Abstract
Background:
The purpose of this meta-analysis is to compare the efficacy of tranexamic acid (TXA) versus placebo after a total shoulder arthroplasty (TSA).
Methods:
In April 2017, a systematic computer-based search was conducted in the databases of PubMed, Embase, Web of Science, Cochrane Library, and Google. Studies comparing TXA versus placebo in reducing blood loss after TSA were included. The endpoints were the need for transfusion, blood loss in drainage, hemoglobin drop, and total blood loss. Stata 12.0 software was used for the meta-analysis.
Results:
Six studies involving a total of 637 patients met the inclusion criteria. The meta-analysis revealed that, compared with control groups, treatment with TXA could decrease the need for transfusion (P < .00001), blood loss in drainage (P = .000), hemoglobin drop (P = .001), and total blood loss (P = .000).
Conclusion:
TXA can decrease the need for transfusion as well as total blood loss in TSA patients. There was a negative correlation between the TXA dose and the need for transfusion and blood loss in drainage. Because the administration route and the dose of TXA were different, more studies are needed in order to identify the optimal dose and route.
Keywords: blood loss, meta-analysis, total shoulder arthroplasty, tranexamic acid
1. Introduction
Total shoulder arthroplasty (TSA), which is a successful surgery option for patients with glenohumeral arthritis, is growing in popularity.[1] TSA is associated with considerable blood loss and subsequent blood transfusion in some patients.[2,3] Overall rates of blood transfusion after TSA have been reported to range from 7.4% to 43%.[2,4,5] As the frequency of TSA increases, the incidence and risk factors for blood transfusion have gained importance.
Tranexamic acid (TXA) is a synthetic antifibrinolytic agent that has been routinely administered in many surgical fields.[6,7] TXA has been used in TSA since 2015, and the efficacy of TXA in TSA has been debated. Friedman et al[8] determined that TXA could decrease the need for transfusion in TSA patients, while Gillespie et al[9] found that there was no significant difference between TXA and controls in terms of the need for transfusion. Along with these disputes, it should be noted that the sample sizes of the studies were limited (ranging from 22 to 106 patients), which may affect the accuracy of relevant conclusions. For these reasons, we applied a meta-analysis to test the robustness of our findings and to obtain the efficacy of TXA in reducing blood loss in TSA. The purpose of the present meta-analysis was to identify whether administration of TXA, when compared with controls, was associated with a significant reduction in blood transfusions, a reduction in total blood loss, and hemoglobin drop and blood loss in drainage.
2. Methods
Meta-analysis is a research tool that gathers relevant data from published papers, so no ethics committee or institutional review board needed to approve the study.
2.1. Search strategy
Electronic databases, including PubMed, Embase, Web of Science, Cochrane Library, and Google database, were searched for relevant studies published from the time these databases were established up to April 2017. Google was used to search for additional articles. Furthermore, the reference lists of all the full-text articles were reviewed to identify any initially omitted studies, and there was no restriction on the language of publication. The keywords used for the searches included “tranexamic acid,” “total shoulder arthroplasty,” “total shoulder replacement,” “TSA,” and “TSR.” In addition, the medical subject heading (Mesh) terms of the studies were used to maximize the specificity and sensitivity of the search. These keywords and Mesh terms were combined with the Boolean operators AND or OR.
2.2. Eligibility criteria and study quality
Study selection was performed according to the following inclusion criteria: published randomized controlled trials (RCTs) and non-RCTs about patients who underwent TSA (including primary TSA and reverse TSA or RTSA); intervention included TXA versus placebo or no treatment; and reported outcomes included need for transfusion, total blood loss, blood loss in drainage, and hemoglobin drop. All of the studies had to be clinical studies, and trials with cadavers, artificial models, or patients with bleeding disorders were excluded. Two reviewers independently scanned the quality of the eligible studies, and discrepancies were resolved by a senior reviewer. The Cochrane Handbook for Systematic Reviews of Interventions was used to evaluate the methodological quality and risk bias, which included a randomization method, allocation concealment, blinding of participants, personnel and assessors, and complete outcome data and other bias. Risk of bias assessment for each included article was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions using the software RevMan 5.30 (The Cochrane Collaboration, Oxford, UK). The quality of non-RCTs was assessed by the Newcastle–Ottawa Scale (NOS).[10]
2.3. Data collection
The following data were extracted and recorded in a sheet: demographic data about the patients in the literature, author's name, publication date, the sample size of the patients, and the ratio of males to females; the dose and method of TXA treatment, including whether the TXA was applied intravenously or topically, as well as thromboprophylaxis to prevent the occurrence of DVT; and postoperative total blood loss, blood loss in drainage, need for transfusion, and hemoglobin drop.
