Abstract
To compare secondary cytoreductive surgery (SCS) plus chemotherapy with chemotherapy alone in Japanese patients with recurrent epithelial ovarian, tubal, or peritoneal cancer (ROC).
From our institutional database, we identified 112 patients who underwent therapy for ROC between 2005 and 2013. Of the 112 patients, 77 received salvage chemotherapy alone (CT group) and 35 received SCS plus chemotherapy (SCS group). To reduce the impact of treatment selection bias on treatment outcomes, propensity score-matching analysis was used.
In the entire cohort, prognostic features were poorer in the CT group than in the SCS group. The platinum-free interval was significantly lower (15.35 months vs 30.77 months), cancer antigen 125 (CA125) level was significantly higher (247.38 IU/mL vs 83.17 IU/mL), and number of solitary recurrence sites was significantly lower in the CT group than in the SCS group. The matched cohort consisted of 29 CT and 29 SCS patients with a median follow-up period of 24 and 58 months, respectively. In the matched cohort, progression-free survival (PFS) was longer in the SCS group than in the CT group (P = .02); however, overall survival did not differ (P = .23).
SCS might be associated with improved PFS in ROC patients. SCS is beneficial in appropriately selected ROC patients.
Keywords: chemotherapy, ovarian cancer, propensity score, secondary cytoreductive surgery, survival
1. Introduction
Epithelial ovarian cancer is the most common cause of death among gynecologic malignancies.[1] The standard first-line treatment is cytoreductive surgery, followed by combination chemotherapy with paclitaxel and a platinum compound.[2] We observed that about 70% to 80% of patients with epithelial ovarian cancer can achieve complete remission. However, more than 75% of patients with epithelial ovarian cancer develop recurrent disease, even when the initial treatment results in complete remission.[3]
As most cases of recurrent ovarian cancer (ROC) are multifocal, there is no doubt that the standard therapy of ROC is chemotherapy. However, there are quite a few resectable recurrence cases. For such cases, there is no evidence on whether chemotherapy alone is better than chemotherapy plus cytoreduction. The possibility that secondary cytoreductive surgery (SCS) is beneficial in patients with recurrence, as well as the fact that macroscopically complete surgical cytoreduction significantly improves survival in patients with ROC, has been suggested previously.[4] The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score was introduced to select appropriate candidates for complete cytoreduction. Harter et al[5] reported that the AGO score can be used not only as a predictor of complete secondary cytoreduction but also as an independent prognostic factor. The ongoing Descriptive Evaluation of preoperative Selection KriTeria for OPerability (DESKTOP) III prospective clinical trial is attempting to more clearly define the role of secondary cytoreduction.
In the present study, using the propensity score matching method, we evaluated the impact of SCS on recurrence and survival in patients with epithelial ovarian, tubal, or peritoneal cancer showing resectable recurrence.
2. Materials and methods
This was a retrospective single institution study, and institutional review board approval was obtained. We identified 232 patients with ROC between 2005 and 2013. Of these, 112 patients were AGO-score positive and met the following criteria: complete cytoreduction at the primary surgery, no ascites at the time of recurrence (<500 mL), and Eastern Cooperative Oncology Group performance status of 0 and 1 at the time of recurrence. These patients were treated in our hospital for the first recurrence. Patients who had a clinical, radiographic, and serologic platinum-free interval (PFI) after the primary surgery and platinum-based first-line chemotherapy were considered for inclusion. Recurrent disease was clinically confined to an intraabdominal or extraabdominal site identified by physical examination and/or radiologic imaging. We excluded patients with no gross disease identified by imaging techniques. Patients who visited our center for second-look operations or palliative surgery were also excluded.
Of the 112 patients, 35 underwent secondary cytoreduction plus chemotherapy and 77 were treated with chemotherapy alone. The treatment decision was made after discussion by a cancer board including gynecologic oncologists. The clinicians presented several treatment options considered on a cancer board, and the patients ultimately selected the treatment.
