Abstract
Bacterial infections are an important cause of mortality in liver failure. However, the type of infection, predictors of infection, and their impact on outcomes in patients with acute-on-chronic liver failure (ACLF) are limited.
A total of 389 patients with ACLF were admitted in this retrospective, corhort study. Once admitted, clinical data including first infection site, type (community-acquired, healthcare-associated, or nosocomial), and second infection occurrence during hospitalization were collected. The outcome was mortality within 90 days. Multivariable logistic regression models were preformed to predict second infection development and 90-day mortality. Survival probability curves were calculated by the Kaplan–Meier method.
Among 389 patients, 316 (81.2%) patients had infection. The 90-day mortality of patients with and without infection was 52.2% and 16.4%, respectively (P <.001). The most common first infection was healthcare associated (51.3%), followed by nosocomial (30.1%) and community-acquired infections (18.7%). Respiratory tract infection, spontaneous bacterial peritonitis, and urinary tract infection were most prevalent. Gram-positive organism was more frequently seen than gram-negative organisms. Of note, fungi accounted for 15.9% of the total infection cases. During hospitalization, 26.6% patients developed second infections. The 90-day mortality of patients developed or did not develop a second infection were 67.9% and 46.6%, respectively (P <.001). Independent predictors of 90-day mortality in infected patients with ACLF were age, white blood cell (WBC) count, model for end-stage liver disease (MELD) score, hepatic encephalopathy (HE), and second infection.
Infections (regardless of first or second infection) can increase the 90-day mortality significantly in patients with ACLF. And age, WBC count, MELD score, HE, and the presence of second infection are independent risk factors affecting 90-day mortality in patients with ACLF showing infection.
Keywords: bacterial infection, liver failure, nosocomial infection, outcome
1. Introduction
Infections are a common and very serious complication associated with advanced liver disease. Reportedly, the rate of bacterial infection is ∼7% in the general hospital population and 40% among patients hospitalized with cirrhosis.[1,2] Owing to impaired immunity and bacterial translocation from the intestine, patients with cirrhosis show an increased susceptibility to infection.[3] An exaggerated inflammatory response triggered by infection can cause multiorgan failure and death. Despite advancements in critical care and prophylactic antibiotic use to prevent the occurrence of infection, infected patients have a high risk of death.[4,5] Patients diagnosed with cirrhosis with concomitant infections demonstrate a 4-fold increase in mortality rate; 30% of such patients die within a month following infection and another 30% within a year.[6]
Infection distinctively determines prognosis of cirrhosis and affects survival regardless of disease severity even after recovery from the infective episode.[7] Infection leads to accelerated deterioration of liver function and is the most common identifiable extrahepatic trigger of acute-on-chronic liver failure (ACLF), which is characterized by organ failure and extremely poor survival (28-day mortality rate 30–40%).[8,9] Systemic inflammation triggered by infection may cause ACLF through complex mechanisms including an exaggerated inflammatory response and systemic oxidative stress via pathogen-or danger/damage-associated molecular patterns and/or alteration of tissue homeostasis as a consequence of inflammation.[10] These features may induce tissue damage (cell dysfunction, apoptosis, or necrosis), organ failure, and even death. Thus, understanding characteristics of infection in patients with ACLF is important.
Recently, several studies have shown that the manner in which infections are acquired (community-acquired [CA] vs healthcare-associated [HCA] vs nosocomial) affect morbidity and mortality in patients with cirrhosis.[11] Second infections independently increase mortality in patients hospitalized with cirrhosis.[12] The rising prevalence of infections caused by multidrug-resistant (MDR) bacteria is an increasing concern because the emergence of such MDR strains of bacteria is associated with increased failure rates of standard empirical antibiotic therapy used to treat infections and a higher risk of death.[13,14] However, a potential relationship between the infection site, type, predictors of infection, and their impact on outcomes in patients with ACLF remain poorly defined.
2. Patients and methods
2.1. Patient selection
A flow chart explaining the patient selection process is presented in Fig. 1. We retrospectively reviewed data obtained from 492 patients hospitalized with cirrhosis and diagnosed with ACLF between March 2008 and July 2014 at Tianjin Third Central Hospital. Among these, 389 patients were recruited in this study. ACLF was defined as acute deterioration of liver function manifesting as jaundice (total bilirubin [TBIL] ≥5 mg/dL or ≥85 μmol/L) and coagulopathy with international normalized ratio of prothrombin time ([INR] ≥1.5 or prothrombin activity [PTA] ≤40%) complicated with ascites and/or hepatic encephalopathy noted within 4 weeks in a patient diagnosed with cirrhosis.[15] Cirrhosis was diagnosed based on a combination of biochemical, radiological, and endoscopic findings if a liver biopsy confirmation was not available. We excluded 103 patients based on the following criteria: those who presented with hepatocellular carcinoma and nonhepatic neoplasia; immunocompromised patients with human immunodeficiency virus infection; those with severe chronic extra-hepatic disease; and those with a hospital stay <48 hours.
Figure 1.
