Abstract
Triple negative breast cancer (TNBC) is a type of breast cancer (BC) that does not express the oestrogen and the progesterone receptors and the human epidermal growth factor receptor type 2 (HER2). Since there are no positive markers to reliably classify TNBC, these tumours are not yet treated with targeted therapies. Perhaps for this reason they are the most aggressive form of breast carcinomas. However, the clinical observation that these patients do not carry a uniformly dismal prognosis, coupled with data coming from pathology and epidemiology, suggests that this negative definition is not capturing a single clinical entity, but several. We critically evaluate this evidence in this paper, reviewing clinical and epidemiological data and new studies that aim to subclassify TNBC. Moreover, evidence on the role of tumour infiltrating lymphocytes (TILs) on TNBC progression, response to chemotherapy and patient outcome have been published. The heterogeneity, observed even at TILs level, highlights the idea that TNBC is much more than a single disease with a unique treatment. The exploration of the immune environment present at the tumour site could indeed help in answering the question ‘How many diseases is TNBC’ and will help to define prognosis and eventually develop new therapies, by stimulating the immune effector cells or by inhibiting immunological repressor molecules.
In this review, we focus on the prospect of the patient’s diverse immune signatures within the tumour as potential biomarkers and how they could be modulated to fight the disease.
Keywords: Triple negative breast cancer, heterogeneity, gene expression profile, tumour infiltrating lymphocytes
Introduction
Breast cancer (BC) is the most common malignancy and the second cause of death in women of high income countries.1 WHO estimates that by 2020 one in every eight women will develop BC.1 The 5-year survival for BC is 98% for localised disease, 84% for regional disease, but only 23% for distant disease.2 A quarter of patients with early BC will relapse and half of the women with axillary lymph node involvement will relapse.2 There are several clinical types of BC, defined by amplification of specific markers. Steroid hormone receptor overexpression (oestrogen and/or progesterone receptors: ER, PgR) define the most abundant type of BC. Roughly 70% of BC is ER-positive and/or PgR-positive3 4 and this type of BC is amenable to hormonal therapy. Human epidermal growth factor receptor type 2 (HER2) amplification, defines a second type, with an incidence of roughly 20%5 and it is responsive to anti-HER2 directed therapy, namely trastuzumab and, more recently, lapatinib, pertuzumab and TDM1.6 HER2+ BC can be either ER+ or ER−, but its dominant biological driver and clinical feature is traceable to HER2 gene amplification, a potent oncogene. The disease-free survival at 5 years for these two BC subtypes is over 95%.2 The ALTTO trial revealed that the addition of lapatinib to trastuzumab in patients with HER2+ BC had no benefit in disease-free survival.7
The remaining cases are termed triple negative BC (TNBC), breast carcinomas that neither express ER nor PgR and do not have overexpression of HER2. TNBC can represent between 10% to 20% of all BC cases8 and it is the subtype with the worse prognosis when compared with OR-positive (and/or PgR-positive) disease and HER2-positive disease.9 10 In fact, half a million women die in the world every year with BC, of which 150 000 are estimated to be TNBC cases,1 representing around 30% of the BC associated deaths. This may be due in part to the fact that it is the only clinical subtype of BC for which there is no approved adjuvant targeted therapy. In fact, in the pre anti-HER2 therapy era, HER2+ BC had an even more dismal survival than TNBC11 12 and currently HER2+ BC has a disease-free survival at 5 years comparable to hormone-positive disease. When faced with TNBC, clinicians are limited to the use of surgery, radiotherapy and chemotherapy. However, not all patients with TNBC respond to chemotherapy13–15 and this exemplifies what our clinical experience and emerging data suggest—TNBC may be more than a single disease.
Indeed, in the past few years there has been an effort to further divide TNBC cases in order to better understand the distinct patient outcome and to develop new specific therapies for each TNBC subclass.16–18 In this review, we will analyse these new classifications and try to explore new targets that can be used in new therapies.
Clinical and epidemiological heterogeneity in TNBC
TNBC presents a considerable heterogeneity concerning the age of diagnosis, prognosis and response to treatment, to name a few. The clinical evidence for such heterogeneity is summarised in table 1. Normally and succinctly, TNBC is associated with African women, younger age, higher grade tumours, high mitotic index, more advanced stage at diagnosis, namely inflammatory BC, aggressive biology and poor prognosis, as shown in figure 1.19 The peak risk of relapse is at the third year and in this scenario the clinical outcome worsens. Also, survival after metastasis relapse is reduced when compared with other BC subtypes.8
Table 1.
