Supplemental Digital Content is available in the text
Keywords: acute pain, gabapentin, meta-analysis, pregabalin, spinal surgery
Abstract
Background:
Gabapentinoid drugs, which include gabapentin and pregabalin, play an established role in the management of neuropathic pain. However, whether preoperative administration of gabapentinoids has a beneficial role in controlling acute pain after spinal surgery is unknown. We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to determine the efficacy and safety of the preoperative use of gabapentinoids (gabapentin and pregabalin) for the treatment of acute postoperative pain following spinal surgery.
Methods:
In March 2017, a systematic computer-based search was conducted in PubMed, EMBASE, Web of Science, Cochrane Library, and Google databases. RCTs comparing gabapentinoids (gabapentin and pregabalin) with placebo in patients undergoing spine surgery were retrieved. The primary endpoint was the visual analogue scale (VAS) score with rest or mobilization at 6, 12, 24, and 48 hours and cumulative morphine consumption at 24 and 48 hours. The secondary outcomes were complications of nausea, vomiting, sedation, dizziness, headache, urine retention, pruritus, and visual disturbances. After tests for publication bias and heterogeneity among studies were performed, data were aggregated for random-effects models when necessary.
Results:
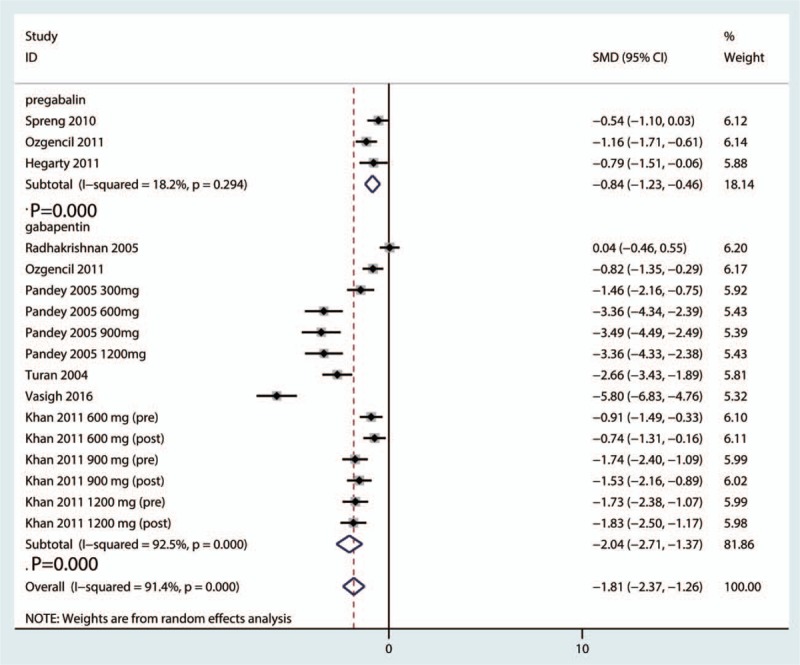
Sixteen clinical studies (gabapentin group n = 8 and pregabalin group n = 8) were ultimately included in the meta-analysis. Gabapentinoids were associated with reduced pain scores at 6, 12, 24, and 48 hours. Similarly, gabapentinoids were associated with a reduction in cumulative morphine consumption at 24 and 48 hours. Furthermore, gabapentinoids can significantly reduce the occurrence of nausea, vomiting, and pruritus. There were no significant differences in the occurrence of sedation, dizziness, headache, visual disturbances, somnolence, or urine retention.
Conclusions:
Preoperative use of gabapentinoids was able to reduce postoperative pain, total morphine consumption, and morphine-related complications following spine surgery. Further studies should determine the optimal dose and whether pregabalin is superior to gabapentin in controlling acute pain after spine surgery.
1. Introduction
Previous studies reported that 80% of patients undergoing spine surgery experience acute postoperative pain.[1,2] Among these patients, 80% describe their pain as severe.[3] The current protocol for pain management is to combine 2 or more analgesic agents to improve the quality of postoperative analgesia and reduce morphine consumption.[4,5] Morphine had been recommended as the first choice for the management of postsurgery pain; however, adverse effects such as nausea and vomiting and poor response to opioids for certain types of pain have limited the routine use of morphine.[6,7] Postoperative pain is not only caused by tissue injury but also associated with inflammatory pain, neuropathic pain, and visceral pain. Peripheral sensitization and central sensitization both contribute to pain. Recently, a greater emphasis has been placed on the administration of nonopioid analgesic drugs and opioid analgesic drugs as components of multimodal anesthesia protocols for relieving pain.[8] The gabapentinoid class of drugs, which includes gabapentin and pregabalin, has an established role in the management of neuropathic pain.[9,10] Gabapentinoids can bind to the alpha2delta subunit of presynaptic voltage-gated calcium channels, thus reducing calcium influx into presynaptic terminals. The effects of gabapentinoids for acute pain control after spinal surgery have not been studied; however, a previous meta-analysis compared gabapentinoids with placebo for pain control in lumbar surgery. In that meta-analysis, the number of the included studies was small,[11] and not all complications were compared. Therefore, we searched for relevant studies through March 2017 and performed a systematic review and meta-analysis to determine whether preoperative administration of a gabapentinoid was associated with lower pain scores, total morphine consumption, and morphine-related complications after spinal surgery.
2. Materials and methods
This meta-analysis was conducted in compliance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions[12] and was written following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) checklist.[13]
2.1. Search strategies
The following databases were searched in March 2017 without language restriction: PubMed (1950–March 2017), EMBASE (1974–March 2017), Web of Science (1950–March 2017), Cochrane Library (September 2017 Issue 3), and Google database (1974–March 2017); (Supplement File 1). The MeSH terms and their combinations used in the search were as follows: “analgesia” OR “pain management” OR “anesthetic agents” OR “lumbar surgery” OR “spinal surgery” OR “lumbar spine surgery” AND “pregabalin” OR “gabapentin” [MeSH terms]. The reference lists of related reviews and original articles were searched for any relevant studies, including randomized controlled trials (RCTs) involving adult humans. When multiple reports describing the same sample were published, the most recent or complete report was used. Because this is a meta-analysis, no ethics committee or institutional review board approval was necessary for the study.
