Abstract
The risk assessment for developing end-stage renal disease (ESRD) remains unclear in patients with lupus nephritis (LN). The purpose of this study was to develop and validate a prediction rule for estimating the individual risk of ESRD in patients with LN using clinical and pathological data.
A total of 599 patients with LN diagnosed by renal biopsy between June 2009 and June 2014 in West China Hospital of Sichuan University were retrospectively followed. Patients were randomly divided into derivation cohort (n = 379) and validation cohort (n = 220). The SLEDAI score was used to evaluate the clinical disease activity. Pathological lesions according to the International Society of Nephrology and the Renal Pathology Society (ISN/RPS) systems were meticulously evaluated. The risk factors for developing ESRD were evaluated using a Cox proportional hazard model with a stepwise backward elimination method.
In the derivation cohort, 100 patients (26.5%) developed ESRD during the average 46.0 ± 21.1 months’ follow-up. The final prediction model included cellular crescents, active index >20, glomerular sclerosis, fibrous crescents, interstitial fibrosis, chronic index >5, nephrotic syndrome, and eGFR <45 mL/min as independent risk factors for developing ESRD. To create a prediction rule, the score for each variable was weighted by the regression coefficients calculated using the relevant Cox model. The prediction rule was validated in the validation cohort. During the follow-up period, 45 patients (21.5%) in validation cohort progressed to ESRD.
This study developed and validated a new prediction rule using clinical measures and pathological changes for developing ESRD in patients with LN.
Keywords: D prediction score system, lupus nephritis, systematic lupus erythematosus
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease that involves multiple organs. Renal involvement in SLE occurs in up to 60% cases and is a major determinant of the outcome. Higher rates of renal involvement were observed in Asians than in white people. Several epidemiologic studies have identified risk factors for poor kidney prognosis in patients with SLE, including age, sex, hypertension, decreased estimated GFR (eGFR), proteinuria, and renal pathologic types.[1] However, most of them were small cohort studies, which cannot provide detailed information about risk factors. The accurate prediction of kidney prognosis in individual cases is important for determining the therapeutic strategy. To date, SLEDAI and BILAG are 2 major scoring systems to evaluate the activity of lupus in clinical studies.[2] However, these systems are not routinely used in medical practice, but for quantification of lupus disease activity primarily for the purpose of clinical trials. Although these 2 systems contain clinical manifestations and laboratory results, renal pathologic changes are not included in either of them. Considering that renal pathologic lesions are crucial to SLE treatment and prognosis prediction, it is reasonable to develop a prediction rule which contains different pathological changes for estimating the risk of ESRD in SLE patients. Herein, we report a new clinical and pathologic risk prediction rule using the new International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification to identify the subgroup of Chinese patients with SLE at high risk of developing ESRD, and we verified the external validity of the score in an independent cohort.
2. Materials and methods
2.1. Study population
A total of 425 adult patients with lupus nephritis (LN), confirmed by kidney biopsy between June 2009 and June 2014 in West China Hospital of Sichuan University, were enrolled in this study as a derivation cohort. Among them, we excluded 17 patients whose biopsy specimen contained <10 glomeruli, 29 patients without available clinical data. Finally, 379 patients were enrolled in this study as a derivation cohort. The patients were followed up until March 31, 2016.
Another 234 adult patients with LN underwent biopsy between June 2009 and June 2014 in West China Hospital of Sichuan University and were followed for at least 1 year as validation cohort. Fourteen patients with biopsy specimens that contained <10 glomeruli were excluded. Therefore, the remaining 220 patients were included in the study as a validation cohort.
This study was approved by the ethics committee of West China Hospital of Sichuan University.
2.2. Clinical measures
Clinical measures were obtained from medical records at the time of the renal biopsy, which included age, sex, BP, serum creatinine, and 24-hour urinary protein excretion or urinary protein-to-creatinine ratio, serum C3 and C4, antinuclear antibodies (ANA), anti-double-stranded DNA antibodies and anti-extractable nuclear antigen (ENA) antibodies, including anti-Sm, anti-RNP, anti-rip antibodies, antineutrophil cytoplasmic antibodies (ANCA), and anticardiolipin antibody (ACA). Hypertension was defined as BP >140/90 mm Hg and/or current use of antihypertensive agents. The eGFR was calculated using the CKD-EPI formula, and the clinical disease activity was assessed by SLEDAI score.
