Abstract
Background:
There are some fertility-sparing treatments in patients with early endometrial cancer (EEC) or atypical complex hyperplasia (ACH), and the objective is to compare them by evaluating the oncologic and reproductive outcomes.
Methods:
We searched the published literature using Medline, Cochrane, EMBASE, and Google Scholar databases up to January 3, 2017, with various combinations of keywords fertility-sparing treatments, progesterone, progestin, intrauterine devices, early endometrial cancer, and atypical complex hyperplasia. The primary endpoint is the complete response (CR) rate, and the secondary endpoints are the partial response (PR) rate, relapse rate (RR), pregnancy rate, and live birth rate.
Results:
Twenty-eight studies containing 1038 women with EEC or ACH were included for review and meta-analysis. The results demonstrated that women with EEC or ACH managed with progestin had a pooled CR rate of 71% (95% confidence interval [CI]: 63–77%). The pooled pregnancy outcomes showed that 34% of women taking progestin treatment for EEC or ACH became pregnant (95% CI: 30–38%); however, only 20% of them delivered live newborns. The pooled CR rate for women using intrauterine device (IUD) was 76% (95% CI: 67–83%), and pooled RR was 9% (95% CI: 5–17%). The pregnancy rate for women whom underwent IUD was 18% (95% CI: 7–37%), and 14% of them delivered live newborns. In patients using progestin plus IUD, the pooled CR rate was 87% (95% CI: 75–93%); among those patients, 40% became pregnant (95% CI: 20–63%), and 35% delivered live newborns. There is no publication bias for the CR rate.
Conclusion:
For patients with EEC and ACH, treatments with progestin, with or without IUD, or IUD alone can reach good CR rate; however, the pregnancy outcomes might be worse in patients treated with IUD alone. Further randomized-controlled studies are warranted to find out a better solution.
Keywords: atypical complex hyperplasia, early endometrial cancer, fertility-sparing treatments, meta-analysis, systematic review
1. Introduction
Endometrial cancer (EC) is the most common gynecological cancer in developed countries.[1] It accounts for 3.6% of all new cancer cases and lead to 1.8% of all cancer deaths in America in 2016.[2] There are 2 major types of endometrial carcinomas: endometrioid carcinoma (type 1) that is related to hormonal imbalance, and serous carcinoma (type 2) that is unrelated to estrogen.[3] Atypical complex hyperplasia (ACH) is the major precursor of type 1 EC and is found in 5% to 10% of premenopausal women with abnormal vaginal bleeding.[4] The pathological appearance of ACH and EEC are similar and sometimes difficult to distinguish. The gold standard of treatment for patients with early endometrial cancer (EEC) and ACH is total hysterectomy with bilateral salpingo-oophorectomy. Although it can achieve good oncologic outcomes, the treatment can destroy fertility. For EC patients at reproductive age and wishes to preserve fertility, fertility-sparing treatments may be considered. At initial staging, magnetic resonance imaging (MRI) is used to exclude cervical and myometrial invasion before fertility-sparing treatment.[5] The criteria for conservative management in premenopausal EC patients are grade 1 well-differentiated tumor; stage FIGO IA tumor without invasion of myometrium on MRI, absence of lymphovascular invasion on specimen, and without intraabdominal disease or adnexal mass.[6,7] Patients should follow-up with hysteroscopy and endometrial sampling after 3 months.[8] ACH and EC that express estrogen and progesterone receptors suggested higher chance of retaining fertility after hormone therapy.[3] The recommended fertility-sparing treatments in the National Comprehensive Cancer Network (NCCN)[9] guidelines and the Society of Gynecologic Oncology's Clinical Practice Endometrial Cancer Working Group[10] included hormone therapy (megestrol and medroxyprogesterone) and levonorgestrel-releasing intrauterine devices (LNG-IUD), but the most common fertility-sparing option is hormone therapy. Progestin is known to suppress the growth of endometrial cancer by downregulating estrogen receptors, activating enzymes in estrogen metabolism, and involving cell cycle regulation by cyclin-dependent kinase (Cdk).[11] Progestin is also known to reinforce p27 (a cyclin E-Cdk2 complex inhibitor) expression, resulting in suppression of the cell cycle.[12] Other fertility-sparing treatments were reported in recent years, such as LNG-IUD combined with progestin, and progestin combined with metformin. It is uncertain which method had favorable outcome. Thus, the objective of this study is to compare the different fertility-sparing treatments on oncologic and reproductive outcomes in patients with EEC or ACH.
