Abstract
Background:
This meta-analysis aimed to evaluate the efficiency and safety of transcutaneous electrical nerve stimulation (TENS) for pain control after total knee arthroplasty.
Methods:
A systematic search was performed in Medline (1966 to June 2017), PubMed (1966 to June 2017), Embase (1980 to June 2017), ScienceDirect (1985 to June 2017), and the Cochrane Library. Only randomized controlled trial (RCT) was included. The fixed/random effect model was used according to the heterogeneity tested by I2 statistic. Meta-analysis was performed using Stata 11.0 software.
Results:
Five RCTs including 472 patients met the inclusion criteria. The present meta-analysis indicated that there were significant differences between groups in terms of visual analogue scale score at 12 hours (average: 3.58 vs 4.34, SMD = −0.260, 95% CI: −0.442 to −0.078, P = .005), 24 hours (average: 3.18 vs 3.52, SMD = −0.244, 95% CI: −0.426 to −0.063, P = .008), and 48 hours (average: 2.70 vs 2.96, SMD = −0.214, 95% CI: −0.395 to −0.033, P = .021) after total knee arthroplasty. Significant differences were found regarding opioid consumption at 12 hours (average: 14.44 vs 18.54, SMD = −0.503, 95% CI: −0.687 to −0.319, P = .000), 24 hours (average: 16.10 vs 18.40, SMD = −0.262, 95% CI: −0.443 to −0.080, P = .005), and 48 hours (average: 12.92 vs 15.12, SMD = −0.183, 95% CI: −0.364 to −0.002, P = .048).
Conclusion:
TENS could significantly reduce pain and opioid consumption after total knee arthroplasty. In addition, there were fewer adverse effects in the TENS groups. Higher quality RCTs are required for further research.
Keywords: meta-analysis, pain control, total knee arthroplasty, transcutaneous electrical nerve stimulation
1. Introduction
Total knee arthroplasty (TKA) has shown well-recognized efficacy in improving functional outcome and reliving pain for patients with knee osteoarthritis.[1] With the aging population, the number of TKAs is increasing. It has been estimated that more than 700,000 TKAs were performed in the United States in 2011 which predicted an increasing trend and requirement for the next few years.[2] However, a majority of patients would suffer a moderate to severe postoperative pain.[3] Effective pain control contributes to early ambulation and maintains motor function. In addition, the risk of thrombotic events and medical costs would be decreased under adequate analgesia. Multiple analgesic strategies have been applied including intravenous opioids, local infiltration analgesia, and peripheral nerve block.[4–6]
Recently, transcutaneous electrical nerve stimulation (TENS) has been proposed for pain management after TKA. It was a physical pain-relief modality based on the gate-control theory of pain and was considered a nonpharmacological, inexpensive, and safe analgesic method.[7] Fundamental research has reported that there were central and peripheral nervous system mechanisms underlying the analgesic action of TENS.[8,9] TENS stimulates the ventrolateral periaqueductal gray matter to activate opioid receptors in the rostroventromedial medulla which reduces the activity of nociceptive dorsal horn neurons of the spinal cord resulting in an analgesic effect.[10,11]
Although shown to be effective in some individual articles,[7,12] it has been unable to show a clear benefit. No meta-analysis has, to the best of our knowledge, investigated the effectiveness and safety of TENS in the setting of postoperative relief of pain after TKA. Thus, there is a lack of scientific evidence. Therefore, we performed a meta-analysis from randomized controlled trials (RCTs) to investigate the effectiveness of TENS, compared with “sham” (i.e., placebo) TENS, for pain control in TKA.
2. Methods
This meta-analysis was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.1. Search strategy
Potentially relevant studies were identified from electronic databases including Medline (1966 to June 2017), PubMed (1966 to June 2017), Embase (1980 to June 2017), ScienceDirect (1985 to June 2017), and the Cochrane Library. Searches included literature dated from database origin to June, 2017. The following key words were used on combination with Boolean operators AND or OR: “total knee replacement OR arthroplasty,” “transcutaneous electrical nerve stimulation,” and “pain control.” No restrictions were imposed on language. The bibliographies of retrieved trials and other relevant publications were cross-referenced to identify additional articles. The search process was performed as presented in Fig. 1.
Figure 1.
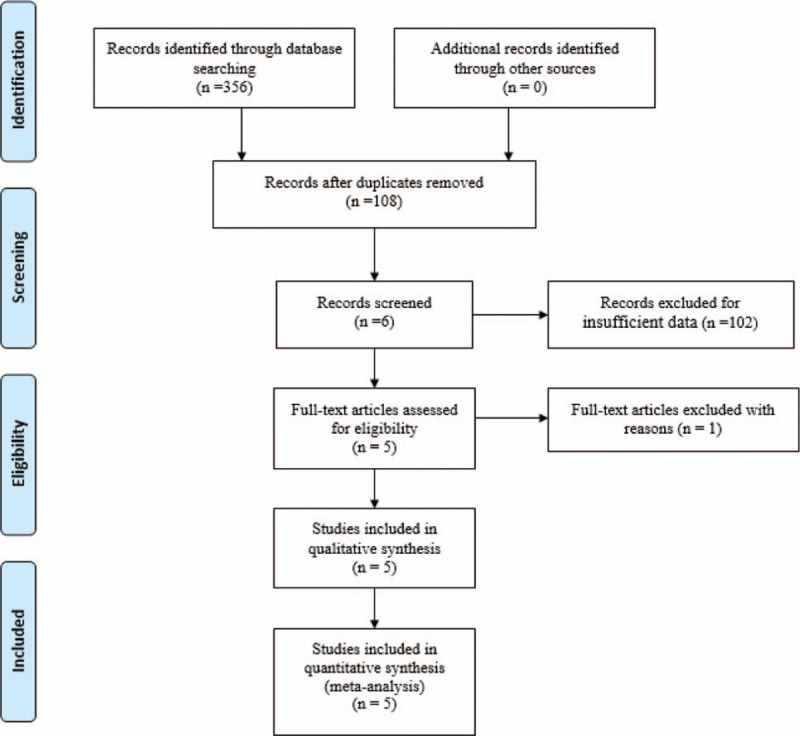
Search results and the selection procedure.
