaf; the latter likely represent artefacts of preservation and not veins. Scale bar = 1 mm. USNM 598188. (B) Cuticle of root-bearing axis showing elongated epidermal cells and a rare stoma (arrow). Scale bar = 200 µm. HPH 330. (C) Detail of stoma from leafy stem. Note area of thinner cuticle surrounding the pore (arrow). Scale bar = 50 µm. HPH 76. (D) Cuticle of leafy stem showing epidermal anatomy. Note the generally isodiametric shape of epidermal cells and the numerous, evenly distributed stomata. Scale bar = 200 µm. HPH 333.
Fig. 3.
Microconchid colonization of Sengelia radicans. (A) Leafy stem colonized by numerous encrusting microconchids (e.g. arrowhead). Scale bar = 10 mm. HPH 245. (B) Horizontal root-bearing axis with microconchids (arrowhead). Note base of lateral root tuft diverging from axis (arrow). Scale bar = 5 mm. HPH 255. (C) Detail of microconchid showing characteristic coiled shell. Scale bar = 1 mm. HPH 3.
Description
Stems.
The body plan of Sengelia radicans consists of three distinct types of axial organs: robust leafy stems, leafless root-bearing axes and roots (Fig. 1). Leafy stems are 3–34 mm wide (mean 15 mm; n = 567) and bear small deltoid leaves (1–7 mm long) (Figs 1A, 2 and 5). Continuous stem fragments recovered on individual rock specimens are up to 25 cm long. The total combined length of all stem fragments used in this study is 35 m. The stems have a narrow (1–2 mm) stele (Figs 1 and 2B, C). The cortex probably lacks sclerenchyma and, even in the largest stems, is preserved as very thin carbonaceous material. Stems were divided into six size classes (Fig. 4A) to examine the distribution of branching across the gradient in stem size. In our sample, stems of intermediate size were more abundant than large and small stems. Microconchids (Fig. 3), encrusting aquatic lophophorates (Zaton et al., 2012), are present on 325 of the stem fragments (60 %), indicating that these were temporarily submerged prior to fossilization (Caruso and Tomescu, 2012).
Leaves.
Leaves are 1–7 mm long, deltoid in profile, and form loose helices of eight to ten leaves per gyre (Fig. 2). Leaf length is positively correlated with stem size (F = 107; P < 0·001; r2 = 0·43) (Fig. 4E). Although larger stems show variation in leaf size, the smallest leaves (1–2 mm) are found only on very small stems or shoot tips (Fig. 5A). The distance between leaf gyres, used as a measure of leaf density, is also positively correlated with stem size (F = 154; P < 0·001; r2 = 0·57) (Fig. 4D). Leaf tips exhibit a darker area consisting of thicker carbonaceous material, which may be related to thicker cuticle or smaller cell size in that region (Fig. 8A). Laterally compressed leaves reveal a vascular trace that enters the leaf at a right angle and terminates at the leaf tip (Fig. 2B–D). These traces are relatively robust, 0·2–0·35 mm thick, and show consistent position and orientation within the leaf. Tracheids recovered from these traces by Tanner (1983) confirm they represent vascular strands. In some specimens two thinner veins appear to converge at the base of the leaf (Fig 2E, F), indicating that, at least in some leaves, the single vein may be formed from the convergence of two smaller ones.
Multiple fine strands of similar size to leaf traces are often preserved in the cortex of stems, sometimes seen diverging from the main stele (Figs 2C and 6F). Some of them, particularly those that are relatively robust, may represent leaf traces, while the thinner and more irregular structures are likely artefacts of compression of the cortex, epidermis, or cuticle (Fig. 2E). Preservation of tracheids is rare in the studied material, and none were recovered from these structures to ascertain the nature of these strands.
Root-bearing axes.
The root-bearing axes are narrow (2–8 mm; mean 4·4 mm; n = 452) and bear lateral dichotomously branching roots (Figs 1B and 3B). For detailed descriptions of the root-bearing axes and discussions of conspecificity, see Matsunaga and Tomescu (2016). Like the leafy stems, root-bearing axes possess a thin central stele (0·5–1·5 mm), and form compressions consisting of very thin carbonaceous material. Root-bearing axes are found preserved in situ horizontally, along bedding planes (Fig. 1B), as well as exposed on vertical breaks of rock specimens, where they extend downwards, crossing the bedding planes (Fig. 7C) (Matsunaga and Tomescu, 2016). Horizontal and vertical root-bearing axes preserved in situ are often arranged parallel to one another and do not intersect (Figs 1B and 7C). Although the root-bearing axes are generally leafless, sparse reduced leaves can be present at their bases, close to their divergence from leafy stems (Fig. 5B). Despite the consistent downward growth of root-bearing axes, the presence of leaves and exogenous development by apical branching (in K-branches) indicate that these axes are stems modified for rooting (Matsunaga and Tomescu, 2016). Colonization by encrusting microconchids is much less common on root-bearing axes compared with leafy stems, with only 109 horizontal axes (30 %) colonized (Fig. 3B). Vertical root-bearing axes, which grew underground, lack microconchids.
Roots.
The roots of Sengelia are found exclusively on root-bearing axes. They are fine (0·4–0·7 mm wide), branch dichotomously and have a thin central stele (Fig. 1C) that is continuous with that of subtending axes (Matsunaga and Tomescu, 2016). The roots exhibit variable arrangement along the root-bearing axes, ranging from alternate to opposite in individual specimens. Roots occasionally possess darkened regions at their tips, which may represent preserved root caps. The largest root tufts observed extend as far as 20 mm laterally from the subtending root-bearing axes and have up to five orders of branching (Matsunaga and Tomescu, 2016).
Branching.
Branching in Sengelia consists exclusively of K-branching and occurs only in leafy stems. Branching has not been observed in any root-bearing axis. K-branching is characterized by two very closely spaced dichotomies, the second of which produces two branches (‘arms’) that diverge at wide angles (∼180°) from one another, generating a K-shaped morphology (Fig. 5). K-branches are supplied by a thick vascular strand that diverges from the stele of the main stem at ∼90°. Branching can be successive along a main axis or reiterative, consisting of several orders of closely spaced K-branches (5B), and occurs with similar frequency on all but the largest stems (Fig. 4B). Branching intervals along individual shoots range from 2 to at least 25 cm; the maximum branching interval is based on the longest recovered Sengelia stem fragment that did not show branching. Size comparisons between main stems and their lateral leafy branches reveal that lateral branches are consistently smaller than the corresponding main stem, at least at the base (Fig. 4F). This indicates that branching of leafy stems produced subordinate (rather than equal) lateral branches, possibly in a pseudomonopodial pattern. Furthermore, in all K-branches one arm is significantly smaller than the other. Because root-bearing axes are consistently narrower than leafy stems, the size inequality of K-branch arms indicates that each K-branch produces a root-bearing axis and a leafy stem.