2. Materials and methods
2.1. Search strategy
We followed the PRISMA guidance for systematic reviews of observational and diagnostic studies.[13] Published literature search was performed using Medline, Cochrane, EMBASE, and Google Scholar databases with various combinations of the following keywords fertility-sparing treatments, progesterone, progestin, intrauterine devices, early endometrial cancer, and atypical endometrial hyperplasia. References in relevant primary publications were hand-searched to identify other eligible trials. The described searches included original literature published up to January 3rd, 2017.
The inclusion criteria were randomized-controlled trials (RCTs), prospective studies, retrospective studies; patients with EEC or ACH; patients undergoing fertility-sparing treatments for EEC or ACH; quantitative outcomes with complete response (CR) rate, partial response (PR) rate, relapse rate (RR), pregnancy rate and live birth rate. The exclusion criteria were letters, comments, editorials, case report, proceeding, personal communication; patients without diagnoses of EEC or ACH; patients with no fertility-sparing treatment for EEC or ACH; studies without quantitative outcomes.
2.2. Data extraction
Data were extracted independently by 2 reviewers (LF and WG). A third reviewer was consulted in the case of disagreements (WZ). We extracted data on study population (number, age, BMI, imaging methods, and percentage of EEC/ACH of subjects, and follow-up time), study design, and the major outcomes.
2.3. Quality assessment
We assessed the quality of the single-arm study using the Modified 18-items Delphi checklist.[14] Quality assessment was performed by 2 independent reviewers (LF and WG), and a third reviewer (WZ) was consulted if no consensus could be reached.
2.4. Statistical analysis
The primary endpoint for this meta-analysis was the CR rate to different fertility-sparing treatments for patients with EEC or ACH. The secondary endpoints were partial response (PR) rate, relapse rate, pregnancy rate, and live birth rate. Event rates with 95% confidence interval (CI) were extracted from each individual study. A χ2-based test of homogeneity was performed and the inconsistency index (I2) and Q statistics were determined. If the I2 statistic were >50%, a random-effects model was used. Otherwise, the fixed-effect model was employed. Pooled effects were calculated, and a 2-sided P value <.05 was considered to indicate statistical significance. Sensitivity analysis for primary outcome was carried out using the leave-one-out approach. In addition, publication bias was assessed on primary endpoint by constructing funnel plots by Egger's test. The absence of publication bias was indicated by the data points forming a symmetric funnel-shaped distribution and 1-tailed significance level P > .05 (Egger's test). All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
2.5. Ethics approval
Ethical approval is not required for the meta-analysis and systematic review.
3. Results
3.1. Basic characteristics of included studies
Using the keyword search, 132 articles were identified. After screening for titles and abstract, 65 articles were kept for full text reviewing. Among these, 18 had no qualitative major outcome, 9 included patients without EC or ACH, 5 were case reports, 2 protocols, 2 letters, and 1 editorial. After considering the inclusion and exclusion criteria, 28 articles were eligible for this review and meta-analysis.[15–42] A flow chart describing the selection of the articles for analysis is presented in Fig. 1.
Figure 1.
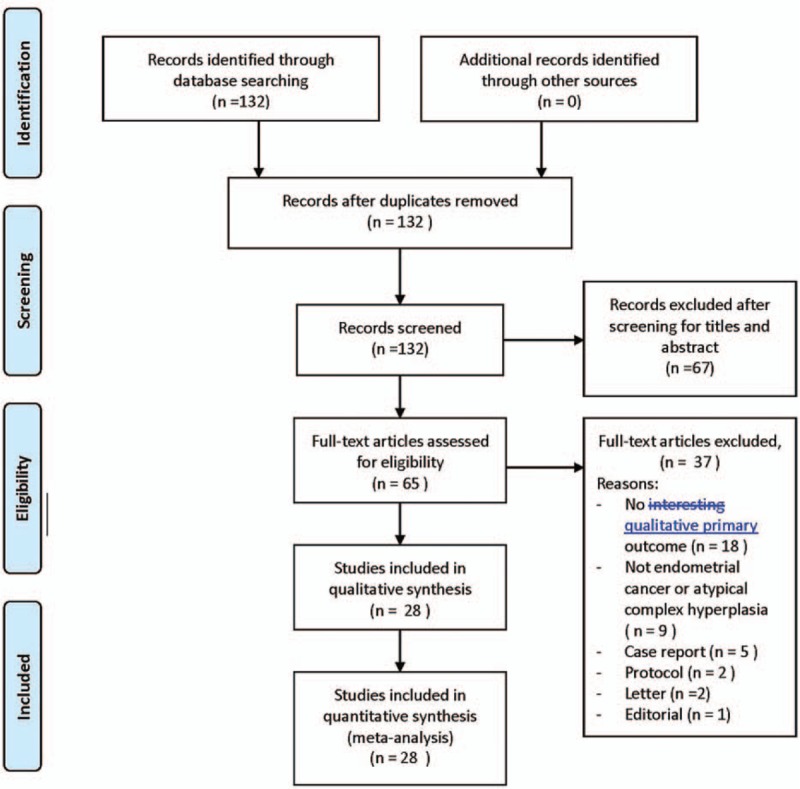
PRISMA flow diagram of study selection.