2.4. Outcome measures and statistical analysis
The main outcomes were the need for transfusion, blood loss in drainage, total blood loss, and hemoglobin drop. Continuous outcomes (total blood loss, blood loss in drainage, and hemoglobin drop) were expressed as the weighted mean difference (WMD) and respective 95% confidence intervals (95% CIs). Dichotomous outcomes (the need for transfusion) were expressed as a risk ratio (RR) with 95% CIs. Statistical significance was set at P < .05 to summarize findings across the trials. The meta-analysis was calculated with Stata, version 12.0 (Stata Corp., College Station, TX). Statistical heterogeneity was tested using the Chi-square test and I2 statistical tests. When there was no statistical evidence of heterogeneity (I2 < 50%, P > .1), the fixed effects model was adopted. Otherwise, a random effect model was chosen. Publication bias was tested using funnel plots. Publication bias was assessed by a funnel plot and quantitatively assessed by Begg test. If the funnel plot was symmetrical and the P value was > .05, we determined there was no publication bias. The relationship between topical TXA dosage and total blood loss was explored using the SPSS software. The correlation coefficient (r) was used to evaluate the relationship between the dosage of TXA and need for transfusion and blood loss in drainage. Power analyses of individual studies and meta-analyses were all conducted using PS software (Power and Sample Size Calculations), version 3.0.43 (Vanderbilt university; State of Tennessee).
3. Results
3.1. Search results
In the initial search, we identified 155 potentially relevant studies, and 79 duplicates were removed by Endnote Software (Version X7; Thompson Reuters, CA). According to the inclusion criteria, 72 studies were excluded after reading the titles and abstracts. Two studies consisted of both TSA and RTSA patients, and thus were divided into 4 studies. Finally, we included 6 clinical trials with a total of 637 patients (292 patients in the TXA group and 345 patients in the control group) in the meta-analysis.[8,9,11,12] The general characteristics of the included studies can be obtained in Table 1. The included studies were published from 2015 to 2017. All of the included studies were published in the United States. The mean age of patients in the included studies ranged from 59.72 to 71 years. Three studies refer to the transfusion criteria and identify as less than 7 g/dL. The power of individual studies ranged from 52.4% to 64.7%. The power of analysis of the total studies was 85.9%, an increase over the range for the power of individual studies (Fig. 1).
Table 1.
The general characteristic of the included studies.
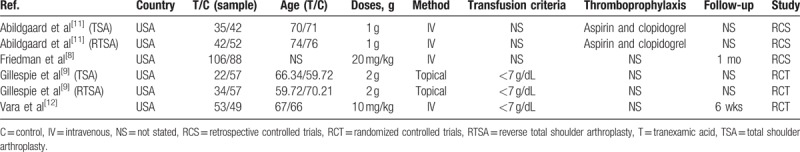
Figure 1.
