Abstract
Background:
It is unknown whether gabapentin is effective in reducing acute pain following laparoscopic cholecystectomy. The purpose of the current meta-analysis was to evaluate the efficacy of gabapentin in reducing pain intensity and postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy.
Methods:
All randomized controlled trials (RCTs) evaluating the efficacy of gabapentin in reducing pain intensity and PONV after laparoscopic cholecystectomy were searched on the following databases: PubMed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Google database, the Chinese Wanfang database, and the China National Knowledge Infrastructure (CNKI). The most recent literature search was conducted on March 21, 2017. Outcomes including visual analog scale (VAS) at 12 and 24 hours, total morphine consumption, and the occurrence of PONV. Continuous outcomes were expressed as the weighted mean difference (WMD) and 95% confidence interval (CI), and the one discontinuous outcome was expressed as risk ratio (RR) and 95% CI. Stata 12.0 software was used for meta-analysis.
Results:
A total of 9 studies involving 966 patients were identified. In total, there were 484 gabapentin subjects and 482 controls. Compared with the control group, gabapentin was associated with lower VAS at 12 hours (WMD = −10.18, 95% CI: −17.36 to −2.80, P = .007) and 24 hours (WMD = −6.33, 95% CI: −8.41 to −4.25, P = .000), which was equivalent on a 110-point VAS scale to 10.18 points at 12 hours and 6.33 points at 24 hours. Compared with the control group, gabapentin was associated with less total morphine consumption by approximately 110.83 mg (WMD = −110.83, 95% CI: −183.25 to −38.42, P = .003). In addition, the occurrence of nausea and vomiting in gabapentin was decreased (25.2% vs 47.6, RR = 0.53, 95% CI: 0.44–0.63, P = .000).
Conclusion:
Gabapentin was efficacious in reducing postoperative pain, total morphine consumption, and morphine-related complications following laparoscopic cholecystectomy. In addition, there was a negative correlation between the gabapentin dosage and the occurrence of nausea and vomiting. The number of included studies is limited, and more studies are needed to verify the effects of gabapentin in laparoscopic cholecystectomy patients.
Keywords: gabapentin, laparoscopic cholecystectomy, meta-analysis
1. Introduction
Postoperative pain, nausea, and vomiting are common complications of laparoscopic cholecystectomy.[1] It is reported that appropriately of 53% to 72% of patients undergoing laparoscopic cholecystectomy require antiemetics after surgery.[2,3] Reduction of postoperative pain can shorten patients’ hospital stays and promote functional recovery.[4] Traditionally, opioids are the first option for the treatment of moderate to severe pain after surgery. However, the side effects of opioid analgesics, such as nausea and vomiting, limit the clinical use of those drugs.[5,6]
Gabapentin is an antiepileptic drug that also has therapeutic effects on diabetic neuropathy and herpes zoster neuropathic pain.[7,8] In addition, gabapentin may reduce postoperative acute pain by reducing central sensitization.[9] Recent meta-analyses show that gabapentin can reduce the amount of opioid use after abdominal hysterectomy, spinal surgery, and orthopedic surgeries.[10–12] Gabapentin has used for pain control in patients following laparoscopic cholecystectomy without conclusive results.[13,14] In addition, there is no systematic review and meta-analysis to assess the efficacy and safety of gabapentin in reducing pain following laparoscopic cholecystectomy. The purpose of the current meta-analysis was to determine whether preoperative treatment with gabapentin is associated with lower pain scores, total morphine consumption, and postoperative nausea and vomiting (PONV) following laparoscopic cholecystectomy.
2. Materials and methods
This is a meta-analysis and thus no ethical approval was necessary.
2.1. Inclusion criteria and exclusion criteria
Inclusion criteria—Study type: clinical RCTs; Participants: patients prepared for laparoscopic cholecystectomy (ASA 3 and 4); Intervention: the experimental group received preoperative oral gabapentin, while the control group received a placebo or blank control. Outcomes: visual analog score (VAS) at 12 and 24 hours, total morphine consumption, and the occurrence of PONV.
Exclusion criteria—Comparison with other drugs (glucocorticoid or pregabalin); Noninclusion of gabapentin drugs; Non-RCTs; Comments, reviews or with no relevant outcomes.
2.2. Search strategies
We systematically searched RCTs that investigated the preoperative use of gabapentin for the treatment of laparoscopic cholecystectomy pain from PubMed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Google database, the Chinese Wanfang database, and the China National Knowledge Infrastructure (CNKI). The most recent literature search was conducted on March 21, 2017. The search terms in the PubMed database were: (((“Cholecystectomy, Laparoscopic”[Mesh]) OR laparoscopic cholecystectomy)) AND gabapentin. There were no restrictions regarding language or publication date. We also manually retrieved reference lists from the identified studies and relevant review studies to identify additional relevant studies.
