Abstract
Background
Some patients with advanced or recurrent, epidermal growth factor receptor (EGFR) mutation-positive (EGFR M+) non-small-cell lung cancer (NSCLC) continue to receive EGFR tyrosine kinase inhibitors (TKIs) beyond radiological progression.
Methods
We analysed a cohort of 577 patients with EGFR M+ NSCLC, who had received a first-line EGFR-TKI. We classified patients according to clinical course and treatment patterns at Response Evaluation Criteria in Solid Tumors (RECIST) progressive disease (PD). We evaluated the period from RECIST PD to TKI discontinuation or clinical PD and also evaluated survival after RECIST PD and compared it between groups.
Results
RECIST PD was documented in 451 cases, of which 283 (62.7%) were clinically stable. 186 (65.7%) discontinued and 97 (34.3%) continued the EGFR-TKI. In those who continued EGFR-TKI, median time between RECIST PD and clinical PD or TKI discontinuation was 5.1 months. Median survival after RECIST PD in patients who discontinued and continued EGFR-TKI after clinically stable RECIST PD was 14.6 and 15.3 months (p=0.5489), respectively. In multivariate analysis, continuing EGFR-TKI therapy, female gender, better performance status and exon 19 deletion subtype were likely positive predictive factors for survival after clinically stable RECIST PD.
Conclusion
Our study suggests that some patients could benefit from receiving an EGFR-TKI beyond radiological progression.
Keywords: non-small-cell lung cancer, epidermal growth factor receptor, tyrosine kinase inhibitors, beyond radiological progression
Key questions.
What is already known about this subject?
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) is the standard treatment of EGFR mutation-positive non-small-cell lung cancer (NSCLC). Some patients may benefit from continuing EGFR-TKI even after radiological progression (beyond progressive disease (PD)). It is unknown how long EGFR-TKI can be continued or who benefits from continuing EGFR-TKI.
What does this study add?
Real-world data of what proportion of patients are continuing EGFR-TKI, how long they are continuing EGFR-TKI and the reason they discontinue EGFR-TKI beyond PD.
How might this impact on clinical practice?
Patients who continued EGFR-TKI beyond PD had no worse survival than patients who did not. Continuing EGFR-TKI therapy beyond radiological progression could thus be a useful strategy in treating patient with EGFR mutation-positive NSCLC.
Introduction
Treating patients with non-small-cell lung cancer (NSCLC) who carry the activating epidermal growth factor receptor (EGFR) gene mutation (EGFR M+) with EGFR tyrosine kinase inhibitors (EGFR-TKIs) is a novel approach when managing advanced NSCLC. Its efficacy, when compared with classical cytotoxic chemotherapy, has been repeatedly shown in phase III randomised studies.1–3 Furthermore, treatment with EGFR-TKIs is well tolerated, with a favourable toxicity to efficacy ratio.
Although initial therapy with EGFR-TKI brings long progression-free survival (PFS) in patients with EGFR M+ NSCLC, other factors are also likely to contribute to their overall survival (OS). In studies evaluating patients undergoing EGFR-TKIs as first-line therapy, postprogression survival was longer than PFS.1–5 Moreover, postprogression survival was even longer than median survival time of patients without the mutation who were treated with cytotoxic drugs.
Discontinuation of EGFR-TKI and switch to chemotherapy with cytotoxic drugs is generally adopted for patients when radiological progressive disease (PD) is observed during treatment with the EGFR-TKI. However, due to the moderate adverse events associated with EGFR-TKIs compared with cytotoxic drugs, EGFR-TKI therapy is continued even after radiological PD in some cases (so-called ‘beyond PD’ administration).6 Several retrospective studies have tried to show the efficacy of EGFR-TKI therapy beyond PD; however, these studies excluded patients whose disease was rapidly progressing and who could not continue EGFR-TKI therapy from the ‘beyond PD’ group.7–10 Therefore, patients receiving an EGFR-TKI could potentially have moderate disease progression and better survival. EGFR-TKIs after radiological PD used in combination with a cytotoxic drug could not show effectiveness.11
In this study, we analysed the clinical management and course of patients with advanced EGFR M+ NSCLC whose cancer had become resistant to first-line EGFR-TKI therapy. We evaluated the impact of the continuation of EGFR-TKIs on the outcomes of the patients who were judged to have Response Evaluation Criteria in Solid Tumors (RECIST)-based, that is, radiological PD.
Materials and methods
Study design
This was an observational prospective cohort study designed to survey target patients in Japan. Treatment and examination were performed in routine clinical practice. Investigators at each of the 31 institutions analysed the clinical records of enrolled subjects. All patients who met inclusion and exclusion criteria and treated with EGFR-TKI during study period were registered and analysed.