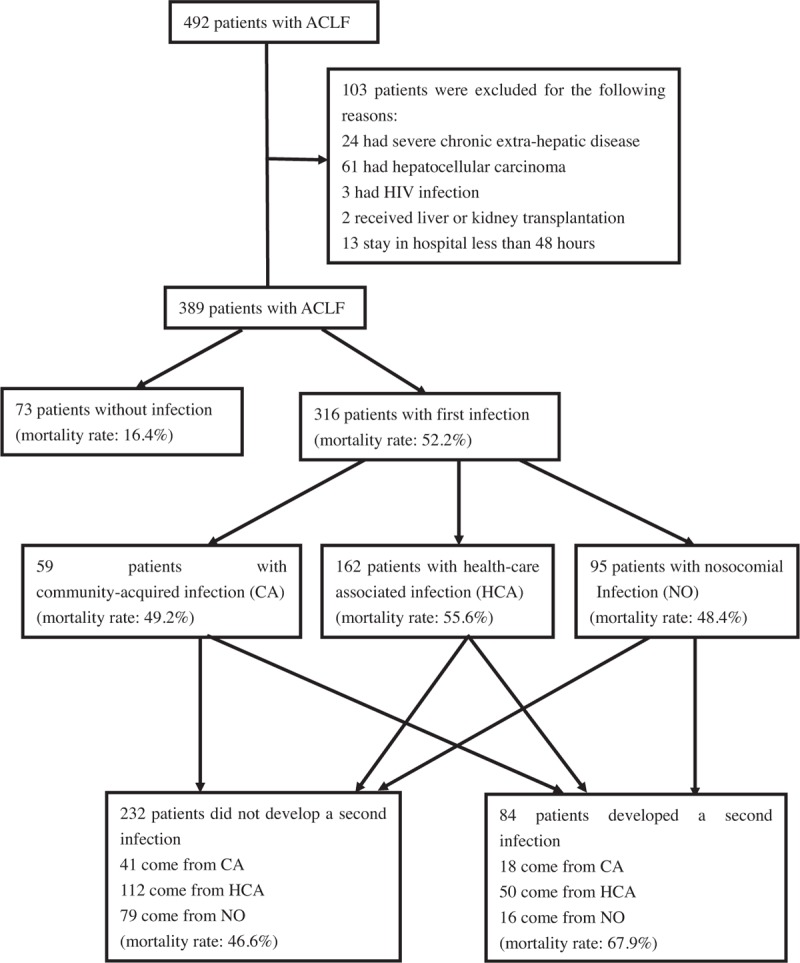
Flow diagram of the study groups selection process. ACLF, acute-on-chronic liver failure.
All patients were hospitalized and received standard supportive treatment. Patients with infections were treated immediately with empiric administration of broad-spectrum antibiotics, based on the site of infection, standard guidelines, and local epidemiology. Antibiotic treatment was later modified based on results of cultures (if available) and in cases showing treatment failure.
Following admission, data were obtained pertaining to patient demographics, vital signs, baseline values of a complete blood count, biochemistry, liver and renal function, and details regarding the infection including medical treatment, type of first infection as well as the development of a second infection. No patients underwent liver transplantation, and no patients were lost to follow-up within the 90-day follow-up period.
2.2. Definitions
Infections were defined as: spontaneous bacteremia: positive blood cultures without a known source of infection; spontaneous bacterial peritonitis (SBP): ascitic fluid showing polymorphonuclear cells >250/uL with/without a positive fluid culture; lower respiratory tract infections: new pulmonary infiltrate in the presence of: at least 1 respiratory symptom such as cough, sputum production, dyspnea, or pleuritic pain with at least 1 finding on auscultation (rales or crepitations), or 1 sign of infection (core body temperature >38°C or <36°C with shivering or leukocyte count >10,000/mm3 or <4000/mm3) in the absence of antibiotics use; infectious diarrhea: diarrhea with one of the following features: a routine stool examination revealing white blood cells; a stool culture positive for Salmonella, Shigella, Yersinia, Campylobacter, or pathogenic Escherichia coli; a positive Clostridium difficile assay; soft-tissue/skin infection: fever with cellulitis; urinary tract infection (UTI): urine white blood cells (WBC) >15/high power field with positive urine Gram stain or culture in a symptomatic patient; intra-abdominal infections: diverticulitis, appendicitis, cholangitis, among others. Other infections: not mentioned above, and fungal infections: classified as a special category.
First infections were defined as CA if they were diagnosed within 48 hours of admission without hospitalizations in the previous 6 months; HCA if they were diagnosed within 48 hours of admission in patients requiring hospitalization for at least 2 days in the previous 6 months; and nosocomial if the infection was diagnosed beyond 48 hours of admission. Second infections were defined as an infection separate from and following the first infection during the same hospitalization.
Hyponatremia was defined as a serum sodium level <130 mmol/L.[16]
The model for end-stage liver disease (MELD) score was calculated using the Malinchoc formula: MELD score = 3.78 × loge [bilirubin (mg/dL) + 11.2 × loge (INR) + 9.57 × loge [creatinine (mg/dL)] + 6.43 × (etiology: 0 if cholestatic or alcoholic, 1 otherwise). [17]
Organ failure was defined based on the chronic liver failure-sequential organ failure assessment (CLIF-SOFA) score created by the European Associated for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium which includes subscores ranging from 0 to 4 for each of the 6 components (liver, kidney, brain, coagulation, circulation, and lungs).[9]
2.3. Ethics statement
The study was performed through chart reviews in adherence with the principle of the Declaration of Helsinki and approved by the Institutional Ethical Committee of Tianjin Third Central Hospital. Due to the retrospective nature of the study, written informed consent could not be obtained from all patients. All data were identified before analysis.