2.3. Quality assessment
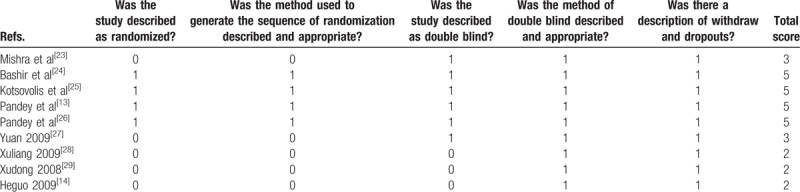
The quality of all included trials was independently assessed by 2 reviewers (LW and YD) on the basis of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (http://handbook.cochrane.org/).[15] The evaluation criteria include 7 items: random sequence generation; allocation concealment; blind method (patients and healthcare providers); blind outcome assessment; incomplete outcome data; selective outcome reporting; and other bias. The criteria were evaluated as low risk, unclear, or high risk. Finally, Review Manager 5.3.0 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) was applied to generate graphics. Meanwhile, the Jadad score was used to quantitatively evaluate the quality of RCTs.[16] The Jadad score including 3 items: random sequence generation, blinding, and dropouts. Low-quality articles have a Jadad score between 1 and 3; high-quality articles have a Jadad score between 4 and 7.[17]
2.4. Data extraction
A specific extraction was conducted to assemble data into a pregenerated standard Microsoft Excel (Microsoft Corporation, Redmond, WA) file. The items extracted from relevant studies were as follows: first author and publication year, sample size of the intervention and control groups, preoperative doses, timing and frequency and the total dose of gabapentin per number of days and follow-ups. Outcomes such as the VAS at 12 and 24 hours and the occurrence of nausea and vomiting were abstracted and recorded in the spreadsheet. Postoperative pain intensity was measured using a 110-point VAS (0 = no pain and 100 = extreme pain). When the numerical rating scale (NRS) was reported, it was converted to a VAS. Additionally, the 11-point VAS was converted to a 110-point VAS.[18] Data in other forms (i.e., median, interquartile range, and mean ± 95% confidence interval (CI)) were converted to the mean ± standard deviation (SD) according to the Cochrane Handbook.[15] If the data were not reported numerically, we extracted these data using the “GetData Graph Digitizer” software from the published figures. All the data were extracted by 2 independent reviewers (L-FW and Y-CD), and disagreements were resolved by discussion.
2.5. Statistical analysis
Statistical analysis was performed using Stata 12.0 software (Stata Corp., College Station, TX). The continuous outcomes (VAS at 12 and 24 hours and total morphine consumption) were expressed as the weighted mean difference (WMD) and 95% CI, and the discontinuous outcome (the occurrence of PONV) was compared using the risk ratio (RR) and 95% CI. The heterogeneity between the studies was assessed using the I2 test; if I2 was less than or equal to 50%, there was no obvious heterogeneity, and a fixed-effect model (Mantel–Haenszel) was applied to the data. We used a random effects model if I2 was more than 50%. Subgroup analysis was conducted according to the dosage of gabapentin (<900 or ≥900 mg/d). The relationship between the gabapentin dosage and the VAS at 12 and 24 hours was explored using GraphPad Prism software (Version 6.0; GraphPad Software, San Diego, CA). The correlation coefficient (r) was used to evaluate the relationship between the dosage of gabapentin and the occurrence of nausea and vomiting. Statistical significance was defined as P < .05. To measure the robustness of the pooled results and avoid a type I error,[19–22] trial sequence analysis (TSA) was performed for the primary outcome (TSA software, version 0.9.5.5 beta; Copenhagen Trial Unit, Copenhagen). Power analyses of individual studies and meta-analyses were all conducted by software PS (Power and Sample Size Calculations, London) version 3.0.43.
3. Results
3.1. Search results
The flow diagram for the included studies can be seen in Fig. 1. According to the search strategy, a total of 688 studies were retrieved (PubMed = 121, Embase = 85, Web of Science = 49, Cochrane Library = 55, Google database = 109, Chinese Biomedical Database (CBM) = 100, VIP database = 38, China Wanfang data = 59, China National Knowledge Infrastructure (CNKI) = 72). Then, the literatures were imported into Endnote software (Version X7, Thompson Reuters, CA) to exclude the duplicates. Then, a total of 418 articles were excluded at the title and abstract level. Finally, a total of 9 RCTs with 966 patients were included in this meta-analysis.[13,14,23–29]
Figure 1.
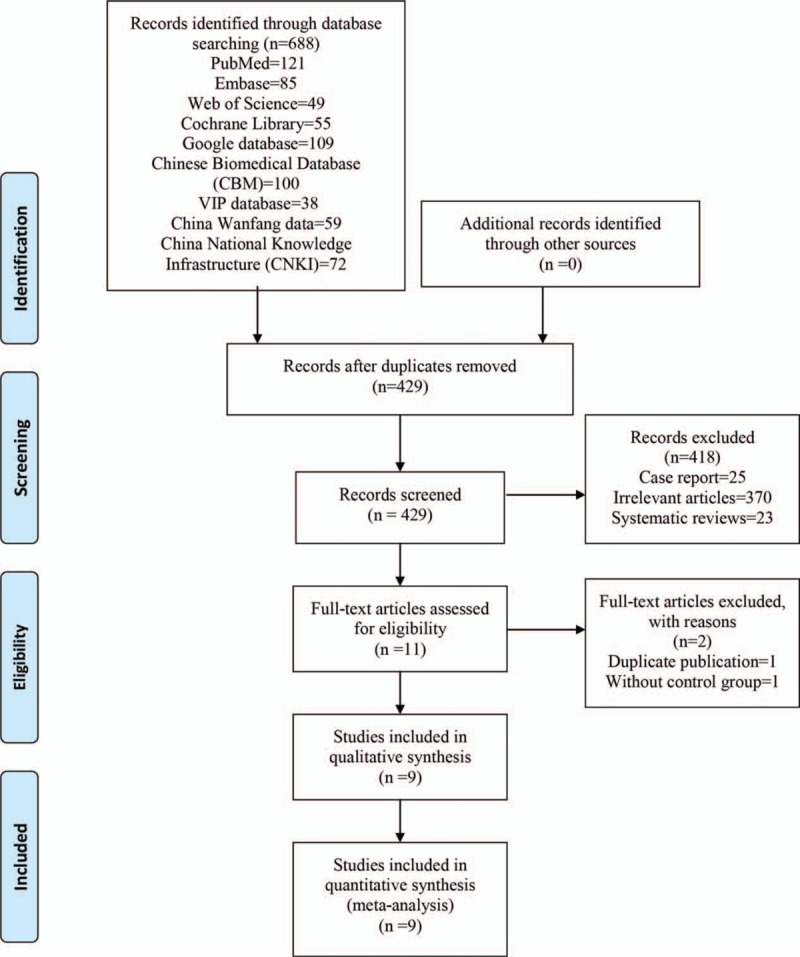
Flowchart of study search and inclusion criteria.
