Abstract
Background
To search for association between ultrasound (US) enthesis abnormalities and disease activity, spine and sacro-iliac joints (SIJ) MRI inflammatory lesions and spine structural changes in a cohort of patients suspected for axial spondyloarthritis (SpA).
Methods
Patients: Of 708 patients included in the DESIR(Devenir des Spondyloarthrites Indifférenciées Récentes) cohort, 402 had an US enthesis assessment and were selected for this study. Imaging: Achilles, lateral epicondyles, superior patellar ligament, inferior patellar ligament entheses were systematically US scanned and abnormalities were summed in US structural and power Doppler (PDUS) scores. Spine radiographs, SIJ and spine MRI scans were centrally scored modified Stoke Ankylosing Spondylitis Spine Score (mSASSS), presence of MRI sacro-iliitis, Spondyloarthritis Research Consortium of Canada and Berlin scores. Analysis: The associations between the US structural/PDUS scores and disease activity, C reactive protein (CRP), MRI SIJ and spine inflammatory lesions and mSASSS were tested by Spearman's correlation tests.
Results
Among the 402 patients included (median age: 33.5 years, males: 48.5%), 55% had US enthesis structural abnormalities while 14% had PDUS abnormalities. There was no association between US scores and Bath Ankylosing Spondylitis Disease Activity Index, CRP or inflammatory lesions on SIJ and spine MRI. There was a correlation between US structural and PDUS scores and the mSASSS (respectively, r=0.151, p=0.005; r=0.143, p=0.007). The proportion of patients with syndesmophytes was higher in the case of US enthesophytes (26% of syndesmophytes vs 6% in the absence of US enthesophytes, p<0.0001).
Conclusion
While the US abnormalities do not seem to be a helpful tool for monitoring disease activity in axial SpA, US enthesophytes, strongly associated with axial syndesmophytes, might be a marker of interest for disease severity.
Trial registration number
NCT01648907, date of registration : 20 July 2012.
Keywords: spondyloarthritis, ultrasound, syndesmophytis, sacro-iliitis
Key messages.
What is already known about this subject?
The association between disease activity and enthesis ultrasound findings is not clear in the literature.
What does this study add?
Ultrasound peripheral enthesitis was not associated with clinical disease activity in patients with early axial spondyloarthritis (SpA).
Ultrasound peripheral enthesitis was not associated with sacroiliitis nor with MRI spine inflammatory lesions.
Ultrasound enthesophyte presence was strongly associated with syndesmophyte presence.
How might this impact on clinical practice?
Ultrasound enthesophyte might be a marker of disease severity in axial SpA.
Enthesitis is the inflammation of insertions of tendons, ligaments and capsules into the bone, and a characteristic sign of spondyloarthritis (SpA). In axial SpA, the frequency of peripheral enthesitis has been found to be within 25%–58%,1 but the real prevalence of this feature depends on the type of assessment (ie, clinical, imaging or histological). Peripheral enthesitis is usually revealed by clinical findings, such as localised pain, tenderness and swelling. Nevertheless, there are no definite clinical criteria for the diagnosis of such manifestations, which may even be asymptomatic. Histological examination of the enthesis is the potential gold standard for evaluation of enthesitis, but is rarely carried out due to ethical and practical constraints.
Ultrasonography (US) has proved to be a sensitive and non-invasive tool to assess the presence of enthesitis, characterised by hypoechogenicity with loss of the tendon fibrillar pattern, tendon thickening, local calcifications, enthesophytes and bone erosions. Moreover, the use of power Doppler US (PDUS) allows the detection of abnormal vascularisation of soft tissues in inflammatory articular diseases.2–7
Recently, it has been suggested that enthesis US could be used for monitoring disease activity and therapeutic response assessment.8–10 However, further studies are needed to confirm the potential associations between US enthesis changes and disease activity or structural axial changes.
In this study, we investigated the prevalence of US enthesis abnormalities, the concordance between US abnormalities and enthesis pain and searched for association between US enthesis abnormalities and disease activity, functional impairment, MRI inflammatory lesions on sacro-iliac joints (SIJ) and spine and radiographic structural changes on spine and pelvis X-rays in a cohort of patients with early inflammatory back pain (IBP) suggestive of axial SpA (Devenir des Spondyloarthrites Indifférenciées Récentes (DESIR) cohort).
Patients and methods
Study population
The DESIR cohort has already been described before.11 Briefly, DESIR is a French prospective longitudinal cohort study monitoring 708 patients (aged between 18 and 50 years) over time with IBP suggestive of axial SpA according to the rheumatologist opinion for ≥3 months and <3 years in 25 centres across France between 2007 and 2010. All patients included in the study had a collection of clinical data, blood samples, pelvis and spine plain X-rays, SIJ and spine MRI at baseline. Among these patients, 402 had also a peripheral enthesis US and were selected for the present study. The present study involved the baseline database of the DESIR cohort.