Patient selection criteria
Our inclusion criteria were: (1) diagnosed as having advanced or postoperative recurrent NSCLC prior to enrolment; (2) diagnosed as carrying the EGFR M+ by each institution (exon 19 deletion, exon 21 L858R mutation and other mutations, but not carrying only the exon 20 insertion or the T790M mutation) and (3) treatment with an EGFR-TKI (gefitinib or erlotinib) as the initial anticancer therapy was started between January 2009 and December 2011. Patients who had received an anticancer drug as local therapy during pleurodesis were also eligible.
Our exclusion criteria were: (1) having active concomitant cancer and (2) treatment with cytotoxic chemotherapy before EGFR-TKI therapy was administered. Patients with postoperative recurrent NSCLC were excluded if they had received postoperative chemotherapy with a platinum product.
The study was conducted according to the Declaration of Helsinki and approved by the institutional review board of each participating institute, as well as by the ethical committee at Public Health Research Foundation. With regard to informed consent, the opt-out method (which provides opportunities to target patients for rejection through information disclosure via posting and publication) was employed, without mandating informed consent from individuals. This policy was based on the Ethical Guidelines for Epidemiological Research in Japan. However, each institution responded by following the instructions from their respective institutional review boards and obtained informed consent from individual patients when it was judged necessary by these institutional review boards.
Survey and clinical outcomes
Each case was surveyed at enrolment, and all surviving patients were surveyed again in December 2013. During the survey, physician measured the clinical outcomes of patients. The main measures of treatment efficacy were best response to EGFR-TKI therapy, PFS and OS. We presumed two progressions where events differed, namely, RECIST PD and clinical PD; RECIST PD was defined as PD established based on RECIST, V.1.1.12 Clinical PD was defined as one or more of the followings: (1) emergence or worsening of clinical symptoms due to disease progression; (2) deterioration of Eastern Cooperative Oncology Group performance status (PS) due to disease progression; (3) any threat to major organs (such as lymphangitis carcinomatosa, spinal cord compression, carcinomatous meningitis or hepatic failure due to liver metastasis) and (4) unequivocal multiorgan progression with or without symptoms. OS was measured from the first day of onset (the start of EGFR-TKI therapy or the diagnosis of RECIST PD) until death or the final day of the follow-up period. Both RECIST PD and clinical PD were diagnosed by attending physicians, and no extramural reviews, such as imaging studies or chart records, were performed. PFS was measured from the first day of EGFR-TKI therapy until RECIST PD or the date on which the patient died, from any cause. The primary end point was the time between RECIST PD and clinical PD or the discontinuation of EGFR-TKI therapy in patients who received the TKI beyond clinically stable RECIST PD. The secondary end points included: the proportion of patients who continued to receive an EGFR-TKI beyond RECIST PD; patients who had a concomitant treatment during EGFR-TKI therapy; patients who had developed ‘disease flare’; survival time since the start of the first-line EGFR-TKI; survival time since the documentation of RECIST PD; survival time since the discontinuation of EGFR-TKI therapy; and the reason(s) for discontinuing EGFR-TKI therapy.
Switching to another EGFR-TKI (eg, gefitinib to erlotinib or vice versa) was considered to be discontinuation of the initial EGFR-TKI therapy, whereas dose or schedule modification of the same EGFR-TKI was included in the continuation category.
Grade 3 or worse adverse events, the reasons for ending EGFR-TKI therapy and the treatments after discontinuing EGFR-TKI therapy were also reported. Disease flare was defined as death or disease state exacerbation under which further treatment was impossible, which occurred within 30 days of discontinuing EGFR-TKI therapy. Cases who were treated with chemotherapy within 30 days of discontinuing EGFR-TKI therapy, and patients who developed an infection or thrombophlebitis not directly related to an aggravation of NSCLC were excluded.13
Statistical analysis
We used the Kaplan-Meier method to estimate survival probability, and we compared the difference between groups with the log-rank test. Factors potentially associated with survival were assessed using univariate and multivariate analyses using the Cox proportional hazards model in groups B and C. Missing data were not imputed. We explored the interaction between prognostic factors and groups. We considered differences to be statistically significant when p<0.05. Analyses was conducted with the SAS/STAT software (V.9.3; SAS institute).
Results
Patient characteristics
A total of 580 patients from 31 institutions were enrolled in the study. Three patients did not meet the inclusion criteria, leaving 577 patients to be analysed further. Patient characteristics are shown in table 1. The majority of the patients (n=529; 91.7%) received gefitinib therapy. In Japan, erlotinib was not registered for first-line use until June 2013. Both age and PS scale were slightly higher compared with previous studies of first-line EGFR-TKI therapy in NSCLC and were close to the actual characteristics of Japanese patients with lung cancer. Patients with exon 19 deletion and exon 21 L858R mutation accounted for 48.4% (n=279) and 47.5% (n=274) of the total patients with EGFR mutations, respectively.
Table 1.