Several developmental stages of K-branching were documented (Matsunaga and Tomescu, 2016): (1) lateral buds, which are K-branch primordia that exhibit two adjacent apices (Fig. 5B); (2) incomplete K-branches, consisting of a fully developed arm with a dormant bud at its base (Fig. 5C); and (3) complete K-branches exhibiting full development of both arms (Fig. 5B). At shoot tips, branching can occur in close succession in the vicinity of the apex, displaying varying degrees of development, with some K-branch arms fully developed and others that remain dormant as buds (Fig. 5A). The distribution of these developmental stages is relatively even among stems of different sizes: buds and complete K-branches occur on stems of all size classes, with complete K-branches being the most common (Fig. 4C). Although relatively rare, incomplete K-branches are documented in stems of all sizes except the largest size class.
Many K-branches, referred to as unresolved K-branches, are incompletely preserved. In some specimens the vascular trace supplying the K-branch, seen in the cortex of the subtending stem, is the only preserved evidence of branching (Fig. 5B). However, in all cases this vascular trace diverges from the main stele at a right angle, consistent with K-branching. In many instances, one or both arms of a K-branch are either not preserved or are covered by other plant material, usually the main stem or other overlapping stems. This is especially frequent in Sengelia stem mat layers, in which the high density of stems obscures details of K-branching and often only the bases of K-branches are visible.
Sporangia.
Sporangia are round to slightly ovoid, 2·3–3·7 mm in diameter, and consist of thick carbonaceous material (Fig. 6). They are cauline on leafy stems, and sessile, and some appear to have a dehiscence slit that runs around the distal margin (Fig. 6G). The sporangia are positioned randomly on stems, in no consistent relationship with the leaves. Despite the large number of specimens examined in this study, sporangia are extremely rare: only 33 were recovered from a total of 20 stem fragments, and 14 of these sporangia came from only three of the 20 stems. Clear or consistent vasculature supplying sporangia was not observed. It is possible that vascular strands were thin like the leaf traces and are not well preserved in the material, or are oriented such that they are obscured as a result of compression of the stems. No spores were recovered, but the consistent size and shape, as well as the presence of a discernable dehiscence slit in some specimens, supports their interpretation as sporangia. Structures interpreted by Tanner (1983) as sporangia are significantly larger (5 × 8 mm) and possess a thick vascular trace identical to those supplying K-branches. We consider these structures to be poorly preserved K-branch buds and not sporangia.
Stratigraphy and in situ plant preservation.
Leafy stems and root-bearing axes of Sengelia are frequently preserved in situ at Cottonwood Canyon in heterolithic deposits of laminated shales and more massive siltstone (Fig. 7) (Matsunaga and Tomescu, 2016). Leafy stems and associated root-bearing axes form densely interwoven mats in layers of dark, finely laminated shale with numerous irregular planes of cleavage (Fig. 7A). The stem mat layers alternate with hard-cemented siltstones that contain transported plant material and other organic detritus. These siltstone layers are penetrated by vertical in situ root-bearing axes (Fig. 7C). Lateral roots borne on these axes extend horizontally and are observed in vertical breaks in the rock, as well as on horizontal breaks along bedding planes (Matsunaga and Tomescu, 2016). Well preserved horizontal root-bearing axes lacking microconchids are also found in the siltstone layers, possibly representing in situ axes growing horizontally underground.
The heterolithic sequence containing in situ plant material reflects cycles of colonization by Sengelia and subsequent burial by flood events on an indefinite time scale (Fig. 7B). A few occurrences provide evidence for smaller-scale floods burying shorter-lived populations. Such specimens consist of several successive layers of Sengelia stems separated by ∼5 mm of sediment (Fig. 7D). Unlike in situ stems in shale layers, these represent a single layer of stems and not multiple layers of matted stems. Vertical axes grow through these layers, and although their organ identity is unclear due to preservation, they are most likely root-bearing axes.
Epidermis and stomata.
Cuticles of leafy stems, root-bearing axes and leaves reveal details of epidermal anatomy and stomata (Fig. 8). Epidermal cells of leafy stems are isodiametric to slightly elongate longitudinally, 46–96 × 37–66 µm (Fig. 8D). Abundant stomata are distributed evenly, with an average density of seven to eight stomata per square millimetre, and oriented longitudinally on the stem. The two reniform guard cells, 45 × 15 µm, possess a thick cuticle that appears darker than that of surrounding epidermal cells. Each guard cell has an area of thinner cuticle surrounding the pore (Fig. 8C). One leaf with recognizable cuticular anatomy was recovered. Leaf epidermal cells are elongate, 100–150 × 40–60 µm, and become smaller and isodiametric near the leaf apex (Fig. 8A). The leaf epidermis shows no stomata. However, regions of it are poorly preserved, so the possibility that some parts of the leaf had stomata cannot be excluded. Sengelia is, hence, interpreted as having rare or no stomata on leaves. Epidermal cells of root-bearing axes are narrow and elongate-rectangular, 95–155 × 22–31 µm (Fig. 8B). When present, stomata of root-bearing axes have the same dimensions as those of the leafy stems, but are sparse and irregularly distributed.
Stomata of leafy stems are anomocytic (Matsunaga and Tomescu, 2016) and surrounded by six or seven epidermal cells, which are sometimes smaller or are shaped differently from other epidermal cells. These cells do not represent subsidiary cells because they do not occur consistently with all the stomata and their size and shape are variable, exhibiting a range in morphology that grades into that of typical epidermal cells (Matsunaga and Tomescu, 2016). Moreover, such cells are not associated with the stomata of root-bearing axes, which are instead surrounded by the elongate cells characteristic of the epidermis of these axes.
Vascular tissues.