2.3. Pathologic measures
Pathologic lesions were evaluated according to the International Society of Nephrology and the Renal Pathology Society (ISN/RPS) systems.[3] Austin system of semiquantitative scores for activity and chronicity was applied.[4]
2.4. Renal outcome
A combined primary endpoint of ESRD, which was defined as the initiation of renal replacement therapy (hemodialysis, peritoneal dialysis, or renal transplantation) or eGFR <15 mL/min, and eGFR decrease >50% of baseline level was applied in this study.
2.5. Statistical analyses
In the present study, normal distribution continuous data are shown as mean ± standard deviation (SD), non-normal distribution continuous data are shown as median and range, categorical data are reported as absolute values and percentages. In the derivation cohort, we performed univariate analyses to estimate the hazard ratios (HRs) with 95% confidence intervals (95% CIs) for each risk factor for the development of ESRD using a Cox proportional hazards model. To build the risk prediction model, a multivariate Cox proportional hazards model with stepwise forward elimination with P < .05 for remaining variables was used. To generate a simple integer-based point score for each variable, we assigned scores by dividing regression coefficients by the value of the smallest coefficient in the model and rounding up to the nearest integer. Statistical analysis was performed using IBM SPSS Statistics 19.0 software.
3. Results
3.1. Characteristics of LN patients enrolled
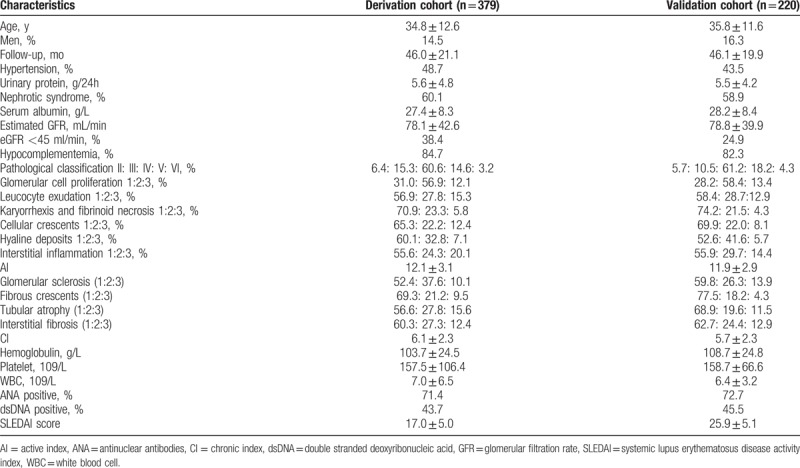
As shown in Table 1, the mean age of patients in the derivation cohort (n = 379) was 34.8 years, and 14.5% of the patients were male. Patients in the validation cohort had a mean age of 35.8 years; 16.3% of them were male. The median follow-up time after renal biopsy was 46.0 ± 21.1 and 46.1 ± 19.9 months in the derivation and validation cohort, respectively.
Table 1.
Baseline characteristics of the patients in the derivation and validation cohorts.
3.2. Development of the risk prediction model for kidney prognosis in the derivation cohort
During the follow-up period, 100 patients (26.5%) in the derivation cohort reached study endpoint. Eight variables (cellular crescents, active index >20, glomerular sclerosis, fibrous crescents, interstitial fibrosis, chronic index >5, nephrotic syndrome, and eGFR <45 mL/min) were significantly associated with a higher risk of incident ESRD in Cox-regression multivariate analysis (Table 2). Cox regression survival curves of these 8 independent risk predictors were listed in Figure 1.
Table 2.
HRs for the development of ESRD in the derivation cohort.