Ethics, consents and permission
The study complied with Good Clinical Practice Guidelines (clinicaltrials.gov: NCTO 164 8907, https://clinicaltrials.gov/ct2/show/NCT01648907) and was approved by the appropriate medical ethic committee (CPP Ile-de-France III, submission number P070302). Patients gave informed consent before the start of the study including permission to publish any individual data treated anonymously.
Clinical and biological data
For the present study, demographic characteristics including age, sex, disease duration and disease activity data were analysed at the outset. The disease activity included the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Ankylosing Spondylitis Disease Activity Score-C reactive protein (ASDAS-CRP), the Bath Ankylosing Spondylitis Functional Index (BASFI), the Bath Ankylosing Spondylitis Metrology Index (BASMI), the Health Assessment Questionnaire for Ankylosing Spondylitis (HAQ-AS), the spondyloarthritis classification criteria including ASAS 2009,12 Amor criteria,13 European Spondylarthropathy Study Group criteria14 or modified New York criteria15 the HLA-B27 status and CRP levels.
Clinical enthesitis presence was assessed according to three different outcomes: 1) presence (yes/no) of an enthesis pain during the physical examination of one of the entheses that had had an US (including Achilles, lateral epicondyle, superior patellar ligament, inferior patellar ligament); 2) history of enthesis pain (yes/no) from one of the entheses that had had an US (same entheses); 3) the Maastricht Ankylosing Spondylitis Entheses Score (MASES: 0–13).16 The data set locked on 30 June 2010 was used for this study.
US measurements
All US were performed in local centres by experienced rheumatologists or radiologists using a 12–16 MHz linear array transducer. Power Doppler settings were standardised with a pulse repetition frequency of 500–800 Hz. The following entheses were scanned along long axis and short axis views: left and right Achilles, lateral epicondyles, superior patellar ligament, inferior patellar ligament. These enthesis sites were selected by the scientific committee of DESIR cohort, based on the literature review. The USs were performed in each patient included in the DESIR cohort blinded to the history of clinical enthesitis.
For each site, the following abnormalities were sought and recorded on a standardised form: presence of abnormal thickness, calcifications, enthesophytes, bony erosions defining structural enthesis changes and the presence of power Doppler signals on the bone-enthesis junction defining inflammatory enthesitis, according to the Outcomes Measures in Rheumatology (OMERACT) definitions.17 Furthermore, a search was also made for any retrocalcaneal bursitis with power Doppler signals.
The US structural changes and inflammatory enthesitis were described according to the each enthesis site. All individual US structural abnormalities (including abnormal thickness, bony erosions, calcifications and enthesophytes, Achilles bursitis—each abnormality observed receiving one point) on each enthesis (eight sites) were summed to get a longitudinal score from 0 to 26 named US structural score as previously proposed.18–20 Inflammatory enthesitis defined by enthesis with an abnormal power Doppler signal at the bone-enthesis junction were also summed in a PDUS score from 0 to 8. As only eight enthesis sites were assessed in the DESIR cohort, previously published scores such as Glasgow Ultrasound Enthesitis Scoring System (GUESS), Bochum Ultrasound Score (BUSES) or Madrid Sonographic Enthesis Index (MASEI)18–20 could not be used for analysis, but the method of adding every structural abnormality of each enthesis site was similar.
Radiograph scoring
Sacro-iliitis on radiographs was centrally assessed according to the modified New York criteria15 by two different blind and trained readers (moderate inter-reader agreement with a kappa about 0.54).21
Assessment of spine structural changes centrally scored by two different blind and trained readers (moderate inter-reader agreement with a kappa about 0.50)22 were analysed for two outcomes: 1) the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS)23 and 2) presence of at least one syndesmophyte on cervical or lumbar spine plain X-rays.
MRI sacro-iliac and spine scoring
An MRI scan of the sacro-iliac joints was performed at the beginning, in each participating centre, using 1.0–1.5 T machines. The acquired sequences were coronal oblique T1-weighted fat spin echo (FSE) and short tau inversion recovery (STIR) with 12–15 semi-coronal slices of 4 mm thickness, parallel to the long axis of the sacrum. All available baseline MRI sacro-iliitis were centrally read independently by the two readers, blinded for all clinical and laboratory data. MRI scans of SIJ were considered positive according to the ASAS definition24: if bone marrow oedema lesions, highly suggestive of SpA, were present, if ≥1 bone marrow oedema lesions on ≥2 consecutive slices or if several bone marrow oedema lesions were visible on a single slice. Inter-reader agreement for the definition of a MRI sacro-iliitis was good (kappa about 0.74). The two readers also scored the MRI scans (intraclass correlation coefficient was about 0.94 for absolute baseline scores) for the presence of inflammatory lesions according to the Spondyloarthritis Research Consortium of Canada (SPARCC) scoring method (range 0–72).25 26
MRI scans of the thoracic spine and lumbar spine were performed using the T1-weighted spin echo and short-tau inversion recovery sequences. Inflammatory lesions were centrally scored by two independent readers blinded to clinical data using Berlin score.27
Statistical analysis
All data (clinical, biological and imaging) were collected the same day, at the baseline visit, as requested by the DESIR protocol.
