Abstract
Background:
Angiogenesis is an essential process in the development and progression of malignant tumors including lung cancer, in which angiopoietin-2 (Ang-2) plays an important role. The objective of this study was to assess the prognostic value of serum Ang-2 levels in patients with lung cancer.
Methods:
A comprehensive systematic electronic search was performed in the Pubmed, Embase, Web of Science, china national knowledge infrastructure, and VIP databases update to October, 2016 (qikan.cqvip.com). Literatures examining the relevance of serum Ang-2 levels to progression and prognosis of lung cancer were eligible for our study. Standardized mean differences (SMD) with 95% confidence interval (95% CI) and a P value were applied to compare continuous variables, and hazard ratio (HR) with 95% CI as well as P value were applied for prognostic role.
Results:
Twenty studies with 1911 patients met the eligibility criteria. Among them, 7 studies with 575 patients with lung cancer assessed the association between expression of serum Ang-2 and prognosis. According to our results, higher levels of serum Ang-2 were associated with the later stage of tumor. Serum Ang-2 levels were significantly lower in stage I than in stage II (SMD: −0.51; 95% CI: −0.75 to −0.27; P < .001), in stage II than in stage III (SMD: −0.52; 95% CI: −0.80 to −0.24; P < .001), in stage III than in stage IV (SMD: −0.58; 95% CI: −0.93 to −0.23; P = .001). In addition, serum Ang-2 levels were higher in patients with lymph node metastasis (SMD: 1.06; 95% CI, 0.57–1.56; P < .001). Meanwhile, patients with lung cancer with higher levels of serum Ang-2 were associated with a significant poorer prognosis when compared to those with lower serum Ang-2 levels (HR: 1.64; 95% CI: 1.20–2.25; P = .002), and this role was further detected when stratified by ethnicity and histological type.
Conclusions:
This systematic review and meta-analysis suggested that serum Ang-2 levels might be a potential predictor for staging, and were associated with prognosis of lung cancer.
Keywords: angiopoietin-2, lung cancer, prognosis, progression
1. Introduction
Lung cancer is the leading cause of cancer death worldwide.[1] Despite of the widely application of low-dose computed tomography in early detection of lung cancer in recent years,[2] a large proportion of patients present with locally advanced or metastatic disease at the time of diagnosis, which results in a poor prognosis.[3,4] Thus, effective predictors for the progression and prognosis of lung cancer are very important.
Angiogenesis is an essential process in the development and progression of malignant tumors including lung cancer,[5,6] which has been proved in many studies to be controlled by a dynamic balance between vessel regression and growth in which angiopoietins plays a critical role along with vascular endothelial growth factor (VEGF).[6,7] Angiopoietin-1 (Ang-1)[8,9] and angiopoietin-2 (Ang-2)[10] have been identified as ligands for Tie2, which is a receptor tyrosine kinase specifically expressed on endothelial cells (ECs). Ang-1 binds to Tie2, and maintains and stabilizes mature vessels by promoting interaction between ECs and surrounding extracellular matrix. Ang-2 antagonizes the stabilizing ability of Ang-1 by competitively binding to Tie2, which resulting in destabilization of vessels. The vessels destabilized by Ang-2 may undergo angiogenic changes in the presence of angiogenic factors such as VEGF; nevertheless, in the absence of VEGF, these destabilized vessels may undergo regression. Therefore, Ang-2 promotes tumor angiogenesis by priming the vasculature and potentiating the effects of VEGF at the front of active neovascularization.[7]
In contrast to predominant expression of Ang-1 and Tie2 in normal lung tissue, Ang-2 is preferentially expressed in lung cancer tissues, which has been implicated as an angiogenic switch in tumorigenesis.[11] Tumors change from an avascular state to an angiogenic state when angiogenic factors for the formation of new blood vessels become dominant over natural angiogenesis inhibitors.[12,13] Subsequently, ECs are activated and lead to Ang-2 expressing intensively, and then the vessels may experience regression. In this situation, the tumor cells might undergo hypoxia, which upregulate VEGF expression to induce tumor angiogenesis conversely. In the presence of VEGF, Ang-2 enables ECs migration, proliferation, and sprouting of new vessels.[14] In addition to this, serum Ang-2 is a useful clinical marker for detecting progression of lung cancer.[15] However, some other studies did report conflicting results that Ang-2 was irrelevant to clinical outcome. Thus, whether serum Ang-2 correlates with prognosis in patients with lung cancer is inconclusive.
Therefore, we performed this systematic review and meta-analysis to assess the clinical significance of serum Ang-2 levels in patients with lung cancer.
2. Materials and methods
2.1. Ethical approval
This present study did not involve human subjects, so ethical approval was not necessary and informed consent was not required.
2.2. Search strategy
We performed a comprehensive systematic electronic search in the Pubmed, Embase, Web of Science, china national knowledge infrastructure (CNKI), and VIP databases update to October 2016 (qikan.cqvip.com). The following terms were used for searching: “Angiopoietin 2” or “Ang-2,” “lung cancer,” “lung carcinoma” or “lung neoplasm,” and all possible combinations. The references reported in the included articles were also considered for completion.