2.4. Statistical analysis
Normally distributed variables were expressed as mean ± SD, and non-normally distributed variables were expressed as a median and interquartile range (IQR). Count and percentages were used to describe categorical variables. Two independent groups were compared using the t-test for continuous normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. If more than 2 groups were compared, we used the one-way analysis of variance and Kruskal–Wallis tests, respectively. For categorical variables, comparisons among groups were made using the χ2 tests or Fisher test as appropriate. The Kaplan–Meier method was used to calculate the 90-day survival probability curves, which were compared with the log-rank test. Multivariate logistic regression analyses, with backward elimination, were used to arrive at a parsimonious model to determine predictors of a second infection. Variables analyzed were age, male, presence/absence of diabetes, use of medications, etiology of cirrhosis, and various parameters assessed at the time of admission including mean arterial pressure, heart rate, INR, TBIL, WBC, platelets (PLT), serum albumin (ALB), serum sodium, creatinine, the MELD score, and complications associated with cirrhosis. The resulting model was then pared down by eliminating, one by one, covariates that were not significant at the .05 level, and a final model was obtained where all covariates significant at the .05 level were identified. Similarly, a multivariate logistic regression model, with backward elimination, was used to arrive at a parsimonious model to determine predictors of death. Variables analyzed were the same as those used for prediction of second infection; however, length of hospital stay and second infection were added.
Two-sided P values of <.05 were considered being statistically significant. All statistical analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL) and figures drawn by GraphPad Prism 5.
3. Results
3.1. Patient characteristics
We analyzed 389 patients with ACLF among which 283 (73.8%) were males, and the mean age of patients was 51.6 ± 11.9 years. The most common etiology of cirrhosis was hepatitis B (53.7%) and alcohol-induced cirrhosis (24.7%). Of note, 24.2%, 44.2%, 64.0%, and 6.2% patients received antibiotics for SBP prophylaxis, lactulose, proton pump inhibitors (PPIs), and beta-blocker therapy, respectively. During hospitalization, 84.1% patients developed ascites, and 24.9%, 35.2%, 56.6% patients had complications such as gastrointestinal bleeding, hepatic encephalopathy, and hyponatremia, respectively (Table 1).
Table 1.
Baseline and characteristics of patients with and without infection.
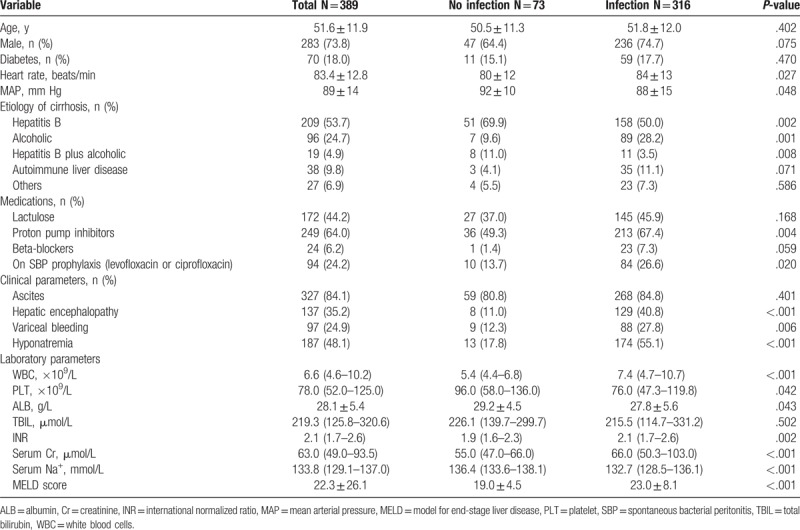
Among 389 patients with ACLF, 316 (81.2%) patients presented with infection. Characteristics of patients with and without infection are shown in Table 1. Complications of cirrhosis such as gastrointestinal bleeding and hepatic encephalopathy were more frequently seen in patients with infection. Additionally, patients with infection showed higher WBC, INR, serum creatinine (Cr), serum sodium values, and a higher MELD score than those without infection.
3.2. First infections
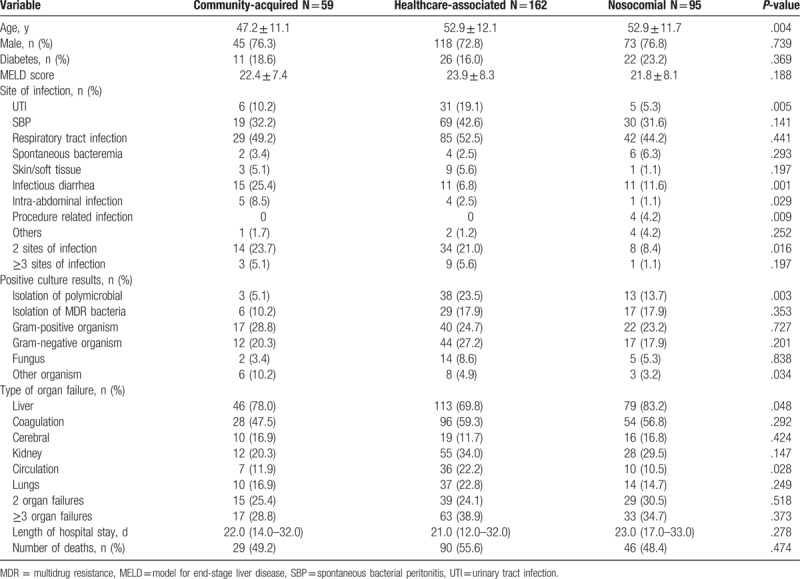
The most common first infection was observed to be respiratory tract infection 156 (49.4%), followed by SBP 118 (37.3%), UTI 42 (13.3%), and infectious diarrhea 37 (11.7%). We found that 247 (78.2%) patients presented with infection at a single site, whereas 69 (21.8%) patients showed >1 site of infection. It was seen that 59 (18.7%) patients had CA, 162 (51.3%) had HCA, and 95 (30.1%) had nosocomial infections. Thus, HCA and nosocomial infection accounted for 81.4% of all cases of infection. The most frequent nosocomial infection was respiratory tract infection (n = 42), followed by SBP (n = 30) and infectious diarrhea (n = 11).