All vegetative organs of Sengelia (leafy stems, root-bearing axes, leaves and roots) are characterized by a single central vascular strand. In leafy stems and root-bearing axes the stele often appears longitudinally ribbed (Figs 1A and 2C), consistent with the lobed cross-sectional stele anatomy reported by Tanner (1983) (Fig. 9A). No secondary vascular tissues are present, even in the largest stems.
Fig. 9.
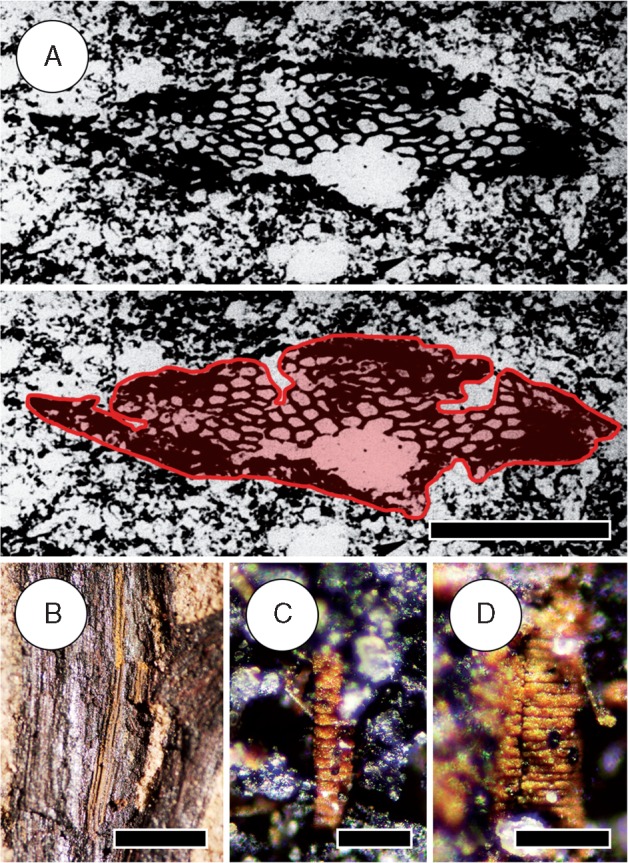
Tracheids of Sengelia radicans. (A) Cross-section of the stele showing lobed outline, traced for clarity (bottom); from Tanner (1983, Fig. 87, unpublished). The stele is slightly compressed and only part of it is preserved. Three lobes are visible, recognized despite some compression by the pattern of tracheid size whereby tracheids towards lobe tips are smaller than those at the centre of the stele. Scale bar = 250 µm. (B) External view of the stele of a leafy stem showing tracheids (longitudinal striations). Note the K-branch trace diverging from it (at bottom). Scale bar = 1 mm. HPH 347. (C, D) Iron oxide–hydroxide lumen casts of metaxylem tracheids exhibiting scalariform secondary wall thickenings. Scale bars = 75 µm. HPH 315.
Samples from the stele of leafy stems consist of tracheids preserved as iron oxide–hydroxide lumen casts (Fig. 9B–D). The majority of tracheids observed exhibit secondary wall thickenings consisting of transverse bars spaced ∼10 µm apart (Fig. 9C, D), indicative of either helical or scalariform thickenings. The abundance of tracheids exhibiting this pattern within the stele indicates that these are metaxylem tracheids. Given their mode of preservation, it is difficult to confidently resolve the type of secondary wall thickening pattern, which could represent scalariform, helical or G-type thickenings.
Justification for a new genus
The Drepanophycales (sensuKenrick and Crane, 1997) include the genera Drepanophycus, Asteroxylon and Baragwanathia. These taxa, sometimes referred to as ‘pre-lycopsids’ (Gensel and Andrews, 1984; Gensel and Berry, 2001), combine characters found in crown group lycophytes with characters found in stem group lycophytes (zosterophylls), such as vascularized or partially vascularized leaves (found in crown lycophytes but not in zosterophylls), K-branching (characteristic of some zosterophylls but not of crown lycophytes), and cauline rather than leaf-borne sporangia (‘cauline’ sporangia on axes in zosterophylls, but adaxial sporangia on sporophylls in crown lycophytes). The presence of these characters in Sengelia suggests that this plant is allied with the Drepanophycales. However, substantial morphological differences between Sengelia and previously described drepanophycaleans, as well as other morphologically similar lycophytes (Table 1), indicate that Sengelia is best treated as a distinct genus. The most notable differences include leaf size and shape, overall branching architecture consisting exclusively of K-branching and, most significantly, a rooting system comprising specialized root-bearing stems.
Table 1.
Morphological comparison between Sengelia and early lycophyte genera
| Sengelia radicans | Drepanophycus | Hueberia | Asteroxylon | Baragwanathia | Kaulangiophyton | Halleophyton | |
|---|---|---|---|---|---|---|---|
| Maximum stem width (mm) | 35 | 40 | 3·4 | 10 | 32 | 9 | 15 |
| Stem branching | Only K-branching | Isotomous–anisotomous; some K-branching | Isotomous–pseudomonopodial; some K-branching | Pseudomonopodial | Anisotomous | Only K-branching | ? |
| Specialized root-bearing axes | Present | Present (only in D. devonicus) | Absent | Absent | Absent | Absent | Absent |
| Maximum leaf length (mm) | 7 | 20 | 2·3 | 5 | 25 | 2 | 5 |
| Mature leaf shape | Deltoid | Deltoid to falcate | Long–triangular to falcate | Narrowly elongate | Linear | Spine-like | Linear |
| Sporangia shape | Round | Round–elliptical | Round | Reniform | Reniform | Ovoid | Round–elliptical |
| Sporangia size (mm) | 2·3–3·7 | 3-5 (up to 8 mm in D. devonicus) | 0·6–0·9 | 1·7–15·2 × 0·6–5·6 | 2 | 6–8 × 4–5 | 1·67–1·87 × 3·13–3·3 |
| Sporangia attachment | Sessile | Stalked | ? Sessile | Stalked | ? | Stalked | ? Sessile |
| cauline | cauline | cauline | cauline | In leaf axils | cauline | cauline | |
| References | this study | Rayner, 1984; Schweitzer and Giesen, 1980; Li and Edwards, 1995; Li et al., 2000 | Xue, 2013 | Kidston and Lang, 1920b; Gensel and Andrews, 1984; Kerp et al., 2013 | Hueber, 1983, 1992 | Gensel et al., 1969 | Li and Edwards, 1997 |
Other Sengelia species
Genus Drepanophycus has historically been broadly defined and poorly circumscribed, with many of the species assigned to this genus based on a limited number of characters and on specimens comprising only vegetative material (Gensel et al., 1969; Gensel and Andrews, 1984; Li et al., 2000). The best understood Drepanophycus species are those described from material that preserves sporangia, branching patterns and occasionally rooting structures: D. spinaeformis (e.g. Schweitzer, 1980; Rayner, 1984; Li et al., 2000), D. qujingensis (Li and Edwards, 1995), D. devonicus (Schweitzer and Giesen, 1980) and D. minor (Xu et al., 2013). When characters of all of these relatively well understood species are taken together, there is significant overlap between genus Drepanophycus and Sengelia. Like Sengelia, many members of Drepanophycus exhibit K-branching, some have small triangular leaves, and in some of them K-branching produces root-bearing axes resembling those of Sengelia. For