Patient characteristics
| Characteristics | Patients (n) (N=577) | % |
| TKI agent | ||
| Gefitinib | 529 | 91.7 |
| Erlotinib | 48 | 8.3 |
| Sex | ||
| Men/women | 177/400 | 30.7/69.3 |
| Age (years) | ||
| Median | 69 | |
| Range | 27–93 | |
| PS | ||
| 0 | 188 | 32.6 |
| 1 | 246 | 42.6 |
| 2 | 84 | 14.6 |
| 3 | 45 | 7.8 |
| 4 | 11 | 1.9 |
| Unknown | 3 | 0.5 |
| Stage | ||
| IIIA | 8 | 1.6 |
| IIIB | 18 | 3.7 |
| IV | 466 | 94.7 |
| Site of metastasis | ||
| Bone | 253 | 23.1 |
| Lung | 240 | 22.0 |
| Brain | 171 | 15.6 |
| Liver | 60 | 5.5 |
| Histology | ||
| Adenocarcinoma | 567 | 98.3 |
| NSCLC (not otherwise specified) | 7 | 1.2 |
| Other | 3 | 0.5 |
| EGFR mutation subtype | ||
| Exon 19 deletion | 279 | 48.4 |
| Exon 21 L858R | 274 | 47.5 |
| Other | 24 | 4.2 |
| Smoking status | ||
| Never smoked | 381 | 66.0 |
| Current smoker | 42 | 7.3 |
| Previous smoker | 152 | 26.3 |
| Unknown | 2 | 0.4 |
| Comorbidity | ||
| COPD | 22 | 3.8 |
| Hepatic disease | 7 | 1.2 |
| Interstitial lung disease | 3 | 0.5 |
| None of the above | 545 | 94.5 |
| Previous treatment | ||
| None | 396 | 68.6 |
| Surgery | 60 | 10.4 |
| Radiation | 101 | 17.5 |
| Surgery+radiation | 20 | 3.5 |
COPD, chronic obstructive pulmonary disease; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung carcinoma; PS, performance status; TKI, tyrosine kinase inhibitor.
Clinical efficacy
The efficacy of EGFR-TKI therapy is summarised in online supplementary table S1. The overall response rate (complete response and partial response) was 69% (n=398). Dose reduction, including change of schedule to reduce days of administration, took place in 192 (33.3%) patients; combined therapy involving surgery, radiotherapy or chemotherapy was performed in 110 (19.1%) patients. Only 14 (2.4%) patients received concomitant chemotherapy during EGFR-TKI therapy, and all of those patients began to receive chemotherapy after the start of EGFR-TKI therapy, with a median delay of 11.8 months.
esmoopen-2017-000214supp001.pdf (229KB, pdf)
Patterns of EGFR-TKI use
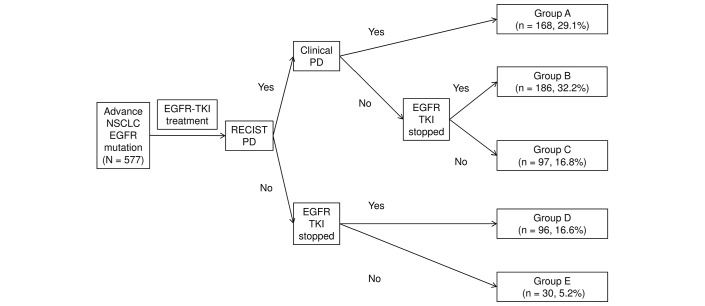
The treatment course is described in figure 1. We defined five groups according to clinical course and pattern of TKI discontinuation. The RECIST PD was documented in 451 patients (groups A, B and C). RECIST PD and clinical PD concurrently manifested in 168 (29.1%) patients. We classified these patients as having aggressive PD and designated them as group A. In this group, patients could have continued (n=22) or discontinued (n=144) EGFR-TKI therapy.
Figure 1.
Clinical courses of patients. Group A: RECIST PD and clinical PD occurred simultaneously; group B: RECIST PD without clinical PD and discontinued EGFR-TKI; group C: RECIST PD without clinical PD and continued EGFR-TKI; group D: no RECIST or clinical PD but discontinued EGFR-TKI; group E: no RECIST or clinical PD and continuing EGFR-TKI. EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung carcinoma; PD, progressive disease; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.
Patients with RECIST PD and no marked clinical deterioration (clinical PD) were assigned to groups B (discontinued EGFR-TKI) and C (continued EGFR-TKI). These patients were considered to demonstrate clinically stable RECIST PD. Group B patients comprised 65.7% of the patients (n=186; 32.2% of all eligible patients) who discontinued EGFR-TKI therapy at or within 30 days of RECIST PD, which is in line with standard treatment conventions. Group C patients continued EGFR-TKI therapy beyond RECIST PD (n=97; 16.8% of all eligible patients).