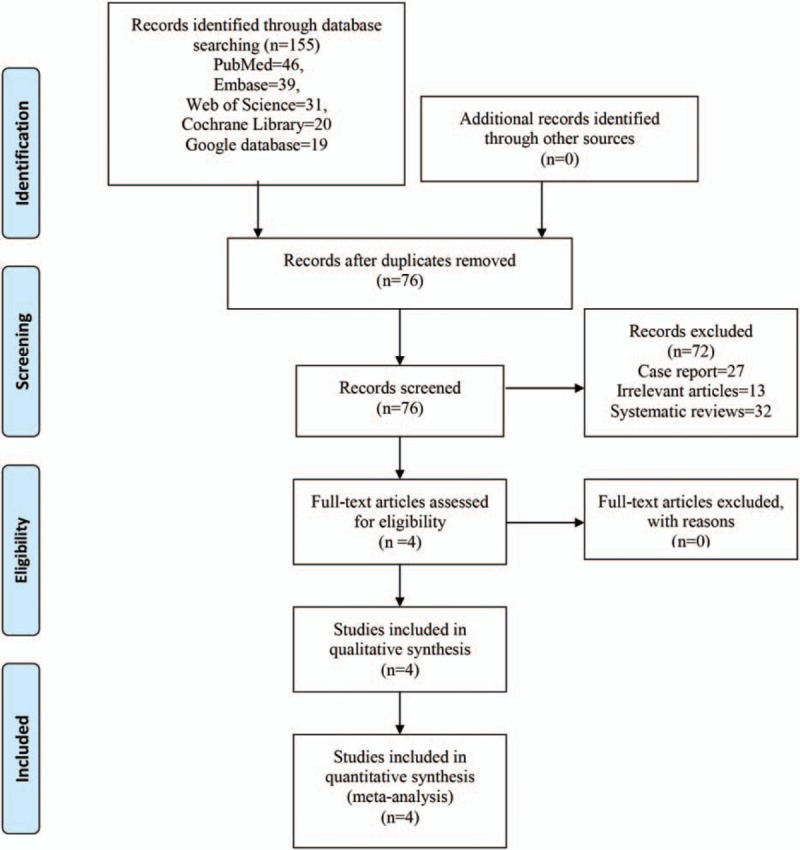
PRISMA flowchart of the included studies.
3.2. Risk of bias of the included studies
A summary and a graph of the risk of bias can be seen in Figs. 2 and 3. The random sequence generation, allocation concealment, blinding of participants, and personnel and blinding to outcome assessment all showed a low risk of bias. The risk of bias of non-RCTs can be seen in Table 2. The MINORS scores of Abildgaard et al[11] and Friedman et al[8] were 18 and 24, respectively. Thus, all of the included studies were high-quality studies.
Figure 2.
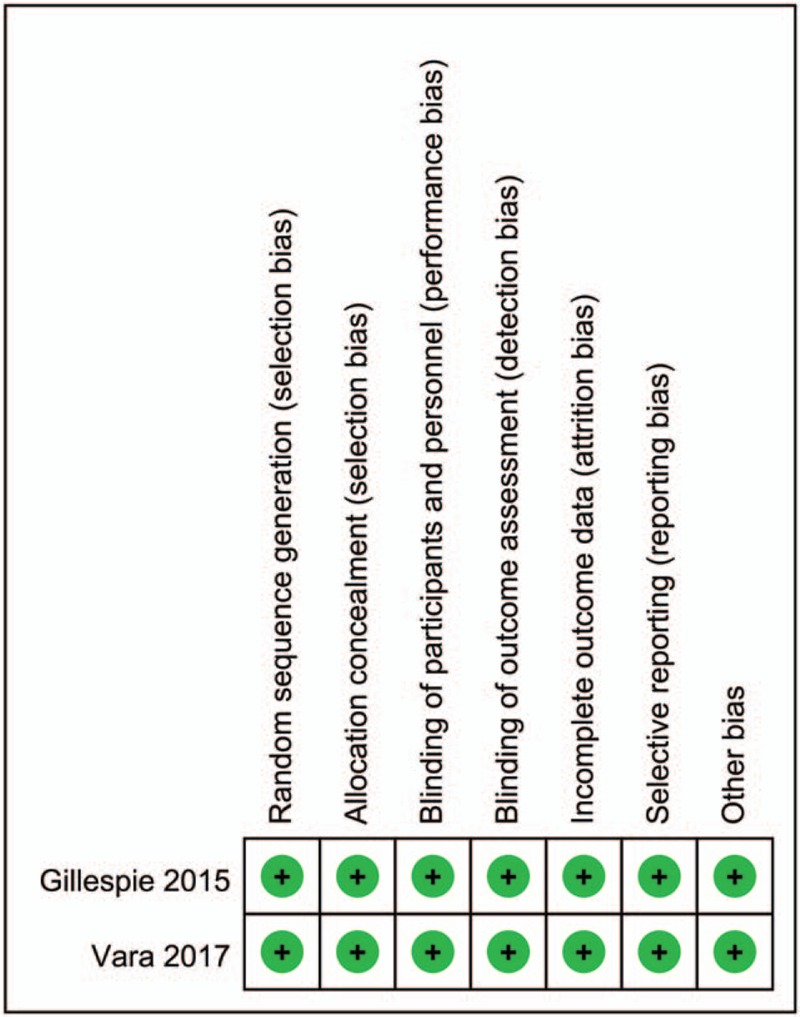
Summary of the risk of bias for the included studies.