Due to the retrospective nature of the study, culture results were available for 167 (52.8%) patients, and 111/167 (66.5%) patients showed a positive culture. In some cases, multiple sites of infection were present, or multiple bacteria were isolated in the same patient, thereby providing 132 positive cultures. The majority of infectious isolates were gram-positive bacteria 79 (59.8%), followed by gram-negative bacteria 73 (55.3%), fungi 21 (15.9%), and others such as mycoplasma 17 (12.9%). Gram-positive organisms were Staphylococcus (n = 36), Enterococcus (n = 29), Streptococcus (n = 14), and Crytococcus (n = 1). Among gram-negative infection-causing agents, E. coli (n = 28) was the most frequent organism, followed by Klebsiella (n = 17), Acinetobacter baumannii (n = 14), Pseudomonas aeruginosa (n = 6), Enterobacter cloacae (n = 6), Stenotrophomonas maltophilia (n = 1), and Pseudomonas fluorescens (n = 1). Extended spectrum beta-lactamase, (ESBL) resistant strain (n = 23), was the most frequently isolated MDR strain, followed by methicillin-resistant Staphylococcus aureus, (MRSA) (n = 19), and vancomycin-resistant enterococcus, (VRE) (n = 5). Among fungal infections, 20 patients were candida-related (18 were pneumonia, 1 was UTI, 1 septicemia) and 1 patient developed pneumonia induced by Trichosporon pullulans infection.
Polymicrobial infections were noted in 54 (48.6%) patients. Prevalence of MDR strains was 52 (46.8%), and there was no difference among CA, HCA, and nosocomial infections (n = 6, 11.5%) versus (n = 29, 55.8%) versus (n = 17, 32.7%) (P = .353) in this respect. There were no differences in type of organism and organ failures in patients among CA, HCA, and nosocomial infections (Table 2).
Table 2.
Clinical characteristics of the first infection.
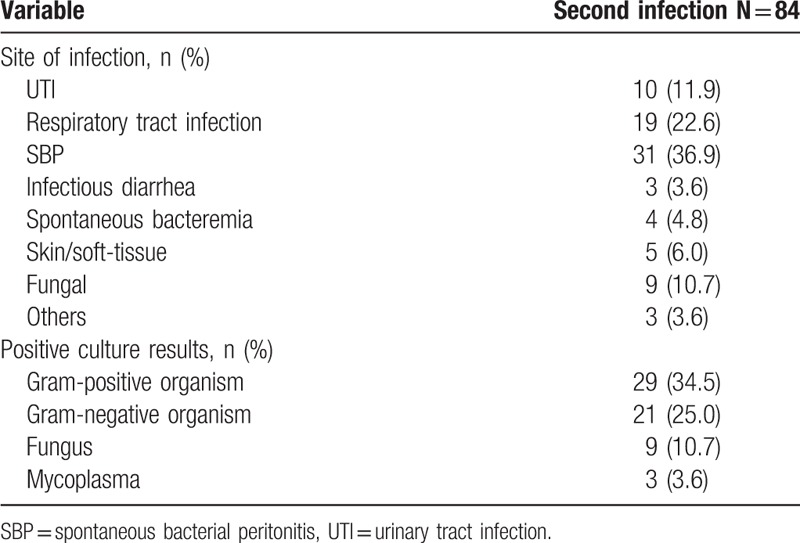
3.3. Second infections
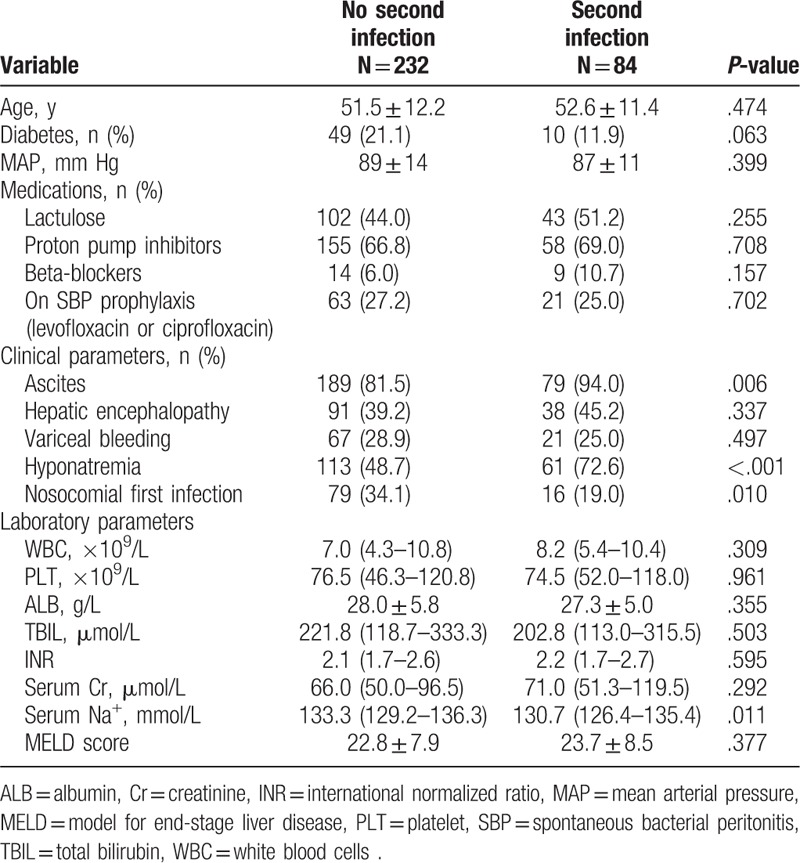
Among 316 infected patients, 84 (26.6%) patients developed a second infection during hospitalization. The majority of second infections were SBP (n = 31, 36.9%), followed by respiratory tract infections (n = 19, 22.6%), UTI (n = 10, 11.9%), fungal (n = 9, 10.7%), skin/soft-tissue infections (n = 5, 6.0%), and others (n = 10, 11.9%). There was no difference between those who developed a second infection and those who did not in terms of gender distribution and etiology of cirrhosis. However, second infections were more frequent in patients in whom the first infection was a nosocomial infection. Second infections were more common in patients with ascites and hyponatremia. There was no significant increase in the rate of development of second infections in patients who demonstrated gastrointestinal bleeding and hepatic encephalopathy (HE) during hospitalization (Table 3).