Group D patients had to stop EGFR-TKI therapy without RECIST PD because of other reasons, such as toxicity (n=96; 16.6%). Group E patients were still taking the EGFR-TKI without progression (n=30; 5.2%).
The patient characteristics are shown in online supplementary table S2, which did not differ among groups. Detail of EGFR-TKI therapy of each study group is summarised in online supplementary table S3. Proportion of patients who had dose reduction of EGFR-TKI was higher in group C, as was that of those who had combined treatment during EGFR-TKI administration.
Clinical efficacy and survival outcomes
In group C (n=97), the median time from RECIST PD to clinical PD or TKI discontinuation was 5.1 months (95% CI 3.5 to 6.0, 25–75% IQR 2.2–9.5; online supplementary figure 1). At 6 and 12 months, 39 (40.8%) and 15 (17.2%) cases in group C continued EGFR-TKI therapy without clinical PD, respectively. Nine patients were censored before clinical PD for a median duration of 14.1 months (RECIST PD to clinical PD).
esmoopen-2017-000214supp002.jpg (343.6KB, jpg)
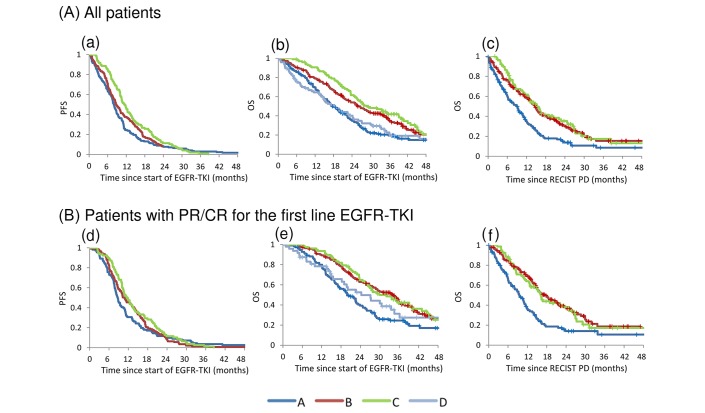
Analysis of survival in our patient cohort is summarised in table 2; the survival curve for PFS and OS since the start of first-line EGFR-TKI therapy is shown in figure 2. For group A, the median OS since starting first-line EGFR-TKI therapy was short at 17.5 (95% CI 15.0 to 20.9) months, which likely reflects the characteristically aggressive tumours in these patients. In groups B and C, the median OS since the start of first-line EGFR-TKI therapy was 25.6 (95% CI 21.7 to 30.8) and 28.9 (95% CI 24.6 to 39.2) months (p=0.22), the median PFS was 8.7 (95% CI 7.7 to 10.1) and 11.5 (95% CI 10.0 to 13.3) months (p=0.04) and the median OS since diagnosis of RECIST PD was 14.6 (95% CI 12.4 to 16.2) and 15.3 (95% CI 12.5 to 20.4) months (p=0.55). A total of 83 patients were lost to follow-up. The median follow-up duration for patients who were either lost to follow-up or who had not died was 32.8, 35.9, 26.7 and 27.1 months for groups A, B, C and D, respectively.
Table 2.
Analysis of survival
| From start of EGFR-TKI to RECIST PD* Median (95% CI) |
From start of EGFR-TKI to death† Median (95% CI) |
From RECIST PD to death† Median (95% CI) |
|
| Months | |||
| All patients | |||
| Group A | 8.0 (7.2 to 8.8) | 17.5 (15.0 to 20.9) | 8.9 (6.2 to 10.7) |
| Group B | 8.8 (7.7 to 10.1) | 25.6 (21.7 to 30.8) | 14.6 (12.4 to 16.2) |
| Group C | 11.5 (10.0 to 13.3) | 28.9 (24.6 to 39.2) | 15.3 (12.5 to 20.4) |
| Group D | – | 18.3 (14.1 to 24.2) | – |
| Patients with PR/CR for the first-line EGFR-TKI | |||
| Group A | 8.7 (7.8 to 9.9) | 19.9 (17.0 to 23.2) | 9.5 (6.9 to 11.4) |
| Group B | 10.8 (9.1 to 13.1) | 33.8 (26.8 to 36.7) | 17.7 (14.6 to 21.6) |
| Group C | 11.7 (10.0 to 13.7) | 29.8 (25.6 to 39.2) | 16.2 (12.7 to 22.8) |
| Group D | – | 24.2 (15.5 to 34.4) | – |
*Patient death from any cause is treated as censored case.
†Patients were censored at their final day of the follow-up.
CR, complete response; EGFR, epidermal growth factor receptor; OS, overall survival; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.
Figure 2.