Figure 3.
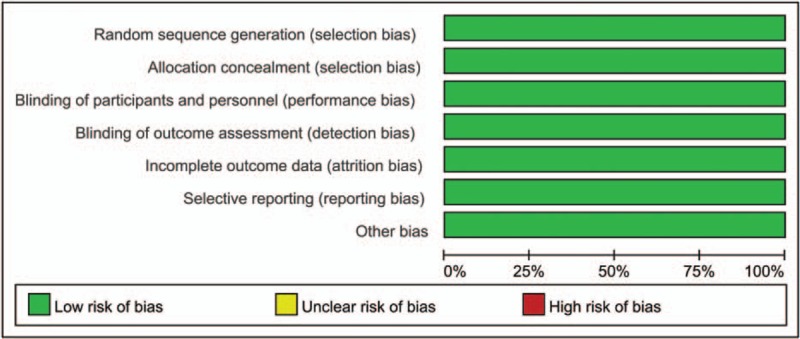
Graph of the risk of bias for the included studies.
Table 2.
The Minors quality score of the non-RCTs.
3.3. Results of meta-analysis
3.3.1. Need for transfusion
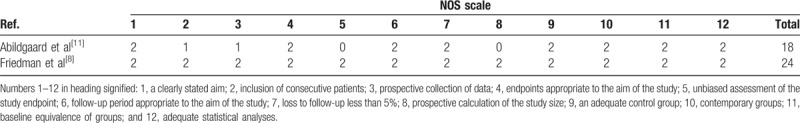
A total of 6 studies (637 patients) provided data regarding the need for transfusion after TSA, and the pooled result indicated that administration of TXA could decrease the need for transfusion by approximately 3.11% (2.40% vs 5.51%, RR = 0.45; 95% CI 0.21–0.99; P = .046, Fig. 4) with no heterogeneity (I2 = 0%, P = .826).
Figure 4.
Forest plot comparing the need for transfusion between the 2 groups.
3.3.2. Blood loss in drainage
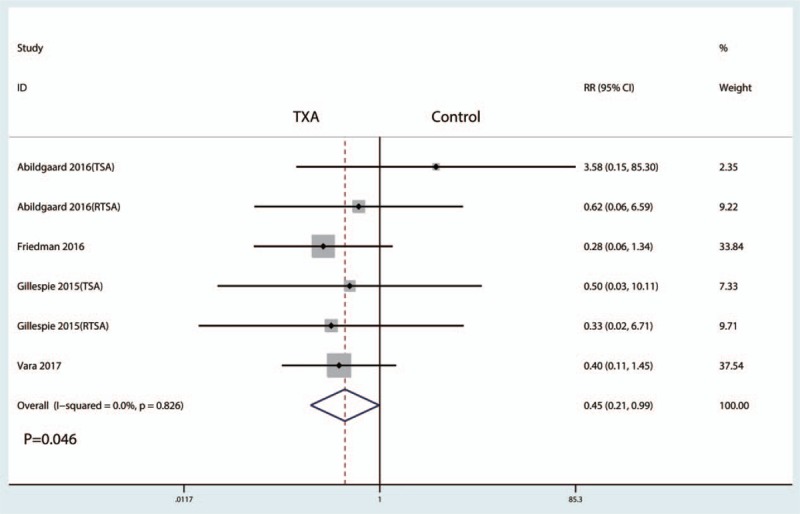
A total of 5 studies (443 patients) provided data about blood loss in drainage after TSA, and the pooled results indicated that administration of TXA could decrease blood loss in drainage after THA (WMD = −119.32; 95% CI −165.56 to −73.08; P = .000, Fig. 5) with high heterogeneity (I2 = 55.4%, P = .062).