2.2. Inclusion criteria and study selection
Patients: adults (age >18 years) undergoing lumbar surgery (lumbar fusion, lumbar laminectomy, or lumbar discectomy); Intervention: perioperative gabapentinoids as an intervention group; Comparison: placebo; Outcomes: visual analogue scale (VAS) at 6, 12, and 24 hours and complications (nausea, vomiting, sedation, dizziness, headache, urine retention, pruritus, and visual disturbances); Study design: RCTs. Two independent reviewers screened the titles and abstracts of the identified studies after removing duplicates in the search results. Any disagreements about the inclusion or exclusion of a study were resolved by discussion or consultation with an expert. The reliability of the study selection was determined by Cohen kappa test; the acceptable threshold value was set at 0.61.[14,15]
2.3. Data abstraction
A specific extraction was performed to collect the following data from the included trials: patients’ general characteristics, country, sample size of the control group and intervention group, preoperative and postoperative doses, and the timing and frequency of gabapentinoid use. Outcomes such as VAS at 6, 12, and 24 hours, cumulative morphine consumption at 24 and 48 hours, and complications (nausea, vomiting, sedation, dizziness, headache, urine retention, pruritus, and visual disturbances) were abstracted and recorded on a form. Postoperative pain intensity was measured by a 100-point VAS. When the numerical rating scale (NRS) was reported, it was converted to a VAS. Additionally, a 10-point VAS was converted to a 100-point VAS.[16] Data in other forms (i.e., median, interquartile range, and mean ± 95% confidence interval (CI)) were converted to the mean ± standard deviation (SD) according to the Cochrane Handbook.[17] If the data were not reported numerically, we extracted these data using the GetData Graph Digitizer software from the published figures. All data were extracted by 2 independent reviewers, and disagreements were resolved by discussion.
2.4. Quality assessment
The methodological quality of all included trials was independently assessed by 2 reviewers using the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (http://handbook.cochrane.org/). A total of 7 items (random sequence generation, allocation concealment, blinding to the participant and personnel, blinding to the outcome assessment, incomplete outcome, selective reporting, and other bias) were measured. Each of the items was measured as “low risk of bias,” “unclear risk of bias,” and “high risk of bias.” The risk of bias summary and risk of bias graph were obtained using Review Manager 5.3.0 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
2.5. Outcome measures and statistical analysis
Continuous outcomes (VAS at 6, 12, and 24 hours and cumulative morphine consumption at 24 and 48 hours) were expressed as the weighted mean differences (WMD) and respective 95% CI. Dichotomous outcomes (occurrence of nausea, vomiting, sedation, dizziness, headache, urine retention, pruritus, and visual disturbances) were expressed as the risk ratio (RR) with 95% CI. Statistical significance was set at P < .05 to summarize the findings across the trials. The meta-analysis was calculated by Stata software, version 13.0 (Stata Corp, College Station, TX). Statistical heterogeneity was tested using the χ2 test and I2 statistic. When there was no statistical evidence of heterogeneity (I2 < 50%, P > .1), a fixed-effects model was adopted; otherwise, a random-effect model was chosen. Publication bias was tested using funnel plots. Publication bias was assessed by funnel plot and quantitatively assessed by Begg test. Subgroup analysis was based on the dose of pregabalin (<300 mg/d was identified as low dose, while ≥300 mg/d was identified as high dose) and gabapentin (<900 mg/d was identified as low dose, while ≥900 mg/d was identified as high dose). Subgroup analysis was also performed based on the category of the drugs (gabapentin and pregabalin). We considered there to be no publication bias if the funnel plot was symmetrical and the P value was > .05. If 1 study comprises 2 or more than 2 doses of gabapentinoid, we divided into corresponding arms to analyses.
3. Results
3.1. Search results
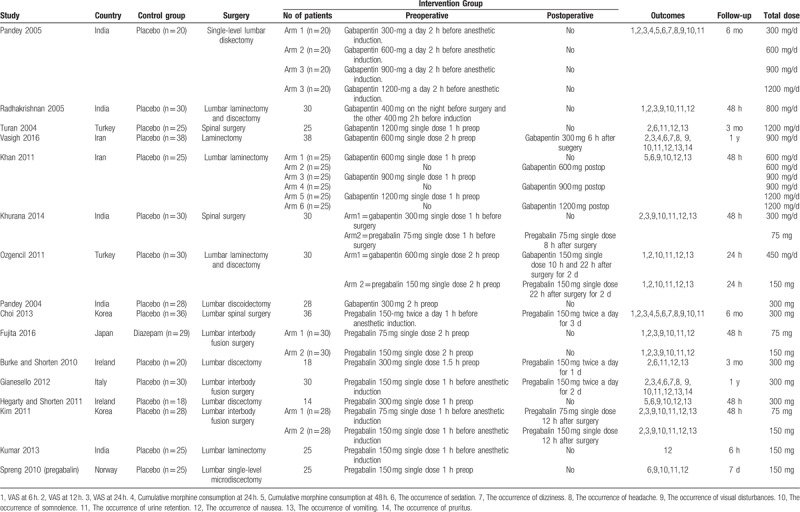
In the initial research, a total of 451 papers were identified from the electronic databases (PubMed = 155, Embase = 67, Web of Science = 82, Cochrane Library = 56, Google database = 91); 3 additional records were identified through other sources. Thus, a total of 454 papers were obtained in the initial search. These bibliographical references were introduced into Endnote Software (Version X7, Thompson Reuters, CA). Duplicates were then removed and 322 papers were reviewed. After screening the titles and abstracts of these 322 studies, 306 papers were excluded because they were irrelevant or did not meet the criteria. Ultimately, 16 clinical studies (gabapentin group n = 8 and pregabalin n = 8) were included in the meta-analysis.[18–33] The flow diagram for the included studies is provided in Fig. 1. Two studies used pregabalin at 2 different doses, and we thus divided the population into 2 arms.[20,24] The general characteristics of the included studies are shown in Table 1.