Secondary surgeries were analyzed for technical resectability, morbidity, and survival. Survival was calculated from the time of secondary cytoreduction to the date of death or last follow-up. Secondary cytoreduction was considered optimal if the largest tumor mass remaining was less than 1 cm in diameter.
In order to reduce the effect of treatment selection bias and simulate the effects of randomization, propensity score matching was performed. Propensity scores were estimated using a logistic regression model based on the statuses of first-line therapy and recurrence. One-to-one matching without replacement was performed using a 0.2 caliper width and the resulting score-matched pairs were used in subsequent analyses as indicated.
Survival curves were constructed using the Kaplan–Meier method and the log-rank test. Continuous data are expressed as mean ± standard deviation. The Chi-square test was used to compare proportions. All statistical analyses were performed using SPSS (ver. 22; IBM Corp., Armonk, NY). A 2-sided P-value < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
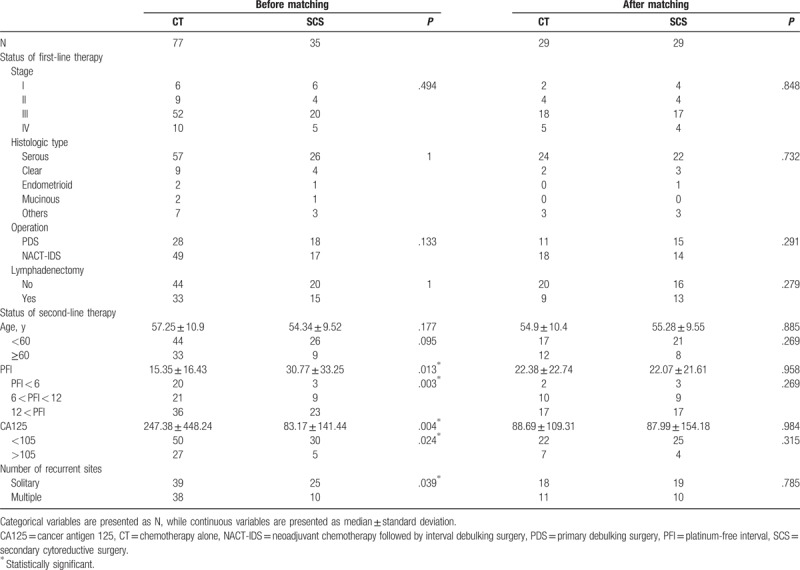
Of the 112 patients included in this study, 35 were treated with SCS plus chemotherapy (SCS group) and 77 were treated with chemotherapy alone (CT group). The baseline characteristics before and after propensity score matching are shown in Table 1. In the entire cohort, the distributions of age, International Federation of Gynecology and Obstetrics (FIGO) stage, histological type, primary therapy, and recurrence site were not significantly different between the 2 groups. However, the cancer antigen 125 (CA125) level at recurrence was significantly higher (247.38 ± 448.24 vs 83.17 ± 141.44, P = .004), progression-free interval was significantly shorter (15.35 ± 16.43 vs 30.77 ± 33.25, P = .013), and number of recurrence sites was significantly lower (P = .039) in the CT group than in the SCS group. There were no significant differences in the baseline characteristics between the 2 treatment groups in the matched cohort (Table 1).
Table 1.
Patient characteristics at the time of primary cytoreductive surgery and recurrence in this study.
3.2. Survival analysis in the entire cohort
In the entire cohort, the median follow-up for survivors was 36.0 months (range, 3–120 months) in the CT group and 58 months (range, 4–110 months) in the SCS group. No patient was lost to follow-up. Of the 112 total patients, all patients relapsed and 73 patients died. Among the entire cohort, the 2-year progression-free survival (PFS) and overall survival (OS) rates were 16.1% and 59.0%, respectively. On comparing the treatment outcomes between the 2 treatment groups, PFS and OS were significantly longer in the SCS group than in the CT group (Fig. 1). These findings indicate that SCS improves both PFS and OS.
Figure 1.