Figure 5.
Forest plot comparing blood loss in drainage between the groups.
3.3.3. Total blood loss
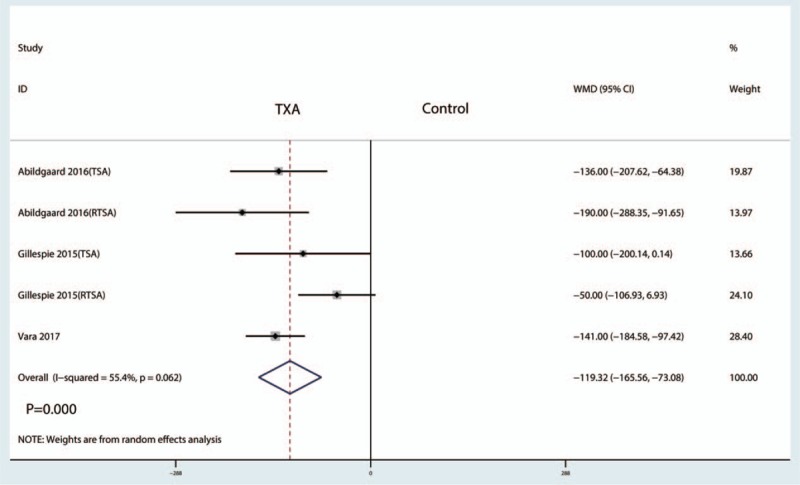
Three studies reported data on total blood loss. The meta-analysis showed that total blood loss after TSA with administration of TXA was reduced by a mean of 267.06 ml (95% CI −385.07 to −149.05, P = .000) compared with the control group (Fig. 6). The meta-analysis involved a fixed-effects model because there was no significant heterogeneity among the studies (I2 = 0.0%).
Figure 6.
Forest plot comparing total blood loss between the 2 groups.
3.3.4. Hemoglobin drop
Six studies reported data on hemoglobin drop. The meta-analysis showed that hemoglobin drop after TSA with administration of TXA was reduced by a mean of 0.53 g/dL (95% CI −0.83 to −0.23, P = .001) compared with the control group (Fig. 7).
Figure 7.
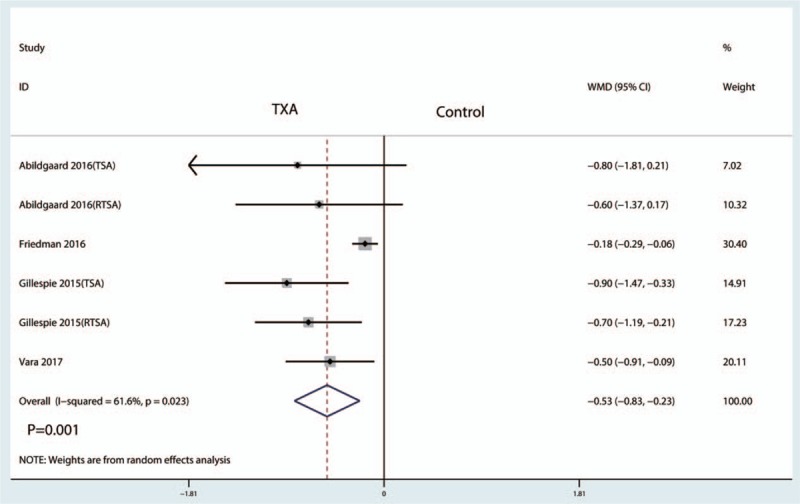
Forest plot comparing the hemoglobin drop between the 2 groups.
