Abstract
Rationale:
Ticagrelor has become one of the first-line antiplatelet agents in acute coronary syndrome (ACS) patients recommend by the guideline due to its more potent and predictable antiplatelet effect. However, bleeding is still a severe drug adverse reaction of ticagrelor therapy. We report a first case on ticagrelor-induced life-threatening bleeding via the cyclosporine-mediated drug interaction.
Patient concerns:
A 58-year-old Chinese male who received cyclosporine 200 mg daily 5 years after renal transplantation. Ticagrelor was added for treating ACS. Unfortunately, gum bleeding and life-threatening bloody stool appeared 8 days later, accompanied with the sudden drop of blood pressure.
Interventions:
Ticagrelor was replaced with clopidogrel. Intravenous injection of proton pump inhibitor and agkistrodon snake venom hemocoagulase were used to stop the bleeding. Meanwhile, packed red blood cells and plasma were continuously transfused to maintain adequate blood volume.
Outcomes:
The patient's bloody stool was well controlled after treatment.
Lessons:
The present case demonstrates that a potential drug–drug interaction (DDI) may lead to a life-threatening drug adverse reaction especially in special subjects. Therefore, regarding DDI, optimizing antiplatelet treatment should be considered for the efficacy and safety of P2Y12 receptor antagonist in this fragile population.
Keywords: adverse drug reaction, bleeding, drug–drug interaction, ticagrelor
1. Introduction
Patients are potentially at risk of further ischemic events after acute coronary syndrome (ACS).[1] Currently, standard therapy for ACS patients is dual antiplatelet agents, comprising aspirin plus P2Y12 receptor antagonist (clopidogrel, prasugrel, or ticagrelor).[2] However, bleeding is a major side effect of antiplatelet agents, being a main cause of noncompliance.
Ticagrelor, a direct-acting P2Y12 receptor antagonist, plays a more rapid and effective platelet inhibition than other antiplatelet drugs (ticlopidin, clopidogrel, and prasugrel).[3] In PLATO trial, compared with clopidogrel, ticagrelor significantly reduced the risk of cardiovascular events in ACS patients with similar total major bleeding, but increased the risk of major bleeding of noncoronary artery bypass grafting.[4] Currently, no article reported ticagrelor-induced serious bleeding event mediated by an inhibitor of metabolic enzymes or efflux transport protein. This is the first report on ticagrelor-induced life-threatening bleeding caused by the drug–drug interaction (DDI) in a renal transplantation patient.
2. Case report
Approval for the study by the local institutional review board was not required because it was a case report. The present patient provided a written informed consent. A 58-year-old Chinese male with a history of renal transplantation, who had been taking cyclosporine 200 mg daily and mycophenolate mofetil 2 g daily for 5 years, presented with recurrent syncope and was admitted to the coronary care unit. Laboratory data on admission showed as follows: white blood cell count 8.5×109/L (reference,3.97–9.15×109/L); neutrophile granulocyte 60.5% (reference, 50–70%); hemoglobin 9.9 g/dL (reference, 13.1–17.2 g/dL); creatinine 186.8 μmol/L (reference, 45–104 μmol/L); creatinine clearance (40.48 mL/min); troponin I 1.2 ng/mL (reference, < 0.04 ng/mL); CK-MB 120 ng/mL (reference, 0–4.3 ng/mL); brain natriuretic protein (BNP) 55 pg/mL (reference, 0–100 pg/mL). Electrocardiogram showed sinus tachycardia and anterolateral myocardial ischemia. The patient was diagnosed with ACS based on clinical symptoms, laboratory data, and electrocardiogram. He underwent coronary angiography after receiving the loading dose of aspirin 300 mg and ticagrelor 180 mg. The coronary angiography results revealed normal left main coronary artery; total occlusion in the proximal segment of the left anterior descending (LAD) coronary artery; intimal flap (sign of plaque rupture) in the proximal segment of the left circumflex (LCX) coronary artery with collateral vessels originating from LCX connecting with the LAD distal to the occlusion; normal but very small right coronary artery. Thus, the patient underwent percutaneous coronary intervention (PCI). A Cordis Vista 6F JL3.5 guiding catheter was inserted into the left coronary artery. After insertion of a guiding wire into the vessel, a Firebird 3.5×23 stent was deployed at the proximal segment of the LCX covering the ruptured plaque, which was then post-dilated with an NC 3.75×15 balloon achieving good expansion. In an attempt to open the occlusion in the LAD, the Pilot 50, Pilot 150, and the Miracle 4.5 guidewires all failed to cross the occlusion, and no further attempts were made considering that the occlusion might be very long and severely calcified. Finally, a repeat angiogram showed no signs of vessel dissection, perforation or thrombus formation. The patient's symptoms and electrocardiogram improved after PCI and was prescribed aspirin (100 mg daily), ticagrelor (90 mg twice daily), atorvastatin (20 mg daily), and olmesartan (20 mg daily) orally for secondary prevention therapy.