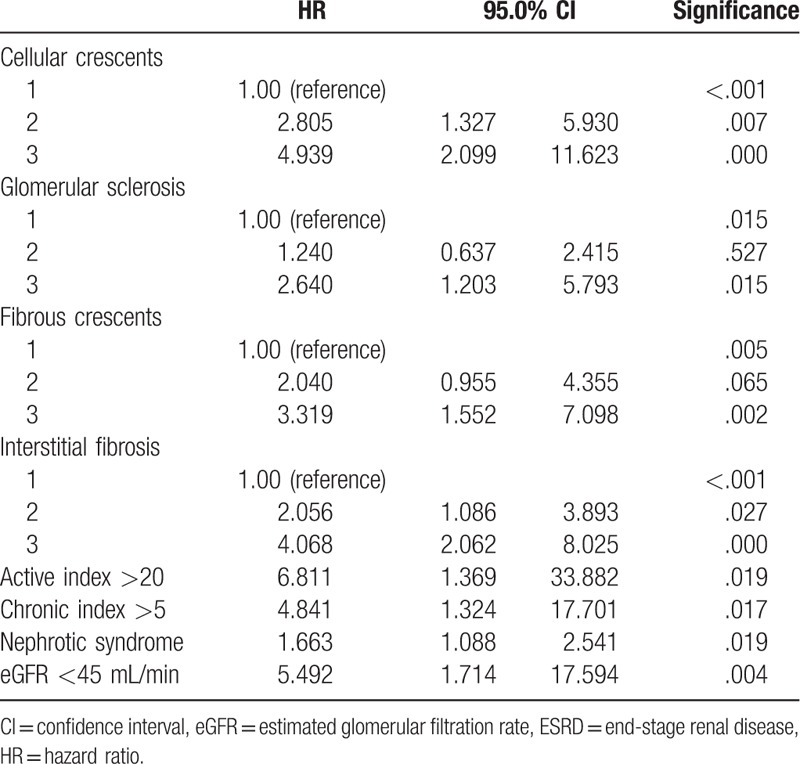
Figure 1.
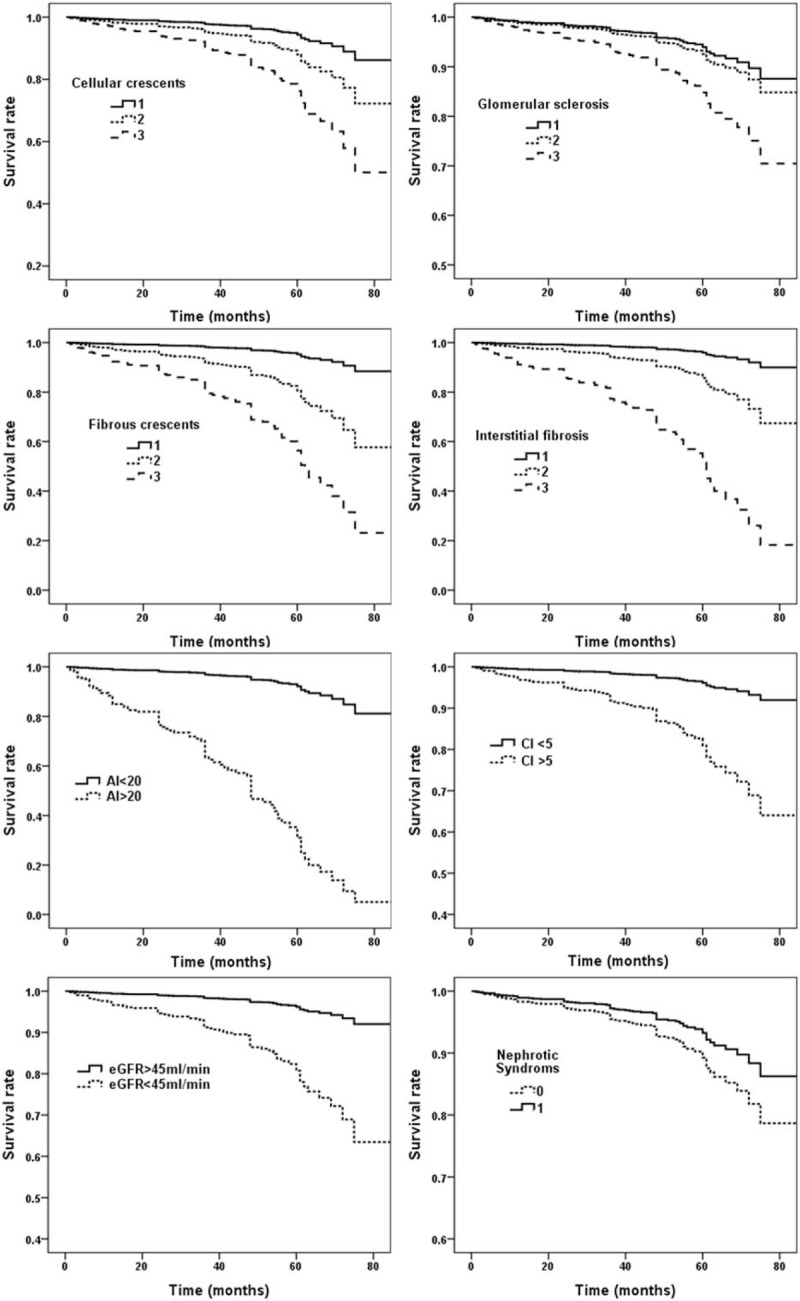
Renal survival curve of independent risk prediction variables.
