Abstract
Although spondylolisthesis was traditionally treated with posterior lumbar interbody fusion (PLIF), minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) was recently proposed as an alternative treatment for spondylolisthesis. However, no studies have focused on the comparison of these 2 techniques’ outcome on spondylolisthesis.
The operative reports and perioperative data of patients who underwent single-level primary open PLIF (n = 29) and MIS-TLIF (n = 26) for I/II spondylolisthesis were retrospectively evaluated. Patients’ demographics, operative blood loss, hospital length of stay, creatine kinase (CK) level, radiographic fusion, complications, and patient-reported outcomes were evaluated. Radiographic fusion was assessed using the Bridwell grading criteria. Preoperative and postoperative patient-reported outcomes included the visual analog scale (VAS) and Oswestry Disability Index (ODI).
Average follow-up was 28 ± 3.6 months (range 24–32 months). Bed rest time, hospital stay, estimated blood loss, and operative time in the MIS-TLIF group were significantly lower than those in the PLIF group (P < .05). The 3-month postoperative ODI and VAS in the MIS-TLIF group were significantly better than the PLIF group (P < .05). However, at the time of the last follow-up, both groups had similar ODI scores and complication, slip reduction, and spinal fusion rates (P > .05).
Compared with PLIF, MIS-TLIF for grade I/II spondylolisthesis can achieve similar reduction and fusion results with better short-term quality of life, shorter hospital stays, less estimated blood loss, and shorter operative times.
Keywords: minimally invasive, posterior lumbar interbody fusion, reduction, spondylolisthesis, transforaminal lumbar interbody fusion
1. Introduction
Spondylolisthesis is the forward slip of a vertebral segment.[1] Of its 5 subtypes, degenerative and isthmic spondylolisthesis are the most common in adults.[2] Spondylolisthesis mostly occurs at the L4-L5 or L5-S1 level, and needs to be treated surgically when conservative management fails.[3] Spondylolisthesis is traditionally treated with posterior lumbar interbody fusion (PLIF), which requires extensive muscle dissection and retraction.[4,5] However, minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) was recently proposed as an alternative treatment for spondylolisthesis. Benefits of MIS-TLIF include smaller operative wounds, reduced trauma to adjacent tissue, and a more rapid postoperative recovery.[6–8]
The effectiveness of MIS-TLIF in the management of spondylolisthesis remains controversial. To the best of our knowledge, no previous studies have directly compared MIS-TLIF and PLIF for spondylolisthesis correction. The purpose of this study was to compare the perioperative factors, clinical outcomes, and radiographic results of MIS-TLIF and PLIF for spondylolisthesis.
2. Methods
2.1. Patient population
We obtained ethical approval from the Ethic Committee of Chinese People's Liberation Army General Hospital for this retrospective study before reviewing the medical records and analyzing the data. As this is a retrospective analysis, our ethic committee did not require patients’ approval. Between March 2012 and March 2014, a total of 55 patients who were managed surgically for spondylolisthesis were retrospectively reviewed. A cohort study (26 MIS-TLIF, 29 PLIF) was undertaken. Mean age at the time of surgery was 48.5 ± 6.6 years. Collection and analysis of radiographic and clinical data was performed by individuals not directly involved in the surgical procedures. Inclusion criteria were as follows: single-level, low-grade (Meyerding grade I or II) isthmic or degenerative spondylolisthesis; and significant back and leg pain that failed conservative management. Patients were excluded if they had multiple-level spondylolisthesis, previous fusion surgery, or severe spinal deformity.
All patients underwent a trial of nonoperative and conservative treatment that included medication, physical therapy, and nerve blocks for at least 3 months before surgery.
2.2. Operative techniques
2.2.1. Bilateral MIS-TLIF with screw fixation
All MIS-TLIF procedures were performed bilaterally. Anteroposterior fluoroscopy was used to identify the disc space and mark the lateral pedicle line, while lateral imaging was used to assist tubular retractor system insertion. After a vertical skin incision of 25 mm for each segment was made along the lateral pedicle line, a 22-mm tubular retractor (MetRx; Medtronic SofamorDanek, Memphis, TN) was introduced into the facet joint under fluoroscopic guidance. A monopolar cautery and pituitary forceps were used to expose the facet joint, and a unilateral total facetectomy and laminectomy was performed with an osteotome and laminectomy punch.