Figure 1.
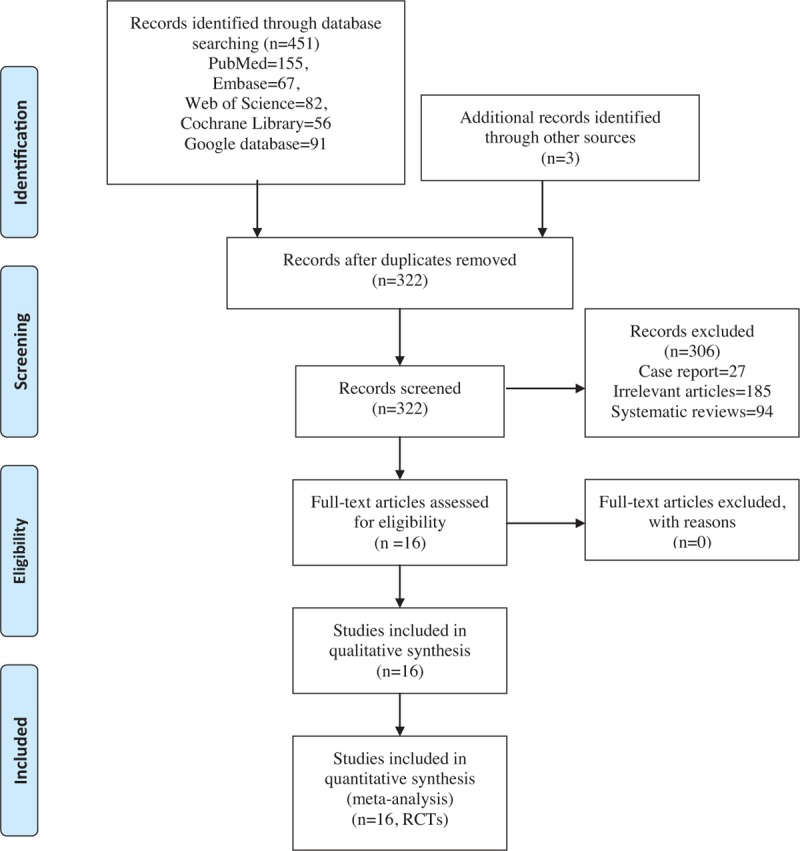
PRISMA flowchart for the included studies. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
Table 1.
The general characteristic of the included studies.
3.2. Quality assessment
The quality assessment of the included studies is summarized in Figs. 2 and 3. The risk of bias of random sequence generation showed unclear risk of bias in 4 studies.[18,20,28,32] Only 1 study failed to describe the blinding of the participants and personnel,[33] and 2 studies had unclear risk of bias for blinding of the outcome assessment.[28,33] The remaining studies all exhibited low risk of bias. Therefore, the overall risk of bias for all studies was low.
Figure 2.
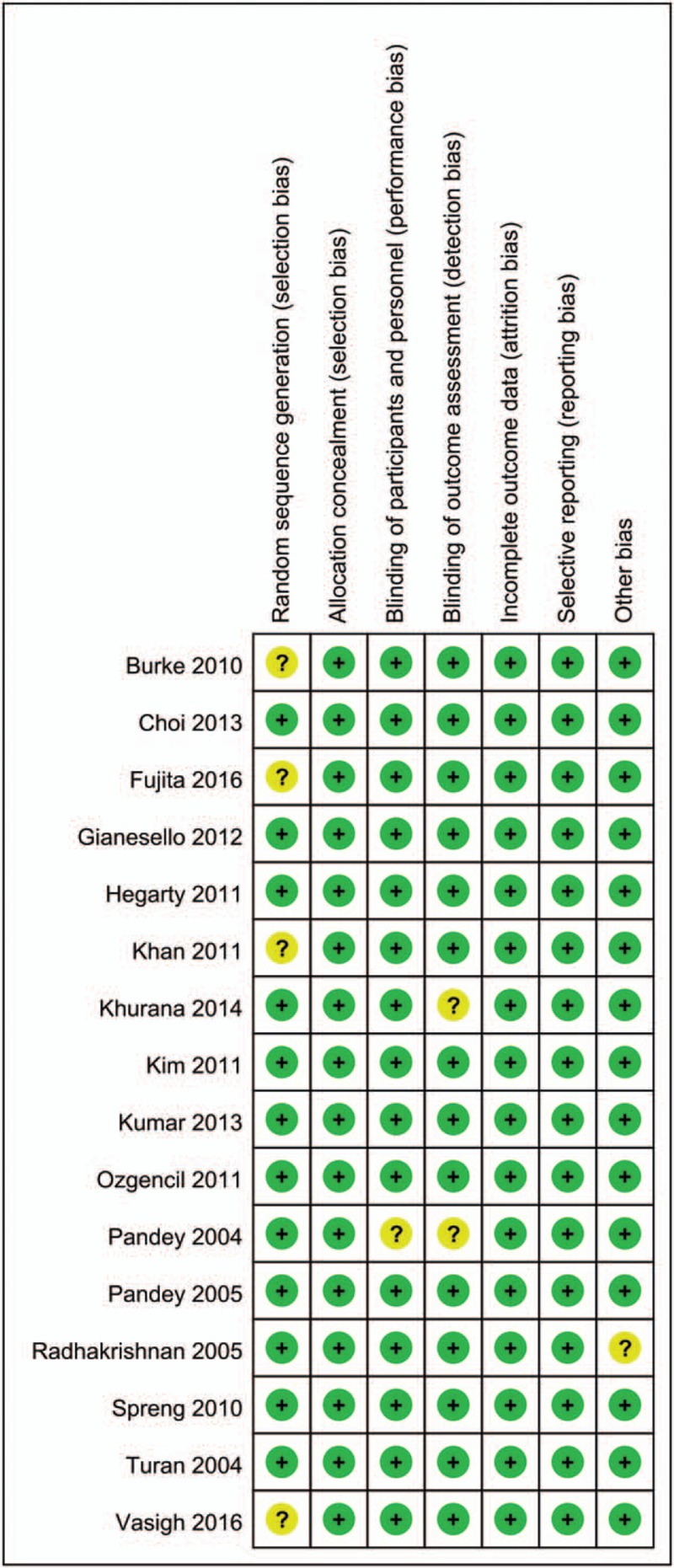
The risk of bias summary for the included studies.