2.2. Inclusion and exclusion criteria
-
1.
Participants: only published articles enrolling adult participants that with a diagnosis of end-stage of knee osteoarthritis and prepared for unilateral TKA.
-
2.
Interventions: the intervention group received transcutaneous electrical nerve stimulation for postoperative pain management after TKA.
-
3.
Comparisons: the control group was received a nonfunctional placebo TENS that seemed to work but provided no stimulus.
-
4.
Outcomes: visual analogue scale (VAS) scores in different periods, opioids consumption, length of stay, and postoperative complications.
-
5.
Study design: only clinical RCTs were regarded as eligible in our study.
2.3. Selection criteria
Two reviewers independently scanned the abstracts of the potential articles identified by the above searches. Subsequently, the full text of the studies that met the inclusion criteria was screened, and a final decision was made. A senior author had the final decision in any case of disagreement regarding which studies to include.
2.4. Date extraction
Two of the authors independently extracted data from the included studies. The corresponding authors were consulted for details of data that were incomplete. The following data were extracted and recorded in a spreadsheet: first author names, publication year, samples size, baseline characteristics, intervention procedures, and outcome parameters. Other relevant data such as parameter of TENS and duration of follow-up were also extracted from individual studies. Primary outcomes were VAS scores and opioids consumption in different periods. Secondary outcomes were length of hospital stay and postoperative complications.
2.5. Quality assessment
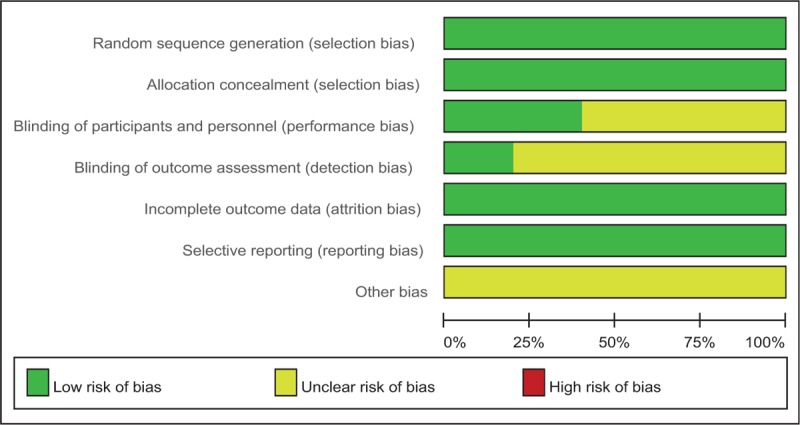
Quality assessment of the included RCTs was assessed by 2 authors independently who used the Cochrane Collaboration's tool. We conducted “risk of bias” table including the following key points: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting and other bias, each item was recorded by “Yes,” “No,” or “Unclear.” Each risk of bias item was presented as a percentage across all the included studies. The percentage indicated the proportion of different levels of risk of bias for each item.
The quality of the evidence for the main outcomes in present meta-analysis was evaluated using the Recommendations Assessment, Development and Evaluation (GRADE) system including the following items: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The recommendation level of evidence is classified into the following categories: high, which means that further research is unlikely to change confidence in the effect estimate; moderate, which means that further research is likely to significantly change confidence in the effect estimate but may change the estimate; low, which means that further research is likely to significantly change confidence in the effect estimate and to change the estimate; and very low, which means that any effect estimate is uncertain.
2.6. Data analysis and statistical methods
Pooling of data was carried out using Stata 11.0 software (The Cochrane Collaboration, Oxford, UK). Statistical heterogeneity was evaluated based on the value of P and I2 using standard χ2 test. When I2 >50%, P < .1 was considered to be significant heterogeneity, the random-effect model was used for meta-analysis. Otherwise, the fixed-effect model was performed. Sensibility analysis is conducted to assess the origins of heterogeneity. The results of dichotomous outcomes (postoperative complications) were expressed as risk difference (RD) with 95% confidence intervals (CIs). For continuous various outcomes (VAS scores, opioids consumption, and length of stay), mean difference or standard mean difference (SMD) with 95% CIs was applied for the assessment. Power analysis for each outcome was also calculated by Power and Precision software.
3. Results
3.1. Search result
A total of 356 studies were identified through the initial search. By scanning the abstracts, 351 reports that did not meet inclusion criteria were excluded from the current meta-analysis. No gray literature was included. Finally, 5 RCTs[13–17] published between 2004 and 2017 were included in the present meta-analysis. These studies included 237 patients in the experimental groups and 235 patients in the control groups.
3.2. Study characteristics
Demographic characteristics, the details about the included studies are summarized in Table 1. The sample size of the included studies ranged from 47 to 246 and the mean age ranged from 75.4 to 67.1. All of them evaluate the analgesic efficiency of TENS in patients undergoing TKA. Four studies performed general anesthesia and 1 paper applied spinal anesthesia. All the articles showed that TKAs were performed by the same teams. All the participants received narcotic medications as an adjunct concomitant pain management. All of them suggest the outcomes for at least 95% of the patients. The follow-up period ranged from 1 to 3 months. The quality assessment of the included studies can be seen in detail in Table 2. Each risk of bias item is presented as the percentage across all the included studies, which indicates the proportion of different levels of risk of bias for each item (Table 3).