Clinical, epidemiological and therapeutic heterogeneity of triple negative breast cancer (TNBC)
| Characteristics | Worse outcome | Better outcome |
| Age of presentation | Young | Old |
| Stage at presentation | Advanced | Early |
| Growth rate | Fast | Slow |
| First site of metastasis | Liver and brain | Lymph nodes and bone |
| Chemotherapy response | Resistant | Sensitive |
| Body mass index | High | Low |
| Ethnicity | African | Caucasian |
Figure 1.
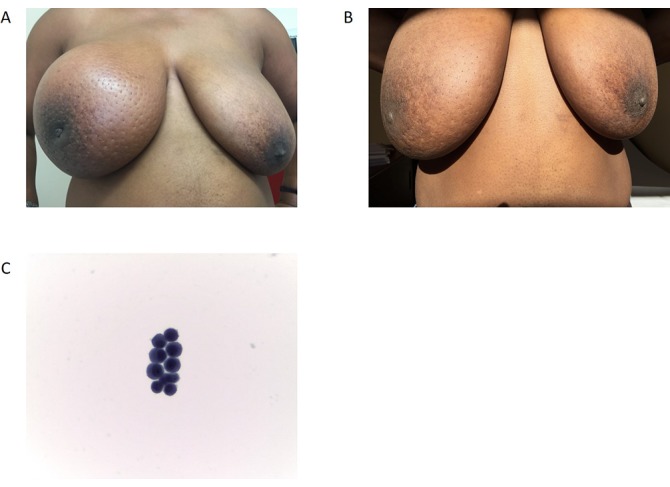
An inflammatory triple negative breast cancer of an African woman before (A) and after (B) treatment. Patients that descend from African ethnicity have a higher probability of developing more aggressive forms of breast cancer with lower curability rates. In fact, this patient, although the breast mass decreased (B), did not respond to the treatment and developed very aggressive meningeal carcinomatosis (C) 6 months after diagnosis while still finishing neoadjuvant chemotherapy with an opening pressure in the lumber tap of 40 mm Hg (normal 15–20 mm Hg) and died within 2 weeks of this diagnosis despite intrathecal treatment.
Considering the age at diagnosis, there is increasing evidence that TNBC may have a bimodal distribution with the first incidence peak in premenopausal patients and a second peak after 70 years of age.20–23 Prognosis of stage-matched premenopausal TNBC is worse than older age TNBC. One can speculate on the underlying biology that explains this difference in outcome. Premenopausal TNBC would be a disease with a few very powerful molecular drivers, more akin to single hit neoplasms; whereas, geriatric TNBC would be a disease of generalised chromosomal instability, a hallmark of ageing tissues and of geriatric cancer.24 In fact, such genomic heterogeneity has been observed in TNBC using deep sequencing.12 25
TNBC has an aggressive behaviour with presentation of de novo metastatic BC, large locally advanced breast lesion or metastatic disease developing shortly after adjuvant chemotherapy.26–28 TNBC frequently metastasises to the viscera, liver, lung or brain.29–32 However, this is not always the case as it may also have an oligometastatic phenotype closer to ER+ BC, with only lymph node and bone disease.33 TNBC is also heterogeneous in terms of time of recurrence (figure 2). Unlike ER+ BC, whose recurrence curve is linear, TNBC has a higher rate of recurrence in the first 5 years and a lower rate of recurrence afterwards; nevertheless, there appears to be two distinct recurrence peaks.23 34 The pattern of late recurrence is generally associated with less aggressive disease, frequently with bone metastases.9 33 The apparent different paths of tumour progression in TNBC might be driven by confounding factors. These differences may be just a proxy for age of incidence;35 additionally, the group of patients that have late recurrence and good prognosis, may represent the 10% of tumours that are false negatives for ER.36
Figure 2.

Heterogeneity in the natural history of triple negative breast cancer. Metastasis develop preferentially in the viscera in patients that relapse more rapidly, leading to a bad prognosis. On the other hand, patients with later relapse present TNBC with a tendency to develop bone metastasis, leading to a better prognosis.