Figure 3.
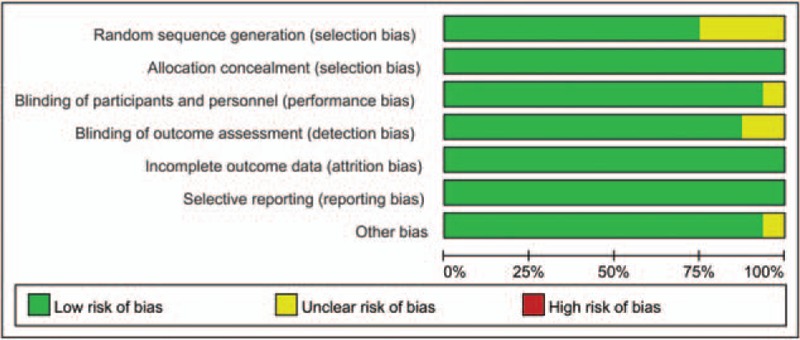
The risk of bias graph for the included studies.
4. Results of meta-analysis
4.1. VAS with rest at 6, 12, and 24 hours
Postoperative VAS scores at 6 hours were reported in 16 studies, and the pooled results indicated that preoperative administration of gabapentinoids was associated with reduced VAS at 6, 12, 24, and 48 hours; this corresponded to a reduction of 10.57 points (WMD = −10.57, 95% CI −14.52, −6.63, P = .020, Fig. 4) at 6 hours, 9.29 points (WMD = −9.29, 95% CI −11.74, −6.85, P = .000, Fig. 5) at 12 hours and 7.19 points at 24 hours (WMD = −7.19, 95% CI −10.45, −3.93, P = .000, Fig. 6) on a 110-point visual analogue score. The funnel plot of the VAS at 6 hours is shown in Fig. 7; the results indicated that there was no potential publication bias between the VAS at 6 hours. The P value obtained from the Begg test was.903 and indicated that there was no publication bias between the VAS at 6 hours (Fig. 8).
Figure 4.
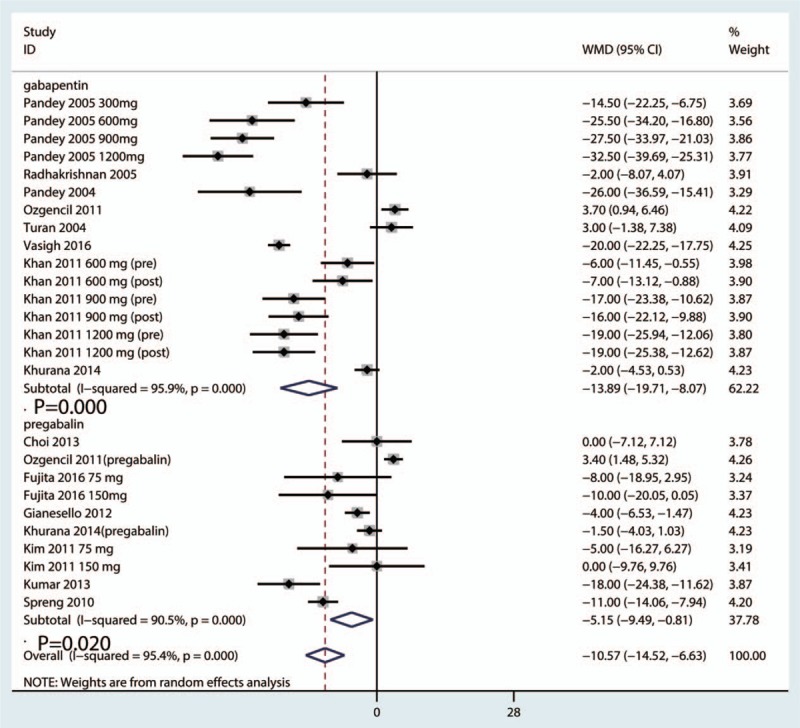
Forest plot comparing VAS at 6 h between the gabapentinoids group and the control group. VAS = visual analogue scale.
Figure 5.
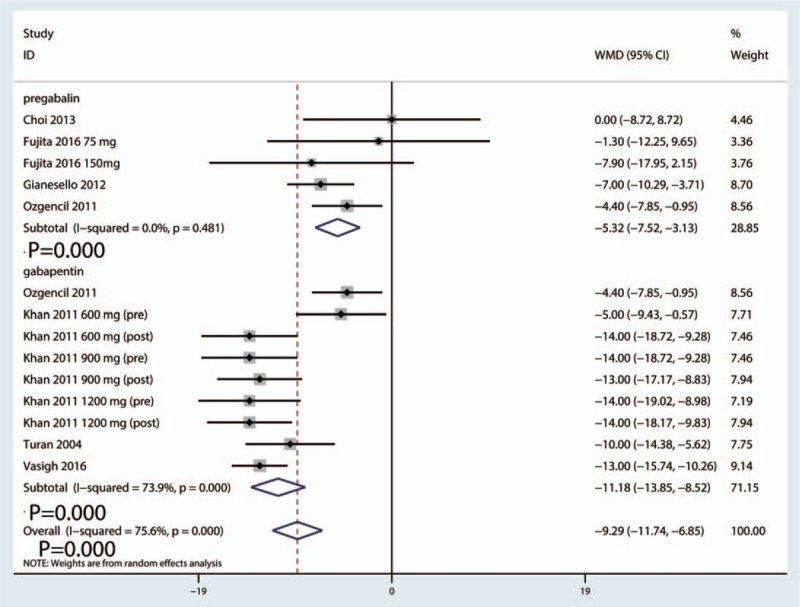
Forest plot comparing VAS at 12 h between the gabapentinoids group and the control group. VAS = visual analogue scale.