Table 1.
Trials characteristics.
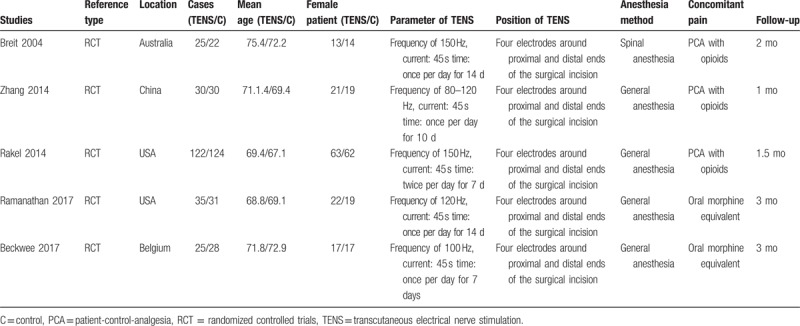
Table 2.
Methodological quality of the randomized controlled trials.
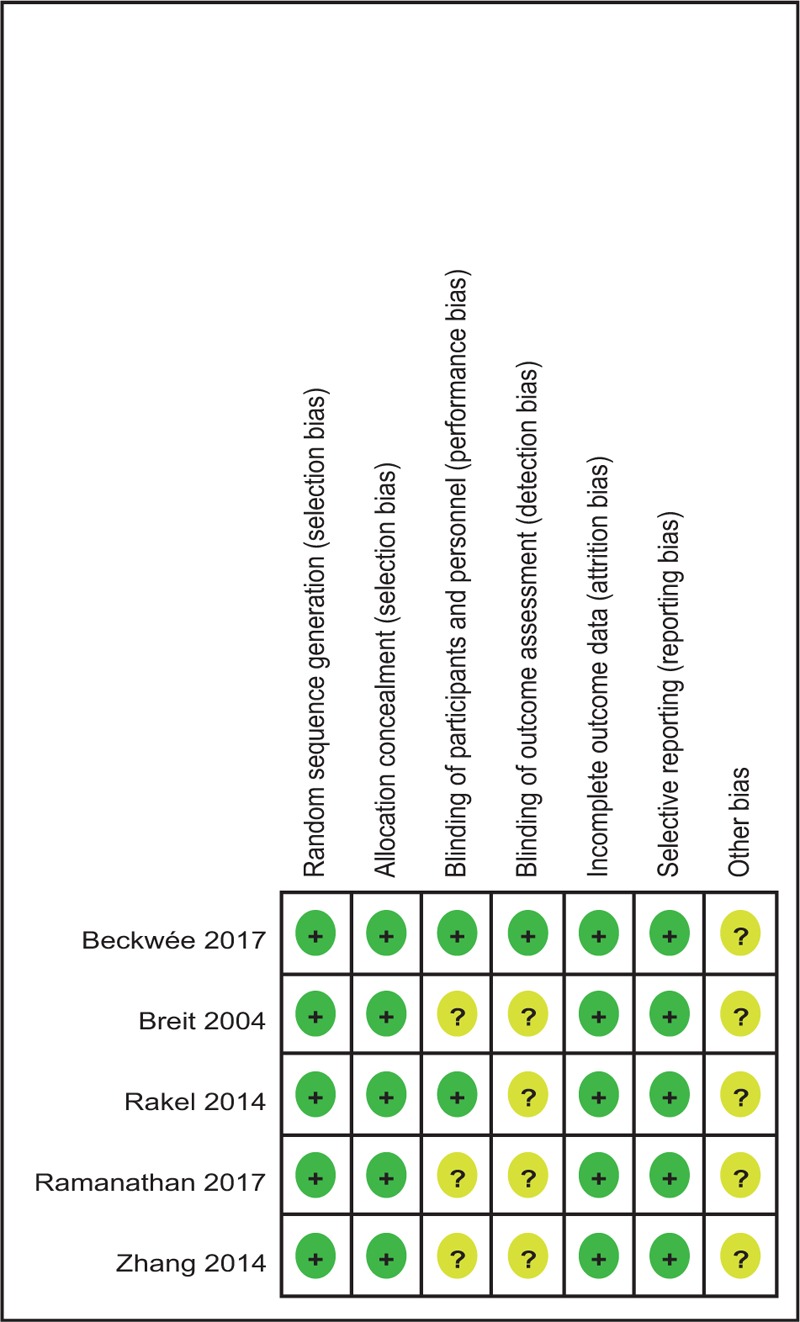
Table 3.
Risk of bias.
3.3. Outcomes for meta-analysis
3.3.1. VAS scores at 12 hours
Five articles[13–17] showed the outcomes of VAS scores at 12 hours after TKA. A fixed-effects model was used because no significant heterogeneity was found among the studies (average: 3.58 vs 4.34, χ2 = 6.68, df = 4, I2 = 40.1%, P = .154). The pooled results demonstrated that significant difference in VAS scores at 12 hours was found between 2 groups (SMD = −0.260, 95% CI: −0.442 to −0.078, P = .005, power = 87.6%; Fig. 2).
Figure 2.