these reasons, the Cottonwood Canyon material assigned here to Sengelia was originally placed in D.devonicus by Tanner (1983). However, two species of Drepanophycus stand out from the rest in terms of their similarity to Sengelia (Table 2). Drepanophycusdevonicus and D. minor both exhibit frequent K-branching, have consistently short leaves, and have root-bearing axes produced by K-branching. If these two species are excluded, the differences between Sengelia and the remaining Drepanophycus species become much more clear-cut: (1) branching in Sengelia is exclusively by K-branching, whereas K-branching is present but not prevalent in Drepanophycus; (2) mature leaves are significantly shorter in Sengelia than in Drepanophycus; and (3) specialized root-bearing axes produced by K-branching are seen in Sengelia but not Drepanophycus. These differences are consistent with Sengelia as a new genus and indicate that D. devonicus and D. minor are more similar to Sengelia than they are to other members of Drepanophycus. We therefore propose that these species be transferred to genus Sengelia: Sengelia devonica (Schweitzer et Giesen) Matsunaga et Tomescu comb. nov., Sengelia minor (Xu, Feng, Jiang et Wang) Matsunaga et Tomescu comb. nov.
Table 2.
Comparative features of Sengelia and Drepanophycus.
| Sengelia radicans | Drepanophycus minor | Drepanophycus devonicus | Other Drepanophycus species | |
|---|---|---|---|---|
| Stratigraphic occurrence | Lower Devonian | Middle Devonian | Middle Devonian | Lower–Upper Devonian |
| (Lochkovian–Pragian; ∼410 Ma) | (Givetian; 387–382 Ma) | (Eifelian; 393–387 Ma) | ||
| Maximum. stem width (mm) | 35 | 14 | 20 | 42 |
| Branching | Only K-branching | Dichotomous and K-branching | Only K-branching | Dichotomous and K-branching |
| Root-bearing axes produced by K-branching | Present | Present | Present | Absent/unknown |
| Maximum width of root-bearing axes (mm) | 8 | 6 | 4·5 | – |
| Branching of root-bearing axes | Not observed | Unknown | Present | – |
| Maximum leaf length (mm) | 7 | 6 | 6 | 20 |
| Leaf shape | Triangular–oblong | Falcate (upcurved) | Triangular | Falcate (recurved) or spine-like |
| Sporangia shape | round–slightly ovoid | reniform | ovoid–reniform | circular–reniform |
| Sporangia size (mm) | 2·3–3·7 | 2·1–3·3 × 1·9–2·5 | 3·5–5 × 5·5–8·0 | 3–5 |
| Sporangia attachment | Sessile | 1 mm stalk | 3–4 mm stalk | 1 mm stalk |
| References | This paper | Xu et al., 2013 | Schweitzer and Giesen, 1980 | Grierson and Banks, 1963; Banks and Grierson, 1968; Schweitzer, 1980; Rayner, 1984; Li and Edwards, 1995; Li et al., 2000 |
Some occurrences of D.spinaeformis are associated with structures that resemble the root-bearing axes of Sengelia. One of these is the Lower Devonian material described by Rayner (1984), which consists of slender leafless axes with lateral root-like structures, preserved at an angle to bedding planes and suggesting in situ preservation. However, these axes were not found connected to the specimens of D. spinaeformis at the locality. Similar root-bearing axes are also associated with D. spinaeformis material described from the Middle Devonian by Schweitzer (1980). However, the relationships of these axes to the rest of the material assigned to D. spinaeformis are unclear in the published descriptions and figured specimens. While these two D. spinaeformis occurrences could represent additional examples of Sengelia-like plants, lack of organic connection and the small numbers of specimens preclude unequivocal taxonomic reassessment of the material.
DISCUSSION
Growth habit of Sengelia radicans
Sengelia is interpreted as forming dense, low-growing mats with a prostrate, creeping habit (Fig. 10), as indicated by morphological features and taphonomy. The plant material forms dense in situ mats of interwoven stems in the shale layers (Fig. 7). The larger Sengelia stems in these in situ populations were unlikely to be self-supporting because, despite their large size (up to 34 mm wide), they form very thin compressions. The absence of any thick carbonaceous material indicates that the cortex of stems was a low-density tissue probably consisting of large, thin-walled cells or much aerenchyma and lacking significant supporting tissue. This, along with the large size of stems and their narrow steles, are inconsistent with a self-supporting habit. These features point towards a creeping growth habit in which only the apical regions of stems were possibly erect, supported by turgor. Furthermore, root-bearing axes were most likely produced by each K-branch along the shoot system, anchoring it to the ground, and K-branches with fully developed root-bearing axes occur on stems of all sizes (Fig. 4C). Together, these suggest that the entire shoot system of Sengelia was closely appressed to the substrate, consistent with a prostrate habit. The abundance of stomata on stems indicates that they were photosynthetic and, given the large size and surface area of stems relative to leaves, probably the main photosynthetic organ. Segments of root-bearing axes growing above ground, along the substrate, may also have been photosynthetic, as some specimens have been documented with stomata (Fig. 8B). The presence of well-preserved horizontal root-bearing axes in siltstone layers indicates that the axes sometimes grew horizontally underground, possibly in response to encountering physical barriers in the sediment such as large fish plates, which occur frequently in these layers.
Fig. 10.