2.3. Inclusion and exclusion criteria
The eligible studies in this meta-analysis met the following criteria: investigating the association between serum Ang-2 levels and progression or prognosis or other clinic pathological characteristics related to lung cancer; data were available for further meta-analysis; published in English or Chinese. Articles were excluded if they were published in the form of case reports, letters, reviews, or conference abstracts; insufficient data were provided for further meta-analysis. For the studies containing overlapping patients, we chose the study with the largest number of events to avoid duplication.
2.4. Quality assessment
Newcastle-Ottawa Scale (NOS) was performed to assess the quality of each available nonrandomized study.[16] The NOS consists of 3 perspectives with a maximum of 9 stars: a maximum of 4 stars for selection, 2 stars for comparability, and 3 stars for the ascertainment of either the exposure or outcome of interest. Studies labeled with 5 or more NOS scores were considered to be high quality.[17]
2.5. Data extraction
The eligible articles were reviewed independently by 2 reviewers and any disagreement would be discussed until a consensus was reached. The following essential information were retrieved from the articles including the author, publication year, ethnicity, the source of patients, study design, histological type, stage, age, sex, status of lymph node metastasis, smoking history, and prognosis data. Because the critical value of serum Ang-2 was different in the included studies, we distinguished the concentration of serum Ang-2 into a relative higher level and lower level.
2.6. Statistical analysis
To assess the relationship between expression of Ang-2 and prognosis, we measured the hazard ratio (HR) and 95% confidence intervals (95% CIs) as the effective value. If the 2 statistics were given explicitly in the articles, we would use the crude ones, but if not, HR was estimated according to Parmar method.[18] Standardized mean difference (SMD) was applied for continuous variables. The P value <.05 was thought to be statistically significant. Chi-square–based I2 test and Q-statistic test were used to assess the statistical heterogeneity. I2 < 50% and P > .10 suggested acceptable heterogeneity and then a fixed-effect model was applied. Otherwise, significant heterogeneity existed and a random effect model was used. Sensitivity analysis was performed by excluded each study sequentially. All of the calculations were performed by STATA version 12.0 (Stata Corporation, College Station, TX) and Review Manager V.5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
3. Results
3.1. Literature selection and study characteristics
There were 413 citations from the Pubmed, Embase, Web of Science, CNKI, and VIP databases (qikan.cqvip.com). The majority of the researches were eliminated after screening the titles and abstracts. A total of 56 articles were left, and the full texts of which were further evaluated in detail. Finally, 20 studies with 1911 patients in total matched the criteria and were included in the present study.[15,19–37] The flow chart summarizing the systematic literature selection process is shown in Figure 1. Based on the NOS score (Table 1), each study was considered high quality. Seven studies in total assessed the association between expression of serum Ang-2 and prognosis.[15,21–25,28] Among them, 2 studies focused on serum Ang2 levels in small cell lung cancer (SCLC),[21,28] whereas the other 5 studied non–small cell lung cancer (NSCLC).[15,22–25] In addition, 4 studies came from Caucasian institutions,[21–23,25] whereas the other 3 from Asian.[15,24,28] Further information of the included studies are presented in Table 1.
Figure 1.
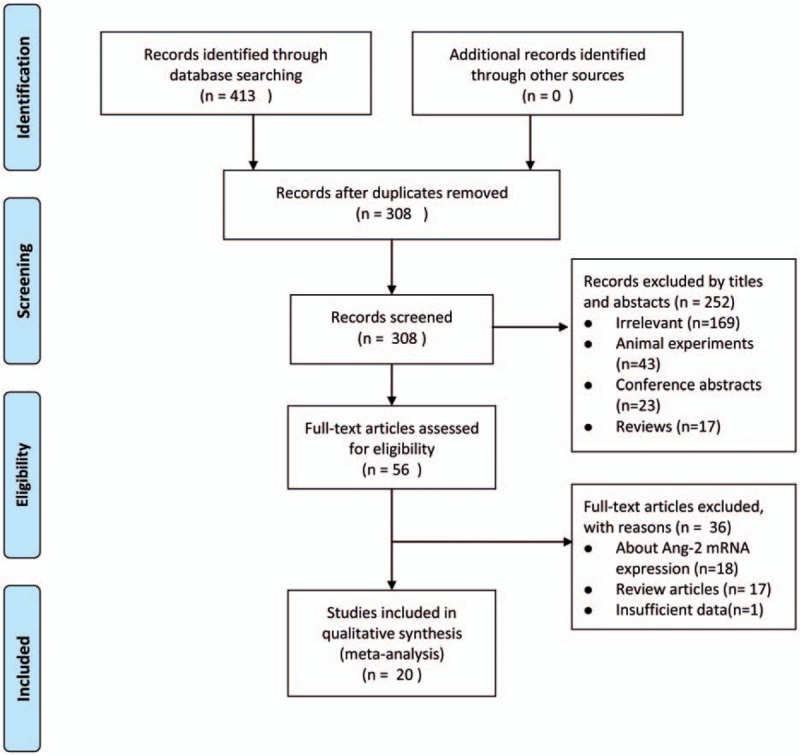
PRISMA flow chart of literature selection. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Baseline information of included articles.