Patients included in this study were all described as were the patients from the DESIR cohort who did not have any US examinations.
The concordance and agreement between enthesis clinical pain or history of enthesis pain and US structural changes or the presence of power Doppler signal abnormalities were assessed for each site with a concordance rate and kappa statistics. Since the frequency of US abnormalities was low, it is possible that a bias in kappa statistics could have been at play; therefore, a prevalence-adjusted and bias-adjusted kappa (PABAK) was also calculated, as previously proposed.28 A correlation between clinical enthesitis assessed by the MASES and the US findings assessed by the US structural score and the PDUS score was searched by a Spearman’s correlation test.
Associations between the US structural score and PDUS score and disease activity assessed by the BASDAI, the ASDAS-CRP, the CRP or functional impairment assessed by the BASFI, the BASMI or the HAQ-AS were sought by carrying out the Spearman's correlation test. Associations between PDUS and non-steroidal anti-inflammatory drug (NSAID) intake were also tested by two different ways: NSAID intake (yes/no) with a Wilcoxon test and NSAID Dougados index with a Spearman's correlation test. Furthermore, as enthesophyte presence, especially on Achilles enthesis can be associated with overweight, the association between BMI and at least one enthesophyte was investigated.
US structural abnormalities (yes/no) and power Doppler presence (yes/no) were compared between patients according to the presence of a MRI sacro-iliitis using a χ2 test. A correlation between US structural score and PDUS score and SIJ MRI bone marrow oedema assessed by the SPARCC score was sought using the Spearman’s correlation test.
An association between US structural score and syndesmophyte presence was investigated. A correlation between the US structural score and the mSASSS was calculated with a Spearman’s correlation test. The mSASSS scores were compared according to the presence of each individual US structural abnormality (each abnormality yes/no) by the Wilcoxon test. The proportions of patients with at least one US enthesophyte as well as with at least one enthesis with a PDUS signal were compared between patients with or without at least one syndesmophyte and also between patients with a mSASSS score of <two points versus patients with mSASSS of at least two points.
Finally, to assess the independence of associations between US structural and PDUS scores and axial structural changes, a multivariate analysis was performed through a logistic regression where the explanatory variable was a mSASSS score of 2 or more. Two models were performed: the first model included as covariates age, sex, BMI, HLA-B27 presence, ASDAS-CRP MRI sacro-iliitis presence, smoking status, disease duration, US structural score and PDUS score. The second model included the same covariates except US structural change that was replaced by the presence of at least one US enthesophyte.
All statistics were performed using STATA software. No missing data imputation was used in these analyses.
Results
Description of the study population
The baseline characteristics of the 402 patients included in this study were similar to the patients who did not have any history of US tests (table 1).
Table 1.
Characteristics of the study population patients compared with the patients who had not had an US at baseline
| Baseline characteristics | Patients with US: n=402 | Patients without US: n=306 |
| Age, year, med (IQR) | 33.5 (26.7–39.4) | 33.1 (26.6–40.6) |
| Male (%) | 195 (48.5) | 132 (43.1) |
| Symptom duration, months, med (IQR) | 17.1 (8.7–27.8) | 16.7 (10.0–24.8) |
| Number of patients with at least one clinical enthesitis (%) | 259 (64.4) | 200 (65.3) |
| BASDAI, med (IQR) | 45 (29–60) | 47 (28–62) |
| ASDAS-CRP, med (IQR) | 2.55 (1.89–3.23) | 2.64 (1.88–3.26) |
| MASES, med (IQR) | 2 (0–4) | 2 (0–4) |
| HLA B27 positivity, number (%) | 233 (58.0) | 177 (57.8) |
| Meeting diagnostic criteria for axial SpA*, n (%) | 375 (93.3) | 275 (89.9) |
| mSASSS, med (IQR) | 0 (0–0) | 0 (0–0) |
| Patients with positive MRI sacro-iliitis†, number (%) | 134 (33.3) | 85 (27.8) |
| SPARCC, med (IQR) | 0 (0–3.5) | 0 (0–2.5) |
| Berlin score, med (IQR) | 0 (0–0) | 0 (0–0) |
| NSAIDs intake, number (%) | 366 (91.0) | 272 (88.9) |
| CRP (mg/L), med (IQR) | 3.5 (2.0–7.3) | 3.1 (2.0–7.0) |
*One criteria set for axial SpA: patients meeting at least one of the following diagnostic criteria set for axial SpA: modified New York criteria, 2009 ASAS criteria, Amor criteria, ESSG criteria.