African patients
The association between African women or women with African ancestry and increased susceptibility to more aggressive BC goes back to the 1990s, before BC subtypes were part of our thought process. At this time, it was known that BC in African women was more frequently ER-negative, affected younger women and, when stage and age matched, had worse prognosis.37–39 Indeed, African women have more frequently premenopausal aggressive TNBC21 40–42 and their risk of developing TNBC is three times higher, independent of other risk factors for BC.43 Moreover, Caucasian and Asian women tend to have TNBC with a later age of onset and less aggressive clinical course,44–46 but still with bad prognosis.47 African women are less responsive to neoadjuvant chemotherapy (NACT) and have worse prognosis when diagnosed with locally advanced TNBC.48
Regarding incidence, TNBC has been defined as a rare disease in northern Europe and in some states of the USA,49 accounting for less than 15% of BC cases.50 Whereas in some US states, where more than 20% of the population is African, the frequency of TNBC is substantially higher. African premenopausal women were twice as likely to have TNBC than Caucasian women in North Carolina (39% vs 14%)40 and Atlanta cohorts (47% vs 22%).21 Additionally, an epidemiological study from Nigeria reported that 59% of BC cases in their country were TNBC.41
A possible explanatory factor for these ethnicity-based differences may be the breast density, which is higher in African women, and the rigidity of the extracellular matrix can be a factor that drives the malignancy.51 52 Also, cancer in dense breasts is harder to diagnose by mammography,51 53 raising the possibility that the worse prognosis in these cases might be due to a delay in diagnosis. However, stage matching in epidemiological studies eliminates this hypothesis.54 55 Additionally, denser mammary glands may have distinct growth factor profiles that predispose women to more aggressive cancer with oestrogen independence. This difference may also be caused by the fact that African women can have a distinct subtype of TNBC that could be more aggressive and more resistant to chemotherapy when compared with women from other ethnicities. This hypothesis will be further explored later in this review.
An analysis of outcome of 25 000 patients with cancer enrolled in the Southwest Oncology Group phase III clinical trial54 showed that ethnicity does not affect outcome of carcinoma of the lung, colon, lymphoma, leukaemia or multiple myeloma; but that it affects sex hormone responsive carcinomas of the breast, ovary and prostate. Biologically, there are data suggesting TNBC in Africans has more signalling through growth factor pathways such as insulin growth factor receptor type 1 (IGF1) and the vascular endothelial growth factor-activated genes,56 although IGF1 signalling is increased in obese individuals, which was not controlled in this study, and African women have higher body mass index than Caucasians. In fact, the link between obesity and TNBC was shown in the Carolina Breast Study.40 Moreover, the stem cell renewal pathway Wnt β-catenin is active in TNBC57 and specifically more active in tumours of African patients.58 The stem cell marker ALDH1 was studied with tissue microarrays in a cohort of 192 TNBCs from Ugandan patients and was found to be present in 48% of the tumours and correlated with high histological and nuclear grades.59
Response to chemotherapy
One of the most striking causes of heterogeneity of TNBC is the different sensitivity to chemotherapy, where patients are either chemoresponsive or chemoresistant.60 On one hand, TNBCs are among the most chemoresponsive BCs: data from neoadjuvant studies show that the fraction of tumours experiencing pathological complete response (pCR) is mostly comprised of TNBC.60–65 On the other hand, TNBCs are frequently chemoresistant tumours as documented by the short survival of patients with metastatic disease in published series.66 67 One could argue that the apparent chemoresponsiveness found in neoadjuvant studies evolves into a chemoresistant phenotype. Nevertheless, this does not seem to be the case since, in large neoadjuvant trials with long follow-up, those women that obtain a pCR consistently maintain survival advantage over the years.68 This suggests that chemosensitivity is a hard-wired feature of a particular tumour.69 Chemoresistance is linked to abundance of stem-like cells that are pluripotent and quiescent and are therefore able to undergo epithelial to mesenchymal transition (EMT) and are resistant to therapy that targets dividing cells. In gene expression studies, these TNBCs enriched in cells that have properties similar to stem cells are classified as claudin low tumours.70
Due to this heterogeneity in response rates to standard chemotherapy, several other therapeutic strategies are being analysed (table 2). These strategies encompass poly (ADP-ribose) polymerase inhibitors (PARPi) and platinum agents (see next section); anti-VEGF drugs,71 72 namely bevacizumab; capecitabine, which is a prodrug that is converted to fluorouracil;72 or ixabepilone that stabilises microtubules.73 74 Although these approaches are still being tested in clinical trials, the most promising one is the use of olaparib (a PARPi) in HER2-negative metastatic disease with germline BRCA mutation (gBRCAm).75 The other clinical trials are still ongoing or haven’t shown significant differences between patient arms.
Table 2.