3.2. Relationship between serum Ang-2 levels and tumor stage as well as other clinical pathological characteristics of lung cancer
There were a total of 9 studies[15,20,29–35] evaluating the correlation of serum Ang-2 levels and stage progression of lung cancer. According to the results of our meta-analysis, higher levels of serum Ang-2 were associated with the later stage of tumor (Table 2). Serum Ang-2 levels were significantly different between stage I and stage II (SMD: −0.51; 95% CI: −0.75 to −0.27; P < .001) (Fig. 2A), stage I and stage III (SMD: −0.83; 95% CI: −1.44 to −0.21; P = .009) (Fig. 2B), stage I and stage IV (SMD: −1.51; 95% CI: −2.59 to −0.42; P = .006) (Fig. 2C), stage II and stage III (SMD: −0.52; 95% CI: −0.80 to −0.24; P < .001) (Fig. 2D), stage II and stage IV (SMD: −1.21; 95% CI: −1.98 to −0.44; P = .002) (Fig. 2E), as well as stage III and stage IV (SMD: −0.58; 95% CI: −0.93 to −0.23; P = .001) (Fig. 2F). Moreover, serum Ang-2 levels were highly associated with lymph node involvement in lung cancer.[24,29,33,34,36] Patients with lymph node metastasis were associated with significant higher serum Ang-2 concentration (SMD: 1.06; 95% CI: 0.57–1.56; P < .001) (Fig. 3). However, we did not find any statistical difference between the serum Ang-2 levels and age, sex, smoking history, and histological type (Table 2).
Table 2.
Pooled results of clinical significance of serum angiopoietin-2 levels in lung cancer.

Figure 2.
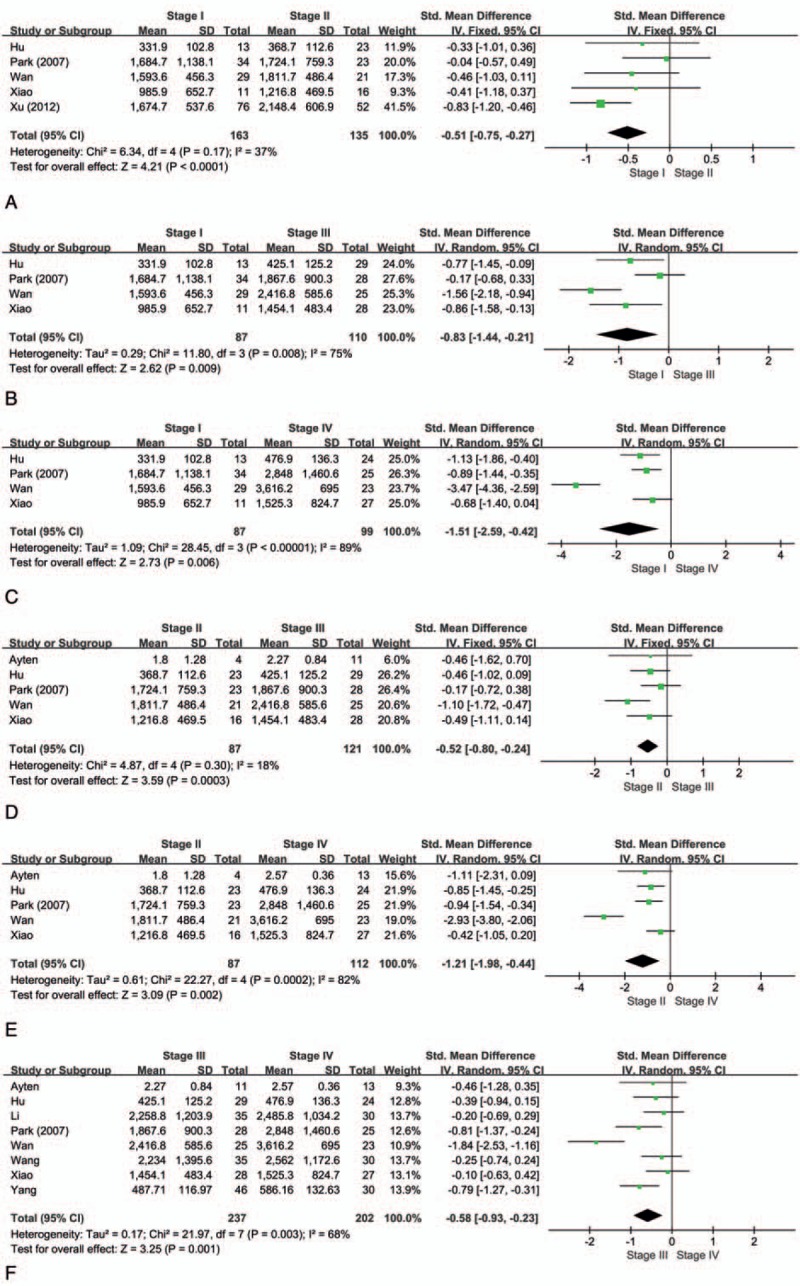
Forest plot of role of SMD and its 95% CI of serum Ang-2 levels between (A) stage I and II, (B) stage I and III, (C) stage I and IV, (D) stage II and III, (E)stage II and IV, and (F) stage III and IV. For each study, the estimate of SMD was plotted with squares, and the size of the square reflects the weight of the study in the meta-analysis. The horizontal line crossing the square represents the 95% CI. The diamond represents the summary SMD and 95% CI. Ang-2 = angiopoietin-2, CI = confidence interval, SMD = standardized mean differences.