†positive sacro-iliac joint MRI according to ASAS definition.
ASDAS-CRP, Ankylosing Spondylitis Disease Activity Score with C reactive protein; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; Berlin score: spine MRI scoring system; ESSG, European Spondylarthropathy Study Group; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; NSAIDs, non-steroidal anti-inflammatory drugs; SPARCC, Spondyloarthritis Research Consortium of Canada scoring system (sacro-iliac joints MRI scoring system); US, ultrasound.
In this study, 58% of patients carried the HLA-B27 allele, one-third had a MRI sacro-iliitis according to the ASAS definition and most of them had no syndesmophyte at baseline, with an mSASSS median about 0. Patients had also an active disease with ASDAS-CRP median about 2.55 and a BASDAI median about 45/100. 91% of patients received NSAIDs.
Description of US abnormalities
The frequency of US structural and inflammatory abnormalities is summarised in table 2.
Table 2.
Description of US enthesis abnormalities: number (%) on 402 patients
| Superior patellar ligament | Inferior patellar ligament | Lateral epicondyle | Achilles enthesis | |
| Abnormal thickness | ||||
| Unilateral Bilateral |
25 (6.5) 15 (3.9) |
24 (6.3) 6 (1.6) |
10 (2.6) 8 (2.1) |
27 (7.1) 12 (3.1) |
| Bone erosions | ||||
| Unilateral Bilateral |
12 (3.1) 5 (1.3) |
14 (3.6) 3 (0.8) |
6 (1.6) 5 (1.3) |
22 (5.5) 4 (1.0) |
| Calcifications | ||||
| Unilateral Bilateral |
18 (4.7) 5 (1.3) |
30 (7.8) 7 (1.8) |
26 (6.8) 10 (2.6) |
18 (4.7) 7 (1.8) |
| Enthesophytes | ||||
| Unilateral Bilateral |
17 (4.4) 4 (1.0) |
16 (4.2) 0 (0.0) |
18 (4.7) 7 (1.8) |
36 (9.0) 37 (9.2) |
| Doppler signal | ||||
| Unilateral Bilateral |
12 (3.1) 1 (0.3) |
21 (5.2) 6 (1.5) |
12 (3.1) 7 (1.7) |
13 (3.2) 2 (0.5) |
| Bursitis | NA | NA | NA | |
| Unilateral Bilateral |
30 (7.5) 11 (2.7) |
NA, not assessed; US, ultrasound.
Two hundred and six patients (55.2%) had at least one structural change on one enthesis site and only 56 patients (14.6%) had a power Doppler signal observed on one enthesis site. The most frequent structural change observed was the presence of enthesophytes on Achilles entheses noted in 36 patients (9.0%) on only one Achilles enthesis and in 37 patients (9.1%) on both Achilles entheses.
Concordance and agreement between current enthesis pain or history of enthesis pain and US findings
There was a moderate to good concordance and agreement between current, or history, of enthesis pain and US abnormalities, at the enthesis level.
Concordance rates between current pain during physical examination or history of enthesis pain and US structural changes were similar—about 74%–88%. Achilles enthesis history of pain was the enthesis site the least associated with US structural change (concordance rate about 74%–77%). The concordance rates between current or history of enthesis pain and power Doppler signal abnormalities were high, contained between 86% and 95%. Agreement between current, or history of, enthesis pain and US structural changes or power Doppler signal abnormalities assessed by the kappa statistics was weak, contained between −0.02 and 0.21. However, the low frequency of structural or inflammatory abnormalities at each site may induce a bias in the kappa statistics estimation and it is thus recommended to calculate PABAK in such a situation.28 The agreement assessed by the PABAK thus revealed a moderate-to-good agreement with a PABAK contained between 0.55 and 0.75 for agreement between current, or history of, enthesis pain and US structural change and a good agreement with PABAK contained between 0.68 and 0.90 for agreement between current, or history of, enthesis pain and pwer Doppler abnormalities (see online supplementary table S1).
rmdopen-2017-000482supp001.docx (15.1KB, docx)
However, at the patient level, neither the US structural score nor the PDUS scores correlated with clinical enthesitis assessed with the MASES (correlation with US structural score: r=0.071, p=0.2; correlation with PDUS score: r=0.059, p=0.2).