3.4. Subgroup analysis
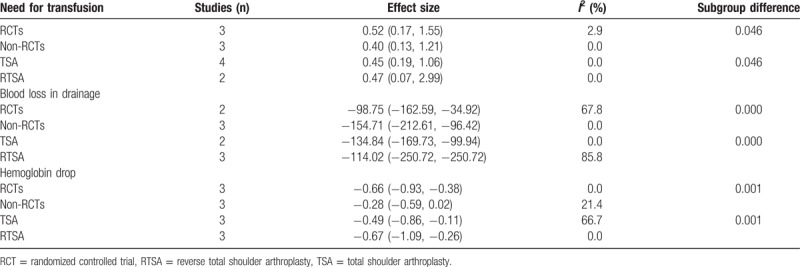
Subgroup analysis was conducted according to the surgery type (TSA or RTSA) and the quality of the included studies (RCTs or non-RCTs). Final results are summarized in Table 3. The results indicated that there was no significant difference between the subgroups in terms of the need for transfusion, blood loss in drainage, and hemoglobin drop.
Table 3.
Subgroup analysis of the need for transfusion, blood loss in drainage, and hemoglobin drop.
3.5. Dose–effect relationship
We plotted the total dose of TXA on the abscissa and the corresponding need for transfusion and blood loss in drainage as the ordinate to generate a scatterplot. The results indicated that the occurrence of need for transfusion and amount of blood loss in drainage tended to decrease as the TXA dose increased. Meanwhile, the linear correlation coefficient (r) was calculated using the Spearman method. A negative correlation was observed between the dosage of TXA and need for transfusion (r = −0.872, P < .001, Fig. 8) and blood loss in drainage (r = −0.512, P = .013, Fig. 9).
Figure 8.
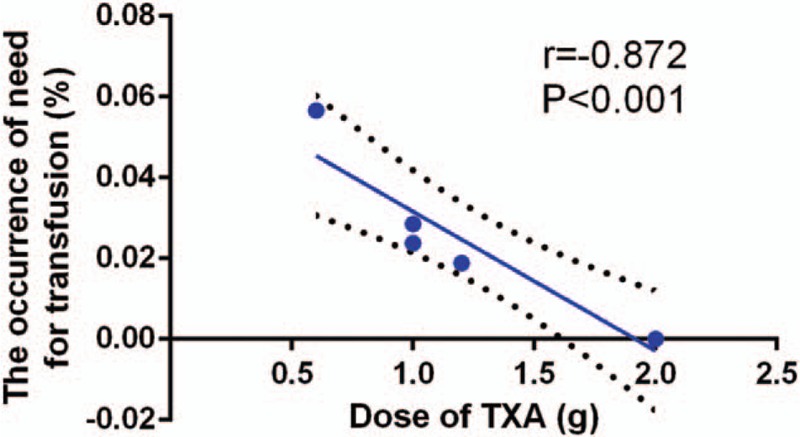
Scatter plot showing the relationship between the dose of TXA and the need for transfusion.
Figure 9.
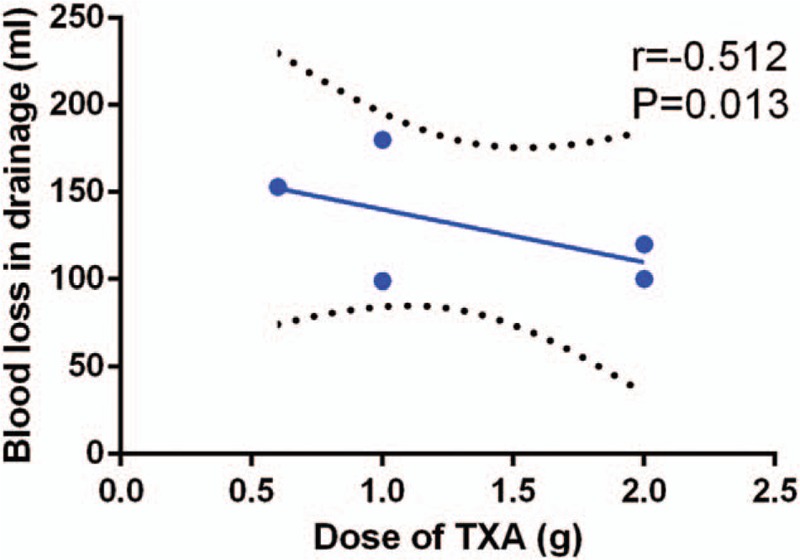
Scatter plot showing the relationship between the dose of TXA and the blood loss in drainage.