(A) Survival time. All patients. (a) PFS since the start of EGFR-TKI (groups A, B and C). (b) OS since the start of EGFR-TKI (groups A, B, C and D). (c) OS since RECIST PD diagnosis (groups A, B and C).(B) Survival time. Patients with PR or CR for first-line EGFR-TKI. (d) PFS since the start of EGFR-TKI (groups A, B and C). (e) OS since the start of EGFR-TKI (groups A, B, C and D). (f) OS since the diagnosis of RECIST PD (groups A, B and C). CR, complete response; EGFR, epidermal growth factor receptor; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.
Reasons for EGFR-TKI therapy discontinuation
In group A, progression of central nervous system (CNS) (brain and/or leptomeningeal) and bone was reported in 56 (33.3%) and 29 patients (17.3%), respectively, at the documentation of radiological and clinical PD. We found progression of brain metastasis in 106 (23.5%) of all patients who experienced RECIST PD (group B: 27 patients, 14.5%; group C: 14 patients, 14.4%). Metastasis only to brain or to localised bone was detected in 9 (brain) and 20 (bone) group A patients, 12 (brain) and 12 (bone) group B patients and 4 (brain) and 4 (bone) group C patients. Five group A patients and eight group C patients continued EGFR-TKI with concomitant use of local radiotherapy. The median OS since diagnosis of RECIST PD was 6.1 months for the five group A patients.
In group C, patients continued the EGFR-TKI after the diagnosis of RECIST PD and stopped the EGFR-TKI mostly because of clinical PD (98.9%). With regard to clinical PD in group C, patients were diagnosed with symptom emergence (44.3%), PS deterioration (35.1%), threat to major organs (14.4%) and unequivocal multiorgan progression (44.3%). New brain metastasis was found in 23 patients (23.7%) at the time of clinical PD diagnosis. The reasons for physicians choosing to discontinue EGFR-TKI are summarised in online supplementary table S4, and the details of clinical progression are shown in online supplementary table S5.
Disease flare was only observed in six patients (1.0% of all 577 patients and 1.1% of the 537 patients who discontinued EGFR-TKI therapy).
Post-EGFR-TKI therapy
A total of 547 patients discontinued first-line EGFR-TKI, and 352 of them received further systemic therapy. Cytotoxic chemotherapy was administered to 184 patients (52.3%), and 168 (47.7%) of these received platinum doublet; rechallenge of EGFR-TKI was administered to 241 patients (68.5%). Change of EGFR-TKI agent immediately after the discontinuation of the first treatment (eg, gefitinib to erlotinib or erlotinib to gefitinib) was carried out in 130 patients (22.5%). In group B (n=186), 29 patients (15.6%) immediately received another EGFR-TKI at RECIST PD. The survival times since RECIST PD of those who did and did not immediately have another EGFR-TKI were 11.5 (4.0–14.4) and 15.2 (13.0–18.6) months, respectively.
Factors associated with improved survival
We carried out a combined analysis of groups B and C, that is, those who remained clinically stable at RECIST PD, for the variables associated with improved survival after RECIST PD (table 3). Female gender, younger age, exon 19 deletion subtype and metastasis to other than CNS were favourable prognostic factors in univariate and multivariate analysis. Smoking status and PS were found to have interaction with treatment groups. Patients of smokers in group B were better than those in group C. Patients with worse PS have poor prognosis both in groups B and C, but the degree was much severe in group B.
Table 3.
Combined analysis of groups B and C (univariate and multivariate analysis of factors associated with better survival after RECIST PD)
| Characteristics | Univariate | Multivariate | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender (men over women) | 1.48 | 1.10 to 2.01 | <0.05 | 2.06 | 1.36 to 3.12 | <0.01 |
| Age (≥70 over others) | 1.30 | 0.98 to 1.74 | .07 | 1.91 | 1.38 to 2.64 | <0.01 |
| EGFR (exon 19 deletion over others) | 0.76 | 0.56 to 1.01 | .06 | 0.71 | 0.52 to 0.97 | .03 |
| Agent (erlotinib over gefitinib) | 0.64 | 0.37 to 1.10 | .10 | 0.54 | 0.30 to 0.95 | .03 |
| Site of metastasis (CNS over others) | 1.32 | 0.97 to 1.79 | .08 | 1.47 | 1.05 to 2.06 | .03 |
| Smoking* at group C | – | – | – | 1.77 | 0.92 to 3.39 | .09 |
| Smoking* at group B | – | – | – | 0.50 | 0.31 to 0.80 | <0.01 |
| PS† at group C | – | – | – | 2.18 | 1.17 to 4.06 | .01 |
| PS† at group B | – | – | – | 4.77 | 3.03 to 7.49 | <0.01 |
*Smoking (current over others).
†PS (2–4 over 0–1).
CNS, central nervous system; EGFR, epidermal growth factor receptor; PD, progressive disease; PS, performance status; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.