Association between US enthesis abnormalities and baseline disease activity and functional impairment
The US structural score was not correlated with disease activity assessed by the BASDAI (r=0.095, p=0.07), the ASDAS-CRP (r=0.075, p=0.2) or CRP (r=0.093, p=0.07). The PDUS score was weakly correlated with the BASDAI (r=0.103, p=0.04), but not with the ASDAS-CRP (r=0.075, p=0.2) nor with the CRP (r=0.053, p=0.304). There was no association between the US structural score or PDUS score and the presence of clinical peripheral arthritis (respectively p=0.5 and p=0.7 for the comparison of scores according to the presence of at least one peripheral arthritis). PDUS was not associated with NSAID intake, taken as a qualitative variable (yes/no; p=0.7) or as a quantitative variable (NSAID Dougados index: Spearman's correlation test: r=−0.09; p=0.08).
The US structural score was not correlated with the BASFI (r=0.064, p=0.2) or the BASMI (r=0.069, p=0.2), but was weakly correlated with the HAQ-AS (r=0.126, p=0.02). The PDUS score was not correlated with any of the BASFI (r=0.092, p=0.07), the BASMI (r=0.101, p=0.06) or with the HAQ-AS (r=0.090, p=0.079). Finally, as expected, the presence of at least one enthesophyte was strongly associated with BMI (BMI median in patients with at least one enthesophyte=24.41 (IQR: 22.27–28.09); BMI median in patients without enthesophytes: 22.54 (IQR: 20.76–25.40); p=0.0001).
Relationship between US enthesis abnormalities and MRI inflammatory lesions
Neither US structural changes nor power Doppler signal abnormalities were related to MRI sacro-iliitis (table 3).
Table 3.
Comparison of US enthesis abnormalities according to the presence of an MRI sacro-iliitis according to the ASAS definition
| Patients with sacro-iliitis, n=134 | Patients without sacro-iliitis, n=262 | p Value | |
| US structural score, med (IQR) | 1 (0–2) | 0 (0–1) | 0.2 |
| US PD score, med (IQR) | 0 (0–0) | 0 (0–0) | 0.4 |
| Number (%) of patients with at least one enthesis abnormal thickness | 33/123 (26.8) | 58/247 (23.5) | 0.5 |
| Number (%) of patients with at least one enthesis bone erosion | 21/126 (16.7) | 38/250 (15.2) | 0.7 |
| Number (%) of patients with at least one enthesis calcification | 31/126 (24.6) | 61/250 (24.4) | 0.9 |
| Number (%) of patients with at least one enthesophyte | 32 (25.4) | 67 (26.7) | 0.8 |
| Number (%) of patients with at least one enthesis abnormal PD signal | 21/126 (16.7) | 35/251 (13.9) | 0.5 |
| Number (%) of patients with at least one Achilles bursitis | 16/134 (11.9) | 25/262 (9.5) | 0.5 |
US structural score, sum of all the entheses with calcification, bone erosions, abnormal thickness, bursitis, enthesophytes; US PD score, sum of all the enthesis with an abnormal power Doppler signal; US, ultrasound.
Neither the US structural score nor the PDUS score correlated with inflammatory lesions on SIJ (correlation between SPARCC score and US structural score: r=0.025, p=0.6; correlation between SPARCC and PDUS score: r=0.005, p=0.9) or the spine (correlation between Berlin score and US structural score: r=−0.022, p=1.0; correlation between Berlin score and PDUS score: r=−0.009, p=0.9).
Relationship between US enthesis abnormalities and radiographic structural changes
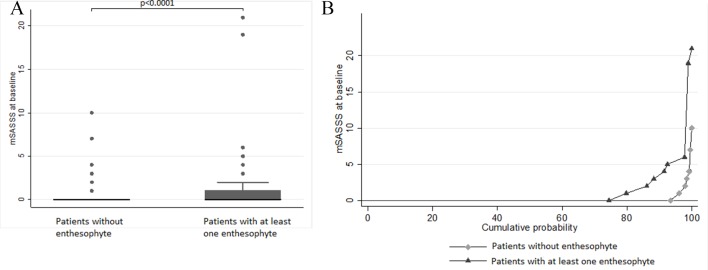
There was a significant correlation between the US structural score and the mSASSS (r=0.151, p=0.005) and between the PDUS score and the mSASSS (r=0.143, p=0.007). When analysing each different abnormality of the structural change, the strongest association between US structural change and mSASSS was observed with the presence of at least one enthesophyte (comparison of the medians of the mSASSS according to the presence of at least one enthesophyte detected by US: p<0.0001, see figure 1A and B). The proportions of patients with syndesmophyte on spine radiographs or with a mSASSS of 2 or more were higher in the presence of enthesophytes observed with US or presence of power Doppler on at least one enthesis (table 4).
Figure 1.