Ongoing clinical trials with PARP inhibitors (PARPi), anti-VEGF, ixabepilone and capecitabine drugs, divided by metastatic, neoadjuvant or adjuvant setting, regimen and efficacy
| Trial ID/number | Phase | Setting | Regimen | Efficacy | Reference |
| OlympiAD NCT00494234 |
III | Metastatic (HER2-negative with gBRCAm) | Olaparib (PARPi) versus chemotherapy | Progression-free survival improved | 75 |
| ABRAZO NCT02034916 |
II | Metastatic (with gBRCAm) | Talazoparib (PARPi) in patients previously exposed to platinum or multiple cytotoxic regimens | Talazoparib is well tolerated and has promising antitumour activity | 143 |
| EMBRACA NCT01945775 |
III | Metastatic | Talazoparib (PARPi) in patients who have received prior chemotherapy for metastasis | Ongoing, no results published | 144 |
| PARTNER NCT03150576 |
II/III | Neoadjuvant (TNBC or gBRCAm) | Adding olaparib (PARPi) to neoadjuvant platinum or multiple prior cytotoxic regimens | Ongoing, no results published | 145 |
| NCT02282345 | II | Neoadjuvant (invasive BC and deleterious BRCAm) | Talazoparib (PARPi) | Ongoing, no results published | 146 |
| NCT00148694 | II | Neoadjuvant (TNBC) | Cisplatin | pCR=22% | 147 |
| NCT02199418 | II | Neoadjuvant | Paclitaxel and cisplatin | pCR=64.7% (in TNBC) | 100 |
| NCT00483223 | II | Metastatic (TNBC) | Cisplatin or carboplatin | ORR=25.6% | 148 |
| NA | NA | Metastatic | Carboplatin and paclitaxel | ORR=56.6% (in TNBC) | 149 |
| BEATRICE NCT00528567 |
III | Adjuvant (TNBC) | Bevacizumab (anti-VEGF) | No differences in overall survival; prior patient selection must be performed. | 71 |
| NCT01069796 | II | Metastatic (TNBC) | Bevacizumab, paclitaxel, capecitabine | Ongoing, no results published | 72 |
| TITAN NCT00789581 |
III | Adjuvant (TNBC) | Ixempra (ixabepilone) versus Taxol | No differences in disease-free survival and overall survival with Ixempra | 73 |
| NCT00633464 | II | Metastatic (TNBC) | Ixabepilone and ixabepilone + cetuximab (EGFR inhibitor) | Time to response improved in combination, progression-free survival similar | 74 |
BC, breast cancer; gBRCAm, germline BRCA mutation; NA, non applicable; ORR, objective response rate; PARPi, poly (ADP-ribose) polymerase inhibitor; pCR, pathological complete response; TNBC, triple negative breast cancer; EGFR, epidermal growth factor receptor.
Moreover, there is increasing evidence that anticancer immune responses may contribute to the control of cancer after conventional chemotherapy.76 Therefore, as we later explore, the fact that TNBC tumours exhibit a high heterogeneous immune microenvironment, may also be associated with the different chemoresistance/chemosensitivity profiles among TNBC.
Due to the different levels of heterogeneity in TNBC here described, it was essential to come up with a subclassification of TNBC in order to develop new targeted therapies, especially in the cases where chemotherapy is not effective.
The triple negative subclassification
In general, BC can be classified into multiple subtypes according to different criteria. TNBC can be further subtyped using histological, developmental and molecular criteria.
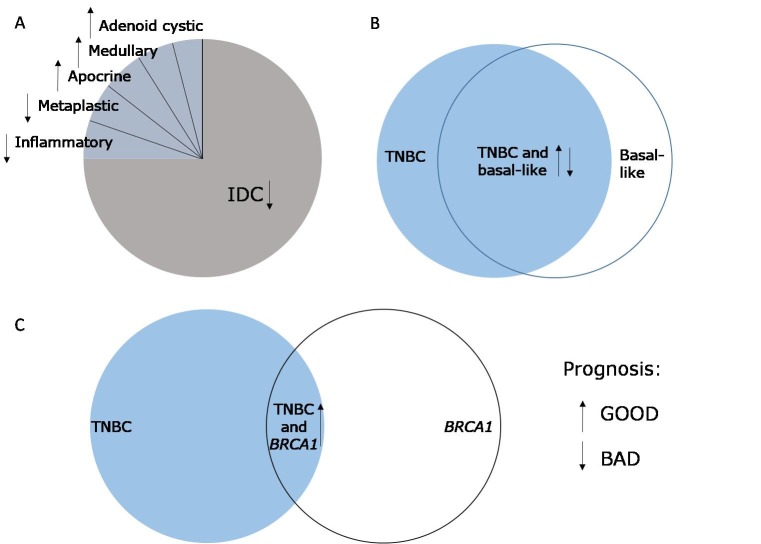
Histologically, the majority of TNBC is grade 3 or poorly differentiated (figure 3).77 78 The few remaining cases are rare histological types like adenoid-cystic, medullary, apocrine, metaplastic or inflammatory BCs.79–82
Figure 3.