Whole-plant reconstruction of Sengelia radicans. The reconstruction shows one Sengelia individual to emphasize the prostrate or creeping habit in which stems are erect only near shoot tips, in accordance with the non-self-supporting nature of larger stems. Frequent and sometimes reiterative K-branching produced an extensive shoot system that formed dense mats of stems on the ground. Root-bearing axes are shown growing either along the substrate before growing downwards, or straight downwards from the base, and have sparse reduced leaves near the base. Dichotomously branching roots extend laterally from the root-bearing axes. Successive K-branching is depicted at variable intervals, with longer intervals on larger stems and with the number of K-branches relative to total stem length matching the morphometric data from this study. Leaf sizes and densities relative to stem size are also consistent with the morphometric data. Sporangia (yellow dots) are sparse and aggregated into loosely defined fertile areas. The underlying stem mat layer (bottom right) represents a previous Sengelia population buried in situ by a major flood event, consistent with the stratigraphic sequence observed at the outcrop at Cottonwood Canyon (Fig. 7B). Root-bearing axes are shown diverging downwards from it. The reconstructed Sengelia plant is depicted colonizing the sediments that buried the previous population. Scale bar = 10 cm.
The contrasting shape of epidermal cells between root-bearing axes and leafy stems suggests different developmental patterns for the two organs (Fig. 8). The comparatively elongate shape of epidermal cells in root-bearing axes indicates that significant lengthening of the axis was achieved by cell elongation. This contrasts with the isodiametric cells of leafy stems, which are consistent with a growth pattern that relies much more on cell division. Elongation during the growth of root-bearing axes may explain the irregular arrangement and sparse distribution of the minute leaves found in proximal regions of these axes. Furthermore, growth and elongation of the axis following bud dormancy could have resulted in telescoping of the axis out from underneath the leaf primordia produced by the initial bifurcation of the lateral branch, prior to dormancy. In this case, leaves of the root-bearing axes would have been produced only in the bud stage, but not upon reactivation of the meristem following dormancy.
Kin recognition mechanisms?
The parallel, non-intersecting trajectories of many in situ root-bearing axes of Sengelia (Figs 1B and 7C) are reminiscent of the effects of root foraging behaviour under constraints imposed by kin recognition and avoidance mechanisms. Studies of extant plants have demonstrated that some species modulate their root foraging behaviour to minimize interference between roots, in order to reduce competition and maximize resource exploitation by conspecifics and closely related or clonal individuals (Mahall and Callaway, 1991; Holzapfel and Alpert, 2002; Dudley and File, 2007; Semchenko et al., 2007; Biedrzycki et al., 2010; Bhatt et al., 2011). The parallel orientation of closely spaced Sengelia root-bearing axes may reflect an avoidance strategy aimed at reducing competition for resources between root-bearing axes of the same or closely related individuals and at optimally exploiting soil resources. Such patterns have been documented in the fossil record, for example in the Permian (252–299 Ma) rooting system of Pinnatiramosus qianensis, in which an avoidance and space-filling strategy has been proposed based on root branches that curve away from one another or follow parallel trajectories (Edwards et al., 2007). Rooting structures of Drepanophycus recently documented from the Early Devonian of China (Xue et al., 2016) show comparable arrangement of vertical axes within the sediment and could provide another source of data for investigating this type of interaction.
Growth environment
Stratigraphy at the fossil locality provides information on the growth environment of Sengelia. The layers of hard-cemented siltstone containing abundant organic debris, which are penetrated by in situ Sengelia root-bearing axes, alternate with dark shales containing in situ mats of Sengelia stems (Fig. 7). This pattern of alternating lithology and fossil content reflects cycles of substrate colonization by Sengelia populations (shale layers containing stem mats) and subsequent burial of these populations by major flood events, which deposited the siltstone layers. The same siltstone layers provided the substrate for establishment of new Sengelia populations, as indicated by the presence of downward-growing root-bearing axes in the siltstones beneath the stem mat layers.
Similar patterns are seen in the late Pragian–early Emsian Xujiachong Formation of China, in which floodplain sediments contain abundant in situ rooting structures belonging to Drepanophycus (Xue et al., 2016). These rooting structures, interpreted as rhizomes, bear a striking resemblance to Sengelia rooting structures documented from the uppermost layer exposed at Cottonwood Canyon (Fig. 7E; see also Matsunaga and Tomescu, 2016, Fig. 9D), in which the rooting horizon is significantly oxidized relative to those preserved in underlying layers (e.g. Fig 7C). These similarities suggest that comparable processes of floodplain colonization and sediment stabilization by drepanophycalean lycophtyes were occurring in geographically distant regions during the Early Devonian, and were potentially widespread.
The presence of encrusting microconchids, sometimes in high numbers, on Sengelia fossils (Fig. 3) indicates that some plant populations were submerged for extended intervals prior to burial, consistent with the stratigraphic evidence for flood episodes. Nevertheless, Sengelia was a fully terrestrial plant and not aquatic or amphibious, as demonstrated by the absence of microconchids from 40 % of stems, which indicates that not all stems experienced extended submergence, and by the numerous stomata found on stems, which are inconsistent with an aquatic habit.
Furthermore, additional stratigraphic information demonstrates that the water table oscillated below the ground surface colonized by Sengelia populations. This is indicated by the presence of a ∼40-cm zone of mottling in the siltstone below the uppermost stem mat layer and its rooting horizon (Fig. 7E). Such mottling is caused by the remobilization of oxides in the sediment due to small fluctuations in the water table (P. Holterhoff, Hess Corporation, USA, pers. comm.). The thickness of the mottled layer indicates the amplitude of the water table oscillations within the sediment, far below the surface colonized by plants, and demonstrates that submergence of stem mats was temporary, as a result of occasional flood events.
Together, the data from microconchids, stomata, taphonomy and stratigraphy indicate that Sengelia was a fully terrestrial plant that grew in environments prone to periodic flooding, such as river floodplains. A floodplain environment is supported by the geometry of deposits of the Beartooth Butte Formation, which is consistent with a river channel fill.