3.2. General characteristic and quality assessment
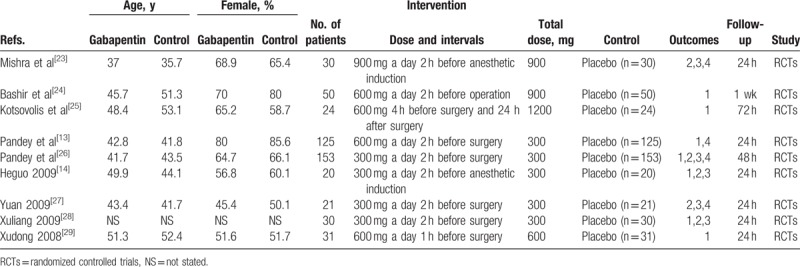
The general information of the patients is shown in Table 1. The publication years ranged from 2004 to 2016. The numbers of gabapentin and control subjects ranged from 20 to 153. The total gabapentin dosages ranged from 300 to 1200 mg per day. Finally, the follow-up times ranged from 24 hours to 1 week. The general characteristic of the included studies were comparable and all studies describe the intent to treat analyses.
Table 1.
The general characteristic of the included studies (1, the occurrence of nausea and vomiting; 2, VAS at 12 hours; 3, VAS at 24 hours, 4, total morphine consumption).
The risk of bias summary and risk of bias graph are provided in Figs. 2 and 3, respectively. The risk of bias for random sequence generation and allocation concealment was unclear in 5 studies[14,27–29] and low in 4 studies.[13,24–26] Blinding of participant and personnel was unclear in 4 studies,[14,27–29] and the rest all had low risk of bias.[13,23–26] We used Jadad score to assess the RCTs, and the results are displayed in Table 2. The Jadad scores were 2 in 3 studies, 3 in 2 studies, and 5 in 4 studies. The quality of all of the studies were acceptable. The individual study's power was ranged from 65.3% to 72.9%. The power of the meta-analysis of the total studies was 86.4%.
Figure 2.
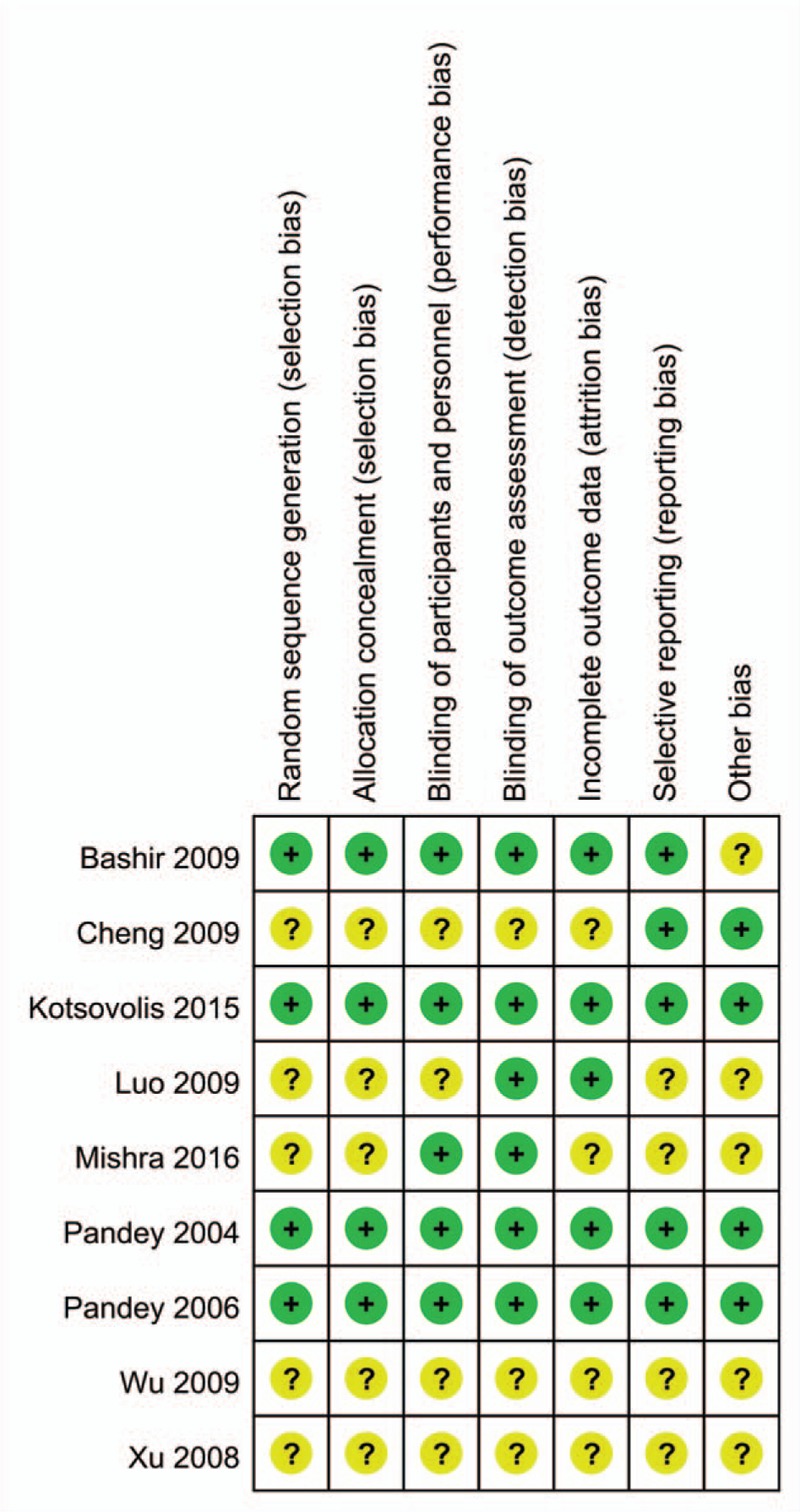
Risk of bias of included randomized controlled trials. +, No bias; −, bias; ?, bias unknown.