Table 3.
Comparing characteristics of patients who did or did not develop a second infection.
Among those who developed a second infection, 62 (73.8%) patients showed a positive culture. A majority of infectious isolates were gram-positive bacteria (n = 29, 34.5%), followed by gram-negative bacteria (n = 21, 25.0%), fungal organisms (n = 9, 10.7%), and mycoplasma (n = 3, 3.6%) (Table 4). Among gram-positive organisms, Staphylococcus (n = 12), Enterococcus (n = 13), and Streptococcus (n = 4) were commonly isolated, whereas among gram-negative organisms, Klebsiella (n = 9), E. coli (n = 7), A baumannii (n = 3), P. aeruginosa (n = 1), and E cloacae (n = 1) were commonly found. VRE (n = 8) was the most frequently isolated MDR strain, followed by MRSA (n = 7) and ESBL (n = 4).
Table 4.
Types of second infection and culture results.
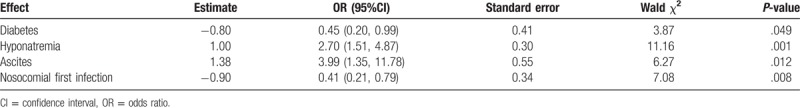
Multivariate logistic regression analysis was used to evaluate independent factors associated with development of a second infection. Based on univariate analysis of parameters presented in Table 3, independent factors associated with the development of a second infection were noted to be diabetes (odds ratio [OR] = 0.45, 95% confidence interval [CI] 0.20–0.99, P = .049), hyponatremia (OR = 2.70, 95%CI 1.51–4.87, P = .001), ascites (OR = 3.99, 95%CI 1.35–11.78, P = .012), and nosocomial first infection (OR = 0.41, 95%CI 0.21–0.78, P = .008) (Table 5).
Table 5.
Risk factors for the development of second infection.
3.4. Impact of infection on 90-day survival
Incidence of 90-day mortality in patients with and without infection was 52.2% and 16.4%, respectively (P <.001). In infected patients, mortality in cases of CA, HCA, and nosocomial infections was 49.2%, 55.6%, and 48.4%, respectively (P = .474). Based on different sites of first infection, mortality rate was the highest in patients with only SBP (55.1%, 38/69) followed by those with respiratory tract infection (53.1%, 60/113), infectious diarrhea (50%, 8/16), other infections (41.7%, 10/24), and UTI (34.8%, 8/23) (P = .330). Mortality in patients with 1, 2, and ≥3 sites of infections was 50.6%, 57.1%, and 61.5%, respectively (P = .378). Of note, multidrug resistance (MDR) did not seem to affect mortality related to the first infection. Mortality in patients with and without MDR infection was 46.2% and 53.2%, respectively (P = .293). Additionally, mortality in patients who did or did not develop a second infection was 67.9% and 46.6%, respectively (P = .001) (Fig. 2).
Figure 2.
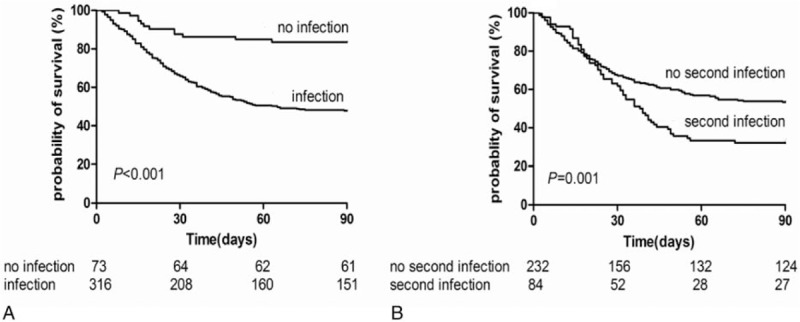
Kaplan–Meier survival curves in patients (A) with and without infection (B) developed or did not develop a second infection during hospitalization.