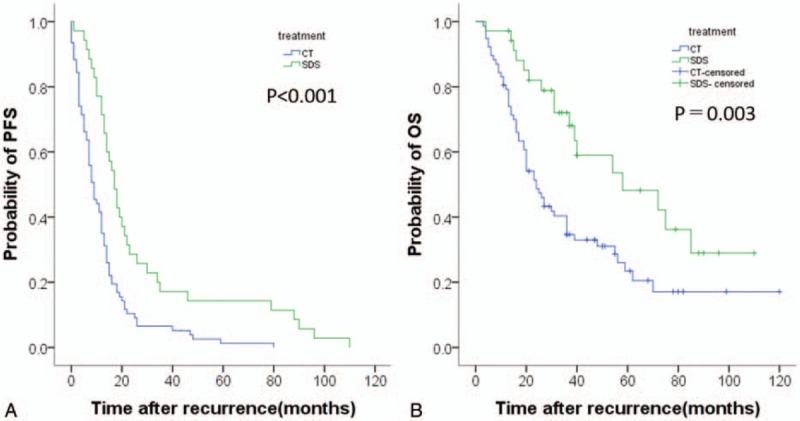
Kaplan–Meier survival curves for estimating the probability of (A) progression-free survival and (B) overall survival after treatment with secondary cytoreductive surgery plus chemotherapy (N = 35) versus chemotherapy alone (N = 77) in the entire cohort.
3.3. Survival analysis in the matched cohort
We performed further analyses in the matched cohort, where the median follow-up durations were 36 and 40 months in all patients and in the surviving patients at the time of analysis, respectively. In the CT and SCS groups, 11 (34.5%) and 8 (21.3%) patients, respectively, were followed up for less than 2 years. Among the 58 total patients, all patients relapsed and 25 patients died. In this cohort, PFS was significantly different between the 2 treatment groups (P = .02). However, there was no significant difference in OS between the 2 treatment groups (P = .23) (Fig. 2).
Figure 2.
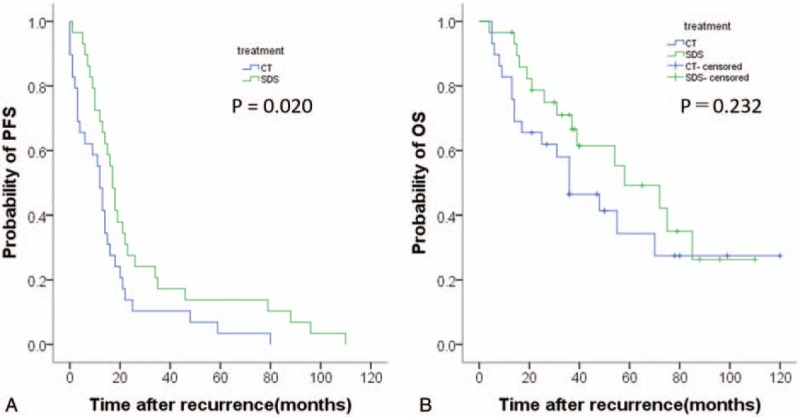
Kaplan–Meier survival curves for estimating the probability of (A) progression-free survival and (B) overall survival after treatment with secondary cytoreductive surgery plus chemotherapy (N = 29) versus chemotherapy alone (N = 29) in a propensity score-matched cohort.