Seventeen studies were retrospective studies and 11 studies were prospective. The 28 studies included a total of 1038 women with EEC or ACH whom wished to preserve fertility; 809 patients were treated with progestin, 170 patients received LNG-IUD therapy, and 59 patients were treated with both progestin and IUD. The basic characteristics of the 28 studies are summarized in Table 1. Patients’ age ranged from 27.5 to 57.5 years old.
Table 1.
Basic data of included studies.

3.2. Meta-analysis of progestin
3.2.1. All progestin
Twenty studies reported treatment of progestin for women with EEC or ACH. Out of which, 18 studies provided CR rates. There was heterogeneity in the CR among the 18 studies; therefore, a random-effect model was used (Q statistic = 40.671, I2 = 58.20%). The result of the meta-analysis revealed that women with EEC and ACH managed with progestin had pooled CR rate of 71% (95% CI: 63–77%, Fig. 2A). In 8 of 20 studies with PR reported, the meta-analysis showed a pooled PR rate of 17% (95% CI: 10–27%). A total of 19 studies reported the relapse rate during the follow up period; the pooled relapse rate was 20% (95% CI: 19–40%). Meta-analysis of the 18 studies reporting pregnancy outcomes showed that 34% of women undergoing progestin treatment for EEC or ACH became pregnant (pooled event rate = 34%; 95% CI: 30–38%); however, only 20% of them delivered live newborns (Table 2).
Figure 2.
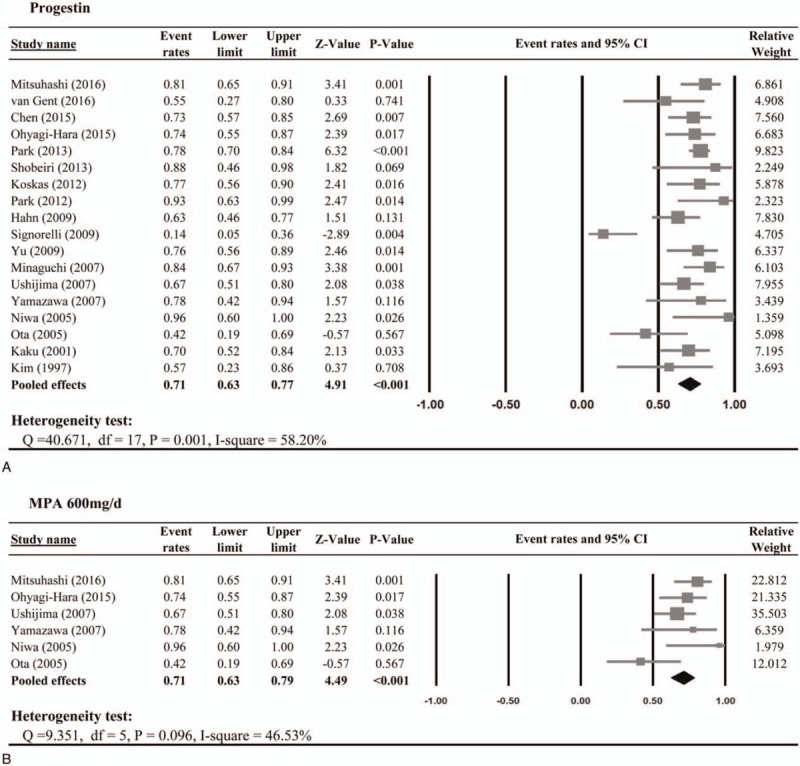
Meta-analysis of the complete response rate to progestin.
Table 2.
Meta-analysis of secondary endpoints.

3.2.2. Medroxyprogesterone acetate (MPA) >400 mg/day
When women treated with higher dose of MPA (>400 mg/day), 71% and 21% of patients achieved CR and PR, respectively (Fig. 2B). During the follow-up period, the pooled relapse rate was 33% (95% CI: 18–53%). In addition, the pooled rates of pregnancy and live birth were 34% (95% CI: 23–46%) and 21% (95% CI: 14–31%), respectively (Table 2).