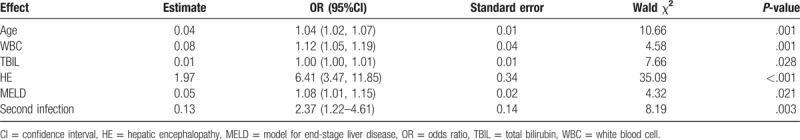
Multivariate logistic regression analysis was used to evaluate independent factors associated with 90-day mortality. Based on univariate analysis of parameters presented in Table 6, independent factors associated with 90-day mortality were age (OR = 1.04, 95%CI 1.02–1.07, P = .001), WBC (OR = 1.12, 95%CI 1.05–1.19, P = .001), MELD (OR = 1.08, 95%CI 1.01–1.15, P = .021), hepatic encephalopathy (OR = 6.41, 95%CI 3.47–11.85, P <.001), and second infection (OR = 2.37, 95%CI 1.22–4.61, P = .003) (Table 7).
Table 6.
Comparison between patients with infection who died or survived.
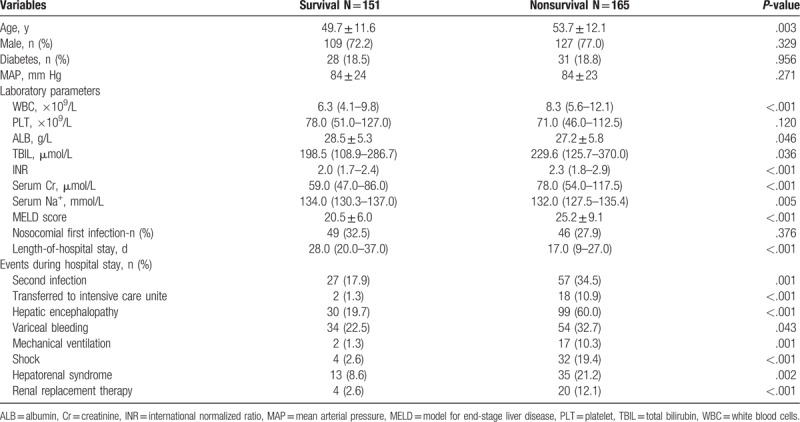
Table 7.
Multivariate logistic regression analysis of independent predictors associated with 90-day mortality in patients with infection.
4. Discussion
Bacterial infections are a major cause of morbidity and mortality among patients with liver failure. The rate of bacterial infection ranges between 50% and 90% and has been linked to worsening of liver function and increasing mortality rate.[18,19] We studied 316 (81.2%) patients with infections and a large majority of first infections were found to be HCA and nosocomial in nature, which accounted for 81.4% of all infected cases, a finding similar to other studies.[20,21] The greater number of HCA and nosocomial infections observed in ACLF patients is attributable to an increased use of invasive procedures and intensive care unit admissions in such patients. Moreover, HCA infection which is potentially preventable accounts for the majority of infections acquired outside hospital. It suggests that patient with a hospital stay over the past 6 months should be followed up for infection. Infections increase mortality >2-fold in patients with ACLF and 52.2% of patients die within 3 months following infection. In infected patients, bacterial are known to express molecular structures called pathogen-associated molecular patterns (PAMPs).[22]Through combination with various pattern-recognition receptors (PRRs), PAMPs are able to stimulate the innate immune system and result in activation of signaling cascades that activate transcription factors and subsequent lead to transcriptional activation of hundreds of anti- and proinflammatory cytokines genes.[10,23] The imbalance between anti- and proinflammatory cytokines triggered by infection may induce multiorgan failure and significantly increase the risk of death.
In our study, the 3 most common first infections were observed to be respiratory tract infection in 156 (49.4%) patients, followed by SBP in 118 (37.3%) and UTI in 42 (13.3%) patients. We found that 247 (78.2%) patients had infection at a single site, whereas 69 (21.8%) patients showed >1 site of infection. Thus, in patients with ACLF, close attention to the detection of lung, abdominal, and UTIs is warranted. A comprehensive physical examination, chest imaging, regularly repeated ascites cultures, and other assessment methods may help with early diagnosis of infection. Moreover, clinicians should also pay close attention to the presence/development of multisite infection.
Furthermore, we found that 90-day of mortality was the highest in patients with SBP (55.1%), followed by those with respiratory tract infection (53.1%), infectious diarrhea (50%), and UTI (34.8%). There was no difference in mortality based on different sites of infection (P = .330). Recent studies have shown that epidemiological and microbiological characteristics of infections differ widely. Gram-positive and MDR pathogens are more prevalent, particularly in HCA and nosocomial settings.[7,11] However, therapy measures did not revise with the change of epidemiological and microbiological character. For example, based on available guidelines, third-generation cephalosporins are recommended as empirical antibiotic treatment for SBP. [16,24] However, epidemiology of SBP in patients with cirrhosis differs based on CA, HCA, or a nosocomial SBP presentation. Patients with nosocomial SBP show a higher prevalence of MDR bacteria and fail to respond to third-generation cephalosporins in up to 33% to 75% of cases. Notably, ineffective first-line empirical antibiotic treatment has been associated with poor survival rates.[13,25] Thus, the choice of antibiotic treatment in patients with cirrhosis based on epidemiological class and local microbiological patterns should be considered.