Figure 6.
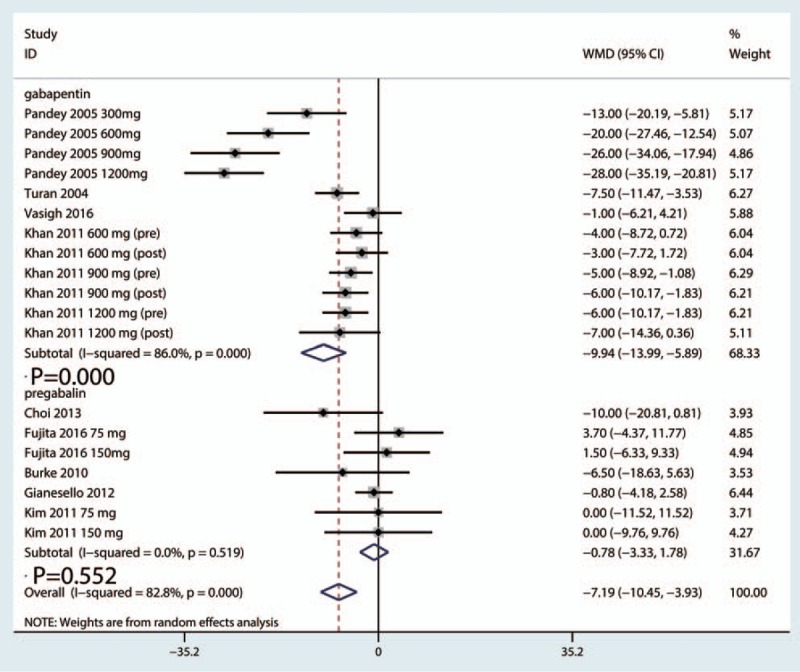
Forest plot comparing VAS at 24 h between the gabapentinoids group and the control group. VAS = visual analogue scale.
Figure 7.
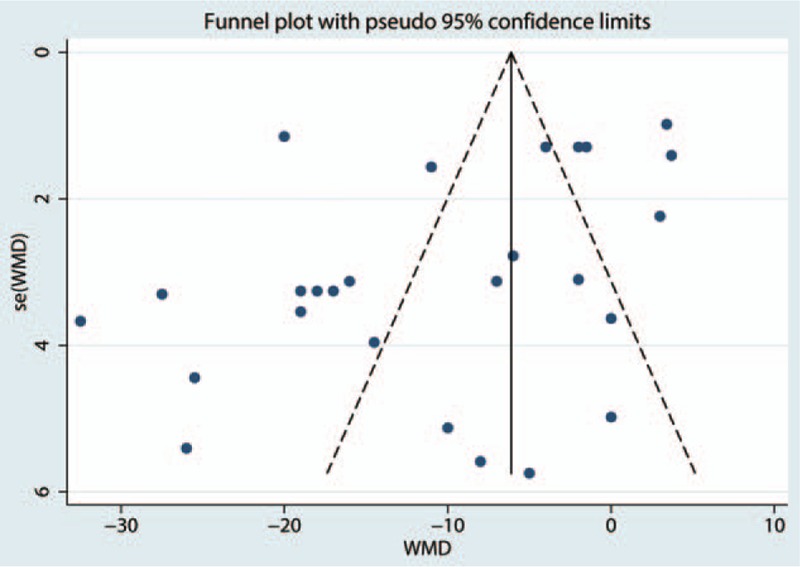
Funnel plot comparing VAS at 6 h between the gabapentinoids group and the control group. VAS = visual analogue scale.
Figure 8.
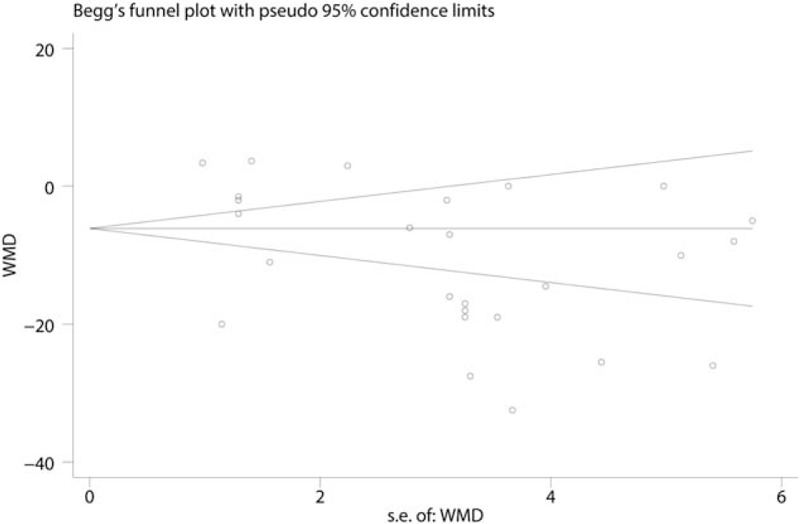
Begg test comparing VAS at 6 h between the gabapentinoids group and the control group. VAS = visual analogue scale.