Figure 3.
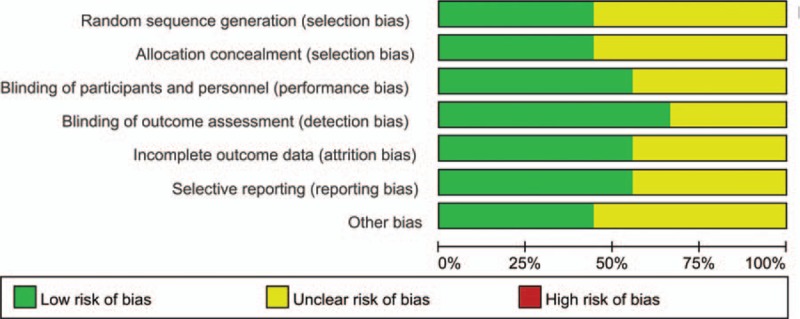
Risk of bias graph.
Table 2.
Jadad score for the evaluation of randomized controlled trials included in this meta-analysis.
3.3. Results of meta-analysis
3.3.1. VAS at 12 hours
Five papers involving 508 patients analyzed the VAS at 12 hours, and there was substantial statistical heterogeneity among the studies (I2 = 95.0%, P = .000). Compared with the control treatments, gabapentin reduced the intraoperative VAS at 12 hours, and the difference was statistically significant (WMD = −10.18, 95% CI: −17.36 to −2.80, P = .007, Fig. 4). We then tested the publication bias using the funnel plot and Begg test; the results are shown in Figs. 5 and 6, respectively. The effect size was found to be symmetrical, and Begg value was 0.952.
Figure 4.
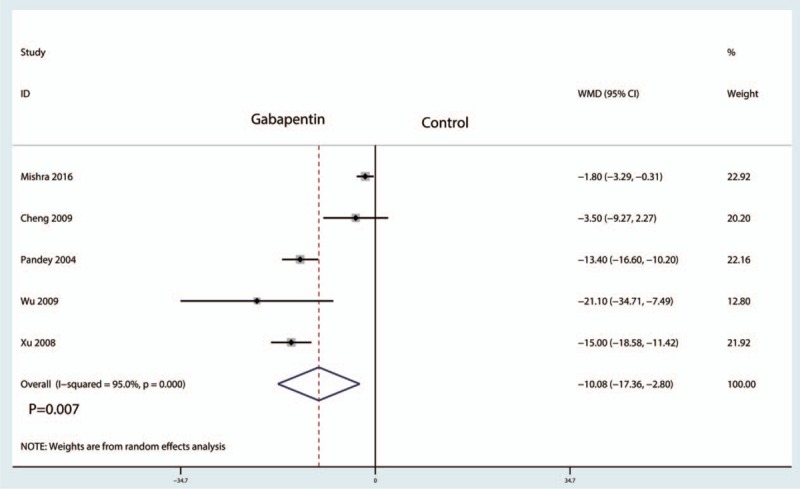
Forest plots of the included studies comparing the VAS at 12 hours.
Figure 5.
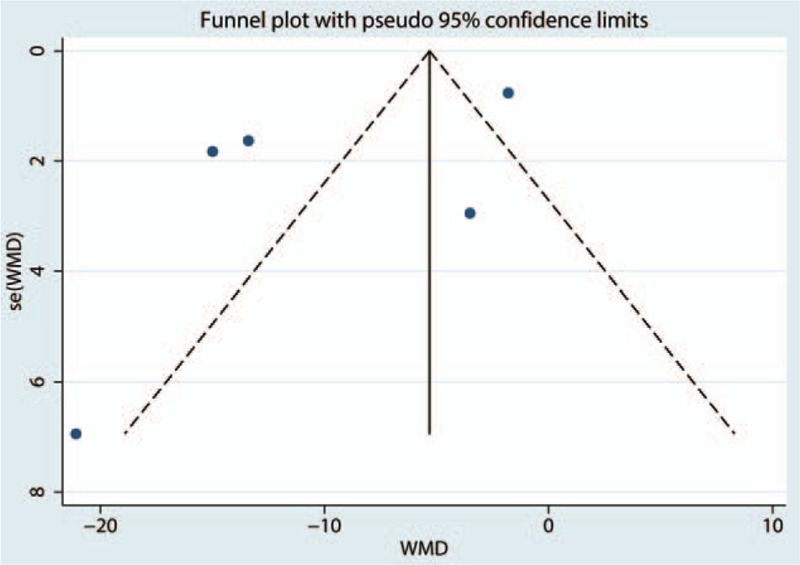
Funnel plot of VAS at 12 hours.
Figure 6.