A score-based prediction rule containing 8 variables selected in multivariate analysis was made using the regression coefficients obtained from the relevant Cox model (Table 3). Then this risk score model was applied to patients in the derivation cohort. As shown in Figure 2A, the incidence rate of ESRD was increased along with the rise of risk score as a nearly linear manner. To simplify the clinical application of this score system, the patients were categorized into 3 groups: low risk (score <10), moderate risk (score 11–20), and high risk (score >20) groups. The incidences of ESRD in these 3 groups were 2.7%, 32.5%, and 79.4%, respectively (Fig. 2B).
Table 3.
Risk scores for the development of ESRD.
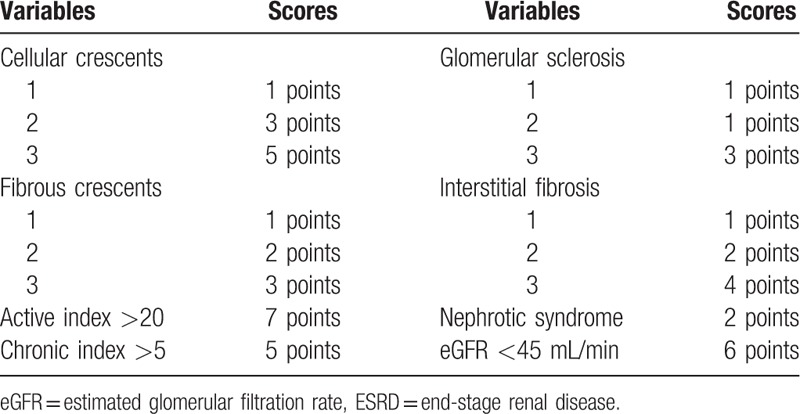
Figure 2.
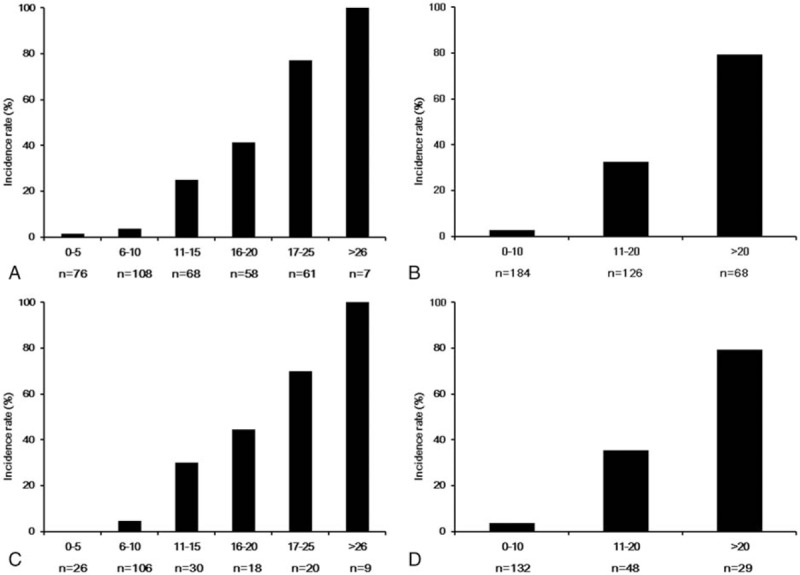
The incidence rate of ESRD by 5-point increments of total risk score and risk stratification in derivation cohort (A, B) and validation cohort (C, D). ESRD = end-stage renal disease.