4. Discussion
This is the first systematic review and meta-analysis comparing TXA in reducing blood loss for TSA patients. Pooled results indicated that administration of TXA could play an effective role in controlling blood loss and reducing the need for transfusion and hemoglobin drop without increasing the occurrence of DVT and infection after TSA. A major strength of this meta-analysis was that we performed a comprehensive search and rigorous calculation regarding TXA for reducing blood loss in TSA. As there was large heterogeneity among the included studies, we performed a subgroup analysis to minimize the heterogeneity and provide a relative stable conclusion.
TXA is a well-tolerated drug that has been widely administered in total knee arthroplasty (TKA), total hip arthroplasty (THA), and spinal surgeries.[13–15] The results of our meta-analysis indicated that administration of TXA was associated with a reduction in the need for transfusion by 3.11% (RR = 0.45; 95% CI 0.21–0.99; P = .046). Shemshaki et al[16] reported that TXA could decrease the need for transfusion by 22.2% in TKA patients. A negative correlation was observed between the dosage of TXA and need for transfusion (r = −0.872, P < .001). There were a total of 2 TXA administration routes (topical or intravenous [IV]) in the included studies, and this difference may cause heterogeneity for the final outcomes. Indeed, topical TXA has similar efficacy for blood loss control to IV TXA in orthopedic surgery. A meta-analysis conducted by Fu et al[17] revealed that there were no significant differences between the need for transfusion, total blood loss, blood loss in drainage, and hemoglobin value at 24 hours post-TKA.
This meta-analysis has shown that TXA can decrease total blood loss by 267.06 mL (95% CI −385.07 to −149.05, P = .000) and blood loss in drainage by 119.32 mL (WMD = −119.32; 95% CI −165.56 to −73.08; P = .000). Weng et al[18] revealed that, compared with a control group, TXA was associated with a reduction of total blood loss by 488.66 mL in TKA patients. The blood loss saving effects of TXA in TSA and TKA differ because blood loss in these 2 surgeries is different. RTSA and TSA also influence the amount of total blood loss; therefore, we performed a subgroup analysis and dose–effect relationship between the TXA dose and blood loss in drainage. The dose–effect relationship showed a negative correlation between the TXA dose and the need for transfusion and blood loss in drainage. A negative correlation was observed between the TXA dose and blood loss in drainage (r = −0.512, P = .013).
In a meta-analysis of 15 trials studying IV TXA use in TKA, Yang et al[19] found no significant difference in activated prothrombin time, DVT, or pulmonary embolism (PE). We did not perform a meta-analysis comparing the occurrence of DVT or PE between the TXA dose and control group. Four studies reported that none of the patients experienced DVT or PE. It is established that TXA does not inhibit fibrinolytic activity in vein walls.[20] Although no patients experienced DVT or PE, small numbers of patients and high-risk patients, such as those with a history of PTE or DVT, were excluded. Thus, more long-term follow-up studies are needed to further determine the safety of TXA in TSA.
There were several potential limitations of this meta-analysis: only 6 studies were included in the meta-analysis, and the sample sizes in each trial were not large, which could affect the final results; 3 non-RCTs were included in the meta-analysis, which could lower the evidence level; the duration of follow-up in some studies was unclear, and long-term follow-up is needed to identify complications; the publication bias that exists in all meta-analyses will influence the results; the methods of TXA administration were different in the included studies (IV or topical), and thus more studies are needed to identify the optimal route for reducing blood loss after TSA; the baseline of TSA patients differed (2 studies refer to clopidogrel administration and the rest do not refer to this), and this difference may cause heterogeneity in the final outcomes; and included studies were published beginning in 2015, and more time is needed to determine the safety of TXA.
5. Conclusion
This systematic review and meta-analysis indicated that TXA was effective in reducing blood loss in patients who underwent TSA. Moreover, the need for transfusion and blood loss in drainage tended to decrease as the TXA dose increased. High-quality RCTs and well-designed trials will still be required in the future in order to determine the therapeutic dose or to detect adverse effects.