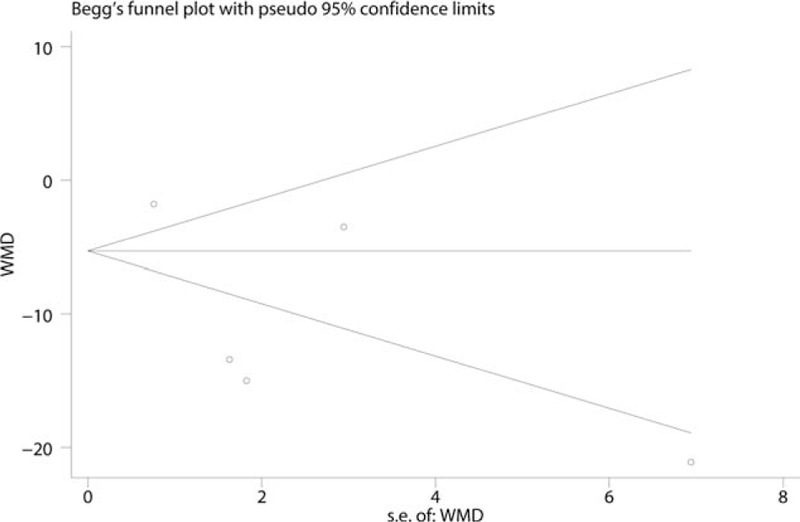
Begg test of VAS at 12 hours.
3.3.2. VAS at 24 hours
Five studies including 508 patients reported the postoperative VAS at 24 hours. There was statistical heterogeneity between the studies (I2 = 55.7%, P = .060), and thus a random effects model was used to perform the meta-analysis. Compared with the control treatments, gabapentin reduced postoperative VAS at 24 hours, and the difference was statistically significant (WMD = −6.33, 95% CI: −8.41 to −4.25, P = .000, Fig. 7).
Figure 7.
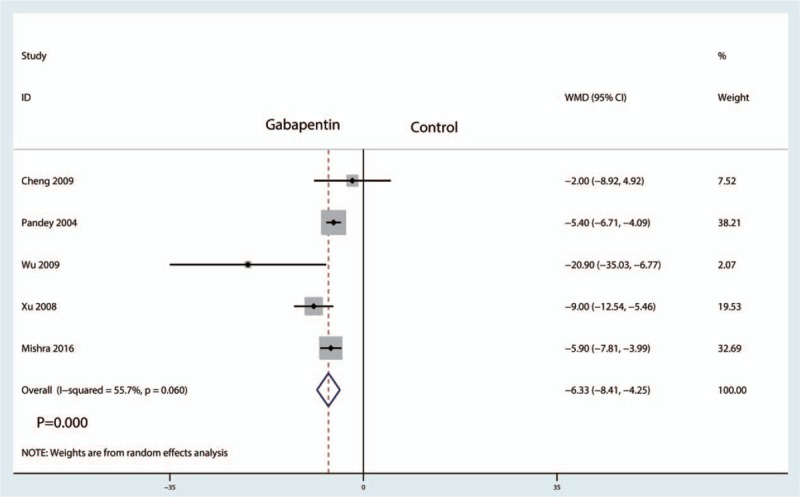
Forest plots of the included studies comparing the VAS at 24 hours.
3.4. Total morphine consumption
Five studies including 706 patients reported the total morphine consumption. There was statistical heterogeneity between the studies (I2 = 99.5%, P = .000), and therefore a random effects model was used to perform the meta-analysis. Compared with the control treatments, gabapentin reduced total morphine consumption after laparoscopic cholecystectomy, and the difference was statistically significant (WMD = −110.83, 95% CI: −183.25 to −38.42, P = .003, Fig. 8).
Figure 8.
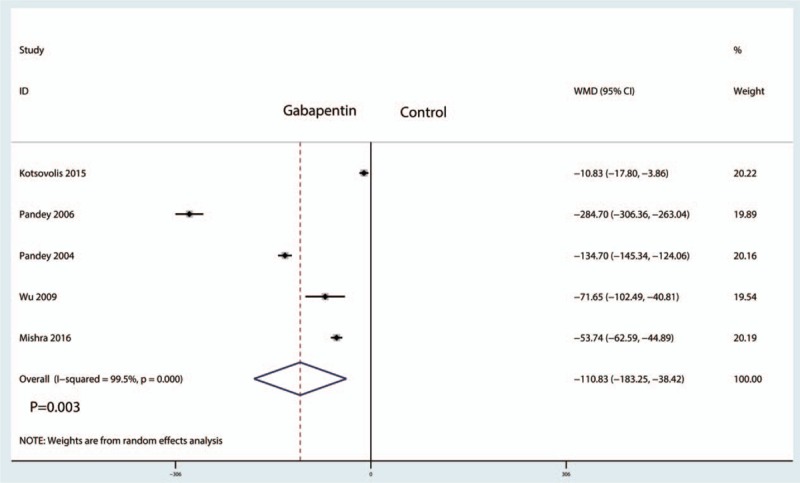
Forest plots of the included studies comparing the total morphine consumption.
3.5. The occurrence of nausea and vomiting
Seven studies including 864 patients analyzed the occurrence of PONV. There was little statistical heterogeneity between the studies (I2 = 33.4%, P = .173), and thus a fixed effect model was used to perform the meta-analysis. Compared with the control group, gabapentin reduced the occurrence of PONV, and the difference was statistically significant (RR = 0.53, 95% CI: 0.44–0.63, P = .000, Fig. 9).
Figure 9.