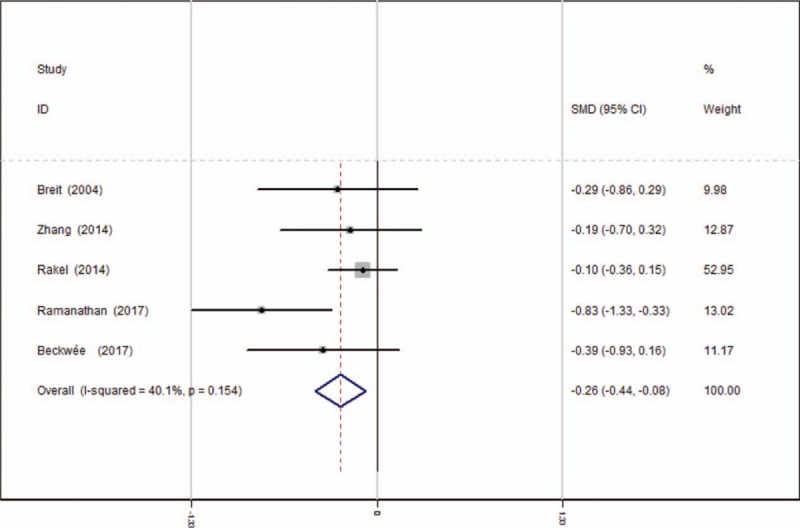
Forest plot diagram showing VAS scores at 12 hours following TKA. TKA = total knee arthroplasty, VAS = visual analogue scale.
3.3.2. VAS scores at 24 hours
Five studies[13–17] reported the outcomes of VAS scores at 24 hours after TKA. A fixed-effects model was used because no significant heterogeneity was found among the studies (average: 3.18 vs 3.52, χ2 = 1.43, df = 4, I2 = 0%, P = .838). The pooled results demonstrated that there was significant difference in VAS scores at 24 hours between groups (SMD = −0.244, 95% CI: −0.426 to −0.063, P = .008, power = 90.3%; Fig. 3).
Figure 3.
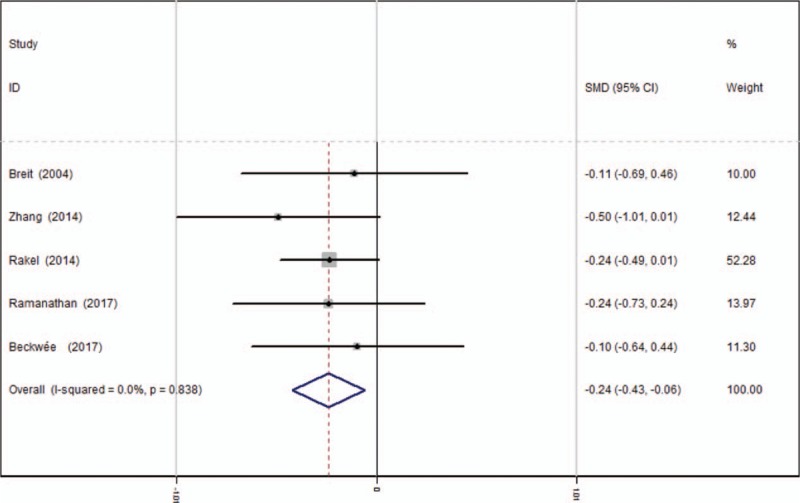
Forest plot diagram showing VAS scores at 24 hours following TKA. TKA = total knee arthroplasty, VAS = visual analogue scale.
3.3.3. VAS scores at 48 hours
Five studies[13–17] reported the outcomes of VAS scores at 48 hours after TKA. A fixed-effects model was used because no significant heterogeneity existed among these studies (average: 2.70 vs 2.96, χ2 = 1.08, df = 4, I2 = 0%, P = .897). The pooled results demonstrated that significant difference in VAS scores at 48 hours was identified between groups (SMD = −0.214, 95% CI: −0.395 to −0.033, P = .021, power = 89.1%; Fig. 4).
Figure 4.
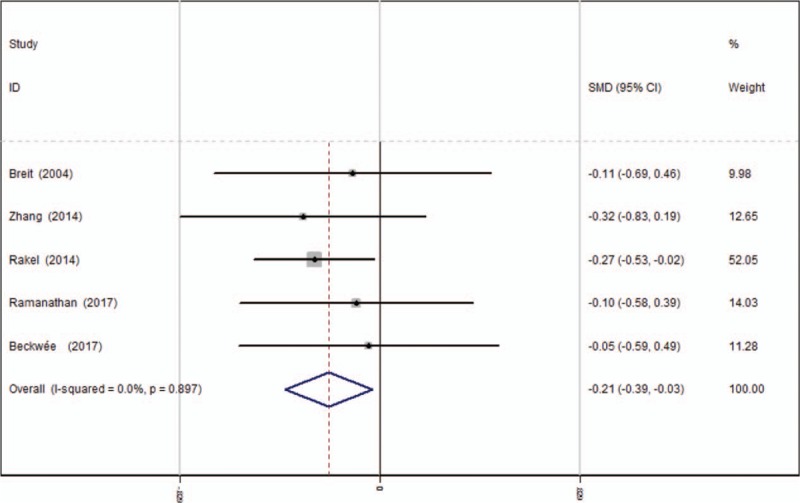
Forest plot diagram showing VAS scores at 48 hours following TKA. TKA = total knee arthroplasty, VAS = visual analogue scale.
3.3.4. Opioids consumption at 12 hours
Opioids consumption at 12 hours after TKA was reported in 5 articles.[13–17] A fixed-effects model was applied because no significant heterogeneity was found among these studies (average: 14.44 vs 18.54, χ2 = 6.71, df = 4, I2 = 40.3%, P = .152). Significant difference was detected in opioids consumption at 12 hours between the 2 groups (SMD = −0.503, 95% CI: −0.687 to −0.319, P = .000, power = 92.2%; Fig. 5).
Figure 5.