Figure 3.
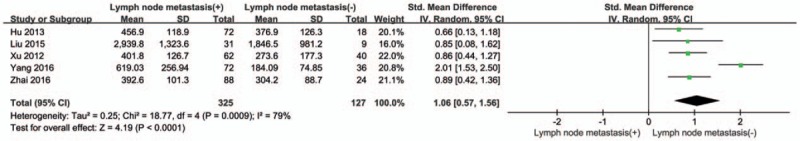
Forest plot of role of SMD and its 95% CI of serum Ang-2 levels between lung cancer patients with and without lymph node metastasis. For each study, the estimate of SMD was plotted with squares, and the size of the square reflects the weight of the study in the meta-analysis. The horizontal line crossing the square represents the 95% CI. The diamond represents the summary SMD and 95% CI. Ang-2 = angiopoietin-2, CI = confidence interval, SMD = standardized mean differences.
3.3. Association between serum Ang-2 levels and overall survival of lung cancer
In this meta-analysis, a total of 7 studies with 575 patients investigated the association between expression of serum Ang-2 levels and prognosis. High heterogeneity was detected (P = .003; I2 = 67.7%), so the random effects model was applied. Patients with lung cancer with higher levels of serum Ang-2 were associated with a significant poorer prognosis when compared to those with lower serum Ang-2 levels (HR: 1.64; 95% CI: 1.20–2.25; P = .002). The results are shown in Figure 4 and Table 3.
Figure 4.
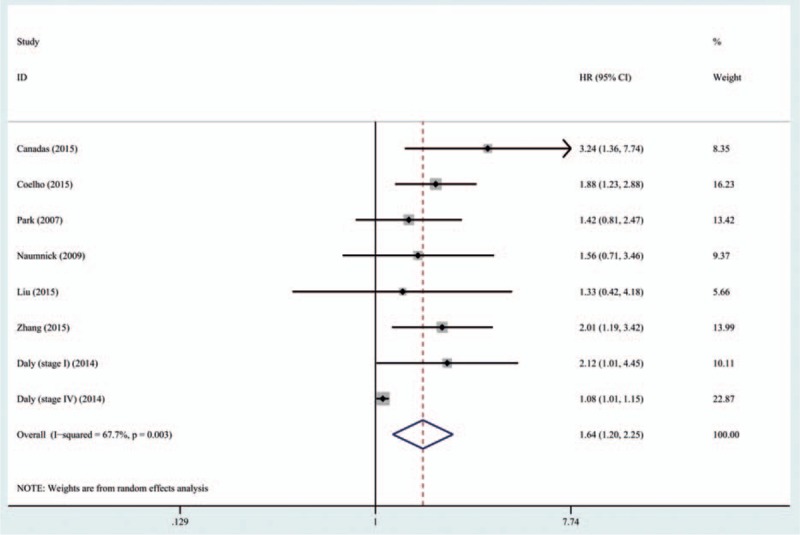
Forest plot of role of serum Ang-2 levels on survival in lung cancer. For each study, the estimate of HR was plotted with squares, and the size of the square reflects the weight of the study in the meta-analysis. The horizontal line crossing the square represents the 95% CI. The diamond represents the summary HR and 95% CI. Ang-2 = angiopoietin-2 , CI = confidence interval, HR = hazard ratio.
Table 3.
Pooled results of prognostic role of serum angiopoietin-2 levels in lung cancer.

In subgroup analysis, higher levels of serum Ang-2 indicated poorer outcomes both in Caucasians (HR: 1.69; 95% CI: 1.10–2.61; P = .017) and Asians (HR: 1.66; 95% CI, 1.16–2.39; P = .006) (Table 3). The heterogeneity of Asian group was acceptable (P = .620, I2 = 0%), whereas it still remained large in Caucasian group (P = .003, I2 = 75.1%) (Fig. 5A). In addition, when stratified by histological type of tumor, higher serum Ang-2 levels were also proved to be a risk factor of poor prognosis in both SCLC (HR: 2.29; 95% CI: 1.46–3.59, P < .001) and NSCLC (HR: 1.44; 95% CI: 1.07–1.93, P = .017) group (Fig. 5B).