3.3. Meta-analysis of LNG-IUD
Two studies[35,36] provided CR for women with EEC and ACH managing with IUD therapy and were included in the meta-analysis. There was no heterogeneity in the CR among the 2 studies; therefore, a fixed-effect model was used (Q statistic = 0.325, I2 = 0%). The result of the meta-analysis revealed that the pooled CR rate was 76% (95% CI: 67–83%) for women undergoing IUD system (Fig. 3). However, only 1 study provided PR rate; thus, meta-analysis was not performed for PR. Two studies reported relapse rate, and the pooled relapse rate was 9% (95% CI: 5–17%). Meta-analysis of the 2 studies reporting pregnancy outcomes showed that 18% of women underwent IUD for EEC or ACH became pregnant (pooled event rate = 18%; 95% CI: 7–37%); however, only 14% of them delivered live newborns (Table 2).
Figure 3.
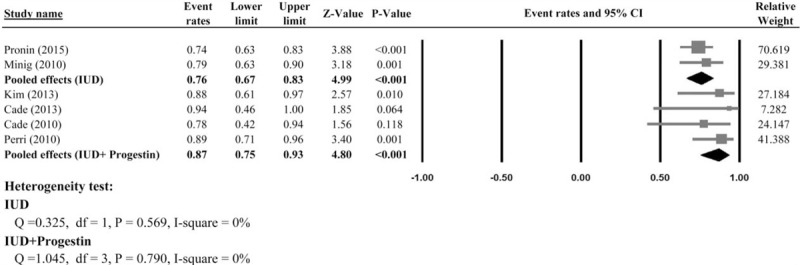
Meta-analysis of the complete response rate to intrauterine device and intrauterine device plus progestin.
3.4. Meta-analysis of Progestin +IUD
Four studies[37–40] provided CR for women with EEC and ACH managed with progestin plus IUD and were included in the meta-analysis. The result of the meta-analysis revealed that the pooled CR rate was 87% (95% CI: 75–93%) for women treated with progestin plus IUD system, and no heterogeneity were found among the 4 studies (Q statistic = 1.045, I2 = 0%, Fig. 3). However, only 1 study provided the PR rate and relapse rate; hence, meta-analysis was not performed for PR and RR. Meta-analysis of the 3 studies reporting pregnancy outcomes showed that 40% of women whom underwent progestin and IUD for EEC and ACH became pregnant (pooled event rate = 40%; 95% CI: 20–63%); however, only 35% of them delivered live newborns (Table 2).
3.5. Meta-analysis of Progestin vs. IUD
Two studies[41,42] provided information on CR rates between patients in the progestin and IUD groups. There was no heterogeneity in the CR among the 2 studies; therefore, a fixed-effect model was used (Q statistic = 0.059, I2 = 0%). The result of the meta-analysis revealed that there was no significant difference in the rate of CR between patients in the progestin and IUD groups (pooled odds ratio (OR) = 0.58, 95% CI: 0.30–1.10, Fig. 4A). Again, the meta-analysis from the 2 studies showed no significant difference in the relapse rate between the 2 groups (pooled OR = 1.09, 95% CI: 0.54–2.20, Fig. 4B).
Figure 4.
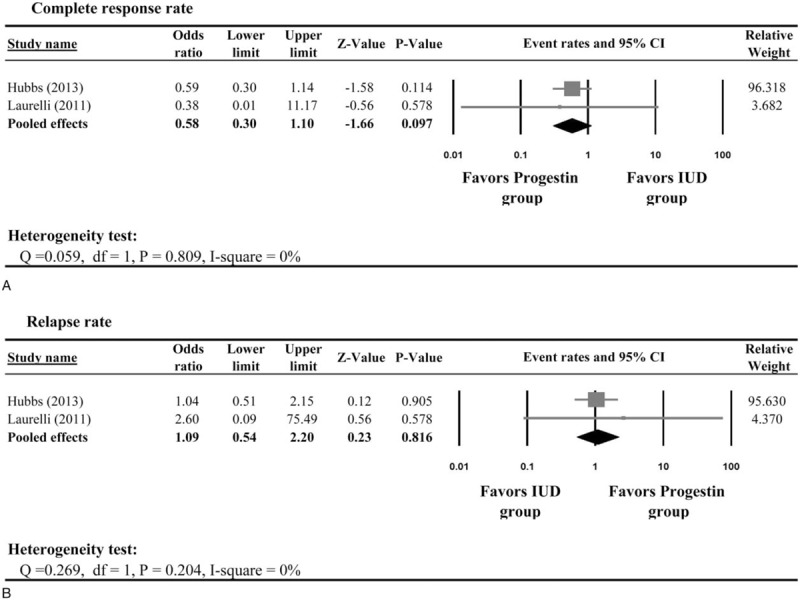
Meta-analysis of the complete response rate between patients treated with progestin and intrauterine device.