The ligamentum flavum was then removed to expose the lateral border of the ipsilateral exiting and traversing nerve roots. An extensive decompression and discectomy was then performed. The same procedure was performed on the contralateral side.
Following decompression and discectomy, an ipsilateral percutaneous pedicle screw system was inserted through the same skin incision under fluoroscopic guidance to reduce the vertebrae usingdouble-upward pull through the screws. Rod fixation was then achieved. Interbody fusion was performed with a polyether ether ketone (PEEK) cage (Capstone; Medtronic SofamorDanek, Memphis, TN) filled autologous bonegraft on each side. The wound was closed in layers without drain placement (Fig. 1). A typical MIS-TLIF case is shown in Fig. 2.
Figure 1.
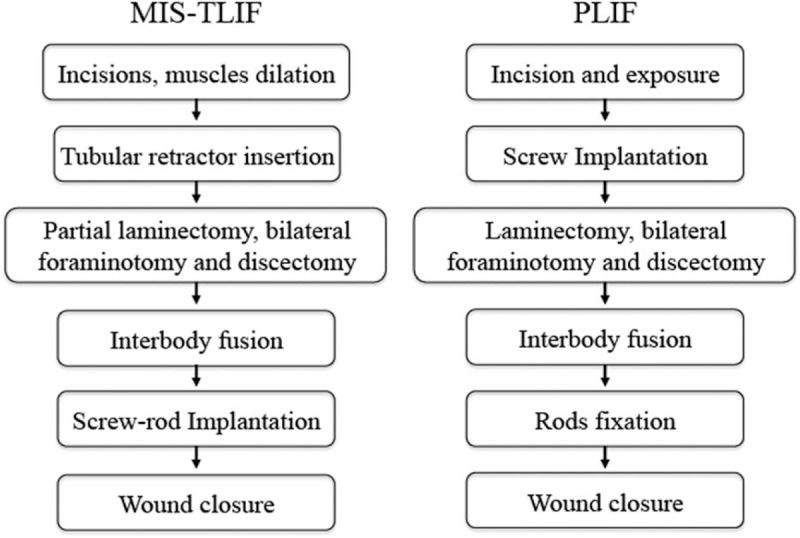
Flow diagram of MIS-TLIF and PLIF.
Figure 2.
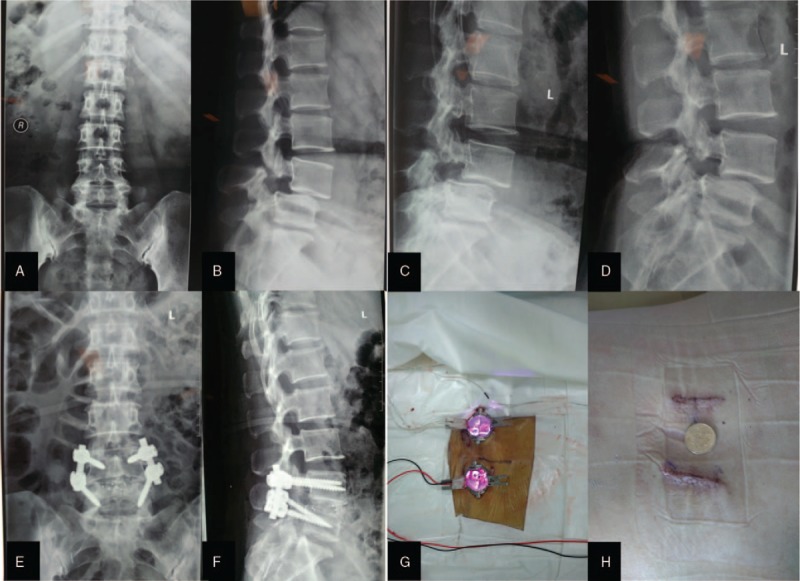
The typical treatment of isthmic spondylolisthesis with MIS-TLIF. A 45-year-old female. (A) Preoperative anteroposterior X-rays, (B) Preoperative lateral X-rays, (C, D) Preoperative flexion and extension X-rays, (E) Postoperative anteroposterior X-rays, (F) Postoperative lateral X-rays, (G) Tubular retractor placement bilaterally, (H) After wound closure.