(A) Box plots of mSASSS medians according to the presence or absence of at least one enthesophytis on ultrasound. (B) Cumulative probability plots of mSASSS according to the presence or absence of at least one enthesophytis on ultrasound. mSASSS, modified Stoke Ankylosing Spondylitis Spine Score.
Table 4.
Relationship between ultrasound abnormalities and spine syndesmophytes
| Presence of syndesmophytes | mSASSS | |||||
| At least one | Absence | p Value | mSASSS≥2 | mSASSS<2 | p Value | |
|
Enthesophytes
At least one Absence |
24 (25.5) 17 (6.4) |
70 (74.5) 247 (93.6) |
<0.0001 | 19 (20.2) 10 (3.8) |
75 (79.8) 254 (96.2) |
<0.0001 |
|
PD signal
At least one enthesis with PD Absence of PD |
11 (28.2) 42 (13.2) |
28 (71.8) 277 (86.8) |
0.01 | 11 (20.8) 16 (5.2) |
42 (19.2) 289 (94.8) |
<0.0001 |
mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; PD, power Doppler.
There was also a significant association between the mSASSS and enthesis abnormal thickness (p=0.005 for comparison of mSASSS medians according to the presence of at least one enthesis with abnormal thickness) and enthesis calcification (p=0.01 for comparison of mSASSS medians according to the presence of at least one enthesis with a calcification).
However, neither US structural score nor PDUS score correlated with the presence of a radiographic sacro-iliitis (respectively p=0.7 and p=0.8 for comparison of US structural score and PDUS scores according to the presence of a radiographic sacroiliitis).
To investigate the independence of the association between the US enthesis changes and the mSASSS, a logistic regression was performed where the explanatory variable was an mSASSS of 2 points or more. In the first model, age, sex, disease duration, HLA-B27 presence, BMI, MRI sacro-iliitis presence, ASDAS-CRP, smoking status, US structural and PDUS scores were the covariates. After backward procedure, the covariates independently associated with a mSASSS of 2 points or more were age (OR=1.08 (95% CI 1.02 to 1.16)), gender (if females: OR=0.33 (95% CI 0.12 to 0.89)), tobacco (OR=2.78 (95% CI: 1.10 to 7.02)) and US structural score (OR=1.19 (95% CI: 1.00 to 1.40)). In the second model, replacing the US structural score by enthesophyte presence enhanced the association between US abnormality and mSASSS (in the case of enthesophyte, probability to observe a mSASSS of 2 points or more: OR=3.83 (95% CI: 1.57 to 9.39)).
Discussion
In this study, about 55% of patients had at least one structural change on one enthesis site and 15% had at least one power Doppler signal observed on one enthesis site assessed by US. There was a moderate-to-good concordance and agreement between current, or history of, enthesis pain and US abnormalities, at the enthesis level. No relationships between US scores and disease activity parameters or functional impairment were observed except a weak association between PDUS score and the BASDAI and between US structural score and the HAQ-AS. Neither the US structural changes nor power Doppler signal abnormalities were associated with MRI sacro-iliitis. However, there was a significant and strong association between US structural score and PDUS score and the mSASSS and especially between the presence of US enthesophytes and syndesmophytes. No relationship between US score and radiographic sacro-iliitis was observed.
To our knowledge, this is the first study investigating the potential relationship between US abnormalities and spine and SIJ MRI inflammatory lesions. Previous studies found controversy results about association between clinical disease activity and US enthesis changes8–10 and in this study there was no correlation between objective inflammatory parameters such as inflammatory lesions on MRI or CRP and US abnormalities do not suggest recommending the use of peripheral enthesis US for monitoring disease activity in early axial SpA.
The most interesting findings of this study was the strong association between peripheral US enthesophytes and early syndesmophytes. To our knowledge, only one recent study already showed such a relationship, but the study was performed on a late disease population of ankylosing spondylitis.29 However, as enthesophytes are not specific to SpA and can also be observed in mechanic diseases these results should be interpreted with caution.
The association between peripheral enthesophyte and syndesmophyte progression remains to be demonstrated by further research.
This work has several limitations. The first one is the absence of reliability study of US findings. As 25 centres participated to DESIR cohort, it was not feasible to organise a reliability study of US scoring. As US is above all a dynamic exam, scoring on scanned images was not used for a centralised scoring. However, only experienced radiologists or rheumatologists participated to the collection of US data in DESIR cohort. The second limit was that only eight sites were examined by US and previous published scores such as GUESS, BUSES or MASEI19 30 could not be used. However, even if the OMERACT definitions of US enthesis abnormality was not published yet,17 the same definition of structural changes and power Doppler abnormality were used in this study and summed in a score as BUSES or MASEI score.
In this study, the US abnormalities were not significantly related to disease activity, functional impairment or MRI inflammatory lesions and did not seem to be a helpful tool to assess disease activity in axial SpA. However, US enthesis changes, and especially enthesophytes, are strongly associated with axial syndesmophytes and might be a marker of interest for disease severity.