3.3. Validation of the prediction rule in the validation cohort
The prediction rule was externally validated in the validation cohort, which was independent from the derivation cohort. During the follow-up period, 45 patients (21.5%) progressed to ESRD in the validation cohort. And the incidence of ESRD also increased linearly with the rise of the total risk score (Fig. 2C). Moreover, as shown in Figure 2D, the incidence of ESRD in different risk subgroups of the validation group was also close to those of the derivation group.
4. Discussion
SLE is an autoimmune disease with multisystem involved; kidney is its most vulnerable target. There still are many unclear aspects in clinical, pathological, and prognostic characteristics about LN. SLEDAI and BILAG are 2 major scoring systems used to evaluate the activity of lupus in clinical studies.[2] Nevertheless, these systems are not routinely used clinical practice, but mostly for quantification of lupus disease activity for the purpose of clinical trials. Moreover, neither of these 2 systems contains renal pathologic changes. Regarding that renal pathologic lesions were crucial to LN treatment and prognosis prediction, these systems could only provide limited help to the decision making and prognosis predicting in LN patients. Therefore, we developed a new clinical and pathologic risk prediction rule using the new International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification to identify the subgroup of Chinese patients with SLE at high risk of developing ESRD. We assume that this novel risk score system is probably of help to the decision making in clinical environment.
To the best of our knowledge, this is the first study of quantification analysis of renal prognosis in LN. In this study, we developed and validated a new prediction rule consisting of 8 variables (cellular crescents, active index >20, glomerular sclerosis, fibrous crescents, interstitial fibrosis, chronic index >5, nephrotic syndrome, and eGFR <45 mL/min). A novel score-based prediction system containing these 8 variables was made using the regression coefficients obtained from Cox regression model, which could predict poor renal prognosis accurately both in derivation and validation cohorts. The incidence of poor prognosis increased along with the rise of score remarkably. To simplify the clinical application of this score system, the patients were categorized into 3 groups: low risk (score <10), moderate risk (score 11–20), and high risk (score >20) groups. We found that the incidence of poor renal prognosis in derivation and validation cohort were relatively mild in low-risk group (2.78% and 3.78%), medium in moderate-risk group (32.5% and 35.4%), and considerately high in high-risk groups (79.4% and 79.3%). These results suggest that our prediction rule is statistically valid and accurate for the risk assessment of poor renal prognosis among Chinese patients with LN. We believe that this score would be useful for determining the initial therapeutic strategies of patients with LN.
A few studies had found that renal pathology subtype (type III, IV, and VI) was independent risk factors of poor renal prognosis in LN. Nevertheless, cox regression analysis in our study did not indicate this correlation. We found that specific pathological changes, but not pathological types, were correlated with renal prognosis. Previously, we found that total remission rate (complete remission and partial remission) in type III and IV LN patients was proximately 80%, which indicated that proliferative LN may not be considered as a risk factor of poor prognosis.[5] In this study, we found that cellular presence of crescents was the only active lesion indicator for poor renal prognosis, consistent with previous studies which reported that crescentic LN had worse treatment response and lower probability of renal survival than those without crescents.[1] Five-year renal survival rate of crescentic LN was only 70.2%.[5] Furthermore, we found that glomerular sclerosis, fibrous crescents, and interstitial fibrosis were poor prognosis-related chronic pathologic lesions. Considering that these pathologic changes suggested loss of functional nephrons, it is easy to understand their correlations with poor prognosis. Besides, these chronic changes had already been reported to related with renal outcome previously.[6,7] Moreover, we also found that active index >20 and chronic index >5 were independent risk factors. Therefore, based on our findings, we speculate that detailed pathologic lesions score is better than pathologic subtypes in predicting renal outcome of LN.