Figure 5.
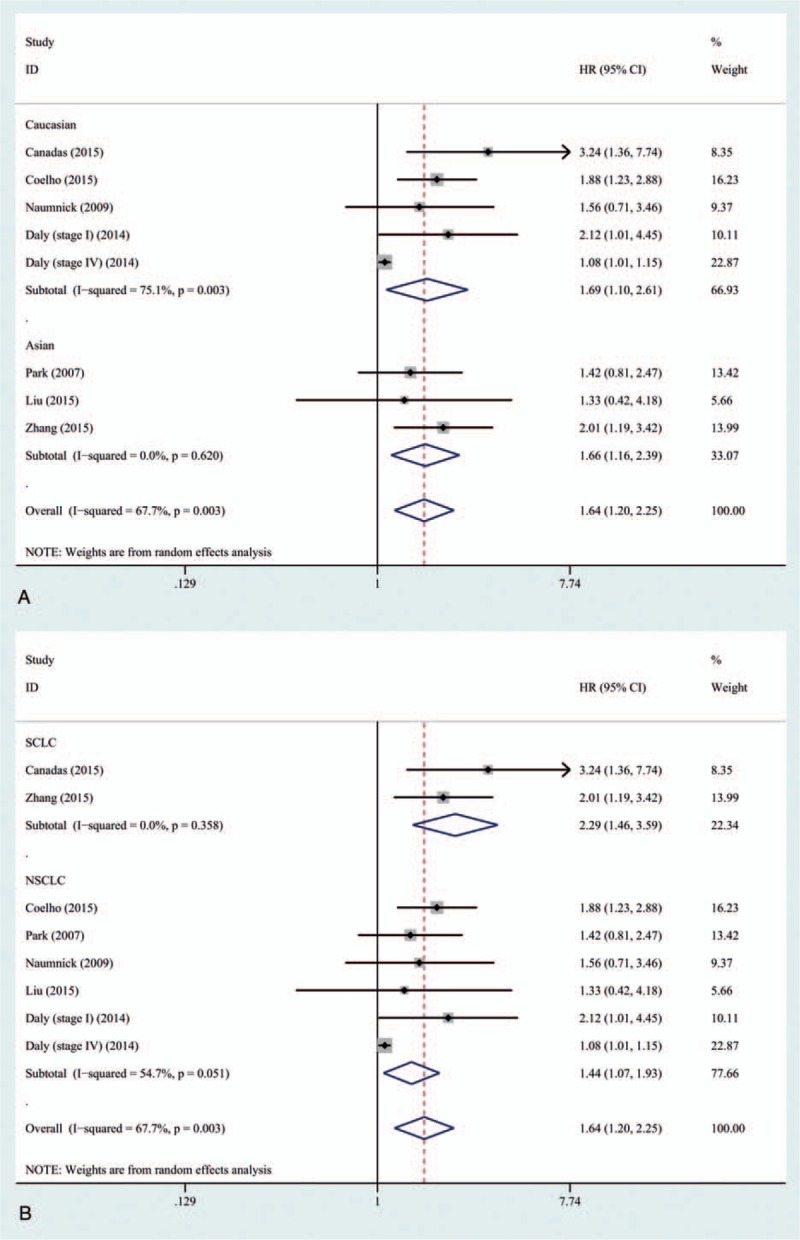
Forest plot of role of serum Ang-2 levels on survival in lung cancer stratified by (A) ethnicity and (B) histological type. For each study, the estimate of HR was plotted with squares, and the size of the square reflects the weight of the study in the meta-analysis. The horizontal line crossing the square represents the 95% CI. The diamond represents the summary HR and 95% CI. Ang-2 = angiopoietin-2 , CI = confidence interval, HR = hazard ratio, NSCLC = non–small cell lung cancer, SCLC = small cell lung cancer.
3.4. Sensitivity analysis
Sensitivity analysis was carried out to evaluate whether the prognosis outcomes were driven by any specific study. No significant changed results were detected by sequential removal of each study based on overall analysis and proved the stability of our findings (Fig. 6).
Figure 6.