Footnotes
Abbreviations: CIs = confidential intervals, DVT = deep venous thrombosis, Mesh = medical subject heading (Mesh), PE = pulmonary embolism, RCT = randomized controlled trials, RR = risk ratio, RTSA = reverse total shoulder arthroplasty, THA = total hip arthroplasty, TKA = total knee arthroplasty, TSA = total shoulder arthroplasty, TXA = tranexamic acid, WMD = weighted mean difference.
The authors report no conflicts of interest.
References
- [1].Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011;93:2249–54. [DOI] [PubMed] [Google Scholar]
- [2].Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:233–9. [DOI] [PubMed] [Google Scholar]
- [3].Ryan DJ, Yoshihara H, Yoneoka D, et al. Blood transfusion in primary total shoulder arthroplasty: incidence, trends, and risk factors in the United States from 2000 to 2009. J Shoulder Elbow Surg 2015;24:760–5. [DOI] [PubMed] [Google Scholar]
- [4].Gruson KI, Accousti KJ, Parsons BO, et al. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg 2009;18:225–30. [DOI] [PubMed] [Google Scholar]
- [5].Jiang JJ, Toor AS, Shi LL, et al. Analysis of perioperative complications in patients after total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1852–9. [DOI] [PubMed] [Google Scholar]
- [6].Mi B, Liu G, Zhou W, et al. Intra-articular versus intravenous tranexamic acid application in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2017;137:997–1009. [DOI] [PubMed] [Google Scholar]
- [7].Liu X, Liu J, Sun G. A comparison of combined intravenous and topical administration of tranexamic acid with intravenous tranexamic acid alone for blood loss reduction after total hip arthroplasty: a meta-analysis. Int J Surg (London, England) 2017;41:34–43. [DOI] [PubMed] [Google Scholar]
- [8].Friedman RJ, Gordon E, Butler RB, et al. Tranexamic acid decreases blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:614–8. [DOI] [PubMed] [Google Scholar]
- [9].Gillespie R, Shishani Y, Joseph S, et al. Neer Award 2015: a randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:1679–84. [DOI] [PubMed] [Google Scholar]
- [10].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [11].Abildgaard JT, McLemore R, Hattrup SJ. Tranexamic acid decreases blood loss in total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:1643–8. [DOI] [PubMed] [Google Scholar]
- [12].Vara AD, Koueiter DM, Pinkas DE, et al. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg 2017;26:1383–9. [DOI] [PubMed] [Google Scholar]
- [13].Evangelista PJ, Aversano MW, Koli E, et al. Effect of tranexamic acid on transfusion rates following total joint arthroplasty: a cost and comparative effectiveness analysis. Orthop Clin N Am 2017;48:109–15. [DOI] [PubMed] [Google Scholar]
- [14].Chiem J, Ivanova I, Parker A, et al. Anaphylactic reaction to tranexamic acid in an adolescent undergoing posterior spinal fusion. Paediatr Anaesth 2017;27:774–5. [DOI] [PubMed] [Google Scholar]
- [15].Ohashi N, Ohashi M, Endo N, et al. Administration of tranexamic acid to patients undergoing surgery for adolescent idiopathic scoliosis evokes pain and increases the infusion rate of remifentanil during the surgery. PLoS One 2017;12:e0173622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shemshaki H, Nourian SM, Nourian N, et al. One step closer to sparing total blood loss and transfusion rate in total knee arthroplasty: a meta-analysis of different methods of tranexamic acid administration. Arch Orthop Trauma Surg 2015;135:573–88. [DOI] [PubMed] [Google Scholar]
- [17].Fu Y, Shi Z, Han B, et al. Comparing efficacy and safety of 2 methods of tranexamic acid administration in reducing blood loss following total knee arthroplasty: a meta-analysis. Medicine 2016;95:e5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weng K, Zhang X, Bi Q, et al. The effectiveness and safety of tranexamic acid in bilateral total knee arthroplasty: a meta-analysis. Medicine 2016;95:e4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 2012;94:1153–9. [DOI] [PubMed] [Google Scholar]
- [20].Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol 1978;67:203–5. [PubMed] [Google Scholar]