Eight days later, the patient suffered from gum bleeding and bright red blood mixed with the stool (50 mL), accompanied with nausea and vomiting the next morning. Blood pressure suddenly dropped from 125/88 to 98/68 mm Hg, and the heart rate increased from 77–81 to 105–110 beats/min. Therefore, oral olmesartan therapy was discontinued, and pantoprazole (80 mg daily) was intravenously injected to stop the gastrointestinal bleeding. The next day, black gelatinous blood (200 mL) was found in the patient's stool in the morning, and the blood (350 mL) turned dark in the evening. Bloody stool (250–350 mL) could be observed about every 20 minutes; then, the hemoglobin level dropped from 9.9 to 4.6 g/dL (Fig. 1). At this juncture, ticagrelor was withdrawn immediately, whereas cyclosporine was maintained as the immunosuppression agent for his renal transplantation. In order to stop the bleeding, the intravenous injection of pantoprazole (80 mg twice daily) and agkistrodon snake venom hemocoagulase (2U daily) were taken. In addition, norepinephrine (2 mg daily) was subsequently administrated through the nasogastric feeding tube. Meanwhile, packed red blood cells (10U total) and plasma (600 mL total) were continuously transfused in an attempt to maintain adequate blood volume. Gastroscopic and enteroscopic examination failed to reveal the location of gastrointestinal hemorrhage. The patient's bleeding was well controlled 5 days after treatment (total bloody stool volume of 920 mL). The hemoglobin level gradually increased (7.8, 8.0, 8.0, and 8.9 g/dL, respectively), with the fecal occult blood test being negative.
Figure 1.
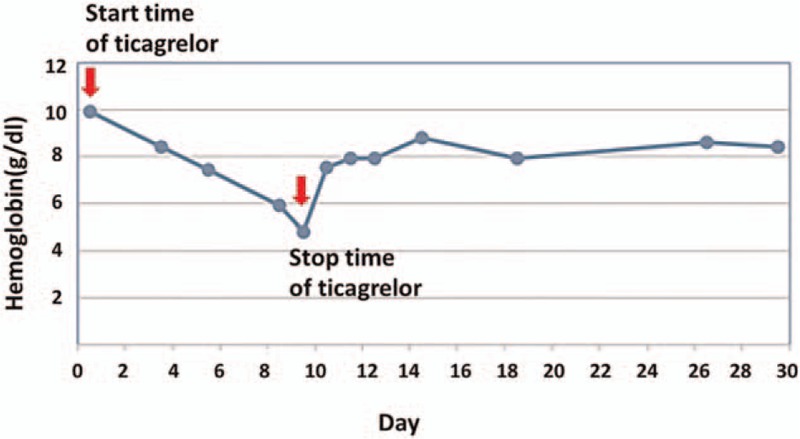
The changes of hemoglobin level after ticagrelor-induced bleeding.