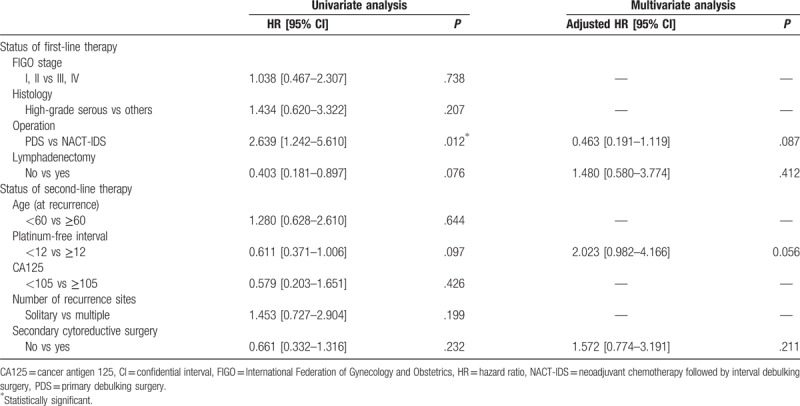
In the matched cohort, serous histologic type, interval debulking surgery at first therapy, and only chemotherapy at recurrence were potential poor prognostic factors for PFS in the univariate analysis. In the multivariate analysis, these three factors were prognostic factors for PFS (Table 2). In contrast, interval debulking surgery at first therapy, lymphadenectomy at the primary surgery, and a short PFI were potential poor prognostic factors for OS in the univariate analysis. In the multivariate analysis, we did not identify any prognostic factors (Table 3). These findings indicate that SCS affects the prognosis for PFS but not for OS.
Table 2.
Univariate and multivariate analyses of progression-free survival.
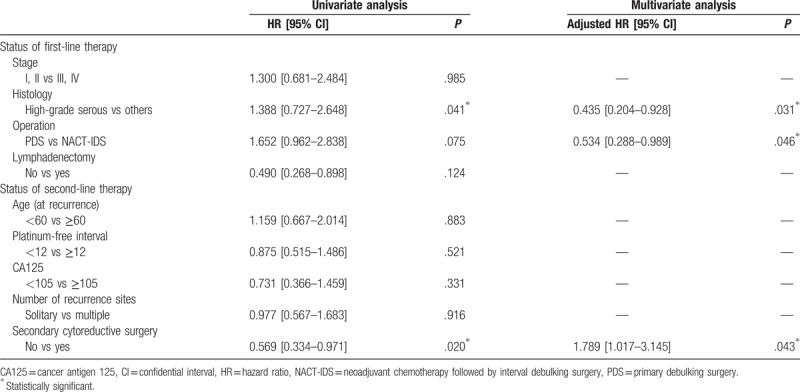
Table 3.
Univariate and multivariate analyses of overall survival.
3.4. Surgical findings at secondary cytoreduction
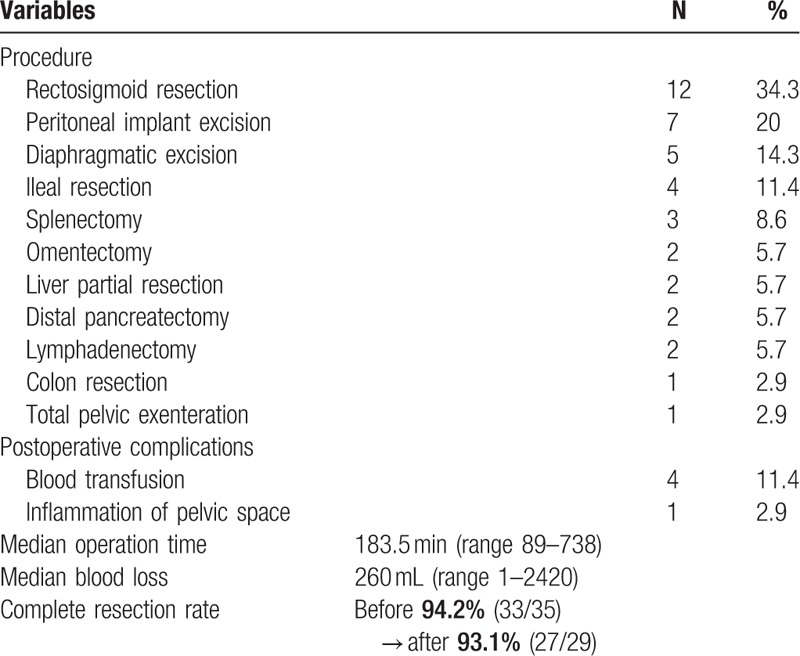
Findings at SCS and the surgical procedures performed are detailed in Table 4. Twenty-five patients (71.4%) had solitary recurrent tumors and 10 patients (28.6%) had multiple recurrent sites. Of the 35 patients who underwent SCS, 33 had no residual disease after surgery. The remaining 2 patients also had optimal resection (<1 cm). Severe operative morbidity in this series included postoperative inflammation of the pelvic space in one patient who underwent total pelvic exenteration. We performed blood transfusion in 4 patients. However, no cases of preoperative death were observed. Therefore, SCS is safe for AGO-positive patients and complete resection can be performed with high probability.