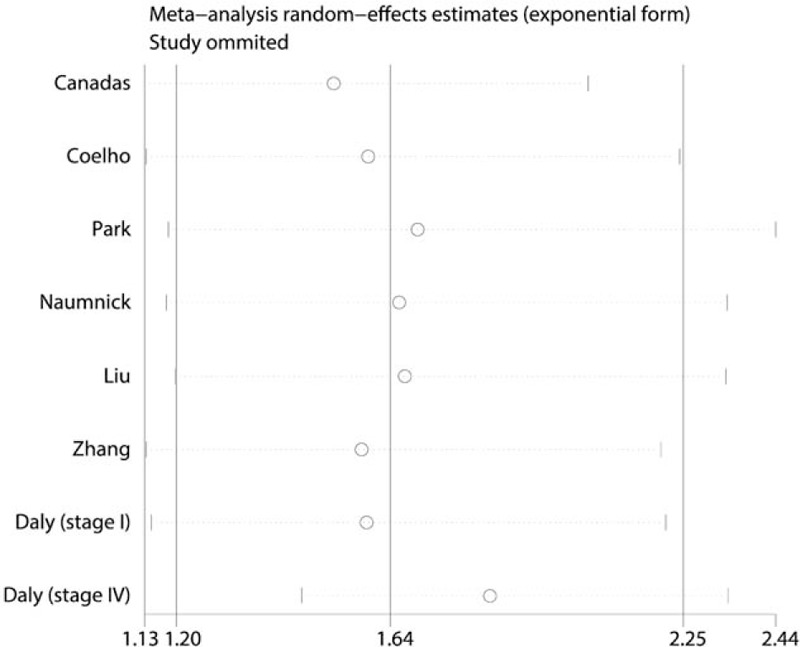
Sensitivity analysis of overall survival. For each study, the circle represents the summary hazard ratio (HR) when excluded this study. The horizontal line crossing the circle represents the 95% confidence interval (CI). The short vertical at the ends of horizontal line represents the lower and upper limit of 95% CI, respectively.
4. Discussion
Lung cancer is one of the most common malignancies, which is the leading cause of cancer-related mortality in the world. In spite of major advances in lung cancer therapeutic strategies, the mortality is still on the rise with unfavorable prognosis and short median overall survival time. Thus, it is essential to identify reliable predictors for lung cancer.
Angiogenesis, which is regulated by a complex balance of proangiogenic and antiangiogenic, plays an important role in carcinogenesis. The degree of angiogenesis was found to be in correlation with the prognosis of patients with lung cancer.[38] Although several preclinical studies addressed some molecules with antiangiogenic activity, the majority of them failed to display efficacy in clinical trials and were abandoned.[39] In recent years, Ang-2 was reported to be a regulator during tumor progression[40] and found to be associated with poor prognosis.[41] Ang-2 expression along with the presence of angiogenic changes in the presence of angiogenic factors such as VEGF at the tumor periphery results in destabilization of vessels, and is associated with promoting further growth of tumor. However, only a few researches documented the correlation between serum Ang-2 and progression and prognosis of lung cancer. A recent meta-analysis from Xuan et al[42] suggested that Ang-2 levels, including in serum, cancer tissues, and mRNA expression, were significantly associated with poor prognosis for patients with NSCLC. Instead, in our current meta-analysis, we reviewed 19 studies and focused on the effect of serum Ang-2 concentration on the role of progression and prognosis in both NSCLC and SCLC.
A linkage between the degree of angiogenesis and metastasis was reported in a variety of malignant tumors, including lung cancer.[38,43] In our meta-analysis, we found the result that for patients with lung cancer, serum Ang-2 levels increased along with the tumor stages. Especially, the result that patients in stage IV had the highest serum Ang-2 levels could corroborate the critical role of Ang-2 in tumor development and metastasis. Meanwhile, significantly higher levels of serum Ang-2 in patients with lung cancer with lymph node metastasis might also reveal Ang-2 to be a potential biomarker in staging. In addition, some previously published studies suggested that the expression of Ang-2 in lung cancer tissues was higher than that in the normal lung tissues.[11,44] These findings aforesaid could plausibly provide theoretical support for that Ang-2 was essential to angiogenesis during tumor progression. Although lung cancer is clinically staged by using whole body imaging currently, patients present with locally advanced or metastatic disease at the time of diagnosis, which also makes it difficult in evaluation of early lung cancer. Thus, determination of serum Ang-2 levels could provide additional information in predicting lung cancer staging, which is noninvasive, convenient, less expensive, and the samples are highly available. Moreover, as current guidelines are to refer patients for imaging examinations only after they have presented with symptoms such as a chronic cough, at which point a typical NSCLC patient generally has fairly advanced and nonresectable tumor. Routine screening of serum Ang-2 levels would be highly advantageous in this group.
For patients with lung cancer, although some evidences have suggested expression of Ang-2 in tumor tissue indicated poor prognosis,[45–47] only a few studies evaluated the levels of Ang-2 in circulation. In 2007, Park et al[15] first reported that in NSCLC, the low Ang-2 group had a better overall survival compared to the high Ang-2 group. Since then, more and more researchers paid attention to the prognostic implications of serum Ang-2 levels in NSCLC. However, there was still no unified conclusion up to now.[15,19,22–25] According to the outcomes of our present meta-analysis, serum Ang-2 levels might be a significantly predictor in lung cancer, and this role extend to both NSCLC and SCLC group. Interestingly, the HR was higher in SCLC group than in NSCLC group. As SCLC typically presents as a more aggressive type of lung cancer, this result might suggest that serum Ang-2 might work as a potential driver of disease aggression. Furthermore, as reported by Zhang et al,[28] “partial response” occurred more frequently among the SCLC patients with the lower serum Ang-2 levels while patients with higher levels were prone to “progressive disease” during combination chemotherapy. This result might interpret the better prognosis in the patients with the lower serum Ang-2 levels compared to the patients with the higher serum Ang-2 levels indirectly.