Life history of Sengelia radicans – clonality and expansion in monospecific stands
The non-self-supporting nature of the leafy stems, coupled with the high density of stems in the shale layers, indicates that Sengelia grew in dense populations with a mat-forming habit. Frequent, sometimes reiterative (Fig. 5B) K-branching and the rarity of sporangia recovered from stem material suggest that vegetative growth and clonality was the primary mode of propagation that allowed populations of this plant to colonize new territory or overlying sediment after burial by flood episodes. Although several other plant taxa have been documented at Cottonwood Canyon (Tanner, 1983), their absence from in situ stem mats indicates that Sengelia formed monospecific stands. These features are consistent with the ‘turfing in’ strategy of space occupation seen in many early land plants, which is thought to have compensated for limited photosynthetic surface area and resource acquisition capacities (DiMichele and Hook, 1992). Additionally, the many dormant buds present on stems could be part of a survival strategy that involved regrowth through overlying sediment after burial by floods (Fig. 5C). A role in regrowth after burial has been suggested for comparable structures of other early vascular plants (Gensel et al., 2001; Kenrick, 2002), many of which occupied flood-prone environments (DiMichele and Hook, 1992; Hotton et al., 2001). Concurrently, the spatial relationships of in situ root-bearing axes suggest subsurface foraging behaviour that may reflect kin recognition and avoidance mechanisms. If explored in more detail, data on this aspect of Sengelia eco-physiology may provide evidence that stem mats consisted of closely related or clonal individuals. All of these features taken together point towards a life history strategy adapted for survival after flooding, dominated by clonality and characterized by infrequent sexual reproduction.
Conclusions
Although aspects of its anatomy have yet to be documented, Sengelia emerges as one of the best characterized early vascular plants to date. The whole-plant concept assembled here (Fig. 10) provides insights not only into the morphology of the shoot and rooting systems, but also into the growth environment and life history of Sengelia. Comparable understanding of these aspects of organismal biology in other Early Devonian plants come from studies of the Emsian (∼405 Ma) Rhynie Chert flora, e.g. Aglaophyton, Rhynia, Nothia, Asteroxylon (Kidston and Lang, 1920a, b, 1921; Edwards, 1980, 1986; Remy and Hass, 1996; Roth-Nebelsick et al., 2000; Kerp et al., 2001, 2013; Daviero-Gomez et al., 2005; Taylor et al., 2005; Wellman et al., 2006).
From an evolutionary perspective, Sengelia contributes to our knowledge of the diversity of lycophyte body plans and rooting systems (Matsunaga and Tomescu, 2016). Although the stem-derived rooting system of Sengelia may seem unusual, rooting structures derived from stems or axes, rather than roots, are seen throughout the lycophyte clade. Zosterophyllum species produced rooting structures derived from one arm of a K-branch (Walton, 1964; Hao et al., 2010). Asteroxylon, a drepanophycalean lycophyte, produced downward-growing branching axes exogenously from rhizomatous stems (Kidston and Lang, 1920b). Phylloglossum, an extant member of the Lycopodiales found only in Australia and New Zealand, produces a downward-growing subterranean stem that is enlarged for starch storage, as well as structures referred to as roots, but which develop exogenously (Bower, 1885). Extant Selaginella produces rhizophores, leafless organs of exogenous origin that grow downward and produce roots at their tip (Gifford and Foster, 1989). Rhizomorphic lycophytes, including extant Isoetes and several extinct lineages, have complex rooting systems (Hetherington et al., 2016) demonstrated as shoot homologues (Rothwell and Erwin, 1985).
Sengelia provides yet another example of this type of body plan, in which stems or axes have a rooting function, in a key position between stem-group lycophytes (zosterophylls) and more derived lineages. This diversity of lycophyte rooting systems raises questions about the evolution of body plans in the clade, while underlying similarities, such as positive gravitropism in stems and undifferentiated axes, suggest shared developmental mechanisms. All of these provide exciting avenues of research for future studies that could elucidate patterns of lycophyte phylogeny and evolution, and may provide insights into the mechanisms driving the tremendous diversity of plant form through time.
ACKNOWLEDGEMENTS
The authors are grateful to Brent Breithaupt, US Bureau of Land Management Regional Paleontologist, for providing permits to collect at Cottonwood Canyon. For access to collections and specimen loans we thank: William DiMichele, Carol Hotton, Nathan Jud, and Jonathan Wingerath (National Museum of Natural History – Smithsonian Institution); Thomas N. Taylor, Edith L. Taylor and Rudolph Serbet (University of Kansas); Kirk Johnson and Ian Miller (Denver Museum of Nature and Science); and Ian Glasspool and Patrick Herendeen (Field Museum of Natural History). We also thank Christopher Steenbock, Joseph Caruso, Richard Tate, James Cornwell, Glenn Shelton, Allison Bronson, Ashley Ortiz, Hannah Barrett-Watson, Jeffery Barrett and Rachel Klassen for assistance in the field and laboratory. Peter Holterhoff provided assistance in the field and helpful information on stratigraphy and depositional environments. This work was supported by graduate student research awards from the Botanical Society of America, Geological Society of America, Paleontological Society (James M. and Thomas J. M. Schopf Award) and Humboldt State University Department of Biological Sciences to K.K.S.M., and by grants from the Humboldt State University Office of Research and Graduate Studies, Humboldt State University Sponsored Programs Foundation and the American Philosophical Society to A.M.F.T.