Table 4.
Surgical procedures of secondary cytoreductive surgery (N = 35).
4. Discussion
We analyzed 112 AGO score-positive patients who were diagnosed with ROC. This study showed a significant improvement in PFS with SCS plus chemotherapy compared to that with chemotherapy alone, after adjusting for other relevant prognostic factors. However, we did not observe an improvement in OS with SCS plus chemotherapy. These findings are the first indications that SCS does not contribute to the improvement in OS in ROC patients.
Complete resection at SCS is an important prognostic factor. There are many reports on the predictive markers of complete cytoreduction in ROC.[6–9] Tian et al[10] reported that performance status, residual tumor at primary debulking surgery, CA125 level at recurrence, ascites at recurrence, and disease-free interval are independent prognostic factors of secondary cytoreduction for ROC. The authors proposed 6 factors (5 factors plus FIGO stage) for the risk score. The complete resection rates were 53.4% and 20.1% in the low- and high-risk score groups, respectively. Additionally, the DESKTOP I trial proposed the score for the prediction of complete cytoreduction in ROC.[8] Resectability was assumed if the following 3 factors were present: complete resection at first surgery, good performance status, and absence of ascites. The complete resection rate was 76% in positive AGO score patients in the only prospective study of ROC patients (DESKTOP II trial).[11] Therefore, as the complete resection rate was higher with the AGO score than with the risk score of Tian et al, we believe that the AGO score is a suitable predictive marker of SCS for ROC.
In our investigation using the AGO score, macroscopically complete SCS was obtained in 94.2% (33/35) of patients whose median survival after salvage surgery was 58.0 months compared to 24.0 months for patients who received chemotherapy alone (P = .003). Secondary resection is technically possible in a significant proportion of ROC patients. However, in previously published series, the technical success rates of secondary cytoreduction varied widely, ranging 37% to 83%[12–15] without an AGO score and 76% to 84% with an AGO score.[11,16] We consider that it is possible to predict complete cytoreduction by using the AGO score, because the use of the AGO score stabilizes the complete resection rate at a higher level.
Surprisingly, SCS was not associated with the prolongation of OS after recurrence in our study. Although unadjusted risk analysis did show increased survival following SCS, this difference was no longer of statistical relevance after risk adjustment. The prolongation of OS observed in unadjusted analysis was clearly due to differences in baseline characteristics and not due to the SCS itself. In a previous study,[15,17–20] SCS improved OS compared to survival in patients who did not undergo surgery. A systematic review in 2010 compared ROC patients who were treated with SCS and chemotherapy with those who were treated with chemotherapy alone; however, there has been no prospective study on this topic.[17] There are few retrospective series and a cancer registry study.[15,18–20] A study with relatively large data has also been reported, but the bias of patient background has not been removed sufficiently.[21] These studies have the most important issue that clinicians operated on the operable ROC patients. Studies on SCS excluding the intention of clinicians have not been reported. We selected ROC patients using the AGO score to rule out the intention of clinicians. However, in our unadjusted analysis, PFI was significantly longer and CA125 level was significantly lower in the SCS group than in the CT group. We believed that we could not completely exclude the intention of clinicians. Therefore, we applied propensity-scoring methods to evaluate the putative impact of SCS on OS and PFS in ROC patients. We eliminated background bias as much as possible using propensity score matching with larger data than that in previous studies. As a result, PFS was longer with SCS plus chemotherapy than with chemotherapy alone; however, it did not prolong OS.