3.6. Sensitivity analysis and publication bias
Sensitivity analyses were performed using the leave-one-out approach with each study removed in turn (Table 3). The direction of combined estimates on CR rate did not vary markedly with the removal of each study, indicating that the meta-analysis had good reliability and the data were not overly influenced by any particular study. Figure 5 illustrated that there was no publication bias for the findings in regard to CR rate via Egger's (t = 0.337, P = .370).
Table 3.
Sensitivity analysis.

Figure 5.
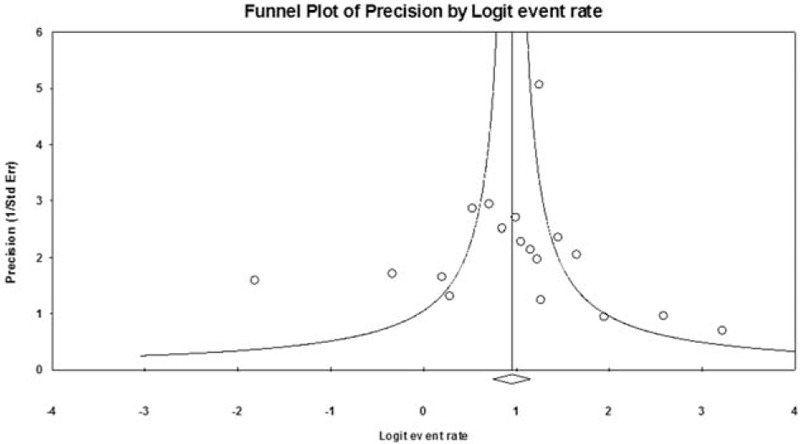
Funnel plots for the complete response rate showing the distribution of published study outcomes.
3.7. Quality assessment
We used Modified 18-items Delphi checklist to evaluate the quality of the included articles, and the results were reported in Table 1. In the 26 single-arm studies, all the included studies stated the aim clearly in the abstract or introduction and described the characteristics of the included participants. The final total scores ranged from 10 to 16 (maximum possible score of 18). Overall, the included studies are of good quality.
4. Discussion
We employed meta-analysis techniques to compare oncologic and reproductive outcomes of fertility-sparing treatments in patients with EEC and ACH. The results showed that patients managed with progestin had a pooled CR rate of 71%. Pooled pregnancy outcomes showed that 34% of women underwent progestin became pregnant; however, only 20% of them delivered live newborns. The pooled CR rate for women underwent IUD system was 76%, and the pooled relapse rate was 9%. Among patients treated with IUD, 18% became pregnant, and 14% of them delivered live newborns. In patients managed with progestin plus IUD, the pooled CR rate was 87%; 40% of them became pregnant, and 35% of them delivered live newborns. It seemed that patients with the IUD system alone had worse reproductive outcomes than patients with progestin, with or without IUD system.
A number of reviews and meta-analysis studies mentioned fertility-sparing treatments in patients with EEC and ACH. First, Gunderson et al[8] conducted a systematic review in 2012, with various outcomes in EEC and ACH patients receiving progestin as fertility-sparing therapy. They concluded that ACH patients might have better response rate to hormone therapy than EEC patients. However there seemed to be no differences in reproductive outcomes. Gallos et al[43] performed a meta-analysis to evaluate the clinical and reproductive outcomes of EEC and ACH with fertility sparing treatment. They concluded that fertility-sparing treatment for EEC and ACH is feasible and may improve live birth rates. The study interventions included oral progestin, hysteroscopic resection, IUD, without subgroup analysis. Koskas et al[44] reviewed 370 patients from 24 articles that underwent fertility-sparing treatments for atypical hyperplasia and endometrial cancer. They concluded that fertility-sparing management should not be contraindicated in older patients with infertility or obesity since the oncologic and reproductive outcomes investigated showed no significant association with age, obesity or previous infertility. Most recently, Carneiro et al[45] reviewed articles for the safety of fertility-preservation in EC. Overall, patients with grade 1 minimally invasive tumor were recommended for conservative management, which is supported by previous studies. In our study, we did subgroup analysis of progestin with or without IUD, and IUD alone, and concluded that both progestin (with or without IUD) and IUD alone could have satisfying CR, but patients with progestin alone might have better reproductive outcomes. Systemic hormone therapy would affect hypothalamic–pituitary–gonadal axis; however, local hormone therapy with IUD could resolve the problem of patient compliance. There is no definite reason for the difference in reproductive outcomes between patients with progestin and IUD.