Discussion
Continuing EGFR-TKIs beyond radiological progression is an attractive strategy when treating patients with EGFR M+. In our study, 40% of patients continued EGFR-TKI therapy after radiological progression, and they could continue it without clinical deterioration for a median of 5.1 months. Although the survival benefit was not apparent between patients who continued or discontinued the EGFR-TKI beyond RECIST PD, results of the multivariate analysis suggested that continuing EGFR-TKI therapy, as well as female gender, better PS and exon 19 deletion subtype, was a likely predictive factor for improved survival.
Moreover, treatment with an EGFR-TKI for several additional months could be beneficial to patients in itself, since patients could have better quality of life compared with cytotoxic chemotherapy.14 On the other hand, patients who immediately switched to another EGFR-TKI after RECIST PD tended to have inferior survival compared with those who changed to other chemotherapies. Unfavourable characteristics, such as brain metastasis, may well lead to a change of EGFR-TKI accounting for the apparent poor outcome.15 16
Continuing EGFR-TKI therapy in patients who progress asymptomatically has been supported by several clinical experiences. In addition, rapid progression after EGFR-TKI cessation (disease flare) is a concern.13 17 In this study, however, the emergence of disease flare-up was far lower than previously reported and was compatible with the values reported in Japanese patients with lung cancer.13 17 18 The time between the end of EGFR-TKI therapy and initiation of the next round of chemotherapy was relatively short (median: 11 days; range: 0–645 days) and might have masked rapid progression.
Maintaining the inhibition of oncogene expression throughout systemic therapy is effective in HER2-positive patients with breast cancer.19 Several studies also retrospectively showed favourable survival in patients using EGFR or anaplastic lymphoma kinase inhibitors beyond RECIST PD.7–9 20 In these studies, too, patients who continued TKI therapy after radiological progression were evaluated. However, these studies failed to separate patients who could not continue TKI therapy because of clear clinical progression from those who discontinued TKI therapy only because of radiological progression. Therefore, a bias for the better prognosis of patients who continue TKI therapy could not be controlled for. In fact, only 54.4% of patients could continue erlotinib on RECIST disease progression in a single-arm, phase II study that prospectively evaluated the efficacy of continuing erlotinib.21 In our study, 37% of patients (group A) were clinically unstable due to disease progression, and the vast majority of them could not continue EGFR-TKI therapy at the time of first RECIST PD.
Adding cytotoxic chemotherapy to EGFR-TKI therapy after progression of EGFR-TKI therapy is another attractive strategy. Early studies, conducted before the discovery of EGFR activating mutations, showed no benefit in adding an EGFR-TKI to standard chemotherapy.22–25 A phase III study that investigated the efficacy of adding cisplatin and pemetrexed after gefitinib failure in patients with EGFR M+ failed to show the benefit of adding EGFR-TKI therapy to chemotherapy in terms of PFS and OS.11 Combining EGFR-TKI with cytotoxic chemotherapy is still in under investigation in first-line chemotherapy, but there is no clear benefit in EGFR-TKI-treated patients.
Understanding the resistant mechanism of EGFR-TKI has led to the development of new, third-generation EGFR-TKIs. Approximately 50% of patients have T790M mutation at progression of gefitinib, erlotinib or afatinib. Third-generation EGFR-TKIs, especially osimertinib, are shown to be effective in patients with T790M mutation.26 After the progression of conventional EGFR-TKI, molecular analysis of resistant should be examined and the use of osimertinib is the standard of care if patients have T790M mutation.27 However, same issues with conventional EGFR-TKI will arise when patients have progression in osimertinib, and one of which is the appropriate time to stop the EGFR-TKI. Findings from this study are thus likely to be applicable even after the use of osimertinib.
Our study had several limitations. It was an observational study and was therefore limited in how it could clarify the benefit of using EGFR-TKIs beyond radiological progression. First, since it was not a prospective randomised study, we could not fully adjust for the bias. Any imbalance in patient condition, such as comorbidity we could not analysed, might have biased the effect of treatment strategy between our groups. Patients with poor PS, for example, could not receive chemotherapy and may thus have had no other choice but continue with EGFR-TKI therapy. Second, follow-up was performed in daily practice and no preplanned interval was assumed. Each investigator conducted the clinical assessment of each individual patient. These two factors may have led to variations in evaluation time and observer bias. Third, the diagnosis of clinical PD solely depended on the judgement of each attending physician without considering the unified objective scores. We also did not perform extramural reviews of radiological and clinical progression indicators. Because our goal was to measure the clinical outcomes in patients treated according to real-world practice, we defined clinical PD as the ‘end’ of the clinical benefit with TKI under the assumption that these patients should be treated with another strategy. In addition, there are no approved or well-used symptom score criteria in Japan. In future analyses, evaluating symptoms by unified criteria will be important for understanding when to correctly discontinue EGFR-TKI therapy. There is a report proposing a local–regional approach for patients with symptom progression only in the brain or a single brain metastasis.28 In our study, there were five patients who met this description, but because the prognosis after RECIST PD was not particularly good, we did not separate them from the other symptomatic patients. However, the clinical conditions of RESIST PD considerably vary from patient to patient, and some patients may benefit from additional local therapy while continuing EGFR-TKI even with symptomatic PD. This should be considered in future analyses.