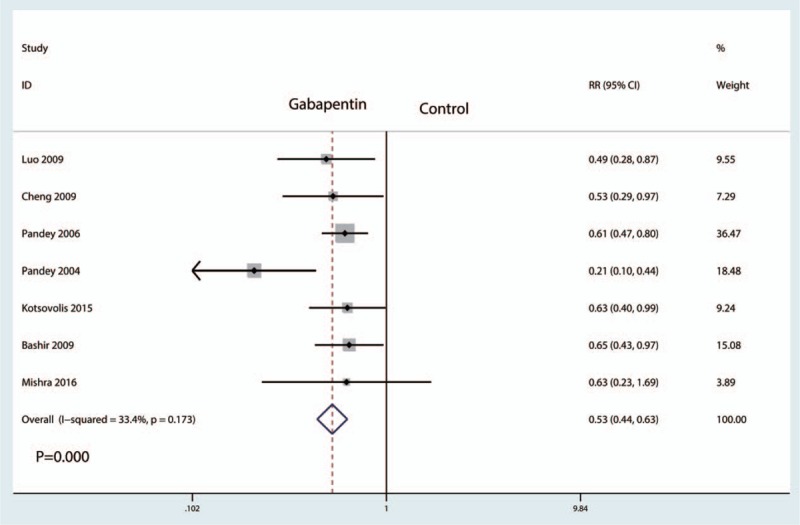
Forest plots of the included studies comparing the occurrence of nausea and vomiting.
3.6. Dose–effect relationship
We plotted the gabapentin dosage on the abscissa, with the corresponding PONV as the ordinate, to generate a scatterplot. In addition, the linear correlation coefficient (r) was calculated. There was a negative correlation between the dosage of gabapentin and PONV (r = −0.754, P = .041, Fig. 10). The occurrence of nausea and vomiting tended to decrease as the gabapentin dosage increased.
Figure 10.
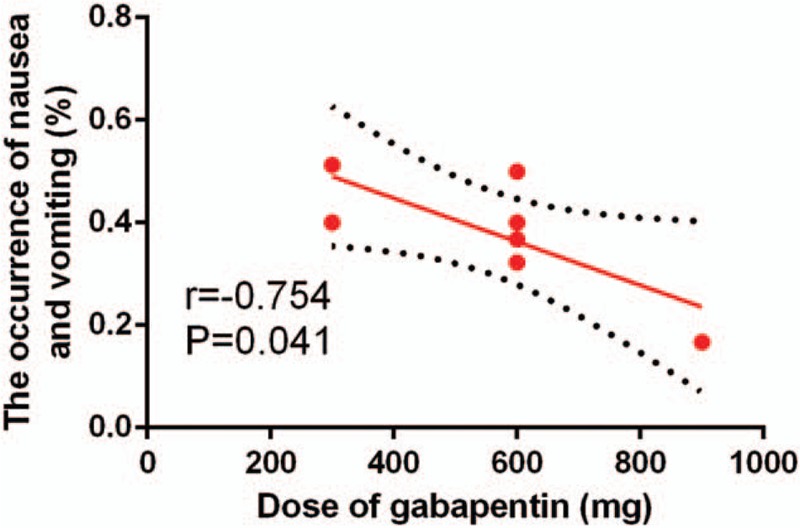
Scatter plot showing the relationship between the dosage of gabapentin and the occurrence of nausea and vomiting.
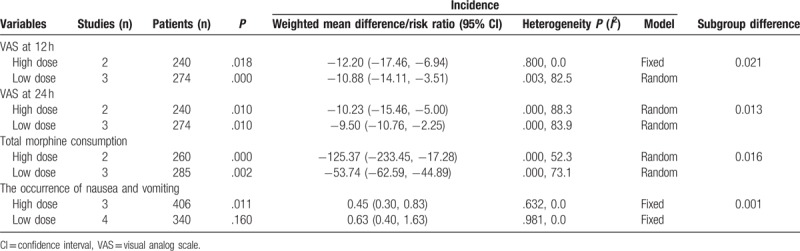
3.7. Subgroup analysis
Subgroup analysis results are available in Table 3. The pooled results indicated that a high dosage of gabapentin was superior to a low dosage in reducing VAS at 12 and 24 hours, total morphine consumption, and the occurrence of nausea and vomiting (P < .05).
Table 3.
Subgroup analysis of the outcomes according to the dose of gabapentin (high dose vs low dose).
3.8. TSA results
TSA results shown that the accumulative Z-curve crossed the trial sequential monitoring boundary for benefit after the trials. A TSA confirmed the VAS at 12 hours (Fig. 11A), VAS at 24 hours (Fig. 11B), total morphine consumption (Fig. 11C), and the occurrence of nausea and vomiting (Fig. 11D). These results provided firm evidence of a significant reduction in VAS at 12 hours, VAS at 24 hours, total morphine consumption, and the occurrence of nausea and vomiting in the gabapentin group. Meanwhile, the sample was sufficient to provide firm conclusion.
Figure 11.