In our study, we attempted to address the fertility-sparing treatment for EEC or ACH according to different intervention, including oral progestin, IUD, and progestin plus IUD. At first, hormone therapy was suggested as a conservative treatment for patients who had EEC or ACH and favored to preserve fertility. In recent years, other choices such as LNG-IUD and oral progestin plus LNG-IUD have emerged. LNG-IUD provides local progestin to the endometrium and spares most of the systemic effects of oral progestin, such as weight gain and increased risk of venous thrombosis.[35] However, according to our results, caution needs to be taken on the use of LNG-IUD alone since it may lead to worse reproductive outcomes.
Recently, the anti-cancer effect of metformin has been acknowledged, which includes preventing cancer recurrence and increasing tumor radiosensitivity.[46] Metformin may be applied in conservative treatment for EC as well. It is reported that obese patients with type I endometrial cancer had less risk of cancer recurrence on metformin.[47] For EC patients, the use of metformin is associated with improved recurrence-free survival and overall survival.[48] In our analysis, 1 study used MPA combined with metformin as fertility-sparing treatment, and the authors concluded that metformin could inhibit disease relapse after the hormone therapy. The application of metformin on fertility-sparing treatment in patients with EC and ACH should be studied in the future.
There were several limitations to this meta-analysis. First, most of the included articles are single-arm studies; hence, it is difficult to compare different interventions directly. We can only observe the pooled oncologic and reproductive outcomes. Second, the protocol for the daily dosage of progestin varied. Although we performed a subgroup analysis on MPA >400 mg daily, the dosage of progestin is still not identical.
In the two 2-arm studies, publication bias may be explained since only patients in Laurelli et al[42] received hysteroscopic resection, but not in Hubbs et al.[41] Also, the overall BMI in Hubbs et al.[41] is higher than that in Laurelli et al.[42] There are many confounding factors, such as age, ethnicity, BMI, comorbidities, gravidity and parity, and study design, which may all contribute to the heterogeneity. The confounding factors should be considered for a detailed subgroup analysis when more studies are available in the future. The definition of partial response varied in the included articles. Finally, according to the previous literature,[5] MRI is recommended for initial staging and follow-up. However, the imaging methods in the included articles were not consistent. Some studies used computed tomography or ultrasound only, which may lead to bias. It may be the future direction to find out the difference in oncologic and reproductive outcomes between EEC and ACH patients.
Fertility counseling and fertility-sparing surgery may benefit reproductive-aged patients with gynecologic malignancies in cases when children may be desired in the future.[49] In conclusion, the results of our meta-analysis indicated that patients with EEC and ACH might have similar oncologic outcomes under fertility-sparing treatments of progestin (either with or without IUD) or IUD alone. However, patients treated with IUD alone seemed to have worse reproductive outcomes than patients treated with progestin. Further well-designed RCTs that compare the different interventions for EEC and ACH patients in preserving fertility are essential.