Histological and molecular heterogeneity of triple negative breast cancer (TNBC). (A) Most TNBCs are infiltrating ductal carcinoma (IDC), leading to bad prognosis. The other types of TNBCs have better prognosis, with the exception of metaplastic and inflammatory. (B) There is a high overlap between TNBC and basal-like breast cancer, however, not all TNBCs are basal-like and vice versa. (C) Some patients with TNBC have a BRCA1 germline mutation, which leads to an improved patient prognosis.
There have been attempts to establish a relationship between normal mammary gland development and occurrence of BC, that is, to map different types of BCs into different stages of the mammary gland development,83 as has been done for acute myeloid leukaemia.84 Some TNBCs would correspond to a more primitive subtype of tumour, closer to the most undifferentiated BC progenitor cell (stem cell). This reasoning is supported by the observation that the putative BC stem cells in in vitro models are ER-negative and that, as they subsequently differentiate into mammary gland luminal cells, acquire ER.85 86 Thus, some TNBCs might be more similar to the BC stem cell phenotype, showing a capacity to undergo EMT and reprogramming of embryonic genes, whereas others would not.87 88 This hypothesis is now being analysed in the new molecular characterisation of TNBC studies16–18 and would be concordant with the data on chemoresistance of some TNBCs. The link between breast development and tumorigenesis is conceptually appealing, however more studies need to be performed on this topic.44 89
Although histological and developmental classifications are informative, most of the progress in TNBC subclassification has been performed by molecular studies.
The first molecular observation was based on BRCA1 mutations; however, only for a small number of TNBC cases, since at the most only 20% of patients with TNBC have this mutation.90 91 A proportion of the women with TNBC carry BRCA1 germline mutations, but this proportion varies with age of diagnosis and family history (figure 3).92 The role of BRCA1 null TNBC arising in non-BRCA1 germline mutation carriers is not yet clear. Furthermore, 80% of the BCs arising in BRCA1 germline mutation carriers are TNBC but with good prognosis.93 Deep sequencing of BC genomes revealed that the BRCA1 null TNBC shows less genomic instability than the non-BRCA null TNBC.12 25 BRCA1 status may thus be suggesting two distinct TNBCs. BRCA1-deficient TNBC, germline or somatic, is likely a different biological entity, deficient in DNA repair, and, therefore, responsive to therapy with PARPi and platinum salts.94 This assumption is being tested in current randomised clinical trials in TNBC. However, clinical trials with PARPi have shown distinct outcomes, as the results are either quite promising95 96 or have failed to achieve any response in these patients.97 98 Platinum salts are also being used in trials with promising results, as depicted in this meta-analysis.99 New data on neoadjuvant platinum trials in TNBC have shown a high pCR100 (table 2). More recently, olaparib and talazoparib, both PARPi, are being used in several clinical trials (table 2) to treat patients with gBRCAm. The OlympiAD—a phase III clinical trial that analyses the use of olaparib versus standard chemotherapy in HER2-negative metastatic BC with gBRCAm, found that progression-free survival was improved in the olaparib arm, the time to second progression was longer and there were less adverse events, increasing the health-related quality of life in these patients.75
Besides BRCA1 mutations, other TNBC categories have been described. For instance, Lehmann et al molecularly divided TNBC in six different subtypes according to their unique gene expression profiles.17 These subtypes were classified as basal-like (BL1 and BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL) and luminal androgen receptor (LAR). The first two subclasses had a basal-like phenotype with higher expression of cell cycle and DNA damage response genes and BL2 also had increased growth factor signalling. The IM subtype had elevated immune cell signalling and cytokine signalling. Mesenchymal and MSL subtypes had augmented gene expression for EMT events, cell motility and differentiation. In addition, the MSL class had lower levels of proliferation genes and increased gene expression related with stem cells. Finally, the LAR subclass had increased levels of androgen receptor (AR) and luminal gene expression patterns. Within these subclasses, the response to therapeutic inputs was distinct, as well as the relapse-free survival and distant metastasis-free survival.