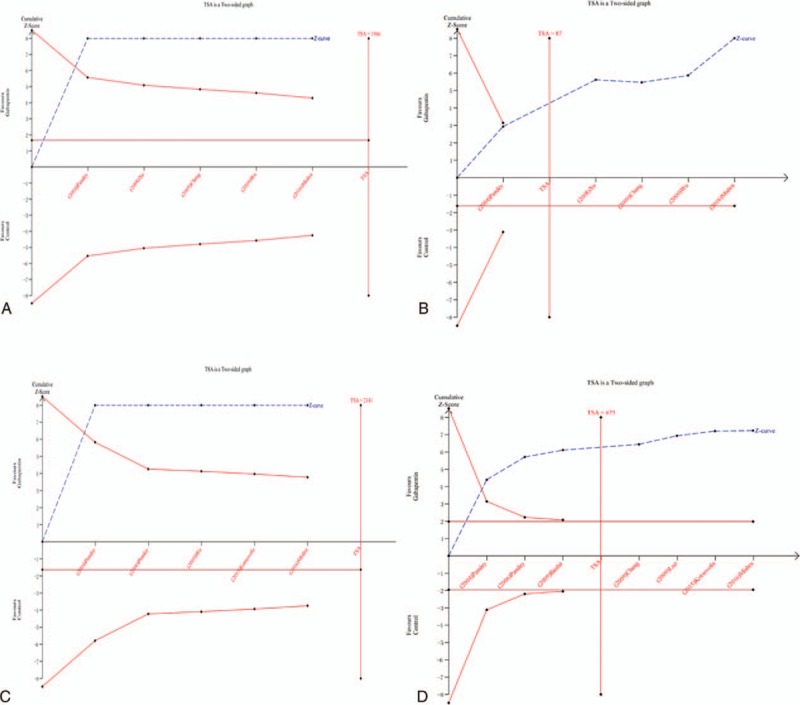
(A) TSA results for VAS at 12 hours, (B) TSA results for VAS at 24 hours, (C) TSA results for total morphine consumption, (D) TSA results for the occurrence of nausea and vomiting.
4. Discussion
This is the first systematic review and meta-analysis to assess the effects of gabapentin on postoperative acute pain and PONV in patients following laparoscopic cholecystectomy. The final results indicated that preoperative treatment with gabapentin was associated with lower pain scores at 12 and 24 hours postoperatively. In addition, gabapentin decreased the total morphine consumption and the occurrence of nausea and vomiting. After a comprehensive search of multiple electronic databases, a total of 9 studies were included in this meta-analysis. A major strength of the current meta-analysis was the statistical rigor with which the outcomes were calculated.
The pooled results indicated that preoperative administration of gabapentin was associated with lower pain scores postoperatively, which was equivalent on a 110-point VAS to 10.18 points at 12 hours and 6.33 points at 24 hours. Arumugam et al[30] performed a meta-analysis involving 17 RCTs with 1793 surgical patients, and their results indicated that gabapentin is an effective analgesic adjunct in patients undergoing elective surgery. Fabritius et al[31] indicated that evidence for the use of gabapentin is lacking as clinically relevant benefits may be absent and there is a major risk of harm, especially when gabapentin is added to multimodal analgesia. Zhai et al[32] conducted a meta-analysis and found that gabapentin was effective in reducing acute postoperative pain in patients undergoing total knee arthroplasty (TKA). These results are contradictory to a previous meta-analysis that compared gabapentinoids (gabapentin or pregabalin) to placebos for managing pain after TKA. In that meta-analysis, the results indicated that no evidence supported the routine use of gabapentin after TKA.[33]
The current meta-analysis indicated that the use of gabapentin can also decrease total morphine consumption in laparoscopic cholecystectomy by 110.83 mg (WMD = −110.83, 95% CI = (−183.25, −38.42), P = .003). These morphine-saving effects have obvious clinical importance, and thus gabapentin should be used as an adjunct to multimodal anesthesia in laparoscopic cholecystectomy. Alayed el al[34] revealed that preemptive administration of gabapentin is effective in decreasing narcotic consumption by appropriately by 55.9 mg.
The most important finding of current meta-analysis was that gabapentin can decrease the occurrence of nausea and vomiting (RR = 0.53, 95% CI = 0.44–0.63, P = .000). In addition, the confidence level of this outcome is relatively high due to the low heterogeneity between the included studies. We then determined the dose–effect relationship between the gabapentin dosage and the occurrence of nausea and vomiting. A negative relation was found between the dosage of gabapentin and the occurrence of nausea and vomiting. In the included studies, we also tried to compare other complications between the gabapentin and control groups. However, there were insufficient data to extend the meta-analysis to other complications.
There were several limitations in the current meta-analysis: the quality of 4 of the articles was low, which likely resulted in selective bias; the heterogeneity of the VAS at 12 and 24 hours and the total morphine consumption was large and made it difficult to draw a definitive conclusion, though subgroups and a random effects model were used to minimize heterogeneity; the supplementary pain control measures in the included studies were different and thus may have introduced heterogeneity; the dosage of gabapentin was different from experiment to experiment; thus, the optimal dosage of gabapentin requires further study; the occurrence of PONV was measured only in 7 studies and also a great limitation.
In conclusion, immediate analgesic efficacy and opioid-sparing effects (PONV) were obtained with the administration of gabapentin. Additionally, the analgesic efficacy and opioid-sparing effects were obvious in the high-dosage gabapentin group. Thus, we recommend routinely administration high dose of gabapentin in reducing acute pain after laparoscopic cholecystectomy. Because the sample size and the number of included studies were limited, a multicenter RCT is needed to clarify the optimal dose and timing of gabapentin in reducing acute pain after laparoscopic cholecystectomy.
Footnotes
Abbreviations: CBM = Chinese Biomedical Database, CENTRAL = the Cochrane Central Register of Controlled Trials, CI = confidence interval, CNKI = China National Knowledge Infrastructure, PONV = postoperative nausea and vomiting, RCTs = randomized controlled trials, RR = risk ratio, TKA = total knee arthroplasty, VAS = visual analog scale, WMD = weighted mean difference.
The authors have no conflicts of interest to disclose.