Footnotes
Abbreviations: ACH = atypical complex hyperplasia, BMI = body mass index, CI = confidence interval, CR = complete response, CT = computed tomography, EC = endometrial cancer, EEC = early endometrial cancer, I2 = inconsistency index, IUD = intrauterine device, LNG-IUD = levonorgestrel-releasing intrauterine device, MA = megestrol acetate, MPA = medroxyprogesterone acetate, MRI = magnetic resonance imaging, OR = odds ratio, PR = partial response, RCT = randomized controlled study, RR = relapse rate.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol 2000;13:295–308. [DOI] [PubMed] [Google Scholar]
- [4].Brownfoot FC, Hickey M, Ang WC, et al. Complex atypical hyperplasia of the endometrium: differences in outcome following conservative management of pre- and postmenopausal women. Reprod Sci 2014;21:1244–8. [DOI] [PubMed] [Google Scholar]
- [5].Butterfield N, Smith JR. Role of imaging in fertility-sparing treatment of gynecologic malignancies. Radiographics 2016;36:2214–33. [DOI] [PubMed] [Google Scholar]
- [6].Eskander RN, Randall LM, Berman ML, et al. Fertility preserving options in patients with gynecologic malignancies. Am J Obstet Gynecol 2011;205:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leblanc E, Narducci F, Ferron G, et al. Indications and teaching of fertility preservation in the surgical management of gynecologic malignancies: European perspective. Gynecol Oncol 2009;1142 suppl:S32–6. [DOI] [PubMed] [Google Scholar]
- [8].Gunderson CC, Fader AN, Carson KA, et al. Oncologic and Reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. [DOI] [PubMed] [Google Scholar]
- [9].National Comprehensive Cancer Network. Uterine Neoplasm (Version 1. 2017). https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed March 3, 2017. [Google Scholar]
- [10].Burke WM, Orr J, Leitao M, et al. SGO Clinical Practice Endometrial Cancer Working Group. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol 2014;134:393–402. [DOI] [PubMed] [Google Scholar]
- [11].Banno K, Kisu I, Yanokura M, et al. Progestin therapy for endometrial cancer: the potential of fourth-generation progestin (review). Int J Oncol 2012;40:1755–62. [DOI] [PubMed] [Google Scholar]
- [12].Shimizu Y, Takeuchi T, Mizuguchi K, et al. Dienogest, a synthetic progestin, inhibits the proliferation of immortalized human endometrialepithelial cells with suppression of cyclin D1 gene expression. Mol Hum Reprod 2009;15:693–701. [DOI] [PubMed] [Google Scholar]
- [13].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Int Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [14].Moga C, Guo B, Schopflocher D, et al. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton AB: Institute of Health Economics; 2012. [Google Scholar]
- [15].Inoue O, Hamatani T, Susumu N, et al. Factors affecting pregnancy outcomes in young women treated with fertility-preserving therapy for well-differentiated endometrial cancer or atypical endometrial hyperplasia. Reprod Biol Endocrinol 2016;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mitsuhashi A, Sato Y, Kiyokawa T, et al. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol 2016;27:262–6. [DOI] [PubMed] [Google Scholar]
- [17].van Gent MDJM, Nicolae-Cristea AR, de Kroon CD, et al. Exploring morphologic and molecular aspects of endometrial cancer under progesterone treatment in the context of fertility preservation. Int J Gynecol Cancer 2016;26:483–90. [DOI] [PubMed] [Google Scholar]
- [18].Emarh M. Cyclic versus continuous medroxyprogesterone acetate for treatment of endometrial hyperplasia without atypia: a 2-year observational study. Arch Gynecol Obs 2015;292:133. [DOI] [PubMed] [Google Scholar]
- [19].Chen M, Jin Y, Li Y, et al. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynecol Obstet 2016;132:34–8. [DOI] [PubMed] [Google Scholar]
- [20].Ohyagi-Hara C, Sawada K, Aki I, et al. Efficacies and pregnant outcomes of fertility-sparing treatment with medroxyprogesterone acetate for endometrioid adenocarcinoma and complex atypical hyperplasia: our experience and a review of the literature. Arch Gynecol Obstet 2015;291:151–7. [DOI] [PubMed] [Google Scholar]
- [21].Park JY, Kim DY, Kim JH, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur J Cancer 2013;49:868–74. [DOI] [PubMed] [Google Scholar]
- [22].Shobeiri MJ, Gharabagh PM, Esmaeili H, et al. Fertility sparing treatment in young patients with early endometrial adenocarcinoma. Pakistan J Med Sci 2013;29:651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koskas M, Azria E, Walker F, et al. Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer Res 2012;32:1037–43. [PubMed] [Google Scholar]