More recently, a new classification was described by Burstein et al.18 In this study, the authors assessed 198 tumour tissues, mainly from Caucasian women, according to their expression profile data. They were able to divide TNBC in four different classes—LAR, mesenchymal (MES), basal-like immunosuppressed (BLIS) and basal-like immune-activated (BLIA). Each subtype had unique gene expression profiles and specific targets. For instance, LAR, which was similar to the subtype described by Lehmann, exhibited AR, ER, prolactin and ErbB4 signalling. MEShad increased cell cycle pathways, mismatch repair of DNA, DNA damage networks and higher IGF1. BLIS had low levels of B cells, T cells and natural killer (NK) cells, reduced immune-regulating pathways and cytokine expression; on the other hand, had elevated expression of SOX1. Opposite of BLIS is BLIA with high levels of immune cells and activation of STAT transcription factor mediator pathways. The BLIS subtype had worst prognosis with low disease-free survival, while BLIA had the better prognosis. Specific targets for each class were found: mucin 1 for LAR; platelet-derived growth factor (PDGF) receptor A and c-Kit for MES; V-Set domain containing T cell activation inhibitor 1 (VTCN1) for BLIS and STAT signal transduction molecules and cytokines for BLIA.
Interestingly, Lehmann recently published another work where he acknowledges the existence of only four subtypes, denominated TNBCtype-4.16 These subtypes were BL1, BL2, M and LAR. In addition to the gene expression profiles that were executed in the first article, histopathological quantification and laser-capture microdissection (LCM) were used. As so, the lymphocytic infiltration was found to be present in all six subtypes, in different percentages, leading to the conclusion that IM is not really an independent subtype but that its components are present in the other classes. Moreover, the authors found that the characteristics of the MSL subtype were mainly derived by the stromal component surrounding the tumour. Again, each of the subtypes had different responses to the same treatment, with 41% of BL1 patients and only 18% of BL2 patients achieving pCR.
Even though the subtypes described by Burstein or by Lehmann are not exactly superimposable, there seems to be a tendency—one class with high expression of AR, another with mesenchymal features and two basal-like. In addition, lymphocytic infiltration appears to be present in distinct proportions across the subtypes, or at least prevalent in two subclasses (BLIS and BLIA). The important message to retrieve from these attempts to classify TNBC is that indeed tumours differ between patients as well as their response to the same treatment and this could be the basis of the heterogeneity found in the clinic that was described in the previous section. It is possible to hypothesise that young women with TNBC can have a subtype that is more aggressive or that doesn’t have a good response to a systemic untargeted treatment, such as BLIS for instance, when compared with menopausal women with TNBC. Moreover, a tumour with mesenchymal phenotype and with stem cell characteristics can be more chemoresistant than a basal-like subtype. This subtype could be more prevalent in African women, since they have a more aggressive phenotype that is more intolerant to treatment.
Another point in common between all classifications is the presence of immune cells within the tumour, either as a subgroup on its own—IM in Lehmann’s first classification or BLIS and BLIA in Burstein’s study, or widespread through all groups as depicted in the second classification published by Lehmann. Moreover, BLIS and BLIA subtypes were the ones with the worst and best prognoses, respectively. Thus, it seems clear that the immune system plays a critical role in the progression of TNBC.
The role of immune cells in TNBC progression
Indeed, a few studies have described the relationship between tumour infiltrating lymphocytes (TILs) and cancer progression and patient survival, namely in melanoma and ovarian, breast, bladder, cervical, colon, prostate, rectum and lung cancers.101–107 TILs enclose cytotoxic CD8+ T cells, CD4+ T helper cells (Th), CD4+/FOXP3+ regulatory T cells (Treg), B cells and NK cells. It was observed that in BC and in the adjacent stroma, the number of TILs is higher when compared with normal breast tissue.108 109 Overall, the presence of TILs in BC seem to lead to a better prognosis and an increased survival rate, since chemotherapeutic drugs are more efficient against tumours implanted in immunocompetent, with respect to immunodeficient hosts.110 111 Severe lymphopenia negatively affects the chemotherapeutic response of multiple distinct solid cancers112 and depletion of CD8+ T lymphocytes in animal models also reduced the efficacy of chemotherapy.110 111 Hence, several reports have been advocating that TILs could serve as a robust marker for predicting pCR rate in respect to NACT.113
In a meta-analysis of studies published with predictive significance of TILs in pCR in cases of BC with NACT,114 the authors observed that an increase in TILs (especially CD8+ and FOXP3+ cells) in biopsies from patients with no previous treatment led to better pCR after NACT. Another meta-analysis observed that an increase in TILs led to a reduction of 30% in the risk of recurrence, a decrease in 22% in the risk of distant recurrence and 34% less risk of death.115
Within patients with BC, the presence of TILs has been associated with increased pCR rates and a decreased risk of mortality in HER2+ and TNBC, but not in ER+ disease.114 116–118 We will now focus on the role of TILs specifically in TNBC.