References
- [1].Wang N, Wang L, Gao Y, et al. Analgesic effect of preoperative pentazocine for laparoscopic cholecystectomy. Cureus 2016;8:e948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Naguib M, el Bakry AK, Khoshim MH, et al. Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron and metoclopramide in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind comparison with placebo. Can J Anaesth 1996;43:226–31. [DOI] [PubMed] [Google Scholar]
- [3].Pertusa V, Bellver J, Marques A, et al. [Antiemetic prophylaxis after laparoscopic cholecystectomy: comparative study of dehydrobenzperidol, metoclopramide, ondansetron and placebo]. Rev Esp Anestesiol Reanim 1996;43:239–42. [PubMed] [Google Scholar]
- [4].Fujii Y. The utility of antiemetics in the prevention and treatment of postoperative nausea and vomiting in patients scheduled for laparoscopic cholecystectomy. Curr Pharm Des 2005;11:3173–83. [DOI] [PubMed] [Google Scholar]
- [5].Park IJ, Kim G, Ko G, et al. Does preoperative administration of gabapentin/pregabalin improve postoperative nasal surgery pain? Laryngoscope 2016;126:2232–41. [DOI] [PubMed] [Google Scholar]
- [6].Dong J, Li W, Wang Y. The effect of pregabalin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Int J Surg 2016;34:148–60. [DOI] [PubMed] [Google Scholar]
- [7].Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014;Cd007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miranda HF, Sierralta F, Jorquera V, et al. Antinociceptive interaction of gabapentin with minocycline in murine diabetic neuropathy. Inflammopharmacology 2017;25:91–7. [DOI] [PubMed] [Google Scholar]
- [9].Agarwal MM, Elsi Sy M. Gabapentenoids in pain management in urological chronic pelvic pain syndrome: gabapentin or pregabalin? Neurourol Urodyn 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [10].Yu L, Ran B, Li M, et al. Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2013;38:1947–52. [DOI] [PubMed] [Google Scholar]
- [11].Achuthan S, Singh I, Varthya SB, et al. Gabapentin prophylaxis for postoperative nausea and vomiting in abdominal surgeries: a quantitative analysis of evidence from randomized controlled clinical trials. Br J Anaesth 2015;114:588–97. [DOI] [PubMed] [Google Scholar]
- [12].Mao Y, Wu L, Ding W. The efficacy of preoperative administration of gabapentin/pregabalin in improving pain after total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord 2016;17:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pandey CK, Priye S, Ambesh SP, et al. Prophylactic gabapentin for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study. J Postgrad Med 2006;52:97–100. [PubMed] [Google Scholar]
- [14].Heguo L, Haitao M, Jianhua C, et al. Antiemetic effect of gabapentin in patients undergoing laparoscopic cholecystectomy. China J Endosc 2009;15:492–4. [Google Scholar]
- [15].Green S, Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Available at: http://handbook.cochrane.org/. 2011. Access 2011. [Google Scholar]
- [16].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [17].Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2017;12:e0172846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang C, Cai X-Z, Yan S-G. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [19].Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009;38:276–86. [DOI] [PubMed] [Google Scholar]
- [20].Thorlund K, Imberger G, Walsh M, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis—a simulation study. PLoS ONE 2011;6:e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763–9. [DOI] [PubMed] [Google Scholar]
- [22].Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287–98. [DOI] [PubMed] [Google Scholar]
- [23].Mishra R, Tripathi M, Chandola HC. Comparative clinical study of gabapentin and pregabalin for postoperative analgesia in laparoscopic cholecystectomy. Anesth Essays Res 2016;10:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bashir F, Mohd K, Qazi S, et al. A randomized, double blind placebo controlled study evaluating preventive role of gabapentin for PONV in patients undergoing laparascopic cholecystectomy. JK Sci J Med Educ Res 2009;11:190–3. [Google Scholar]
- [25].Kotsovolis G, Karakoulas K, Grosomanidis V, et al. Comparison between the combination of gabapentin, ketamine, lornoxicam, and local ropivacaine and each of these drugs alone for pain after laparoscopic cholecystectomy: a randomized trial. Pain Pract 2015;15:355–63. [DOI] [PubMed] [Google Scholar]
- [26].Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth 2004;51:358–63. [DOI] [PubMed] [Google Scholar]
- [27].Yuan C, Yuanchang X. Effect of gabapentin on postoperative pain during anaesthetic period of laparoscopic cholecystectomy. Acad J Second Mil Med Univ 2009;30:972–4. [Google Scholar]
- [28].Xuliang W, Xiaoning Y, Ling Q. Application of preemptive analgesia of gabapentin in laparoscopic cholecystectomy. J Southeast China Natl Defence Med Sci 2009;11:50–2. [Google Scholar]
- [29].Xudong X. The efficacy of gabapentin in the laparoscopic cholecystectomy. J Clin Anesthesiol 2008;24:1083–7. [Google Scholar]
- [30].Arumugam S, Lau CS, Chamberlain RS. Use of preoperative gabapentin significantly reduces postoperative opioid consumption: a meta-analysis. J Pain Res 2016;9:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fabritius ML, Geisler A, Petersen PL, et al. Gabapentin for post-operative pain management—a systematic review with meta-analyses and trial sequential analyses. Acta Anaesthesiol Scand 2016;60:1188–208. [DOI] [PubMed] [Google Scholar]
- [32].Zhai L, Song Z, Liu K. The effect of gabapentin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hamilton TW, Strickland LH, Pandit HG. A meta-analysis on the use of gabapentinoids for the treatment of acute postoperative pain following total knee arthroplasty. J Bone Joint Surg Am 2016;98:1340–50. [DOI] [PubMed] [Google Scholar]
- [34].Alayed N, Alghanaim N, Tan X, et al. Preemptive use of gabapentin in abdominal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol 2014;123:1221–9. [DOI] [PubMed] [Google Scholar]