- [24].Park H, Seok JM, Yoon BS, et al. Effectiveness of high-dose progestin and long-term outcomes in young women with early-stage, well-differentiated endometrioid adenocarcinoma of uterine endometrium. Arch Gynecol Obstet 2012;285:473–8. [DOI] [PubMed] [Google Scholar]
- [25].Hahn HS, Yoon SG, Hong JS, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer 2009;19:1068–73. [DOI] [PubMed] [Google Scholar]
- [26].Signorelli M, Caspani G, Bonazzi C, et al. Fertility-sparing treatment in young women with endometrial cancer or atypical complex hyperplasia: a prospective single-institution experience of 21 cases. BJOG 2009;116:114–8. [DOI] [PubMed] [Google Scholar]
- [27].Yu M, Yang JX, Wu M, et al. Fertility-preserving treatment in young women with well-differentiated endometrial carcinoma and severe atypical hyperplasia of endometrium. Fertil Steril 2009;92:2122–4. [DOI] [PubMed] [Google Scholar]
- [28].Minaguchi T, Nakagawa S, Takazawa Y, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett 2007;248:112–22. [DOI] [PubMed] [Google Scholar]
- [29].Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol 2007;25:2798–803. [DOI] [PubMed] [Google Scholar]
- [30].Yamazawa K, Hirai M, Fujito A, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod 2007;22:1953–8. [DOI] [PubMed] [Google Scholar]
- [31].Niwa K, Tagami K, Lian Z, et al. Outcome of fertility-preserving treatment in young women with endometrial carcinomas. BJOG 2005;112:317–20. [DOI] [PubMed] [Google Scholar]
- [32].Ota T, Yoshida M, Kimura MKK. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int J Gynecol Cancer 2005;15:657–62. [DOI] [PubMed] [Google Scholar]
- [33].Kaku T, Yoshikawa H, Tsuda H, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett 2001;167:39–48. [DOI] [PubMed] [Google Scholar]
- [34].Kim YB, Holschneider CH, Ghosh K, et al. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer 1997;79:320–7. [DOI] [PubMed] [Google Scholar]
- [35].Pronin SM, Novikova OV, Andreeva JY, et al. Fertility-sparing treatment of early endometrial cancer and complex atypical hyperplasia in young women of childbearing potential. Int J Gynecol Cancer 2015;25:1010–4. [DOI] [PubMed] [Google Scholar]
- [36].Minig L, Franchi D, Boveri S, et al. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol 2011;22:643–9. [DOI] [PubMed] [Google Scholar]
- [37].Kim MK, Seong SJ, Kim YS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol 2013;209: 358.e1-4. [DOI] [PubMed] [Google Scholar]
- [38].Cade TJ, Quinn MA, Rome RM, et al. Long-term outcomes after progestogen treatment for early endometrial cancer. Aust N Z J Obstet Gynaecol 2013;53:566–70. [DOI] [PubMed] [Google Scholar]
- [39].Cade T, Quinn M, Rome R, et al. Progestogen treatment options for early endometrial cancer. BJOG 2010;117:879–83. [DOI] [PubMed] [Google Scholar]
- [40].Perri T, Korach J, Gotlieb WH, et al. Prolonged conservative treatment of endometrial cancer patients. Int J Gynecol Cancer 2011;21:72–8. [DOI] [PubMed] [Google Scholar]
- [41].Hubbs JL, Saig RM, Abaid LN, et al. Systemic and local hormone therapy for endometrial hyperplasia and early adenocarcinoma. Obstet Gynecol 2013;121:1172–80. [DOI] [PubMed] [Google Scholar]
- [42].Laurelli G, Di Vagno G, Scaffa C, et al. Conservative treatment of early endometrial cancer: preliminary results of a pilot study. Gynecol Oncol 2011;120:43–6. [DOI] [PubMed] [Google Scholar]
- [43].Gallos ID, Yap J, Rajkhowa M, et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2012;207:266e1–2. [DOI] [PubMed] [Google Scholar]
- [44].Koskas M, Uzan J, Luton D, et al. Prognostic factors of oncologic and reproductive outcomes in fertility-sparing management of endometrial atypical hyperplasia and adenocarcinoma: Systematic review and meta-analysis. Fertil Steril 2014;101:785–94. [DOI] [PubMed] [Google Scholar]
- [45].Carneiro MM, Lamaita RM, Ferreira MCF, et al. Safe fertility-preserving management in endometrial cancer: is it feasible? Review of the literature. J Gynecol Surg 2012;28:399–404. [Google Scholar]
- [46].Han MS, Lee HJ, Park SJ, et al. The effect of metformin on the recurrence of colorectal adenoma in diabetic patients with previous colorectal adenoma. Int J Colorectal Dis 2017;32:1223–6. [DOI] [PubMed] [Google Scholar]
- [47].Hall C, Stone RL, Gehlot A, et al. Use of metformin in obese women with type i endometrial cancer is associated with a reduced incidence of cancer recurrence. Int J Gynecol Cancer 2016;26:313–7. [DOI] [PubMed] [Google Scholar]
- [48].Ko EM, Walter P, Jackson A, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol 2014;132:438–42. [DOI] [PubMed] [Google Scholar]
- [49].Chan JL, Letourneau J, Salem W, et al. Regret around fertility choices is decreased with pre-treatment counseling in gynecologic cancer patients. J Cancer Surviv 2016;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