Despite use of guidelines to reduce in-patient infection, nosocomial and potential preventable second infections continue to occur in patients with ACLF. Nosocomial infections comprise 30.1% of all infection cases. Potentially preventable second infections in patients with liver failure occur in 26.6% cases. The majority of second infections were noted to be SBP (36.9%), followed by respiratory tract infections (22.6%), UTI (11.9%), fungal infections (10.7%), and skin/soft-tissue infections (6.0%). A low threshold for institution of diagnostic paracentesis and prompt initiation of antibiotics and albumin therapy may be helpful to reduce the occurrence of SBP. Respiratory tract infection remains a significant cause of second infection which can be potentially prevented by careful airway monitoring in patients with cirrhosis with altered mental status and those with upper gastrointestinal bleeding. Effective management strategies should be aimed at limiting the use of instrumentation and antibiotics and encouraging early mobilization and airway protection to prevent aspiration. UTI can be prevented by reducing unnecessary instrumentation in the urinary tract such as catheterization, by cleaning the urethra, promoting urination and correction of chronic diseases such as diabetes.
We found that the occurrence of hyponatremia (OR = 2.7) and ascites (OR = 3.99) were independent predictors for the development of second infection. Hyponatremia alters cell membrane permeability, cell dysfunction, apoptosis or necrosis, tissue damage, organ failure, and can cause various metabolic disorders, especially influencing the cardiovascular and nervous systems.[26] Previous studies have showed that this subgroup of patients shows a significantly higher incidence of hepatic encephalopathy (OR = 3.40, 95%CI 2.35–4.92) and SBP (OR = 2.36, 95%CI 1.41–3.93).[27] Furthermore, the presence of ascites indicates a deterioration of liver function and the presence of portal hypertension in patients with cirrhosis. Ascites can reduce the effective arterial volume and lead to progressive cirrhotic cardiomyopathy, which in turn leads to sodium and water retention and further ascites. Bacterial infection is an important cause of circulatory dysfunction, which can increase cytokine production, activation of coagulation pathways, reduced protein C production, increased intestinal permeability, and intestinal bacterial translocation by altering the structure of the intestinal mucosa and predisposing to development of the infections in patients with cirrhosis.[3] Thus, timely correction of hyponatremia and prevention of ascites may help to reduce the incidence of second infections.
In this study, 111 (66.5%) patients showed a positive culture result. Gram-positive organisms were the microorganisms most frequently isolated, followed by gram-negative organisms and fungi in cases of first infection. Detection rate of pathogens (66.5%) did not correlate with the high incidence of infection (81.2%) suggesting that conditions under which the culture was performed and the widespread use of antibiotics could have impacted the results. Therefore, a clinical diagnosis remains the primary means to diagnose infection. Previous studies have described bacterial infections in patients with cirrhosis being primarily caused by gram-negative microorganisms. However, in the past decade, infections induced by gram-positive microorganisms have increased owing to long-term antibiotic prophylaxis using quinolones and other antibiotics, which prevent gram-negative but not gram-positive bacterial infections.[28,29]
Recently, several studies have shown that antibiotic resistance has increased among patients hospitalized with cirrhosis.[13,14] Our study showed a high prevalence of MDR strains at 52 (46.8%), which might be related to the large numbers of HCA (51.3%) and nosocomial infections (30.1%) diagnosed, because patients with ACLF are frequently in need of hospitalization. It is also reasonable to deduce that continuous administration of antibiotics, for treatment or prophylaxis, may lead to the development of greater number of resistant pathogens. Additionally, previous studies have shown that HCA and nosocomial infection are caused more frequently by antibiotic-resistant bacteria and are associated with a more severe clinical course than CA infections.[11,20] Surprisingly, we found that in patients with ACLF, there were no differences in the prevalence of antibiotic-resistant pathogens, severe clinical course, and poor outcome (90-day mortality was 49.2% in CA, 55.6% in HCA, and 48.4% in nosocomial infections). This finding may be due to the breadth size of our sample or because of the generally high use of antibiotics.
There were also several limitations in our study. Firstly, it is retrospective in nature. Thus, specimens were sent for culture based on the clinical judgment of the team caring for the patients enrolled. The proportion of culture-positive infections was found to be lower than in previous prospective studies.[12,28] Therefore, we cannot exclude the possibility that occurrence of antibiotic resistance may have been under-or overestimated in our cohort. Secondly, it was a single-center study performed on patients at Tianjin Third Central Hospital with a small sample size. Finally, diagnosis of infection could have been influenced by differences between individual clinician skills and thus in some cases, it may have been delayed, potentially affecting our findings. Thus, more prospective randomized studies are needed for further investigation.
In conclusion, infections (regardless of first or second infection) can increase the 90-day mortality significantly in patients with ACLF. Age, WBC, MELD score, HE, and the presence of second infection are independent risk factors affecting 90-day mortality in patients with ACLF showing infection. Most first infections (HCA and nosocomial infection) as well as second infections are potentially preventable. In patients with ACLF, close attention to polymicrobial, multisite infections, as well as the emergence of antibiotic-resistant bacteria is warranted. Prevention of the occurrence of infections, early diagnosis and timely treatment of infections is the key to reducing/managing infections related to mortality in patients diagnosed with liver failure.