2.2.2. PLIF
A midline skin incision was used. The fascia was incised and the paravertebral muscles were dissected from the spine. Radiographs were used to confirm the appropriate vertebral level. Bilateral pedicle screw-rod constructs were inserted and a laminectomy was then performed at that level. This was followed by bilateral foraminotomy and discectomy, and interbody graft placement. Cartilaginous material was removed from the endplates using an endplate scraper. Interbody fusion was performed with a PEEK cage (Capstone; Medtronic SofamorDanek, Memphis, TN) filled with autologous bone graft on each side. A final fluoroscopy was performed as necessary to confirm pedicle screw fixation and cage placement. The wound was copiously irrigated and closed in layers (Fig. 1). Drains were removed when output volume was less than 100 mL per day.
2.3. Perioperative clinical and radiographic assessments
We recorded perioperative factors related to the operative procedure, including patient demographics, operative blood loss, surgical time, length of hospital stay, creatine kinase (CK) level, and complications. The Oswestry Disability Index (ODI) functional questionnaire and visual analog pain scale (VAS) were administered preoperatively and postoperatively. The same clinical factors together with a neurologic examination were recorded at follow-up visits 3, 6, and 12 months after surgery.
Radiographic evaluation included preoperative X-rays (standard lumbar anteroposterior/lateral, flexion/extension, and whole spine anteroposterior/lateral views), computed tomography (CT), and magnetic resonance imaging (MRI). All patients underwent repeat X-ray and CT scan 1 year postoperatively. Spondylolisthesis degree (slippage rate) was measured before and 1 year after surgery. Slip reduction was defined as the difference between the pre- and postoperative spondylolisthesis. Reduction rate was calculated as follows: [(preoperative slippage distance − postoperative slippage distance) / preoperative slippage distance] × 100%.
Radiographic fusion was assessed with the grading criteria of Bridwell et al[9]: Grade I, fused with remodeling and trabeculae present; Grade II, graft intact but not fully remodeled and incorporated, with no lucencies above or below; Grade III, graft intact but with a definite lucency at the top or bottom of the graft; and Grade IV, definitely not fused, with resorption of bone graft and collapse. Both Grades I and II were considered radiographic signs of solid fusion, and the fusion condition at the last follow-up was collected into analysis.
2.4. Statistical analysis
SPSS 17.0 (IBM, Armonk, NY) was used for statistical analysis. Continuous variables were demonstrated as mean ± standard deviation (SD) and analyzed using independent t test. Categorical measurements were analyzed using Fisher exact tests. The distribution of spondylolisthesis level was analyzed with Wilcoxon signed rank test. P values < .05 were considered statistically significant.
3. Results
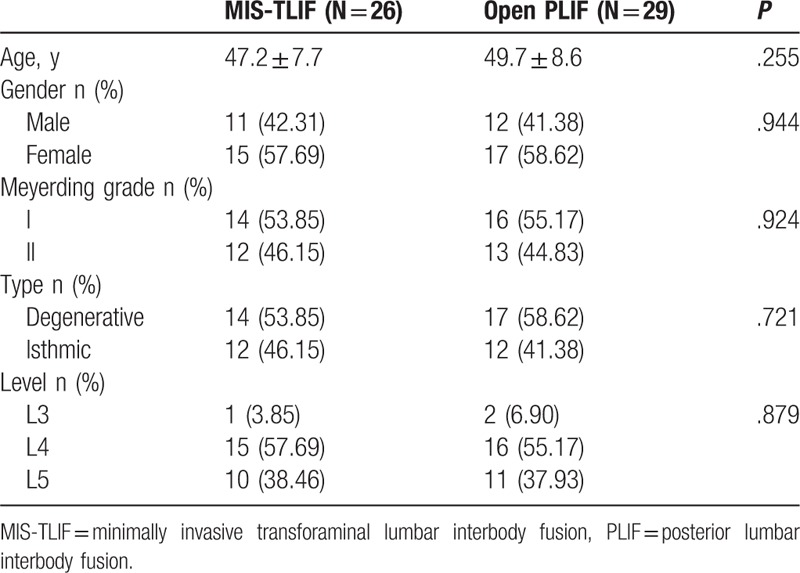
The average follow-up period was 28 ± 3.6 months (range 24–32 months). The most common spondylolisthesis level was L4-L5 and L5-S1 (94.5%). Before surgery, both groups had equivalent average age, sex distribution and cause, degree, and distribution of spondylolisthesis (Table 1).