4.2. Cumulative morphine consumption at 24 and 48 hours
The pooled results indicated that gabapentinoids can reduce the cumulative consumption of morphine at 24 hours (WMD = −18.55, 95% CI −23.52, −13.57, P = .000, Fig. 9). Because only the patients in the pregabalin group had reports of the cumulative consumption of morphine, pooled results suggested that pregabalin can reduce the cumulative consumption of morphine at 48 hours (WMD = −6.52, 95% CI −7.78, −5.25, P = .000, Fig. 10).
Figure 9.
Forest plot comparing cumulative morphine consumption at 24 h between the gabapentinoids group and he control group.
Figure 10.
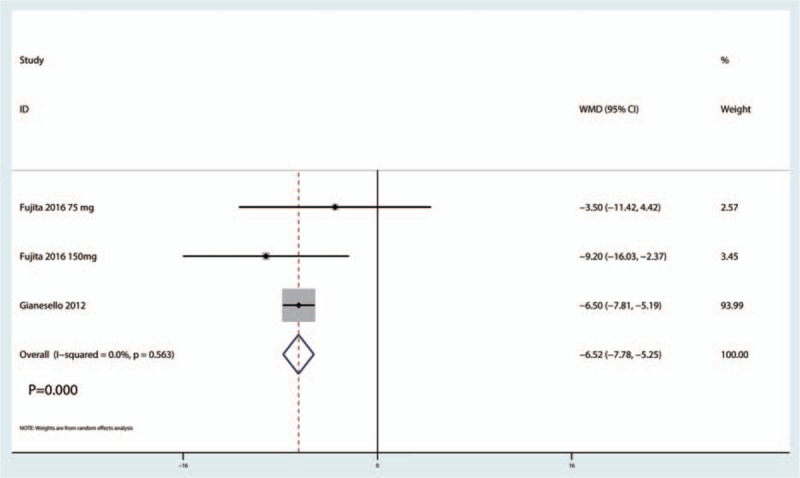
Forest plot comparing cumulative morphine consumption at 48 h between the gabapentinoids group and the control group.
4.3. Complications
There were no significant differences between the groups in the occurrence of sedation (RR = 1.29, 95% CI 0.73, 2.28, P = .541, Supplement Figure S1). There was no significant difference in the occurrence of dizziness (RR = 1.44, 95% CI 1.05, 1.99, P = .086, Supplement Figure S2), headache (RR = 1.10, 95% CI 0.70, 1.73, P = .431, Supplement Figure S3), visual disturbances (RR = 1.76, 95% CI 0.76, 4.04, P = .142, Supplement Figure S4), somnolence (RR = 1.45, 95% CI 0.90, 2.34, P = .142, Supplement Figure S5), or urine retention (RR = 0.61, 95% CI 0.38, 0.97, P = .142, Supplement Figure S6).
Gabapentinoids can significantly reduce the occurrence of nausea (RR = 0.69, 95% CI 0.54, 0.88, P = .004, Supplement Figure S7). Gabapentinoids can also significantly reduce both the occurrence of vomiting (RR = 0.51, 95% CI 0.34, 0.76, P = .004, Supplement Figure S8) and pruritus (RR = 0.34, 95% CI 0.22, 0.55, P = .001, Supplement Figure S9).
4.4. Subgroup analysis
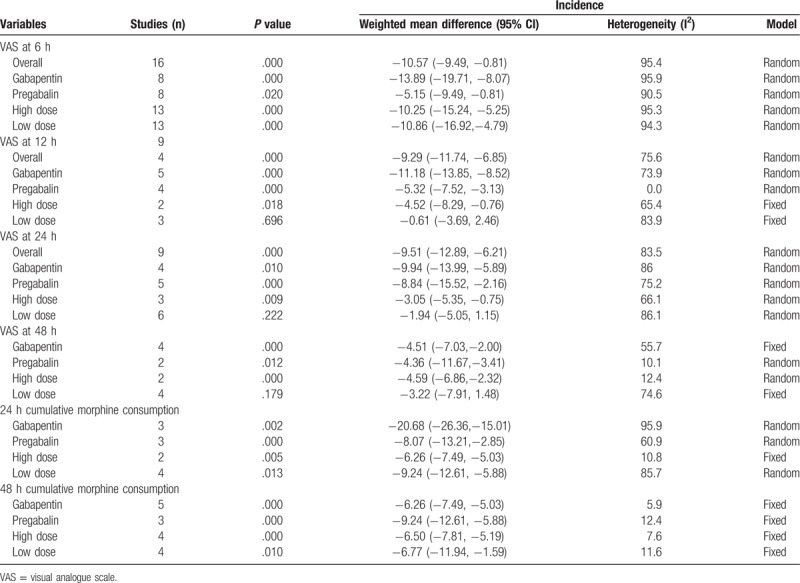
Subgroup analyses were conducted based on the dose and type of gabapentinoids; detailed results are shown in Table 2. Subgroup results indicated that pregabalin was superior to gabapentin in reducing both acute pain and cumulative morphine use at 24 and 48 hours. Furthermore, a high dose of gabapentinoids was superior to a low dose of gabapentinoids in reducing acute pain and cumulative morphine consumption following spinal surgery.
Table 2.
Subgroup analysis of the included studies according to the drug type (gabapentin or pregabalin) and the dose of gabapentinoids.
5. Discussion
The current meta-analysis demonstrated that the use of gabapentinoids is associated with reduced pain scores at 6, 12, and 24 hours, which is equivalent on a 110-point VAS to 10.57 points at 6 hours, 9.29 points at 12 hours, and 7.19 points at 24 hours. The cumulative morphine consumption at 24 and 48 hours was reduced in the gabapentinoids group by approximately 18.55 and 6.52 mg, respectively. The most important finding of this meta-analysis was that gabapentinoids can reduce the occurrence of nausea, vomiting, and pruritus after spine surgery. There was no significant difference in terms of sedation, dizziness, headache, visual disturbances, somnolence, and urine retention.