Acknowledgments
The DESIR cohort is conducted under the control of Assistance Publique-Hôpitaux de Paris via the Clinical Research Unit Paris-Centre and under the umbrella of the French Society of Rheumatology and the Institut National de la Santé et de la Recherche Médicale. The database management is performed within the Department of Epidemiology and Biostatistics (Professor Jean-Pierre Daurès, D.I.M., Nîmes, France). An unrestricted grant from Wyeth Pharmaceuticals was allocated for the first 5 years of the monitoring of the recruited patients. The authors also wish to thank the different regional participating centres: Professor Maxime Dougados (Paris—Cochin B), Professor André Kahan (Paris—Cochin A), Professor Olivier Meyer (Paris—Bichat), Professor Pierre Bourgeois (Paris—La Pitié-Salpetrière), Professor Francis Berenbaum (Paris—Saint Antoine), Professor Pascal Claudepierre (Créteil), Professor Maxime Breban (Boulogne Billancourt). The DESIR cohort was sponsored by the Département de la Recherche Clinique et du Développement de l'Assistance Publique–Hôpitaux de Paris. This study is conducted under the umbrella of the French Society of Rheumatology and INSERM (Institut National de la Santé et de la Recherche Médicale). The database management is performed within the Department of Epidemiology and Biostatistics (Professor Paul Landais, D.I.M., Nîmes, France). An unrestricted grant from Pfizer was allocated for the 10 years of the follow-up of the recruited patients. The authors thank the different regional participating centres: Professor M Dougados (Paris—Cochin B), Professor A Kahan (Paris—Cochin A), Professor O Meyer, Professor P Dieudé (Paris—Bichat), Professor P Bourgeois, Professor L Gossec (Paris—La Pitié Salpetrière), Professor F Berenbaum (Paris—Saint Antoine), Professor P Claudepierre (Créteil), Professor M Breban (Boulogne Billancourt), Dr B Saint-Marcoux (Aulnay-sous-Bois), Professor P Goupille (Tours), Professor J-F Maillefert (Dijon), Dr X Puéchal, Dr E Dernis (Le Mans), Professor D Wendling (Besançon), Professor B Combe (Montpellier), Professor L Euller-Ziegler (Nice), Professor P Orcel, Dr P Richette (Paris—Lariboisière), Professor P Lafforgue (Marseille), Dr P Boumier (Amiens), Professor M Soubrier (Clermont-Ferrand), Dr N Mehsen (Bordeaux), Professor D Loeuille (Nancy), Professor R-M Flipo (Lille), Professor A Saraux (Brest), Dr S Pavy (Le Kremlin Bicêtre), Professor A Cantagrel (Toulouse), Professor O Vittecoq (Rouen). The authors also thank URC-CIC Paris Centre for the coordination and monitoring of the study.
Footnotes
Contributors: ARW was responsible for the concept of the study, data interpretation, manuscript writing. DN was responsible for the statistics and manuscript approval. AC, AC, YD, DL were responsible for the concept of the study, results interpretation and manuscript approval.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: CPP Ile-de-France III, submission number P070302.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data from this study.
References
- 1. Olivieri I, Barozzi L, Padula A. Enthesiopathy: clinical manifestations, imaging and treatment. Baillieres Clin Rheumatol 1998;12:665–81. 10.1016/S0950-3579(98)80043-5 [DOI] [PubMed] [Google Scholar]
- 2. D’agostino MA, Aegerter P, Jousse-Joulin S, et al. How to evaluate and improve the reliability of power Doppler ultrasonography for assessing enthesitis in spondylarthritis. Arthritis Rheum 2009;61:61–9. 10.1002/art.24369 [DOI] [PubMed] [Google Scholar]
- 3. D’Agostino MA. Ultrasound imaging in spondyloarthropathies. Best Pract Res Clin Rheumatol 2010;24:693–700. 10.1016/j.berh.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 4. D’Agostino MA. Role of ultrasound in the diagnostic work-up of spondyloarthritis. Curr Opin Rheumatol 2012;24:375–9. 10.1097/BOR.0b013e328354612f [DOI] [PubMed] [Google Scholar]
- 5. D’Agostino MA, Aegerter P, Bechara K, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis 2011;70:1433–40. 10.1136/ard.2010.138701 [DOI] [PubMed] [Google Scholar]
- 6. D’Agostino MA, Conaghan PG, Naredo E, et al. The OMERACT ultrasound task force -- Advances and priorities. J Rheumatol 2009;36:1829–32. 10.3899/jrheum.090354 [DOI] [PubMed] [Google Scholar]
- 7. Wakefield RJ, D'Agostino MA, Iagnocco A, et al. The OMERACT Ultrasound Group: status of current activities and research directions. J Rheumatol 2007;34:848–51. [PubMed] [Google Scholar]