Table 1.
Patient baseline characteristics.
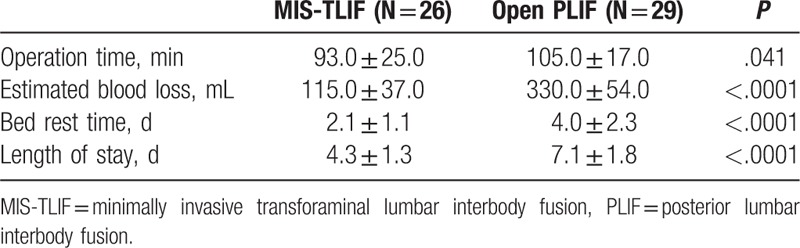
Operative time (MIS-TILF93 vs PLIF 105 minutes, P = .041) was significantly different (Table 2). Intraoperative blood loss in the MIS-TLIF group was significantly less than in the PLIF group (115 vs 330 mL, P < .01). Compared with the PLIF group, the MIS-TLIF group had a shorter bed-rest time and hospital stay (P < .01).
Table 2.
Surgical outcomes.
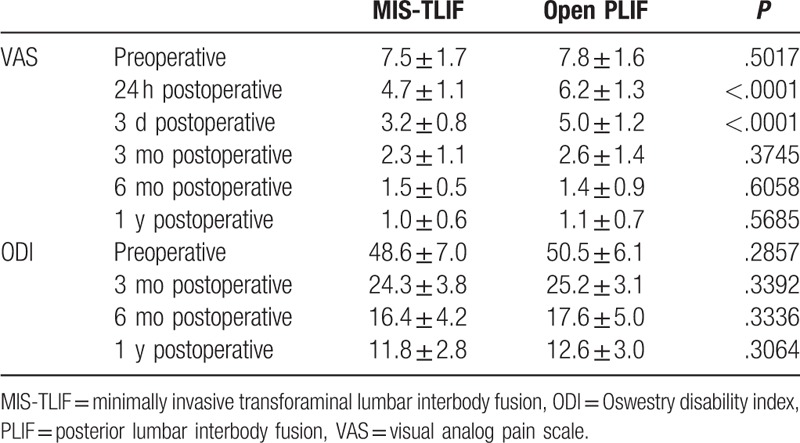
The CK level in venous blood, which can reflect muscle damage, was equivalent between the MIS-TLIF and PLIF groups preoperatively, but significantly lower in the MIS-TLIF group 24 hours, 3 days, and 5 days after surgery (Table 3). Back VAS was measured on a scale of 0 (no pain) to 10 (worst pain imaginable). Mean VAS in the both groups decreased after operation (Table 4). The VAS scores of the MIS-TLIF group were lower than those of the PLIF group 24 hours and 3 days after surgery (P < .01). There were no significant differences in VAS between the 2 groups at any other time points (Table 4). The ODI was measured on a scale of 0% (no disability) to 100% (complete disability). There were no significant differences in ODI between the 2 groups at any time point (Table 4).
Table 3.
Pre- and postoperative creatine kinase level (μmol/L).