The lack of a significant difference in OS after recurrence between the SCS group and the CT group does not imply that SCS is unnecessary for the treatment of ROC. Although SCS did not contribute to the prolongation of OS, the OS at 3 years was 46.4% for patients who did not undergo surgery, and SCS reduced the risk of death by 34.6% and improved OS at 3 years to 71.0%. Additionally, SCS contributed to the significant improvement in PFS (P = .02). Plotti et al[22] indicated that both surgery followed by chemotherapy and chemotherapy alone have a negligible impact on quality of life (QOL) in the QLQ-C30 and EORTC QLQ-OV28 questionnaires. Clinicians generally select systemic chemotherapy in ROC patients, regardless of whether the condition is symptomatic or asymptomatic. However, we demonstrated that complete resection was achieved in 94.2% of AGO-positive ROC patients. We consider that this approach is beneficial for QOL as it creates a cancer-free state in ROC patients. Also, several heterogenous studies have recorded the outcomes of patients who had intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) after SCS for ROC. However, Baiocchi et al described that there was no impact on survival in ROC when HIPEC was added to SCS. Furthermore, HIPEC was more significantly associated with morbidity.[23] Therefore, ROC patients had a benefit through surgical resection followed by systemic chemotherapy as compared with only systemic chemotherapy. Although aggressive surgical treatment combined with systemic chemotherapy does not prolong OS, some of these patients might achieve long-term PFS. Therefore, our results indicate that SCS is a valid treatment option in ROC patients.
The present study has several strengths. It has reported results that are consistent with those of previous studies on the benefit of SCS plus chemotherapy compared with chemotherapy alone and has included more patients than most previous comparative series. Further, data on many variables at recurrence were collected before SCS, allowing adjustment for the most important prognostic factors at recurrence. Finally, the selection bias was statistically reduced by matching resected and unresected patients using propensity scores.
Nevertheless, there were some limitations. First, our analyses were performed retrospectively, which is a major limitation of our study. Second, this retrospective study was limited to a single institution and may not be reflective of other practices. Additionally, this study was unable to adequately address the type and timing of chemotherapy with regard to the relationship with cytoreduction for ROC. Third, the exclusion of patients in the propensity score-matched analysis resulted in a loss of statistical power. These limitations, especially selection bias, would be properly addressed in prospective trials such as DESKTOP III (NCT01166737), GOG213 (NCT00565851), and SOCceR (NTR3337), which are ongoing.
In conclusion, SCS plus chemotherapy may prolong PFS. However, Japanese ROC patients with a positive AGO score may be less likely to benefit from SCS. When we consider that conservation of QOL and tolerability of patients with ROC improved, prolongation of the PFS period contributes to the clinical benefit of patients. However, we could not find evidence that SCS improves the OS rate, and it is unclear which patients actually benefit from SCS. Patients with poor clinical status and a high risk for surgical complications are certainly not good candidates for SCS; however, selecting only patients without any factors of a poor prognosis for surgery might exclude those with few such factors who might benefit from surgery. The value of SCS in these patients should also be determined in prospective randomized trials.