Recently, several cohort studies provided useful information about very long-term prognosis of LN. It was reported that clinicopathological characteristics, treatment responses, and long-term outcomes differ remarkably in LN patients according to gender and pathological subtypes. Hypertension, serum creatinine level, hypocomplementemia, renal proliferative lesion, SLEDAI score, age, male gender, and proteinuria were identified as independent risk factors for poor prognosis of LN patients in different studies.[8–10] In the present study, we found that several clinical indexes, that is, nephrotic syndrome and low eGFR at diagnosis, were also risk predicting factors. These findings were consistent with results from observational studies.[10–12] Proteinuria was a well-known risk factor of poor renal prognosis in variety glomerular diseases. Persistent large amount of proteinuria, that is, nephrotic range proteinuria, will cause damage to glomeruli and tubules and will lead to renal fibrosis. Previous studies found that increased sCr level was related to poor prognosis of LN. In this study, we chose eGFR <45 mL/min in the Cox regression model, because it indicated severe loss of renal function and apparent chronic changes of kidney tissue. Moreover, many studies have already shown that treatment effect as well as renal prognosis of LN patients with GFR <45 mL/min was remarkably poor. In some small observational studies, male gender, hypertension, old age, and hypocomplementemia were also related to renal prognosis of LN patients. Nevertheless, our study did not found these relations.
Our study is a retrospective analysis based on a single center database, therefore the limitations are inevitable. Further validation with prospective multicenter data is needed.
5. Conclusion
In conclusion, we have developed a new prediction score system for poor renal prognosis in patients with LN and verified its validity in an independent cohort. Both clinical and pathologic measures were taken into account in the risk assessment for kidney prognosis. This prediction score system provides a useful tool to estimate the individual risk for ESRD in patients with LN and may be effective at identifying those at high risk for the future development of ESRD.
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, ACA = anticardiolipin antibody, ANA = antinuclear antibodies, ANCA = antineutrophil cytoplasmic antibodies, BILAG = British Isles Lupus Assessment Group, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR = estimated glomerular filtration rate, ENA = anti-extractable nuclear antigen, ESRD = end-stage renal disease, HR = hazard ratios, ISN/RPS = International Society of Nephrology/Renal Pathology Society, LN = lupus nephritis, SD = standard deviation, SLE = systemic lupus erythematosus, SLEDAI = systemic lupus erythematosus disease activity index.
The authors report no conflicts of interest.
References
- [1].Zhang W, Yuan M, Hong L, et al. Clinical outcomes of lupus nephritis patients with different proportions of crescents. Lupus 2016;25:1532–41. [DOI] [PubMed] [Google Scholar]
- [2].Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- [3].Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- [4].Austin HA, III, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 1983;75:382–91. [DOI] [PubMed] [Google Scholar]
- [5].Chen S, Tang Z, Zhang H, et al. Prediction of renal outcomes in patients with crescentic lupus nephritis. Am J Med Sci 2015;349:298–305. [DOI] [PubMed] [Google Scholar]
- [6].Mahmoud GA, Zayed HS, Ghoniem SA. Renal outcomes among Egyptian lupus nephritis patients: a retrospective analysis of 135 cases from a single centre. Lupus 2015;24:331–8. [DOI] [PubMed] [Google Scholar]
- [7].Alsuwaida AO. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus 2013;22:1446–54. [DOI] [PubMed] [Google Scholar]
- [8].Tang Y, Zhang X, Ji L, et al. Clinicopathological and outcome analysis of adult lupus nephritis patients in China. Int Urol Nephrol 2015;47:513–20. [DOI] [PubMed] [Google Scholar]
- [9].Vozmediano C, Rivera F, Lopez-Gomez JM, et al. Risk factors for renal failure in patients with lupus nephritis: data from the Spanish registry of glomerulonephritis. Nephron Extra 2012;2:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang J, Liang D, Zhang H, et al. Long-term renal outcomes in a cohort of 1814 Chinese patients with biopsy-proven lupus nephritis. Lupus 2015;24:1468–78. [DOI] [PubMed] [Google Scholar]
- [11].Dall’Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol 2015;67:1305–13. [DOI] [PubMed] [Google Scholar]
- [12].Kono M, Yasuda S, Kato M, et al. Long-term outcome in Japanese patients with lupus nephritis. Lupus 2014;23:1124–32. [DOI] [PubMed] [Google Scholar]