Footnotes
Abbreviations: ACLF = acute-on-chronic liver failure, CA = community-acquired infection, HCA = healthcare-associated infection, HE = hepatic encephalopathy, MELD = model for end-stage liver disease, SBP = spontaneous bacterial peritonitis, UTI = urinary tract infection, WBC = white blood cells.
Funding: This work was supported by the National 13th 5-year Plan for Hepatitis Research (grant number 2016ZX10002008-007) and Graduate Innovation Fund of Education Department of Hebei Province (grant number CXZZBS2017104).
The authors have no conflict of interest to disclose.
References
- [1].Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalized patients in the Uinted States. Aliment Pharmacol Ther 2014;40:105–12. [DOI] [PubMed] [Google Scholar]
- [2].Sargenti K, Prytz H, Nilsson E, et al. Predictors of mortality among patients with compensated and decompensated liver cirrhosis: the role of bacterial infections and infection-related acute-on-chronic liver failure. Scand J Gastroenterol 2015;50:875–83. [DOI] [PubMed] [Google Scholar]
- [3].Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014;60:1310–24. [DOI] [PubMed] [Google Scholar]
- [4].Piano S, Fasolato S, Salinas F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology 2016;63:1299–309. [DOI] [PubMed] [Google Scholar]
- [5].Merli M, Lucidi C, Di Gregorio V, et al. An empirical broad spectrum antibiotic therapy in health-care-associated infections improves survival in patients with cirrhosis: a randomized trial. Hepatology 2016;63:1632–9. [DOI] [PubMed] [Google Scholar]
- [6].Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246–56. 1256. e1–5. [DOI] [PubMed] [Google Scholar]
- [7].Dionigi E, Garcovich M, Borzio M, et al. Bacterial infections change natural history of cirrhosis irrespective of liver disease severity. Am J Gastroenterol 2017;112:588–96. [DOI] [PubMed] [Google Scholar]
- [8].Shi Y, Yang Y, Hu Y, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 2015;62:232–42. [DOI] [PubMed] [Google Scholar]
- [9].Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37. 1437. e1–9. [DOI] [PubMed] [Google Scholar]
- [10].Arroyo V, Moreau R, Jalan R, et al. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol 2015;621suppl:S131–43. [DOI] [PubMed] [Google Scholar]
- [11].Sargenti K, Prytz H, Strand A, et al. Healthcare-associated and nosocomial bacterial infections in cirrhosis: predictors and impact on outcome. Liver Int 2015;35:391–400. [DOI] [PubMed] [Google Scholar]
- [12].Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology 2012;56:2328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012;55:1551–61. [DOI] [PubMed] [Google Scholar]
- [14].Alexopoulou A, Papadopoulos N, Eliopoulos DG, et al. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int 2013;33:975–81. [DOI] [PubMed] [Google Scholar]
- [15].Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int 2014;8:453–71. [DOI] [PubMed] [Google Scholar]
- [16].European Association for the Study of the liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397–417. [DOI] [PubMed] [Google Scholar]
- [17].Ruf AE, Kremers WK, Chavez LL, et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 2005;11:336–43. [DOI] [PubMed] [Google Scholar]
- [18].Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology 2003;125:755–64. [DOI] [PubMed] [Google Scholar]
- [19].Albillo A, Martínez J. Prognostic value of bacterial infection in acute and chronic liver failure. Liver Int 2016;36:1090–2. [DOI] [PubMed] [Google Scholar]
- [20].Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol 2010;8:979–85. [DOI] [PubMed] [Google Scholar]
- [21].Baijal R, Amarapurkar D, Praveen Kumar HR, et al. A multicenter prospective study of infections related morbidity and mortality in cirrhosis of liver. Indian J Gastroenterol 2014;33:336–42. [DOI] [PubMed] [Google Scholar]
- [22].Wang XW, Gao J, Xu YH, et al. Novel pattern recognition receptor protects shrimp by preventing bacterial colonization and promoting phagocytosis. J Immunol 2017;198:3045–57. [DOI] [PubMed] [Google Scholar]
- [23].Gandoura S, Weiss E, Rautou PE, et al. Gene- and exon-expression profiling reveals an extensive LPS-induced response in immune cells in patients with cirrhosis. J Hepatol 2013;58:936–48. [DOI] [PubMed] [Google Scholar]
- [24].Runyon BA. AASLD. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087–107. [DOI] [PubMed] [Google Scholar]
- [25].Ariza X, Castellote J, Lora-Tamayo J, et al. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol 2012;56:825–32. [DOI] [PubMed] [Google Scholar]
- [26].Cárdenas A, Solà E, Rodríguez E, et al. Hyponatremia influences the outcome of patients with acute-on-chronic liver failure: an analysis of the CANONIC study. Crit Care 2014;18:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Angeli P, Wong F, Watson H, et al. Hyponatremia in cirrhosis: results of a patients population survey. Hepatology 2006;44:1535–42. [DOI] [PubMed] [Google Scholar]
- [28].Wong F, O’Leary JG, Reddy KR, et al. New consensus definition of acute kidney injury accurately predictor 30-day mortality in patients with cirrhosis and infection. Gastroenterology 2013;145:1280–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006;131:1049–56. [DOI] [PubMed] [Google Scholar]