Nevertheless, we must acknowledge several limitations of our studies. First, the majority of the articles included in our study were retrospective studies (n = 19). The period of follow-up was different among these studies and some follow-up data were derived from description of patient, which probably led to information bias. Second, the inclusion and exclusion criteria differed among studies, so that significant large heterogeneity was detected in statistical outcomes. A possible reason might be the inclusion of patients in stage IV of Daly et al's study,[23] among which the differences of serum Ang-2 levels were not noticeable. Third, since the critical value of serum Ang-2 was different in the included studies, we distinguished the concentration of serum Ang-2 into a relative higher level and lower level. In the future, large-scale randomized studies are still needed to prove the prognostic role of serum Ang-2 levels with an accurate critical value in lung cancer.
In conclusion, this present systematic review and meta-analysis could provide evidence that serum Ang-2 levels might serve as a potential predictor for staging of lung cancer and was significantly associated with prognosis of patients with lung cancer.
Footnotes
Abbreviations: Ang-1 = angiopoietin-1, Ang-2 = angiopoietin-2, CI = confidence interval, CT = computed tomography, EC = endothelial cell, HR = hazard ratio, NOS = Newcastle-Ottawa Scale, NSCLC = non-small cell lung cancer, SCLC = small cell lung cancer, SMD = standardized mean difference, VEGF = vascular endothelial growth factor.
This work was supported by the Key Science and Technology Program of Sichuan Province, China (Grant number 2013SZ0005, 2014SZ0148 to Dr. Lunxu Liu).
YX and YZ contributed equally to this work.
The authors have no conflict of interest or financial ties to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- [4].Choi SH, Chae EJ, Shin SY, et al. Comparisons of clinical outcomes in patients with and without a preoperative tissue diagnosis in the persistent malignant-looking, ground-glass-opacity nodules. Medicine (Baltimore) 2016;95:e4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Folkman J, Watson K, Ingiber D, et al. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989;339:58–61. [DOI] [PubMed] [Google Scholar]
- [6].Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242–8. [DOI] [PubMed] [Google Scholar]
- [7].Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999;284:1994–8. [DOI] [PubMed] [Google Scholar]
- [8].Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996;87:1161–9. [DOI] [PubMed] [Google Scholar]
- [9].Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996;87:1171–80. [DOI] [PubMed] [Google Scholar]
- [10].Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997;277:55–60. [DOI] [PubMed] [Google Scholar]
- [11].Wong MP, Chan SY, Fu KH, et al. The angiopoietins, tie2 and vascular endothelial growth factor are differentially expressed in the transformation of normal lung to non-small cell lung carcinomas. Lung Cancer 2000;29:11–22. [DOI] [PubMed] [Google Scholar]
- [12].Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69suppl. 3:4–10. [DOI] [PubMed] [Google Scholar]
- [13].Wheatley-Price P, Shepherd FA. Targeting angiogenesis in the treatment of lung cancer. J Thorac Oncol 2008;3:1173–84. [DOI] [PubMed] [Google Scholar]
- [14].Augustin HG, Koh GY, Thurston G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 2009;10:165–77. [DOI] [PubMed] [Google Scholar]
- [15].Park JH, Park KJ, Kim YS, et al. Serum angiopoietin-2 as a clinical marker for lung cancer. Chest 2007;132:200–6. [DOI] [PubMed] [Google Scholar]
- [16].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 18, 2017. [Google Scholar]
- [17].Gu WJ, Wang F, Tang L, et al. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest 2015;147:335–46. [DOI] [PubMed] [Google Scholar]
- [18].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [19].Akin Kabalak P, Çiledağ A, Demir N, et al. Prognostic significance of serum vascular endothelial growth factor and angiopoietin-2 in patients with lung cancer. Tuberk Toraks 2015;63:71–7. [DOI] [PubMed] [Google Scholar]
- [20].Ayten O, Tas D, Demirer E, et al. Angiopoietin 2 levels in serum and bronchial lavage fluids and their relationship with cancer stages in lung cancer patients. Thorac Cancer 2013;4:20–6. [DOI] [PubMed] [Google Scholar]
- [21].Cañadas I, Taus Á, Villanueva X, et al. Angiopoietin-2 is a negative prognostic marker in small cell lung cancer. Lung Cancer 2015;90:302–6. [DOI] [PubMed] [Google Scholar]