A study observed that in TNBC samples, the presence of CD8+ cells within the tumour was associated with a reduction of 28% in mortality and the presence of these cells in the stroma led to a decrease of 21%.116 The presence of TILs correlates with pCR, and the quantity of TILs in the tumour microenvironment is also important, as it was found that the higher the number of CD8+ T cells present at the tumour site, the better the prognosis of patients with TNBC.119–121 In the past few years, a number of clinical trials in BC, namely in TNBC, started to implement the quantification of TILs and the possible association with survival. For instance, in ECOG 2197 and ECOG 1199 trials, it was observed that for each 10% increment in TILs (within the stroma), there was a 19% reduction in the risk of death.91 Loi et al observed that each 10% increase in TILs was associated with 27% reduction in the risk of death (BIG 02–98 clinical trial)117 and 13% reduction in the relative risk of distant recurrence (FinHER trial).122 If the patients were treated with chemotherapy, this reduction was of 18%.122 Thus, it is possible that the clinical efficacy of therapeutic regimens commonly employed to treat TNBC, namely chemotherapy, is largely determined by T lymphocyte-dependent immune response. Indeed, in a prospective study, it was found that an increase in TILs in BC supports a better response to anthracycline/taxane-based NACT.123
However, not all studies corroborate this association between presence of TILs and patient outcome. In fact, and just as an example, Liu et al, determined that there was no association between CD8+ TILs infiltration and improved survival in TNBC that showed no expression of basal markers.120
Moreover, CD8+ T cells are present in immune infiltrates within the tumour, and other T cells, such as CD4+ Th and FOXP3 are also present in the tumour microenvironment. CD4+ Th may be correlated with good prognosis (higher Th1) and with poor prognosis (higher Th2) in BC, but not specifically in TNBC.124 Treg effect on prognosis is controversial, with different studies in TNBC affirming distinct consequences from the presence of Tregs in tumours. For instance, Mahmoud et al described that Treg is not an independent prognostic factor,119 while Lee et al claimed that improved survival in patients with TNBC was associated with highly infiltrating Treg.125 It was also described that the presence of FOXP3 + in patients post-NACT was found to be a predictive marker for a low pCR rate.114 Interestingly, it was described that a ratio of CD8+/FOXP3+ ≥3 in primary BC led to an improved overall survival, whereas if this ratio is <3 in metastatic BC (at first relapse), it also led to improved overall survival.126 In metastatic TNBC, the levels of CD3+, CD4+, CD8+ and FOXP3+ TILs were inferior than the matched primary tumour, and metastatic TNBCs had fewer TILs compared with metastatic HER2+, ER+ or PgR+ tumours.126
Besides CD4+, CD8+ and FOXP3+ TILs, other cells from the immune system can have a pivotal role in TNBC progression, as tumour associated macrophages (TAMs), dendritic cells (DCs), tumour associated neutrophils (TANs), myeloid derived suppressor cells (MDSCs) and NK cells.127–129 Nevertheless, few studies have been developed that focus on these cells from the immune system and their possible association with patient outcome.
The information input that is used nowadays in the clinic for patients with TNBC is not sufficient to distinguish between patients who have a high chance to have a pCR when treated with conventional NACT from patients who cannot achieve a pCR and could benefit more from alternative immunotherapies. The information provided by the clinical measure of TILs pretreatment could be an answer to help in decision making regarding the treatment of different patients with TNBC. Stratification of patients based on the presence of TILs, and more specifically the subtype and the phenotype of TILs, will be paramount in the future to provide quality treatment to non-responders to conventional systemic therapy. Indeed, immunotherapy is not yet a reality for patients with TNBC, since the clinical trials implemented so far had a low response rate,130 probably due to poor characterisation of the immune environment and subsequent suboptimal patient stratification.