A major strength of the current meta-analysis was that we comprehensively searched the electronic databases (PubMed, EMBASE, Web of Science, Cochrane Library, and Google database) and calculated the relevant outcomes in a statistically rigorous method. We included RCTs and excluded non-RCTs, and thus the selective risk of bias was largely eliminated. The quality of the included RCTs was high or moderate. The only factor that reduced the level of evidence was the heterogeneity between the studies, which was caused by the different doses and time intervals of the gabapentinoids used. The type of gabapentinoids was also a source of heterogeneity. In the end, we performed a subgroup analysis to reduce the heterogeneity.
Pooled results indicated that preoperative administration of gabapentinoids was associated with a significant reduction of acute pain at 6, 12, and 24 hours following spinal surgery. These results were in contrast to the Hamilton et al study,[34] in which the authors found no evidence to support the routine use of gabapentinoids in the management of acute pain following total knee arthroplasty (TKA). Mao et al[35] performed a meta-analysis and found that gabapentinoids were associated with lower pain scores, morphine consumption, and postoperative nausea and vomiting (PONV) following total hip arthroplasty. Eipe et al[36] included 43 clinical studies in a meta-analysis and found that pregabalin use is uncommon and primarily restricted to surgical procedures associated with pro-nociceptive mechanisms. Furthermore, pregabalin was most likely to be efficacious in conditions associated with chronic pain. Choi et al[19] reported that the occurrence of chronic pain after spine surgery was approximately 20%; this incidence is lower than the 32% reported following discectomy and up to 85% reported after amputation.[37–39] If the theory proposed by Eipe et al[36] is correct, then the lower incidence of chronic pain after spine surgery may explain why pregabalin failed to demonstrate efficacy in patients undergoing spine surgery.[24] Arumugam et al[40] included 17 RCTs and found that the administration of preoperative gabapentin reduced the consumption of opioids during the initial 24 hours following surgery.
In a previous meta-analysis of the use of gabapentinoids in the management of postoperative pain after lumbar spinal surgery, the findings suggested that both gabapentin and pregabalin were efficacious in reducing postoperative pain.[11] However, only 2 studies included in the meta-analysis compared pregabalin with placebo for spinal surgery. Jiang et al[41] reported that preoperative use of pregabalin was efficacious in the reduction of postoperative pain, total morphine consumption, and the occurrence of nausea following spine surgery. However, the sample size and the number of included studies were limited. Dong et al[42] performed a meta-analysis of the use of pregabalin for reducing pain after TKA and found that pregabalin was effective in reducing pain intensity after TKA.
Morphine-related complications were also compared between the gabapentinoid and control groups. Significant reductions were found in the incidence of postoperative nausea, vomiting, and pruritus following spinal surgery. Grant et al[43] conducted a meta-analysis and found that preoperative pregabalin was associated with a significant reduction in PONV; however, postoperative analgesia did not improve accordingly. There were no significant differences in sedation, dizziness, headache, visual disturbances, somnolence, and urine retention.
There were several limitations to this meta-analysis: other perioperative pain management protocols were used in all of the studies, and thus heterogeneity existed in the final outcomes; spine function outcomes were not reported in the included studies and whether better pain control was correlated with preferable spine outcomes was unknown; the dosage and interval of gabapentinoid administration differed between the studies, the optimal dose of gabapentinoids required further study; we only identified the published papers about the gabapentinoids versus control groups, so unpublished papers may influence the final results; and only 2 studies directly compared gabapentin with pregabalin in spinal surgery. Direct studies to determine whether pregabalin was superior to gabapentin need further study.
6. Conclusion
This is the first meta-analysis to compare the preoperative use of gabapentinoids versus a placebo for the management of pain after spine surgery. Analgesic efficacy and opioid-sparing effects were observed with the administration of gabapentinoids. Additionally, a significant decrease in the risk of nausea, vomiting, and pruritus was associated with the use of gabapentinoids. The optimal dose and dosing intervals of gabapentinoids will require further study.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, NRS = numerical rating scale, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled trials, RR = risk ratio, SD = standard deviation, VAS = visual analogue scale, WMD = weighted mean differences.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cohen BE, Hartman MB, Wade JT, et al. Postoperative pain control after lumbar spine fusion. Patient-controlled analgesia versus continuous epidural analgesia. Spine (Phila Pa 1976) 1997;22:1892–6. [DOI] [PubMed] [Google Scholar]
- [2].Gottschalk A, Freitag M, Tank S, et al. Quality of postoperative pain using an intraoperatively placed epidural catheter after major lumbar spinal surgery. Anesthesiology 2004;101:175–80. [DOI] [PubMed] [Google Scholar]
- [3].Melemeni A, Staikou C, Fassoulaki A. Gabapentin for acute and chronic post-surgical pain. Signa Vitae 2007;2suppl 1:42–51. [Google Scholar]
- [4].Kim HJ, Park JH, Kim JW, et al. Prediction of postoperative pain intensity after lumbar spinal surgery using pain sensitivity and preoperative back pain severity. Pain Med 2014;15:2037–45. [DOI] [PubMed] [Google Scholar]
- [5].Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003;97:534–40. [DOI] [PubMed] [Google Scholar]
- [6].Pavlin DJ, Chen C, Penaloza DA, et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg 2002;95:627–34. [DOI] [PubMed] [Google Scholar]
- [7].Sun XL, Zhao ZH, Ma JX, et al. Continuous local infiltration analgesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krych AJ, Baran S, Kuzma SA, et al. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 2014;22:843–7. [DOI] [PubMed] [Google Scholar]