LITERATURE CITED
- Bateman RM, Hilton J.. 2009. Palaeobotanical systematics for the phylogenetic age: applying organ-species, form-species and phylogenetic species concepts in a framework of reconstructed fossil and extant whole-plants. Taxon 58: 1254–1280. [Google Scholar]
- Banks HP, Grierson JD.. 1968. Drepanophycus spinaeformis Göppert in the early Upper Devonian of New York State. Palaeontographica Abt. B 123: 113–120. [Google Scholar]
- Bhatt MV, Khandelwal A, Dudley SA.. 2011. Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs. New Phytologist 189: 1135–1142. [DOI] [PubMed] [Google Scholar]
- Biedrzycki ML, Jilany TA, Dudley SA, Bais HP.. 2010. Root exudates mediate kin recognition in plants. Communicative and Integrative Biology 3: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower FO. 1885. On the development and morphology of Phylloglossum drummondii. Philosophical Transactions of the Royal Society of London 176: 665–678. [Google Scholar]
- Caruso JA, Tomescu AMF.. 2012. Microconchid encrusters colonizing land plants: the earliest North American record from the Early Devonian of Wyoming, USA. Lethaia 45: 490–494. [Google Scholar]
- Daviero-Gomez V, Kerp H, Hass H.. 2005. Nothia aphylla: the issue of clonal development in early land plants. International Journal of Plant Sciences 166: 319–326. [Google Scholar]
- Denison RH. 1956. A review of the habitat of the earliest vertebrates. Fieldiana Geology 11: 357–457. [Google Scholar]
- DiMichele WA, Hook RW.. 1992. Paleozoic terrestrial ecosystems In: Behrensmeyer AK, Damuth JD, DiMichele WA, Potts R, Sues HD, Wing SL, eds. Terrestrial ecosystems through time. Chicago, USA: University of Chicago Press, 205–325. [Google Scholar]
- Dorf E. 1933. A new occurrence of the oldest known terrestrial vegetation, from Beartooth Butte, Wyoming. Botanical Gazette 95: 240–257. [Google Scholar]
- Dorf E. 1934. Stratigraphy and paleontology of a new Devonian formation at Beartooth Butte, Wyoming. Journal of Geology 42: 720–737. [Google Scholar]
- Dudley SA, File AL.. 2007. Kin recognition in an annual plant. Biology Letters 3: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DS. 1980. Evidence for the sporophytic status of the Lower Devonian plant Rhynia gwynne-vaughanii Kidston and Lang. Review of Palaeobotany and Palynology 29: 177–188. [Google Scholar]
- Edwards DS. 1986. Aglaophyton major, a non-vascular land-plant from the Devonian Rhynie chert. Botanical Journal of the Linnean Society 93: 173–204. [Google Scholar]
- Edwards D, Wang Y, Bassett MG, Li CS.. 2007. The earliest vascular plant or a later rooting system? Pinnatiramosus qianensis from the marine Lower Silurian Xiushan Formation, Guizhou Province, China. Palaios 22: 155–165. [Google Scholar]
- Elliot DK, Johnson HG. . 1997. Use of vertebrates to solve biostratigraphic problems: examples from the Lower and Middle Devonian of Western North America. Geological Society of America Special Paper 321: 179–188. [Google Scholar]
- Fiorillo AR. . 2000. The ancient environment of the Beartooth Butte Formation (Devonian) in Wyoming and Montana: combining paleontological inquiry with federal management needs In: McCool SF, Cole DN, Borrie WT, O’Loughlin J, eds. Wilderness science in a time of change conference , Vol.3: Wilderness as a place for scientific inquiry; 1999 May 23–27;Missoula, MT: USDA Forest Service Proceedings RMRS-P-15 3: 160–167. [Google Scholar]
- Gensel PG, Andrews HN.. 1984. Plant life in the Devonian. New York: Praeger. [Google Scholar]
- Gensel PG, Berry CM.. 2001. Early lycophyte evolution. American Fern Journal 91: 74–98. [Google Scholar]
- Gensel PG, Kasper A, Andrews HN.. 1969. Kaulangiophyton, a new genus of plants from the Devonian of Maine. Bulletin of the Torrey Botanical Club 96: 265–276. [Google Scholar]
- Gensel PG, Kotyk ME, Basinger JF.. 2001. Morphology of above- and below-ground structures in Early Devonian (Pragian-Emsian) plants In: Gensel PG, Edwards D, eds. Plants invade the land. New York: Columbia University Press, 83–102. [Google Scholar]
- Gifford EM, Foster AS.. 1989. Morphology and evolution of vascular plants, 3rd edn New York: W.H. Freeman. [Google Scholar]
- Grierson JD, Banks HP.. 1963. Lycopods of the Devonian of New York State. Palaeontographica Americana4: 221–295. [Google Scholar]
- Hao SG, Xue JZ, Guo DL, Wang DM.. 2010. Earliest rooting system and root:shoot ratio from a new Zosterophyllum plant. New Phytologist 185: 217–225. [DOI] [PubMed] [Google Scholar]
- Hetherington AJ, Berry CM, Dolan L.. 2016. . Networks of highly branched stigmarian rootlets developed on the first giant trees. Proceedings of the National Academy of Sciences of the USA 113: 6695–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel C, Alpert P.. 2003. Root cooperation in a clonal plant: connected strawberries segregate roots. Oecologia 134: 72–77. [DOI] [PubMed] [Google Scholar]
- Hotton CL, Hueber FM, Griffing DH, Bridge JS.. 2001. Early terrestrial plant environments: an example from the Emsian of Gaspé, Canada In: Gensel PG, Edwards D, eds. Plants invade the land: evolutionary and environmental perspectives. New York: Columbia University Press, 179–212. [Google Scholar]
- Hueber FM. 1972. Rebuchia ovata, its vegetative morphology and classification with the Zosterophyllophytina. Review of Palaeobotany and Palynology 14: 113–127. [Google Scholar]
- Hueber FM. 1983. . A new species of Baragwanathia from the Sextant Formation (Emsian) Northern Ontario, Canada. Botanical Journal of the Linnean Society 86: 57–79. [Google Scholar]
- Hueber FM. 1992. . Thoughts on the early lycopsids and zosterophylls. Annals of the Missouri Botanical Garden 79: 474–499. [Google Scholar]
- Kenrick P. 2002. The origin of roots In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots: the hidden half, 3rd edn. New York: Marcel Dekker, 1–13. [Google Scholar]
- Kenrick P, Crane PR.. 1997. The origin and early diversification of land plants. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Kerp H, Hass H, Mosbrugger V.. 2001. New data on Nothia aphylla Lyon 1964 ex El-Saadawy et Lacey 1979, a poorly known plant from the Lower Devonian Rhynie chert In: Gensel PG, Edwards D, eds. Plants invade the land: evolutionary and environmental perspectives. New York: Columbia University Press, 52–82. [Google Scholar]
- Kerp H, Wellman CH, Krings M, Kearny P, Hass H.. 2013. . Reproductive organs and in situ spores of Asteroxylon mackiei Kidston and Lang, the most complex plant from the Lower Devonian Rhynie Chert. International Journal of Plant Sciences 174: 293–308. [Google Scholar]