Appropriate criteria for the best use of an agent beyond progression are still being developed.21 Our study did reflect the real-world treatment practices and outcomes, which we believe would be informative to clinical decision-making.29 30
In conclusion, our prospective cohort study of patients with EGFR M+ suggested that some patients could clinically benefit from receiving EGFR-TKI therapy beyond radiological progression. Patients who continued EGFR-TKI beyond radiological progression had no worse survival than patients who did not, including those who immediately switched to chemotherapy. Due to the better tolerability of EGF-TKIs, continuing EGFR-TKI therapy beyond radiological progression could thus be a useful strategy in treating patients with EGFR M+.
Footnotes
Contributors: YG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YG, KY, YH, CT, YO and HK. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: YG. Clinical revision of the manuscript for important intellectual content: all authors. Statistical analysis: YO. Obtained funding: HK. Administrative, technical or material support: HK. Study supervision: HK. Read and approved the final manuscript: all authors.
Funding: The Comprehensive Support Project for Oncology Research (CSPOR) of Public Health Research Foundation conducted this study. CSPOR received funding support from an AstraZeneca-Investigator-sponsored study. AstraZeneca had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation or approval of the manuscript but reviewed it before submission.
Competing interests: YG has received personal fees as honoraria from Pfizer Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim Japan, Ono Pharmaceutical, Novartis Pharma, GlaxoSmithKline and Bristol-Myers Squibb. His institution has received research funding from Merck Serono, Pfizer Japan, Taiho Pharmaceutical, Eisai, Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim Japan, Ono Pharmaceutical, Novartis Pharma, Daiichi Sankyo, GlaxoSmithKline, Yakult Honsha, Quintiles, Astellas Pharma and Bristol-Myers Squibb. KY has received personal fees as honoraria from Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Taiho Pharmaceutical, Boehringer Ingelheim Japan, Bristol-Myers Squibb, Ono Pharmaceutical and Pfizer Japan. YH has received personal fees as honoraria from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb and Boehringer Ingelheim Japan. TK has received personal fees as honoraria from Chugai Pharmaceutical, Roche, Boehringer Ingelheim Japan, Ono Pharmaceutical, Eli Lilly Japan, AstraZeneca, Taiho Pharmaceutical, Pfizer Japan and Kyowa Hakko Kirin. His institution has received research funding from Chugai Pharmaceutical, MSD, Takeda Pharmaceutical, Kyowa Hakko Kirin, Daiichi Sankyo, Yakult Honsha, Boehringer Ingelheim Japan, Pfizer Japan, Taiho Pharmaceutical, Shionogi, AstraZeneca, Eli Lilly Japan and Bristol-Myers Squibb. MN has received personal fees as honoraria from Pfizer Japan, Chugai Pharmaceutical, Eli Lilly Japan, Taiho Pharmaceutical, Nichirei Biosciences, Elekta, AstraZeneca, Sanofi, Bristol-Myers Squibb and Ono Pharmaceutical and consulting fees from Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, Taiho Pharmaceutical, Daiichi Sankyo and Pfizer Japan. His institution has received research funding from Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol-Myers Squibb, Takeda Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Eli Lilly Japan and Pfizer Japan. YHK has received personal fee as Speakers’ Bureau from Chugai Pharmaceutical, AstraZeneca and Boehringer Ingelheim Japan. AI has received personal fee as honoraria from AstraZeneca. YH has received personal fees as honoraria from AstraZeneca, Chugai Pharmaceutical and Boehringer Ingelheim Japan. His institution has received research funding from AstraZeneca, Chugai Pharmaceutical and Boehringer Ingelheim Japan. HI institution has received research funding from Ono Pharmaceutical, Takeda Pharmaceutical and MSD. KM institution has received research funding from Ono Pharmaceutical and Chugai Pharmaceutical. KY has received personal fees as honoraria from Pfizer Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Ono Pharmaceutical and Bristol-Myers Squibb. YO has stock in Statcom and received personal fees as executive salaries from Statcom; honoraria from Sanofi and Eisai; consulting fees from Chugai Pharmaceutical, Taiho Pharmaceutical, Shionogi, Kowa and Eisai; and travel expenses from Yakult Honsha and Takeda Pharmaceutical. His institution has received research funding from Eisai. HK has received personal fees as honoraria from AstraZeneca, Taiho Pharmaceutical, Sanofi, Johnson & Johnson, Eli Lilly Japan, Ono Pharmaceutical, Chugai Pharmaceutical, Pfizer Japan, Boehringer Ingelheim Japan, MSD, Dainippon Sumitomo, Daiichi Sankyo, Bristol-Myers Squibb, Novo Nordisk Pharma, Nippon Chemiphar, Nihon Medi-Physics, Kyorin Pharmaceutical, Nippon Kayaku and Kyowa Hakko Kirin and travel expense from Taiho Pharmaceutical. No other disclosures are reported.