- [9].Dolgun H, Turkoglu E, Kertmen H, et al. Gabapentin versus pregabalin in relieving early post-surgical neuropathic pain in patients after lumbar disc herniation surgery: a prospective clinical trial. Neurol Res 2014;36:1080–5. [DOI] [PubMed] [Google Scholar]
- [10].Meng FY, Zhang LC, Liu Y, et al. Efficacy and safety of gabapentin for treatment of postherpetic neuralgia: a meta-analysis of randomized controlled trials. Minerva Anestesiol 2014;80:556–67. [PubMed] [Google Scholar]
- [11].Yu L, Ran B, Li M, et al. Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2013;38:1947–52. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JPT, G. S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. Available at: http://www.cochrane-handbook.org. Access 2011. [Google Scholar]
- [13].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1997;33:363–74. [PubMed] [Google Scholar]
- [15].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [16].Wang C, Cai XZ, Yan SG. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [17].GS H. J. Cochrane handbook for systematic reviews of interventions version 5.1.0[updated March 2011]. 2011. [Google Scholar]
- [18].Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg 2010;110:1180–5. [DOI] [PubMed] [Google Scholar]
- [19].Choi YS, Shim JK, Song JW, et al. Combination of pregabalin and dexamethasone for postoperative pain and functional outcome in patients undergoing lumbar spinal surgery: a randomized placebo-controlled trial. Clin J Pain 2013;29:9–14. [DOI] [PubMed] [Google Scholar]
- [20].Fujita N, Tobe M, Tsukamoto N, et al. A randomized placebo-controlled study of preoperative pregabalin for postoperative analgesia in patients with spinal surgery. J Clin Anesth 2016;31:149–53. [DOI] [PubMed] [Google Scholar]
- [21].Gianesello L, Pavoni V, Barboni E, et al. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol 2012;24:121–6. [DOI] [PubMed] [Google Scholar]
- [22].Hegarty DA, Shorten GD. A randomised, placebo-controlled trial of the effects of preoperative pregabalin on pain intensity and opioid consumption following lumbar discectomy. Korean J Pain 2011;24:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khurana G, Jindal P, Sharma JP, et al. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine (Phila Pa 1976) 2014;39:E363–368. [DOI] [PubMed] [Google Scholar]
- [24].Kim JC, Choi YS, Kim KN, et al. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine (Phila Pa 1976) 2011;36:428–33. [DOI] [PubMed] [Google Scholar]
- [25].Kumar KP, Kulkarni DK, Gurajala I, et al. Pregabalin versus tramadol for postoperative pain management in patients undergoing lumbar laminectomy: a randomized, double-blinded, placebo-controlled study. J Pain Res 2013;6:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ozgencil E, Yalcin S, Tuna H, et al. Perioperative administration of gabapentin 1,200 mg day-1 and pregabalin 300 mg day-1 for pain following lumbar laminectomy and discectomy: a randomised, double-blinded, placebo-controlled study. Singapore Med J 2011;52:883–9. [PubMed] [Google Scholar]
- [27].Spreng UJ, Dahl V, Raeder J. Effect of a single dose of pregabalin on post-operative pain and pre-operative anxiety in patients undergoing discectomy. Acta Anaesthesiol Scand 2011;55:571–6. [DOI] [PubMed] [Google Scholar]
- [28].Khan ZH, Rahimi M, Makarem J, et al. Optimal dose of pre-incision/post-incision gabapentin for pain relief following lumbar laminectomy: a randomized study. Acta Anaesthesiol Scand 2011;55:306–12. [DOI] [PubMed] [Google Scholar]
- [29].Pandey CK, Navkar DV, Giri PJ, et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blind, placebo-controlled study. J Neurosurg Anesthesiol 2005;17:65–8. [DOI] [PubMed] [Google Scholar]
- [30].Radhakrishnan M, Bithal PK, Chaturvedi A. Effect of preemptive gabapentin on postoperative pain relief and morphine consumption following lumbar laminectomy and discectomy: a randomized, double-blinded, placebo-controlled study. J Neurosurg Anesthesiol 2005;17:125–8. [DOI] [PubMed] [Google Scholar]
- [31].Turan A, Karamanlioglu B, Memis D, et al. Analgesic effects of gabapentin after spinal surgery. Anesthesiology 2004;100:935–8. [DOI] [PubMed] [Google Scholar]
- [32].Vasigh A, Jaafarpour M, Khajavikhan J, et al. The effect of gabapentin plus celecoxib on pain and associated complications after laminectomy. J Clin Diagn Res 2016;10:Uc04–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pandey CK, Sahay S, Gupta D, et al. Preemptive gabapentin decreases postoperative pain after lumbar discoidectomy. Can J Anaesth 2004;51:986–9. [DOI] [PubMed] [Google Scholar]
- [34].Hamilton TW, Strickland LH, Pandit HG. A meta-analysis on the use of gabapentinoids for the treatment of acute postoperative pain following total knee arthroplasty. J Bone Joint Surg Am 2016;98:1340–50. [DOI] [PubMed] [Google Scholar]
- [35].Mao Y, Wu L, Ding W. The efficacy of preoperative administration of gabapentin/pregabalin in improving pain after total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord 2016;17:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Eipe N, Penning J, Yazdi F, et al. Perioperative use of pregabalin for acute pain—a systematic review and meta-analysis. Pain 2015;156:1284–300. [DOI] [PubMed] [Google Scholar]
- [37].Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Parker SL, Xu R, McGirt MJ, et al. Long-term back pain after a single-level discectomy for radiculopathy: incidence and health care cost analysis. J Neurosurg Spine 2010;12:178–82. [DOI] [PubMed] [Google Scholar]
- [39].Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res 2013;6:121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arumugam S, Lau CS, Chamberlain RS. Use of preoperative gabapentin significantly reduces postoperative opioid consumption: a meta-analysis. J Pain Res 2016;9:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang HL, Huang S, Song J, et al. Preoperative use of pregabalin for acute pain in spine surgery: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dong J, Li W, Wang Y. The effect of pregabalin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Int J Surg 2016;34:148–60. [DOI] [PubMed] [Google Scholar]
- [43].Grant MC, Betz M, Hulse M, et al. The effect of preoperative pregabalin on postoperative nausea and vomiting: a meta-analysis. Anesth Analg 2016;123:1100–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