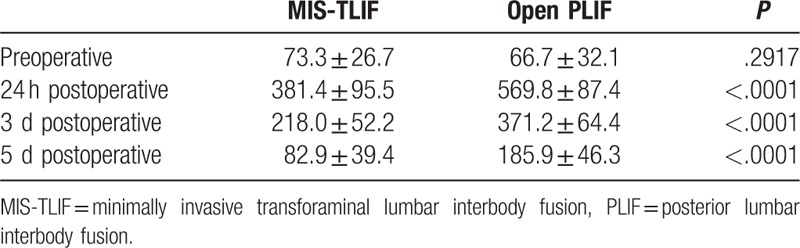
Table 4.
Pre- and postoperative back VAS score and ODI score.
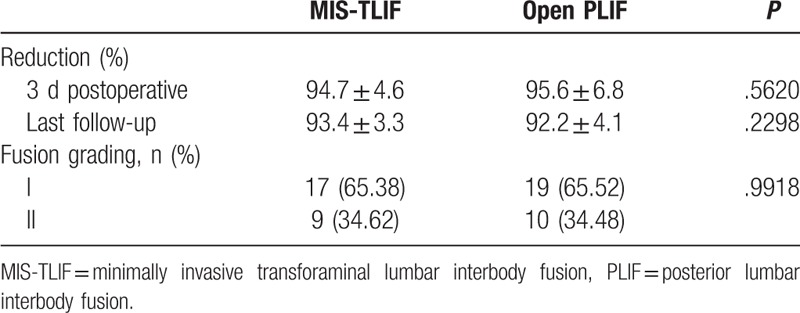
Reduction and fusion rates are listed in Table 5. Spondylolisthesis slip reduction rate was 93.4% in the MIS-TLIF group and 92.2% in the PLIF group (P = .23). Solid fusion (Bridwell fusion grade I or II) was achieved in all patients. In total, 17 of 26 patients (65.4%) in the MIS-TLIF group and 19 of 29 (65.5.0%) patients in the PLIF group achieved grade I fusion, and all others achieved grade II fusion. The fusion rate of both groups was similar after 1 year (P = .99).
Table 5.
Reduction and fusion grading.
No perioperative complications in either group required revision surgery. In the MIS-TLIF group, 1 patient experienced a dural tear without neurologic symptoms. A superficial infection was diagnosed in 1 patient in the PLIF group, which was treated conservatively. There was no difference in the complication rate between groups.
4. Discussion
Of the 5 spondylolisthesis subtypes, degenerative and isthmic are the most common.[2] Both can lead to compression and instability, which result in radicular and low back pain.[2–5] The basic goals of the surgical treatment of spondylolisthesis are decompression and stabilization.
Adequate decompression of the neural elements and definitive stabilization of the unstable mobile segment with interbody fusion can be accomplished using several fusion techniques available. PLIF has been shown to be a safe and effective method for the treatment of low-grade spondylolisthesis, with high fusion and low complication rates.[10,11] Unlike the anterior lumbar interbody fusion (ALIF), PLIF avoids complications related to anterior abdominal structures such as vascular injuries, sympathetic nerve injuries, and injuries to retroperitoneal and peritoneal structures.[12] However, PLIF requires retraction of the dura or nerve roots. Epidural scarring must be avoided. Multiple studies have reported on the impact of the extensive muscle dissection and retraction required in conventional PLIF.[13,14]
Benefits of MIS-TLIF include smaller incisions, reduced trauma to paraspinal muscles, and quicker postoperative recovery.[13] These factors may also lead to reduced estimated blood loss, shorter hospital stays, and decreased surgical site infection rates.[15,16] Multiple studies have reported favorable results after MIS-TLIF for spondylolisthesis.[17,18] Schwender et al[19] reported a 100% fusion rate and significantly improved outcomes in 49 patients (22 with spondylolisthesis) at 1 year postoperatively. Park et al[20] reported good clinical and fusion results of 124 patients who underwent MIS-TLIF (35 with isthmic and 40 with degenerative spondylolisthesis) after a minimum of 5 years of follow-up. Kim et al[21] reported that the outcomes of MIS-TLIF for either isthmic or degenerative spondylolisthesis were equivocal.