Pharmacist considered that a DDI might exist between ticagrelor and cyclosporine, which could lead to an increase in the serum concentration of ticagrelor and result in serious bleeding. Thus, ticagrelor was replaced with clopidogrel (75 mg daily) after the resolution of his serious bleeding. The patient's Naranjo Adverse Drug Reaction Probability Scale score being 7 showed that his life-threatening bleeding was probably caused by ticagrelor (Table 1). The Naranjo's ADR algorithm is a 10-point questionnaire for determining the possibility of drug-related adverse reaction, in which terms such as definite (>8 points), probably (4–8 points), possible (1–4 points), and doubtful (0 points) are calculated.
Table 1.
Adverse drug reaction probability scale of the present case.

3. Discussion
In this case, the patient's life-threatening bleeding was probably caused by ticagrelor according to the high score of Naranjo Adverse Drug Reaction Probability Scale. To be our best knowledge, this is the first report on ticagrelor-induced serious bleeding in a renal transplantation patient due to a potential DDI. Cyclosporine, in this case, might be the factor that raised the concentration of ticagrelor, contributing to the development of ticagrelor-induced bleeding.
Ticagrelor, a reversibly binding P2Y12 receptor antagonist, exhibits linear and predictable pharmacokinetics over a wide dose range.[5,6] After rapid absorption, it is converted to the major active metabolite, AR-C124910XX, via CYP3A enzymes.[7] Like ticagrelor, AR-C124910XX has similar potency in the inhibition of platelet aggregation.[8,9] In a vitro metabolism study, it is clarified that ticagrelor is a substrate and weak inhibitor of cytochrome P450 3A4 isozyme (CYP3A4), as well as a substrate and weak inhibitor of P-glycoprotein (P-gp).[8] Meanwhile, cyclosporine is a potent inhibitor of P-gp and other drug transporters (organic anion transporting polypeptide, and breast cancer resistance protein).[10] Of note, cyclosporine is a probe inhibitor of P-gp recommended by US FDA. As ticagrelor and cyclosporine was co-administered in the present case, a DDI may occur. A pharmacokinetic interaction study has revealed an increased ticagrelor area under the curve (AUC) and maximum concentration (Cmax) of 183% and 130% in the presence of cyclosporine.[11] The similar results were observed in its active metabolite (AR-C124910XX).[11] It is noteworthy that the combination of ticagrelor and cyclosporine was generally well tolerated in healthy volunteers. However, in the present case, the plasma concentration of ticagrelor in a patient with renal insufficiency was likely increased to a higher degree with co-administration. It is possible that P-gp inhibition mediated by cyclosporine is the main mechanism resulting in an increased bleeding risk of ticagrelor. P-gp, an efflux transport that is present in the apical side of the intestinal epithelium, prevents intracellular accumulation of its substrates and could alter the plasma concentration of the drugs by pumping them into the intestinal lumen.[12] P-gp exports pharmacologically and structurally diverse hydrophobic compounds from cells and may be affected by some inhibiting drugs.[10,13] The inhibiting agents usually have similar features to the P-gp substrates or may themselves be substrates of P-gp. For example, cyclosporine is a P-gp substrate as well as a strong inhibitor of P-gp.
In this case, the patient suffered a bleeding with a decline in the hemoglobin level of 5.3 g/dL and received transfusion of 10 units of packed red blood cells, which meet the definition of major bleeding in the PLATO study (a decline in the hemoglobin level of 5.0 g/dL or more, or the need for transfusion of 4 or more units of packed red blood cells).[4] Also, gastrointestinal bleeding was excluded by gastroscopy and enteroscopy, meaning that aspirin was less likely as the culprit for the major bleeding.[14] Because of high score of Naranjo Adverse Drug Reaction Probability Scale, patient's life-threatening bleeding therefore was probability caused by ticagrelor.
Ticagrelor's adverse effect on major bleeding is relatively small and was not associated with a significantly higher risk compared with clopidogrel.[4] However, when regarding Asian population, ticagrelor showed a numerically higher risk of major bleeding compared with clopidogrel.[4] Meanwhile, multivariate analysis indicated that several factors (creatinine clearance under 60 mL/min, the hemoglobin level under 150 g/L, female, and treatment with ticagrelor) were the independent predictors of major bleeding.[4] According to the findings in the PLATO study, the patient in this case report had 3 above independent risk factors, including decreased creatinine clearance (40.48 mL/min), decreased hemoglobin level (99 g/L on admission), and ticagrelor treatment, which contribute to a high bleeding risk.