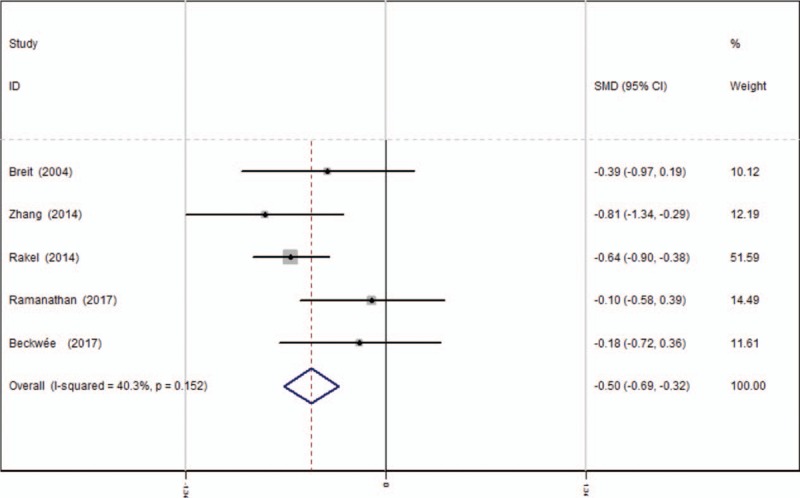
Forest plot diagram showing opioid consumption at 12 hours following TKA. TKA = total knee arthroplasty.
3.3.5. Opioids consumption at 24 hours
Opioids consumption at 24 hours after TKA was provided in 5 studies.[13–17] A fixed-effects model was used because no significant heterogeneity was found among these studies (average: 16.10 vs 18.40, χ2 = 1.46, df = 4, I2 = 0%, P = .834). The pooled results demonstrated that there was significant difference in opioids consumption at 24 hours between groups (SMD = −0.262, 95% CI: −0.443 to −0.080, P = .005, power = 90.8%; Fig. 6).
Figure 6.
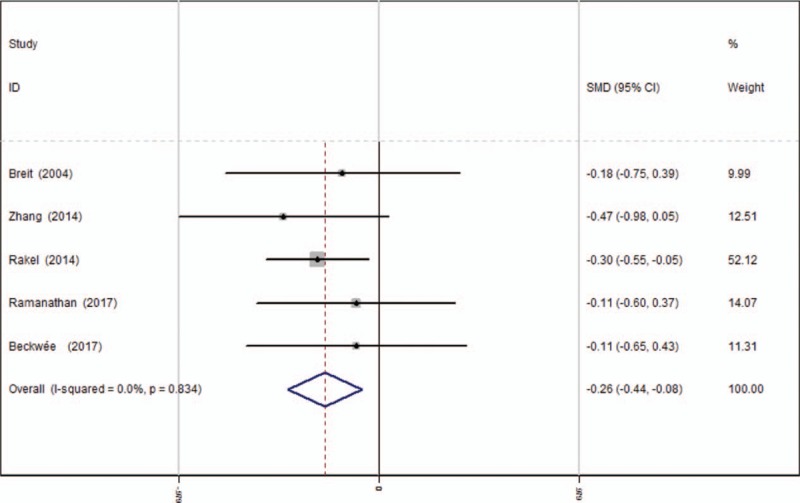
Forest plot diagram showing opioid consumption at 24 hours following TKA. TKA = total knee arthroplasty.
3.3.6. Opioids consumption at 48 hours
Five articles[13–17] reported the outcomes of opioids consumption at 48 hours after TKA. A fixed-effects model was used because no significant heterogeneity was found among the pooled data (average: 12.92 vs 15.12, χ2 = 1.20, df = 4, I2 = 0%, P = .879). Significance difference in opioids consumption at 48 hours was observed between the 2 groups. (SMD = −0.183, 95% CI: −0.364 to −0.002, P = .048, power = 91.7%; Fig. 7).
Figure 7.
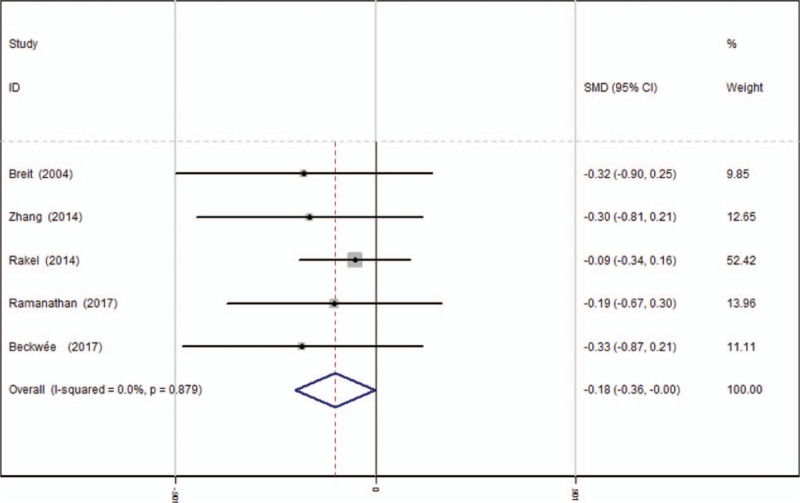
Forest plot diagram showing opioid consumption at 48 hours following TKA. TKA = total knee arthroplasty.
3.3.7. Length of hospital stay
Five studies[13–17] reported the length of hospital stay for the groups. A random-effects model was used because significant heterogeneity was identified in the pooled results (average: 3.24 vs 3.26, χ2 = 25.56, df = 4, I2 = 84.4%, P = .000). No significant difference in the length of hospital stay was observed between the 2 groups (SMD = 0.178, 95% CI: −0.005 to 0.362, P = .057, power = 96.6%; Fig. 8).