Acknowledgment
The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Abbreviations: AGO = The Arbeitsgemeinschaft Gynaekologische Onkologie, CA125 = cancer antigen 125, DESKTOP = Descriptive Evaluation of preoperative Selection KriTeria for OPerability, HIPEC = hyperthermic intraperitoneal chemotherapy, NACT-IDS = neoadjuvant chemotherapy followed by interval debulking surgery, OS = overall survival, PDS = primary debulking surgery, PFI = platinum-free interval, PFS = progression-free survival, ROC = recurrent ovarian cancer, SCS = secondary cytoreductive surgery.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334:1–6. [DOI] [PubMed] [Google Scholar]
- [3].Usami T, Kato K, Taniguchi T, et al. Recurrence patterns of advanced ovarian, fallopian tube, and peritoneal cancers after complete cytoreduction during interval debulking surgery. Int J Gynecol Cancer 2014;24:991–6. [DOI] [PubMed] [Google Scholar]
- [4].Rose PG, Nerenstone S, Brady MF, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med 2004;351:2489–97. [DOI] [PubMed] [Google Scholar]
- [5].Harter P, Beutel B, Alesina PF, et al. Prognostic and predictive value of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score in surgery for recurrent ovarian cancer. Gynecol Oncol 2014;132:537–41. [DOI] [PubMed] [Google Scholar]
- [6].Onda T, Yoshikawa H, Yasugi T, et al. Secondary cytoreductive surgery for recurrent epithelial ovarian carcinoma: proposal for patients selection. Br J Cancer 2005;92:1026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bristow RE, Peiretti M, Gerardi M, et al. Secondary cytoreductive surgery including rectosigmoid colectomy for recurrent ovarian cancer: operative technique and clinical outcome. Gynecol Oncol 2009;114:173–7. [DOI] [PubMed] [Google Scholar]
- [8].Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol 2006;13:1702–10. [DOI] [PubMed] [Google Scholar]
- [9].Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer 2011;105:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tian WJ, Chi DS, Sehouli J, et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Ann Surg Oncol 2012;19:597–604. [DOI] [PubMed] [Google Scholar]
- [11].Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer 2011;21:289–95. [DOI] [PubMed] [Google Scholar]
- [12].Jänicke F, Hölscher M, Kuhn W, et al. Radical surgical procedure improves survival time in patients with recurrent ovarian cancer. Cancer 1992;70:2129–36. [DOI] [PubMed] [Google Scholar]
- [13].Vaccarello L, Rubin SC, Vlamis V, et al. Cytoreductive surgery in ovarian carcinoma patients with a documented previously complete surgical response. Gynecol Oncol 1995;57:61–5. [DOI] [PubMed] [Google Scholar]
- [14].Eisenkop SM, Friedman RL, Wang HJ. Secondary cytoreductive surgery for recurrent ovarian cancer. A prospective study. Cancer 1995;76:1606–14. [DOI] [PubMed] [Google Scholar]
- [15].Güngör M, Ortaç F, Arvas M, et al. The role of secondary cytoreductive surgery for recurrent ovarian cancer. Gynecol Oncol 2005;97:74–9. [DOI] [PubMed] [Google Scholar]
- [16].Janco JM, Kumar A, Weaver AL, et al. Performance of AGO score for secondary cytoreduction in a high-volume U.S. center. Gynecol Oncol 2016;141:140–7. [DOI] [PubMed] [Google Scholar]
- [17].Galaal K, Naik R, Bristow RE, et al. Cytoreductive surgery plus chemotherapy versus chemotherapy alone for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2010;6:CD007822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kuhn W, Schmalfeldt B, Pache L, et al. Disease-adapted relapse therapy for ovarian cancer: results of a prospective study. Int J Oncol 1998;13:57–63. [PubMed] [Google Scholar]
- [19].Matsumoto A, Higuchi T, Yura S, et al. Role of salvage cytoreductive surgery in the treatment of patients with recurrent ovarian cancer after platinum-based chemotherapy. J Obstet Gynaecol Res 2006;32:580–7. [DOI] [PubMed] [Google Scholar]
- [20].Xu X, Chen X, Dai Z, et al. Secondary cytoreduction surgery improves prognosis in platinum-sensitive recurrent ovarian cancer. J Exp Clin Cancer Res 2013;32:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].da Costa AA, Valadares CV, Mantoan H, et al. The value of secondary cytoreductive surgery in recurrent ovarian cancer and application of a prognostic score. Int J Gynecol Cancer 2016;26:449–55. [DOI] [PubMed] [Google Scholar]
- [22].Plotti F, Scaletta G, Aloisi A, et al. Quality of life in platinum-sensitive recurrent ovarian cancer: chemotherapy versus surgery plus chemotherapy. Ann Surg Oncol 2015;22:2387–94. [DOI] [PubMed] [Google Scholar]
- [23].Baiocchi G, Ferreira FO, Mantoan H, et al. Hyperthermic intraperitoneal chemotherapy after secondary cytoreduction in epithelial ovarian cancer: a single-center comparative analysis. Ann Surg Oncol 2015;23:1294–301. [DOI] [PubMed] [Google Scholar]