- [22].Coelho AL, Araújo AM, Gomes MP, et al. Combined Ang-2 and VEGF serum levels: holding hands as a new integral biomarker in non-small-cell lung cancers. Future Oncol 2015;11:3233–42. [DOI] [PubMed] [Google Scholar]
- [23].Daly S, Kubasiak JC, Rinewalt D, et al. Circulating angiogenesis biomarkers are associated with disease progression in lung adenocarcinoma. Ann Thorac Surg 1968;98:1968–75. discussion 1975. [DOI] [PubMed] [Google Scholar]
- [24].Liu YY, Wang SW. Expression and significance of VEGF and Ang-2 in serum of patients with NSCLC. J Mod Oncol 2015;23:2774–7. [Google Scholar]
- [25].Naumnik W, Chyczewska E, Ossolinska M. Serum levels of angiopoietin-1, angiopoietin-2, and their receptor Tie-2 in patients with nonsmall cell lung cancer during chemotherapy. Cancer Invest 2009;27:741–6. [DOI] [PubMed] [Google Scholar]
- [26].Park JH, Choi H, Kim YB, et al. Serum angiopoietin-1 as a prognostic marker in resected early stage lung cancer. Lung Cancer 2009;66:359–64. [DOI] [PubMed] [Google Scholar]
- [27].Xu C, Wang W, Wang Y, et al. Serum angiopoietin-2 as a clinical marker for lung cancer in patients with solitary pulmonary nodules. Ann Clin Lab Sci 2016;46:60–4. [PubMed] [Google Scholar]
- [28].Zhang CL, Ge SL, Yang N, et al. Elevated serum level of angiopoietin-2 as a potential marker for poor prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:305–9. [DOI] [PubMed] [Google Scholar]
- [29].Hu HD, Zou J, Zhang YQ. The clinical significance of serum angiopoietin-2 level in patients with lung cancer. J Clin Pulm Med 2013;18:2149–51. [Google Scholar]
- [30].Li MQ, Shi XK, You Q, et al. Clinical significance of tumor Ang-2 for lung cancer detection. Heilongjiang J Med 2015;28:17–9. [Google Scholar]
- [31].Wan RG, Chen DB, Zhou SH. The detection and clinical significance of serum angiopoietin-2 levels in non-small cell lung cancer. Clin J Lab Med 2008;31:540–2. [Google Scholar]
- [32].Xiao SH, Rong F, Huang J, et al. Relationship between dynamic changes of serum Ang2 and VEGF in NSCLC. Canc Res Prev Treat 2011;38:83–6. [Google Scholar]
- [33].Xu CH, Yu LK, Zhang Y. The levels and clinical significance of angiopoietin-2 in serum of patients with non-small cell lung cancer. J Bas Clin Oncol 2012;25:114–6. [Google Scholar]
- [34].Yang H, Luo SX. Six kinds of tumor markers in diagnosis of lung cancer and its clinical application value. J Mod Oncol 2016;24:577–81. [Google Scholar]
- [35].Wang YX, Wang HJ, Li MQ, et al. Application of new tumor marker, angiopoietin-2 detections in diagnosis of lung cancer. Int J Immunol 2012;35:160–2. [Google Scholar]
- [36].Zhai NL, Liu JP, Gao FQ. Expression of serum VEGF, Ang-2 and survivin in lung carcinoma and their correlation. J Binzhou Med Univ 2016;39:23–5. [Google Scholar]
- [37].Fawzy A, Gaafar R, Kasem F, et al. Importance of serum levels of angiopoietin-2 and survivin biomarkers in non-small cell lung cancer. J Egypt Natl Canc Inst 2012;24:41–5. [DOI] [PubMed] [Google Scholar]
- [38].Macchiarini P, Fontanini G, Hardin MJ, et al. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 1992;340:145–6. [DOI] [PubMed] [Google Scholar]
- [39].Sessa C, Guibal A, Del Conte G, et al. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat Clin Pract Oncol 2008;5:378–91. [DOI] [PubMed] [Google Scholar]
- [40].Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011;19:512–26. [DOI] [PubMed] [Google Scholar]
- [41].Huang H, Bhat A, Woodnutt G, et al. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer 2010;10:575–85. [DOI] [PubMed] [Google Scholar]
- [42].Xuan ZX, Zhang S, Yuan SJ, et al. Prognostic value of angiopoietin-2 in non-small cell lung cancer patients: a meta-analysis. World J Surg Oncol 2016;14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yuan A, Yang PC, Yu CJ, et al. Tumor angiogenesis correlates with histologic type and metastasis in non-small-cell lung cancer. Am J Respir Crit Care Med 1995;152:2157–62. [DOI] [PubMed] [Google Scholar]
- [44].Takanami I. Overexpression of Ang-2 mRNA in non-small cell lung cancer: association with angiogenesis and poor prognosis. Oncol Rep 2004;12:849–53. [PubMed] [Google Scholar]
- [45].Etoh T, Inoue H, Tanaka S, et al. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res 2001;61:2145–53. [PubMed] [Google Scholar]
- [46].Zhou L, Lan H, Zhou Q, et al. Plasma angiopoietin-2 is persistently elevated after non-small cell lung cancer surgery and stimulates angiogenesis in vitro. Medicine (Baltimore) 2016;95:e4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tanaka F, Ishikawa S, Yanagihara K, et al. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res 2002;62:7124–9. [PubMed] [Google Scholar]