Figure 8.
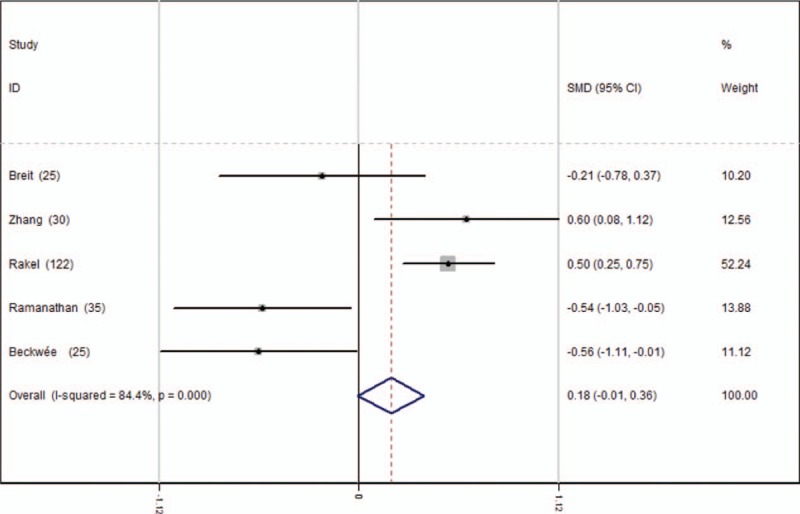
Forest plot diagram showing length of stay following TKA. TKA = total knee arthroplasty.
3.3.8. Nausea
Five studies[13–17] reported the postoperative complications of nausea. A fixed-effects model was used because no significant heterogeneity was found among these studies (χ2 = 0.38, df = 4, I2 = 0%, P = .984). Significant difference in the incidence of nausea was found between the 2 groups (RD = −0.098, 95% CI: −0.180 to −0.016, P = .020, power = 85.0%; Fig. 9).
Figure 9.
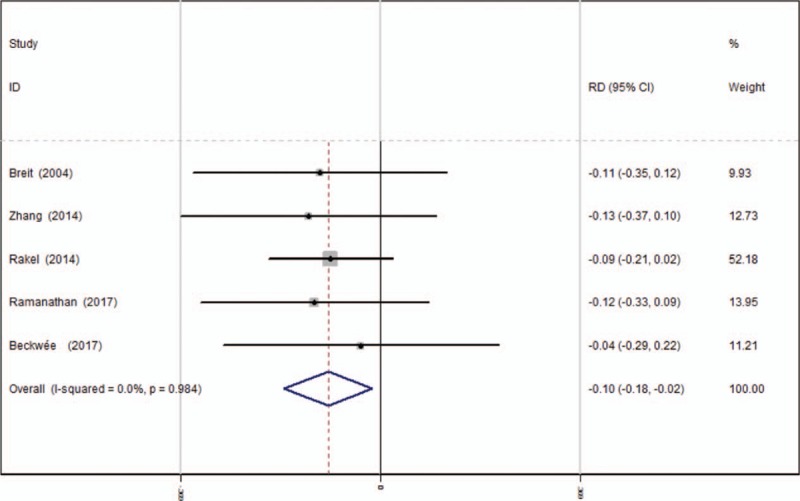
Forest plot diagram showing incidence of nausea following TKA. TKA = total knee arthroplasty.
3.3.9. Vomiting
Five articles[13–17] reported the postoperative complications of vomiting following TKA. A fixed-effects model was used due to the low significant heterogeneity among these studies (χ2 = 0.62, df = 4, I2 = 0%, P = .961). Significant difference was found in terms of the incidence of vomiting between the groups (RD = −0.094, 95% CI: −0.173 to −0.016, P = .018, power = 92.2%; Fig. 10).
Figure 10.
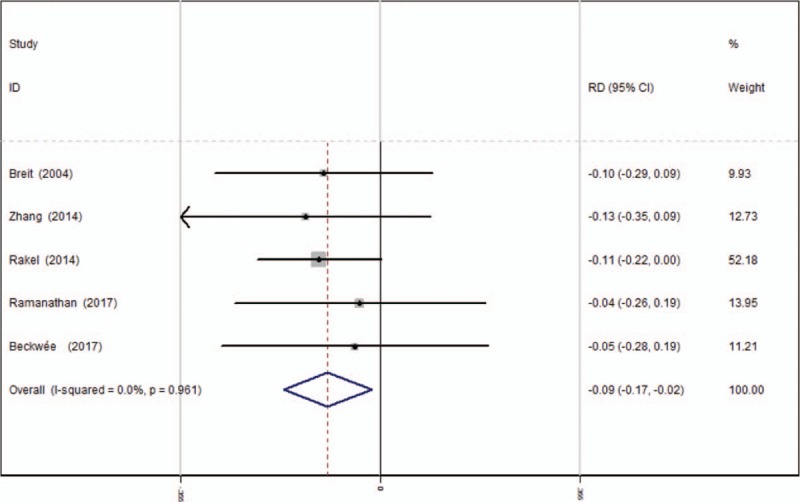
Forest plot diagram showing incidence of vomiting following TKA. TKA = total knee arthroplasty.
4. Discussion
To the best of our knowledge, this is the first meta-analysis to evaluate the efficiency and safety of transcutaneous electrical nerve stimulation for pain control in total knee arthroplasty. The most important finding of the present meta-analysis was that the application of TENS could significantly reduce the VAS scores and opioid consumption within the first 48 hours after TKA. Moreover, there is a decreased risk of nausea and vomiting in experimental groups compared with control groups. All the outcomes in this meta-analysis were evaluated using the GRADE system. The evidence quality for each outcome was high (Table 4) which means that further research is unlikely to change confidence in the effect estimate.