4. Conclusion
Co-administration of ticagrelor with cyclosporine increased exposure to ticagrelor and its metabolite mediated by P-gp, leading an enhanced risk of bleeding in these fragile patients with renal transplantation. Therefore, potential DDI is worthy of clinical attention and optimal treatment strategy should be considered to ensure the safety of drugs.
Footnotes
Abbreviations: ACS = acute coronary syndrome, AUC = area under the curve, Cmax = maximum concentration, CYP3A4 = cytochrome P450 3A4 isozyme, DDI = drug–drug interaction, P-gp = P-glycoprotein.
CZ, LS, and MC contributed equally to the study as first authors.
Funding: This work was supported by Science Fund of Hospital Pharmacy of Shanghai Jiao Tong University, School of Medicine (JDYX2016ZD003), Program for Key Discipline of Clinical Pharmacy of Shanghai (2016), Program for Key but Weak Discipline of Shanghai Municipal Commission of Health and Family Planning (Clinical Pharmacy, 2017) and National Natural Science Foundation of China (81502991).
The authors have no conflicts of interest to disclose.
References
- [1].Pu J, Mintz GS, Brilakis ES, et al. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J 2012;33:372–83. [DOI] [PubMed] [Google Scholar]
- [2].Sabouret P, Taiel-Sartral M. New antiplatelet agents in the treatment of acute coronary syndromes. Arch Cardiovasc Dis 2014;107:178–87. [DOI] [PubMed] [Google Scholar]
- [3].Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- [4].Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2011;32:2933–44. [DOI] [PubMed] [Google Scholar]
- [5].Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol 2010;70:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].RT KB. AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect, in healthy subjects. Can J Clin Pharmacol 2008;15:1. [Google Scholar]
- [7].Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug–drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics. Drug Metab Dispos 2011;39:703–10. [DOI] [PubMed] [Google Scholar]
- [8].Teng R, Butler K. The effect of ticagrelor on the metabolism of midazolam in healthy volunteers. Clin Ther 2013;35:1025–37. [DOI] [PubMed] [Google Scholar]
- [9].Teng R, Mitchell PD, Butler KA. Pharmacokinetic interaction studies of co-administration of ticagrelor and atorvastatin or simvastatin in healthy volunteers. Eur J Clin Pharmacol 2013;69:477–87. [DOI] [PubMed] [Google Scholar]
- [10].Yigitaslan S, Erol K, Cengelli C. The effect of P-gp inhibition and activation on the absorption and serum levels of cyclosporine and tacrolimus in rats. Adv Clin Exp Med 2016;25:237–42. [DOI] [PubMed] [Google Scholar]
- [11].Teng R, Kujacic M, Hsia J. Pharmacokinetic interaction study of ticagrelor and cyclosporine in healthy volunteers. Clin Drug Investig 2014;34:529–36. [DOI] [PubMed] [Google Scholar]
- [12].Hamman JH, Demana PH, Olivier EI. Targeting receptors, transporters and site of absorption to improve oral drug delivery. Drug Target Insights 2007;2:71–81. [PMC free article] [PubMed] [Google Scholar]
- [13].Krishna R, Bergman A, Larson P, et al. Effect of a single cyclosporine dose on the single-dose pharmacokinetics of sitagliptin (MK-0431), a dipeptidyl peptidase-4 inhibitor, in healthy male subjects. J Clin Pharmacol 2007;47:165–74. [DOI] [PubMed] [Google Scholar]
- [14].Valkhoff VE, Sturkenboom MC, Kuipers EJ. Risk factors for gastrointestinal bleeding associated with low-dose aspirin. Best Pract Res Clin Gastroenterol 2012;26:125–40. [DOI] [PubMed] [Google Scholar]