Our study showed that MIS-TLIF had shorter operative times and less intraoperative blood loss than PLIF. This is likely because the tubular retractor system used in MIS-TLIF passes through the intermuscular space without dissecting the paravertebral muscles from the spine. Avoiding muscle dissection not only reduced operative time and decreased blood loss but also reduced muscular injury, while, in PLIF, paravertebral muscles were dissected from the spine and lamina was resected, which caused greater trauma. Postoperative CK level and VAS were used to evaluate the extent of intraoperative muscle injury. In the 5 days after surgery, patients with MIS-TLIF had a lower CK level and a less incisional pain, which may have contributed to the reduced bed-rest time and hospital stays of these patients.
With respect to clinical and radiographic outcomes, we observed equivalent patient-reported outcomes, spinal fusion rates, and complications between MIS-TLIF and PLIF. The rationale behind MIS-TLIF is to combine the advantages of minimally invasive techniques with an effective direct neural decompression of the central canal, bilateral exiting roots, and traversing roots. This was achieved through facetectomy and hemi-laminectomy followed by disc height restoration and spondylolisthesis reduction with grafted cage application. Although the supraspinous ligament, interspinous ligament, and part of the ligamentum flavum were not released, patients with MIS-TLIF had satisfactory decompression, reduction, and fusion outcomes. The ligaments of patients with spondylolisthesis are abnormally relaxed and therefore do not interfere with slip reduction. The main factors that can block vertebral reduction include the hyperplasia and concentration of the facet joint, proliferative fibrocartilaginous tissue, and the intervertebral disc itself, all of which can be released during MIS-TLIF.
Previous studies have reported that using 1 or 2 cages during intervertebral fusion had no impact on overall reduction and fusion rates.[20] However, to achieve a better intervertebral release result and avoid the loss of unilateral intervertebral height, we used 2 cages during bilateral MIS-TLIF, each placed as anteriorly as possible to restore sagittal balance. Considering the biomechanics of the spine, 2 cages might achieve better balance. At our last follow-up, no re-slips occurred and all fusions were solid. On the basis of these results, we suggest that minimally invasive transforaminal lumbar interbody arthrodesis can be a reasonable treatment option for properly selected patients with spondylolisthesis.
Limitations of this study include its retrospective nature and the lack of cases with more serious spondylolisthesis (Meyerding grade III or IV). However, this is the first study that compares the clinical and radiographic outcomes of MIS-TLIF and PLIF for spondylolisthesis. Future studies that include longer-term follow-up periods and a larger number of patients should be performed.
5. Conclusion
This study demonstrates that MIS-TLIF can achieve similar reduction and fusion rates to PLIF in the management of grade I/II spondylolisthesis and better short-term quality of life, shorter hospital stays, less estimated blood loss, and reduced operative time.
Footnotes
Abbreviations: ALIF = anterior lumbar interbody fusion, CK = creatine kinase, CT = computed tomography, MIS-TLIF = minimally invasive transforaminal lumbar interbody fusion, MRI = magnetic resonance imaging, ODI = Oswestry Disability Index, PEEK = polyether ether ketone, PLIF = posterior lumbar interbody fusion, VAS = visual analog scale.
The authors report no conflicts of interest.