Table 4.
The GRADE evidence quality for main outcome.
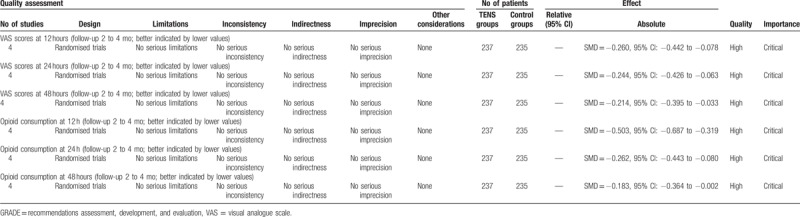
With the aging population, the incidence of knee osteoarthritis is increasing. TKA is an excellent surgical procedure for patients with painful arthritic knees. However, TKA was usually associated with moderate to severe postoperative pain. Postoperative pain after TKA is usually intense, and immediate postoperative opioid consumption can be high. Consensus has been reached that effective postoperative analgesia improved patient outcomes by allowing early ambulation and rehabilitation. The optimal analgesic strategy remains controversial and pain control after TKA is an interesting topic in the field of orthopedic surgery. Multimodal pain management following TKA was recommended to improve pain relief and reduce opioid consumption.[18,19]
Usually, TENS was applied to treat chronic pain syndrome in previous studies. Recently, TENS was used for the treatment of acute pain, such as postoperative situation, and provided positive results. TENS has recognized as an alternative treatment for pain control. Two or more electrodes were connected to the skin. Nerves were stimulated by electric current that was created by a device to provide analgesia efficacy.[20] It was believed that pain relief was achieved by the gate control theory of pain. Interneurons was activated by non-nociceptive afferent that inhibits the biologic activity of nociceptive projection neurons.[21] Previous studies have reported that TENS was effective and safe for treating chronic pain syndrome,[22,23] however, TENS still remains controversial in reducing acute pain. Although recent studies reported the use of TENS in the setting of postoperative pain after TKA, the sample size of the current study was small, and the conclusion remains controversial. Thus, we performed the present meta-analysis from published RCTs to assess the utility of TENS. A VAS score was used for the measurement of postoperative pain. The present meta-analysis indicated that the application of TENS could significantly reduce VAS scores at 12 hours (SMD = −0.260, 95% CI: −0.442 to −0.078, P = .005), 24 hours (SMD = −0.244, 95% CI: −0.426 to −0.063, P = .008), and 48 hours (SMD = −0.214, 95% CI: −0.395 to −0.033, P = .021) after TKA.
It was reported that there was approximately 30% to 60% of patients who suffered moderate to severe pain in the first 2 days following TKA. Additional opioids were used as an adjunct to concomitant pain control. The personal control aspect of patient-control-analgesia (PCA) and the rapid onset were preferred by patients. Numerous articles have reported that the application of the PCA device was associated with a high level of patient's satisfaction.[24,25] In our study, opioid consumption was considered an objective means to measure pain. Morphine-related adverse effects including nausea, vomiting, respiratory depression, and pruritus were well known and drew our attention.[26,27] Besides the side effects, drug dependence should be considered, as it is also an important issue that is related to opioid administration. It was crucial to minimize the opioid consumption for patients and improve their recovery and satisfaction. However, the clinical use of TENS after TKA for reducing opioid consumption was seldom reported. Meta-analysis can strengthen statistical power and enlarge sample size by pooling results of published studies, which could point out stronger evidence. Five high-quality RCTs were included in our meta-analysis. The present meta-analysis indicated that the application of TENS could significantly reduce opioid consumption at 12 hours (SMD = −0.503, 95% CI: −0.687 to −0.319, P = .000), 24 hours (SMD = −0.262, 95% CI: −0.443 to −0.080, P = .005), and 48 hours (SMD = −0.183, 95% CI: −0.364 to −0.002, P = .048) after TKA.
Analgesia efficacy is not the only concern when evaluating the analgesic effect of TENS. Nausea and vomiting are known adverse effects that are frequently associated with PCA opioids. Decreased morphine consumption can subsequently and effectively avoid such complications. The overall incidence of nausea was 59/237 in the TENS groups compared with 82/235 in the control groups (P < .05). The use of TENS could significantly decrease the risk of postoperative complications. Due to the small number of included articles, large sample sizes of high-quality RCTs are further needed.
Several potential limitations of this study should be noted. Only 5 RCTs were included, and the sample size was relatively small. Some important outcome parameters such as range of motion were not fully described and could not be included in the meta-analysis. The methods of blinding were unclear or not described in some included studies which may influence our result. Short-term follow-up may lead to the underestimation of complications. Publication bias is an inherent weakness that exists in all meta-analyses.
Despite the limitations above, this is the first meta-analysis from RCTs to evaluate the efficiency and safety of TENS for pain control in patients undergoing TKA. Higher quality RCTs are required for further research.
5. Conclusion
TENS could significantly reduce pain and opioid consumption after total knee arthroplasty. In addition, there were fewer adverse effects in the TENS groups. Higher quality RCTs are required for further research.
Footnotes
Abbreviations: LOS = length of stay, RCT = randomized controlled trials, TSA = total shoulder arthroplasty, VAS = visual analogue scale.
JL and YS conceived the design of the study. YS performed and collected the data and contributed to the design of the study. JL finished the manuscript. All authors read and approved the final manuscript.
JFL and YZS contributed equally to the study.
The authors have no conflicts of interest to disclose.