Patient consent: Obtained.
Ethics approval: The institutional review board of each participating institute, as well as by the ethical committee at Public Health Research Foundation.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Mok TS, Wu YL, Thongprasert S, et al. . Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 2. Mitsudomi T, Morita S, Yatabe Y, et al. . Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Kobayashi K, et al. . Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 4. Han JY, Park K, Kim SW, et al. . First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–8. 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Yang JC, Yamamoto N, et al. . Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 6. Oxnard GR, Arcila ME, Sima CS, et al. . Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22. 10.1158/1078-0432.CCR-10-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishie K, Kawaguchi T, Tamiya A, et al. . Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol 2012;7:1722–7. 10.1097/JTO.0b013e31826913f7 [DOI] [PubMed] [Google Scholar]
- 8. Weickhardt AJ, Scheier B, Burke JM, et al. . Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807–14. 10.1097/JTO.0b013e3182745948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu HA, Sima CS, Huang J, et al. . Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346–51. 10.1097/JTO.0b013e31827e1f83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo PC, Dahlberg SE, Nishino M, et al. . Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer 2015;121:2570–7. 10.1002/cncr.29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soria JC, Wu YL, Nakagawa K, et al. . Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990–8. 10.1016/S1470-2045(15)00121-7 [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13. Chaft JE, Oxnard GR, Sima CS, et al. . Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298–303. 10.1158/1078-0432.CCR-11-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thongprasert S, Duffield E, Saijo N, et al. . Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol 2011;6:1872–80. 10.1097/JTO.0b013e31822adaf7 [DOI] [PubMed] [Google Scholar]
- 15. Katayama T, Shimizu J, Suda K, et al. . Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415–9. 10.1097/JTO.0b013e3181b62572 [DOI] [PubMed] [Google Scholar]
- 16. Lee E, Keam B, Kim DW, et al. . Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069–74. 10.1097/JTO.0b013e318294c8e8 [DOI] [PubMed] [Google Scholar]
- 17. Riely GJ, Kris MG, Zhao B, et al. . Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res 2007;13:5150–5. 10.1158/1078-0432.CCR-07-0560 [DOI] [PubMed] [Google Scholar]
- 18. Akamatsu H, Ono A, Shukuya T, et al. . Disease flare after gefitinib discontinuation. Respir Investig 2015;53:68–72. 10.1016/j.resinv.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 19. Slamon DJ, Leyland-Jones B, Shak S, et al. . Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 20. Goldberg SB, Oxnard GR, Digumarthy S, et al. . Chemotherapy with Erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. Oncologist 2013;18:1214–20. 10.1634/theoncologist.2013-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park K, Yu CJ, Kim SW, et al. . First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: The ASPIRATION study. JAMA Oncol 2016;2:305–12. 10.1001/jamaoncol.2015.4921 [DOI] [PubMed] [Google Scholar]
- 22. Giaccone G, Herbst RS, Manegold C, et al. . Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol 2004;22:777–84. 10.1200/JCO.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 23. Herbst RS, Giaccone G, Schiller JH, et al. . Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial— INTACT 2. J Clin Oncol 2004;22:785–94. 10.1200/JCO.2004.07.215 [DOI] [PubMed] [Google Scholar]
- 24. Gatzemeier U, Pluzanska A, Szczesna A, et al. . Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007;25:1545–52. 10.1200/JCO.2005.05.1474 [DOI] [PubMed] [Google Scholar]
- 25. Herbst RS, Prager D, Hermann R, et al. . TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892–9. 10.1200/JCO.2005.02.840 [DOI] [PubMed] [Google Scholar]
- 26. Jänne PA, Yang JC, Kim DW, et al. . AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689–99. 10.1056/NEJMoa1411817 [DOI] [PubMed] [Google Scholar]
- 27. Mok TS, Wu Y-L, Ahn M-J, et al. . Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40. 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandara DR, Li T, Lara PN, et al. . Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clin Lung Cancer 2014;15:1–6. 10.1016/j.cllc.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol 2012;30:4215–22. 10.1200/JCO.2012.41.6701 [DOI] [PubMed] [Google Scholar]
- 30. Tanaka S, Tanaka S, Kawakami K. Methodological issues in observational studies and non-randomized controlled trials in oncology in the era of big data. Jpn J Clin Oncol 2015;45:323–7. 10.1093/jjco/hyu220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000214supp001.pdf (229KB, pdf)
esmoopen-2017-000214supp002.jpg (343.6KB, jpg)