- 8. Aydin SZ, Karadag O, Filippucci E, et al. Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: an ultrasound study. Rheumatology 2010;49:578–82. 10.1093/rheumatology/kep410 [DOI] [PubMed] [Google Scholar]
- 9. Falcao S, Castillo-Gallego C, Peiteado D, et al. Can we use enthesis ultrasound as an outcome measure of disease activity in spondyloarthritis? A study at the Achilles level. Rheumatology 2015;54:1557–62. 10.1093/rheumatology/keu399 [DOI] [PubMed] [Google Scholar]
- 10. Ruta S, Acosta Felquer ML, Rosa J, et al. Responsiveness to therapy change of a global ultrasound assessment in spondyloarthritis patients. Clin Rheumatol 2015;34:125–32. 10.1007/s10067-014-2673-4 [DOI] [PubMed] [Google Scholar]
- 11. Dougados M, Etcheto A, Molto A, et al. Clinical presentation of patients suffering from recent onset chronic inflammatory back pain suggestive of spondyloarthritis: The DESIR cohort. Joint Bone Spine 2015;82:345–51. 10.1016/j.jbspin.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 12. Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. 10.1136/ard.2009.108217 [DOI] [PubMed] [Google Scholar]
- 13. Amor B, Dougados M, Mijiyawa M. [Criteria of the classification of spondylarthropathies]. Rev Rhum Mal Osteoartic 1990;57:85–9. [PubMed] [Google Scholar]
- 14. Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27. 10.1002/art.1780341003 [DOI] [PubMed] [Google Scholar]
- 15. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 16. Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. 10.1136/ard.62.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terslev L, Naredo E, Iagnocco A, et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise. Arthritis Care Res 2014;66:741–8. 10.1002/acr.22191 [DOI] [PubMed] [Google Scholar]
- 18. Balint PV, Kane D, Wilson H, et al. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis 2002;61:905–10. 10.1136/ard.61.10.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milutinovic S, Radunovic G, Veljkovic K, et al. Development of ultrasound enthesitis score to identify patients with enthesitis having spondyloarthritis: prospective, double-blinded, controlled study. Clin Exp Rheumatol 2015;33:812–7. [PubMed] [Google Scholar]
- 20. Muñoz-Fernández S, de Miguel E, Cobo-Ibáñez T, et al. Enthesis inflammation in recurrent acute anterior uveitis without spondylarthritis. Arthritis Rheum 2009;60:1985–90. 10.1002/art.24636 [DOI] [PubMed] [Google Scholar]
- 21. van den Berg R, Lenczner G, Feydy A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol 2014;66:2403–11. 10.1002/art.38738 [DOI] [PubMed] [Google Scholar]
- 22. de Hooge M, Claudepierre P, Feydy A, et al. FRI0245 How reliable is the scoring of msasss in clinical practice in centers participating in desir? Comparison with the gold standard central reading: Table 1. Ann Rheum Dis 2014;73(Suppl 2):471.3–2. 10.1136/annrheumdis-2014-eular.1522 [DOI] [Google Scholar]
- 23. Creemers MC, Franssen MJ, van't Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. 10.1136/ard.2004.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rudwaleit M, Jurik AG, Hermann KG, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. 10.1136/ard.2009.110767 [DOI] [PubMed] [Google Scholar]
- 25. Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. 10.1002/art.21337 [DOI] [PubMed] [Google Scholar]
- 26. Navarro-Compán V, Ramiro S, Landewé R, et al. Disease activity is longitudinally related to sacroiliac inflammation on MRI in male patients with axial spondyloarthritis: 2-years of the DESIR cohort. Ann Rheum Dis 2016;75:874–8. 10.1136/annrheumdis-2015-207786 [DOI] [PubMed] [Google Scholar]
- 27. Braun J, Baraliakos X, Golder W, et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003;48:1126–36. 10.1002/art.10883 [DOI] [PubMed] [Google Scholar]
- 28. Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol 1993;46:423–9. 10.1016/0895-4356(93)90018-V [DOI] [PubMed] [Google Scholar]
- 29. Aydin SZ, Can M, Alibaz-Oner F, et al. A relationship between spinal new bone formation in ankylosing spondylitis and the sonographically determined Achilles tendon enthesophytes. Rheumatol Int 2016;36:397–404. 10.1007/s00296-015-3360-8 [DOI] [PubMed] [Google Scholar]
- 30. de Miguel E, Muñoz-Fernández S, Castillo C, et al. Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis 2011;70:434–9. 10.1136/ard.2010.134965 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2017-000482supp001.docx (15.1KB, docx)