References
- [1].Yan CH, Chiu KY, Ng FY. Total knee arthroplasty for primary knee osteoarthritis: changing pattern over the past 10 years. Hong Kong Med J 2011;17:20–5. [PubMed] [Google Scholar]
- [2].Hadzic A, Houle TT, Capdevila X, et al. Femoral nerve block for analgesia in patients having knee arthroplasty. Anesthesiology 2010;113:1014–5. [DOI] [PubMed] [Google Scholar]
- [3].Singelyn FJ, Ferrant T, Malisse MF, et al. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous femoral nerve sheath block on rehabilitation after unilateral total-hip arthroplasty. Reg Anesth Pain Med 2005;30:452–7. [DOI] [PubMed] [Google Scholar]
- [4].Bendtsen TF, Moriggl B, Chan V, et al. The optimal analgesic block for total knee arthroplasty. Reg Anesth Pain Med 2016;41:711–9. [DOI] [PubMed] [Google Scholar]
- [5].McCartney CJ, McLeod GA. Local infiltration analgesia for total knee arthroplasty. Br J Anaesth 2011;107:487–9. [DOI] [PubMed] [Google Scholar]
- [6].Wang F, Zhou Y, Sun J, et al. Influences of continuous femoral nerve block on knee function and quality of life in patients following total knee arthroplasty. Int J Clin Exp Med 2015;8:19120–5. [PMC free article] [PubMed] [Google Scholar]
- [7].DeSantana JM, Walsh DM, Vance C, et al. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep 2008;10:492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barnett DC, Hair B. Use and effectiveness of transcutaneous electrical nerve stimulation in pain management. J Neurosurg Nurs 1981;13:323–5. [DOI] [PubMed] [Google Scholar]
- [9].Bilgili A, Cakir T, Dogan SK, et al. The effectiveness of transcutaneous electrical nerve stimulation in the management of patients with complex regional pain syndrome: a randomized, double-blinded, placebo-controlled prospective study. J Back Musculoskelet Rehabil 2016;29:661–71. [DOI] [PubMed] [Google Scholar]
- [10].DeSantana JM, Da Silva LF, De Resende MA, et al. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 2009;163:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tashani O, Johnson M. Transcutaneous electrical nerve stimulation (TENS) a possible aid for pain relief in developing countries? Libyan J Med 2009;4:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lison JF, Amer-Cuenca JJ, Piquer-Marti S, et al. Transcutaneous nerve stimulation for pain relief during office hysteroscopy: a randomized controlled trial. Obstet Gynecol 2017;129:363–70. [DOI] [PubMed] [Google Scholar]
- [13].Beckwee D, Bautmans I, Lefeber N, et al. Effect of transcutaneous electric nerve stimulation on pain after total knee arthroplasty: a blind randomized controlled trial. J Knee Surg 2017;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [14].Breit R, Van der Wall H. Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty. J Arthroplasty 2004;19:45–8. [DOI] [PubMed] [Google Scholar]
- [15].Rakel BA, Zimmerman MB, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo-controlled trial. Pain 2014;155:2599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ramanathan D, Saleh A, Klika AK, et al. The use of transcutaneous electrical nerve stimulation after total knee arthroplasty: a prospective randomized controlled trial. Surg Technol Int 2017;30:425–34. [PubMed] [Google Scholar]
- [17].Zhang Q, Zhang JH, Tong PJ. [Application of transcutaneous electrical nerve stimulation to multimodal analgesia after total knee arthroplasty]. Zhongguo Gu Shang 2014;27:283–6. [PubMed] [Google Scholar]
- [18].Maheshwari AV, Blum YC, Shekhar L, et al. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin Orthop Relat Res 2009;467:1418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meftah M, Wong AC, Nawabi DH, et al. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics 2012;35:e660–4. [DOI] [PubMed] [Google Scholar]
- [20].Moeller Joensson I, Hagstroem S, Siggaard C, et al. Transcutaneous electrical nerve stimulation increases rectal activity in children. J Pediatr Gastroenterol Nutr 2015;61:80–4. [DOI] [PubMed] [Google Scholar]
- [21].Johnson MI, Mulvey MR, Bagnall AM. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database Syst Rev 2015;8:CD007264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cheing GL, Hui-Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch Phys Med Rehabil 1999;80:305–12. [DOI] [PubMed] [Google Scholar]
- [23].Jauregui JJ, Cherian JJ, Gwam CU, et al. A meta-analysis of transcutaneous electrical nerve stimulation for chronic low back pain. Surg Technol Int 2016;28:296–302. [PubMed] [Google Scholar]
- [24].Hida T, Yukawa Y, Ito K, et al. Intrathecal morphine for postoperative pain control after laminoplasty in patients with cervical spondylotic myelopathy. J Orthop Sci 2016;21:425–30. [DOI] [PubMed] [Google Scholar]
- [25].Kunopart M, Chanthong P, Thongpolswat N, et al. Effects of single shot femoral nerve block combined with intrathecal morphine for postoperative analgesia: a randomized, controlled, dose-ranging study after total knee arthroplasty. J Med Assoc Thai 2014;97:195–202. [PubMed] [Google Scholar]
- [26].Bonnet MP, Mignon A, Mazoit JX, et al. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain 2010;14:894.e1–9. [DOI] [PubMed] [Google Scholar]
- [27].Tassinari D, Sartori S, Tamburini E, et al. Adverse effects of transdermal opiates treating moderate-severe cancer pain in comparison to long-acting morphine: a meta-analysis and systematic review of the literature. J Palliat Med 2008;11:492–501. [DOI] [PubMed] [Google Scholar]