References
- [1].Lastfogel JF, Altstadt TJ, Rodgers RB, et al. Sacral fractures following stand-alone L5-S1 anterior lumbar interbody fusion for isthmic spondylolisthesis. J Neurosurg Spine 2010;13:288–93. [DOI] [PubMed] [Google Scholar]
- [2].Butt MF, Dhar SA, Hakeem I, et al. In situ instrumented posterolateral fusion without decompression in symptomatic low-grade isthmic spondylolisthesis in adults. Int Orthop 2008;32:663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agabegi SS, Fischgrund JS. Contemporary management of isthmic spondylolisthesis: pediatric and adult. Spine J 2010;10:530–43. [DOI] [PubMed] [Google Scholar]
- [4].Wu CH, Wong CB, Chen LH, et al. Instrumented posterior lumbar interbody fusion for patients with degenerative lumbar scoliosis. J Spinal Disord Tech 2008;21:310–5. [DOI] [PubMed] [Google Scholar]
- [5].Sivaraman A, Altaf F, Jalgaonkar A, et al. Prospective study of posterior lumbar interbody fusion with either interbody graft or interbody cage in the treatment of degenerative spondylolisthesis. J Spinal Disord Tech 2015;28:E467–71. [DOI] [PubMed] [Google Scholar]
- [6].Virdee JS, Nadig A, Anagnostopoulos G, et al. Comparison of peri-operative and 12-month lifestyle outcomes in minimally invasive transforaminal lumbar interbody fusion versus conventional lumbar fusion. Br J Neurosurg 2017;31:167–71. [DOI] [PubMed] [Google Scholar]
- [7].Djurasovic M, Rouben DP, Glassman SD, et al. Clinical outcomes of minimally invasive versus open TLIF: a propensity-matched cohort study. Am J Orthop 2016;45:E77–82. [PubMed] [Google Scholar]
- [8].Goldstein CL, Phillips FM, Rampersaud YR. Comparative effectiveness and economic evaluations of open versus minimally invasive posterior or transforaminal lumbar interbody fusion: a systematic review. Spine 2016;41Suppl 8:S74–89. [DOI] [PubMed] [Google Scholar]
- [9].Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine: do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine 1995;20:1410–8. [PubMed] [Google Scholar]
- [10].Kim KT, Lee SH, Suk KS, et al. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine 2006;31:712–6. [DOI] [PubMed] [Google Scholar]
- [11].Kuraishi S, Takahashi J, Mukaiyama K, et al. Comparison of clinical and radiological results of posterolateral fusion and posterior lumbar interbody fusion in the treatment of L4 degenerative lumbar spondylolisthesis. Asian Spine J 2016;10:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Udby PM, Bech-Azeddine R. Clinical outcome of stand-alone ALIF compared to posterior instrumentation for degenerative disc disease: a pilot study and a literature review. Clin Neurol Neuros 2015;133:64–9. [DOI] [PubMed] [Google Scholar]
- [13].Asil K, Yaldiz C. Retrospective comparison of radiological and clinical outcomes of PLIF and TLIF techniques in patients who underwent lumbar spinal posterior stabilization. Medicine (Baltimore) 2016;95:e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine 2010;35:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park Y, Ha JW, Lee YT, et al. The effect of a radiographic solid fusion on clinical outcomes after minimally invasive transforaminal lumbar interbody fusion. Spine J 2011;11:205–12. [DOI] [PubMed] [Google Scholar]
- [16].Patel AA, Zfass-Mendez M, Lebwohl NH, et al. Minimally invasive versus open lumbar fusion: a comparison of blood loss, surgical complications, and hospital course. Iowa Orthop J 2015;35:130–4. [PMC free article] [PubMed] [Google Scholar]
- [17].Scheer JK, Auffinger B, Wong RH, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis in 282 patients: in situ arthrodesis versus reduction. World Neurosurg 2015;84:108–13. [DOI] [PubMed] [Google Scholar]
- [18].Tay KS, Bassi A, Yeo W, et al. Intraoperative reduction does not result in better outcomes in low-grade lumbar spondylolisthesis with neurogenic symptoms after minimally invasive transforaminal lumbar interbody fusion-a 5-year follow-up study. Spine J 2016;16:182–90. [DOI] [PubMed] [Google Scholar]
- [19].Schwender JD, Holly LT, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech 2005;18Suppl:S1–6. [DOI] [PubMed] [Google Scholar]
- [20].Park SJ, Lee CS, Chung SS, et al. Postoperative changes in pelvic parameters and sagittal balance in adult isthmic spondylolisthesis. Neurosurgery 2011;682 Suppl Operative:355–63. discussion 362–353. [DOI] [PubMed] [Google Scholar]
- [21].Kim JY, Park JY, Kim KH, et al. Minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis: comparison between isthmic and degenerative spondylolisthesis. World Neurosurg 2015;84:1284–93. [DOI] [PubMed] [Google Scholar]