- Kidston R, Lang WH. . 1920a. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part II. Additional notes on Rhynia gwynne-vaughani, Kidston and Lang; with descriptions of Rhynia major, n. sp., and Hornea lignieri, n. g., n. sp. Transactions of the Royal Society of Edinburgh 52: 603–627. [Google Scholar]
- Kidston R, Lang WH. . 1920b. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part III. Asteroxylon mackiei, Kidston and Lang. Transactions of the Royal Society of Edinburgh 52: 643–680. [Google Scholar]
- Kidston R, Lang WH.. 1921. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion of their bearing on the general morphology of the Pteridophyta and the origin of the organisation of land-plants. Transactions of the Royal Society of Edinburgh 52: 831–854. [Google Scholar]
- Klymiuk AA, Stockey RA, Rothwell GW.. 2011. . The first organismal concept for an extinct species of Pinaceae: Pinus arnoldii Miller. International Journal of Plant Sciences 172: 294–313. [Google Scholar]
- Lamsdell JC, Legg DA.. 2010. An isolated pterygotid ramus (Chelicerata: Eurypterida) from the Devonian Beartooth Butte Formation, Wyoming. Journal of Paleontology 84: 1206–1208. [Google Scholar]
- Lamsdell JC, Selden PA.. 2013. Babes in the wood – a unique window into sea scorpion ontogeny. BMC Evolutionary Biology 13: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-S, Edwards D. . 1995. A re-investigation of Halle's Drepanophycus spinaeformis Goepp. from the Lower Devonian of Yunnan Province, southern China. Botanical Journal of the Linnean Society 118: 163–192. [Google Scholar]
- Li C-S, Edwards D.. 1997. . A new microphyllous plant from the Lower Devonian of Yunnan Province, China. American Journal of Botany 84: 1441–1448. [PubMed] [Google Scholar]
- Li C-S, Hueber FM, Hotton CL.. 2000. A neotype for Drepanophycus spinaeformis Göppert 1852. Canadian Journal of Botany 78: 889–902. [Google Scholar]
- Mahall BE, Callaway RM.. 1991. Root communication among desert shrubs. Proceedings of the National Academy of Sciences of the USA 88: 874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga KKS, Tomescu AMF.. 2016. Root evolution at the base of the lycophyte clade: insights from an Early Devonian lycophyte. Annals of Botany 117: 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RJ. 1984. New finds of Drepanophycus spinaeformis Göppert from the Lower Devonian of Scotland. Transactions of the Royal Society of Edinburgh: Earth Sciences 75: 353–363. [Google Scholar]
- Remy W, Hass H.. 1996. New information on gametophytes and sporophytes of Aglaophyton major and inferences about possible environmental adaptations. Review of Palaeobotany and Palynology 90: 175–193. [Google Scholar]
- Roth-Nebelsick A, Grimm G, Mosbrugger V, Haas H, Kerp H.. 2000. Morphometric analysis of Rhynia and Asteroxylon: testing functional aspects of early land plant evolution. Paleobiology 26: 405–418. [Google Scholar]
- Rothwell GW. 1999. Fossils and ferns in the resolution of land plant phylogeny. Botanical Review 65: 188–218. [Google Scholar]
- Rothwell GW, Erwin DM.. 1985. The rhizomorph apex of Paurodendron: implications for homologies among the rooting organs of Lycopsida. American Journal of Botany 72: 86–98. [Google Scholar]
- Sandberg CA. 1961. Widespread Beartooth Butte Formation of Early Devonian age in Montana and Wyoming and its paleogeographic significance. Bulletin of the American Association of Petroleum Geologists 45: 1301–1309. [Google Scholar]
- Sandberg CA. 1967. Measured sections of Devonian rocks in northern Wyoming. Geological Survey of Wyoming Bulletin 52. Laramie: University of Wyoming. [Google Scholar]
- Schultes RE, Dorf E. . 1938. A sphenopsid from the Lower Devonian of Wyoming. Harvard University Botanical Museum Leaflets 7: 21–34. [Google Scholar]
- Schweitzer H-J. 1980. Uber Drepanophycus spinaeformis Goeppert. Bonner Palaobotanische Mitteilungen 7: 1–29. [Google Scholar]
- Schweitzer H-J, Giesen P. . 1980. Uber Taeniophyton inopinatum, Protolycopodites devonicus und Cladoxylon scoparium aus dem Mitteldevon von Wuppertal. Palaeontographica B 173: 1–25. [Google Scholar]
- Semchenko M, John EA, Hutchings MJ.. 2007. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytologist 176: 644–654. [DOI] [PubMed] [Google Scholar]
- Steenbock CM, Tomescu AMF.. 2013. Resurrecting Sphondylophyton as a rhodophyte alga from the Early Devonian. International Journal of Plant Sciences 174: 1171–1181. [Google Scholar]
- Tanner WR. . 1982. A new species of Gosslingia (Zosterophyllophytina) from the Lower Devonian Beartooth Butte Formation of northern Wyoming. Proceedings of the Third North American Paleontological Convention 2: 541–546. [Google Scholar]
- Tanner W. 1983. A fossil flora from the Beartooth Butte Formation of Wyoming. PhD Thesis, Southern Illinois University, USA.
- Taylor TN, Kerp H, Hass H.. 2005. Life history biology of early land plants: deciphering the gametophyte phase. Proceedings of the National Academy of Sciences of the USA 102: 5892–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlie OE. 2007. Like father, like son? Not amongst the eurypterids (Chelicerata) from Beartooth Butte, Wyoming. Journal of Paleontology 81: 1423–1431. [Google Scholar]
- Walton J. 1964. On the morphology of Zosterophyllum and some other Early Devonian plants. Phytomorphology 14: 155–160. [Google Scholar]
- Wellman CH, Kerp H, Hass H.. 2006. Spores of the Rhynie chert plant Aglaophyton (Rhynia) major (Kidston and Lang) D.S. Edwards, 1986. Review of Palaeobotany and Palynology 142: 229–250. [Google Scholar]
- Xu HH, Feng J, Jiang Q, Wang Y.. 2013. Report of Drepanophycus Göppert (Lycopsida) from the Middle Devonian of Xinjian, China. Journal of Systematics and Evolution 51: 765–772. [Google Scholar]
- Xue JZ. 2013. . New material of Hueberia zhichangensis Yang, Li and Edwards, a basal lycopsid from the Early Devonian of Yunnan, China. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen 267: 331–339. [Google Scholar]
- Xue J, Deng Z, Huang P, Huang K, et al.2016.Belowground rhizomes in paleosols: The hidden half of an Early Devonian vascular plant. Proceedings of the National Academy of Sciences 113: 9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaton M, Wilson MA, Vinn O. . 2012. . Redescription and neotype designation of the Middle Devonian microconchid (Tentaculita) species ‘Spirorbis’ angulatus Hall, 1861. Journal of Paleontology 86: 417–424. [Google Scholar]