Potential new targeted therapies for TNBC considering its immune microenvironment
The success of NACT may be interrupted by distinct strategies employed by tumour cells to induce anergy/exhaustion of effector CD8+ T cells and Th1 cells, namely by defective antigen presentation, by engaging the T cell receptor in the absence of co-stimulation, by shifting the balance from Th1 to Th2 (immune deviation), by expressing inhibitory molecules like PD-L1 which bind the inhibitory receptor PD-1 in effector T lymphocytes, inducing negative regulatory pathways that limit the activity of these cells.131 132 Other inhibitory immune checkpoints, such as CTLA-4, Tim-3 or LAG-3 and the secretion of extrinsic immunosuppressing molecules such as interleukin (IL) 10, TGF-β, indoleamine 2,3-dioxygenase (IDO) or arginase could also directly or indirectly (by recruiting MDSCs and Tregs) negatively impact effector T cells’ actions.131 132 CTLA-4 is also expressed at the surface of T cells and can suppress their function by binding to B7 ligands. Tumour cells can express these ligands on their surface to escape the immune system.133 Tim-3 is a cell surface receptor present in CD4+ and CD8+ T cells with an inhibitory function.134 Tests performed in mouse models of BC found a high number of CD8+ T cells with Tim-3 expression infiltrating the tumour120 and the blockage of Tim3 led to an increase in antitumour immunity.120 121 IDO is an immunosuppressive enzyme present in DCs, macrophages and tumour cells.122 By using an IDO inhibitor, it would be possible to increase the level of maturation of these cells that are able to present tumour-associated antigens to effector cells. Another interesting approach to boost the antitumour response would be by the use of Ox40 agonists, since Ox40 is a co-stimulatory receptor present in T cells.123 124 Moreover, several other molecules with immune checkpoint properties are now being discovered and studied in different malignancies, such as CD39, CD73, TIGIT, CD96 and CD47.135–137
Blocking these immunosuppressing mechanisms to augment T lymphocyte function seems a promising approach to treat cancer.131 132 138 Indeed, these proteins are beginning to be used in anticancer therapeutics, as FDA approved one antibody against CTLA-4 (ipilimumab), two against PD-1 (pembrolizumab and nivolumab) for the treatment of melanoma and also two against PD-L1—atezolizumab and avelumab, for bladder cancer and squamous non-small cell lung cancer treatment, respectively.139
As TNBC appears to have high infiltration of cells from the immune system, it would be interesting to analyse the level of these immune checkpoints on TNBC samples. In fact, the PD-1 inhibitor pembrolizumab is being tested on patients with TNBC in a phase II clinical trial with some promising results.125 Pembrolizumab is also being analysed as an addition to standard NACT in two different trials—KEYNOTE 173 and I-SPY 2. In the first clinical trial, TNBC pCR rates are 90% (NACT+pembrolizumab) and 100% with carboplatin addition.140 In the I-SPY trial, pCR rates were 2%.141 In a metastatic setting (KEYNOTE 86), it was possible to observe that patients that had increased response to pembrolizumab had lower LDH and no liver nor visceral metastasis.142 Thus, it seems that immunotherapy in a metastatic setting has a higher response in less aggressive cases.
A growing body of evidence shows that the type of immune response influences the efficiency of several chemotherapeutic drugs116 and therefore selective immunotherapeutic interventions alone or in synergism with more conventional anticancer agents are now more appealing.
The blockade antibodies against T cell checkpoint molecules including CTLA-4 and the PD-1/PD-L1 axis in monotherapy or combination therapies have begun to revolutionise the current standard cancer treatment in various cancer types, such as melanoma, lung cancer, bladder cancer and Hodgkin’s lymphoma.132 This treatment is still not used in TNBC, because the genetic instability of tumour cells, which is frequent in this type of disease, make it a bad candidate for the success of targeted immunotherapies. However, the implementation of patient stratification by evaluating TILs and their phenotype in the clinic may improve the use of immunotherapies in this subtype of BC. Indeed, with the evaluation of the tumour-immune microenvironment it would be possible to distinguish patients that benefit from standard chemotherapy alone from those who would benefit more from these new monoclonal antibodies alone or in combination with chemotherapeutic agents.
Besides these immune checkpoint inhibitors, other approaches could be interesting for the development of new therapies. For instance, the BLIS subtype described by Burstein has an immunosuppressed environment that is translated to the gene expression profile of these tumours. There are some genes important for the regulation and activation of the immune system effectors that are downregulated in BLIS samples compared with controls. Since this subtype is the one with the worst prognosis it is of foremost importance to discover new ways to treat these patients. The external upregulation of these genes may be one of the methods to overcome the immunosuppression in this subtype. Interestingly, some of these genes are upregulated in BLIA, which demonstrates their importance in the activation of the immune environment. Among these genes are CXCL9, CXCL13, GZMB, GZMA, CD2, CD69, PTPRC and TLR8.
Conclusions
TNBC is still a problem in the clinic, due to the lack of targeted therapies. Additionally, patients with TNBC have a high level of divergence between them, concerning age of diagnosis, the prognosis and the response to treatment. Thus, we are convinced from our clinical observations, laboratory data and from the literature that TNBC is not a single disease and that the subclassification of this clinical entity with clinical phenotype correlates is an unmet clinical need in BC.
In order to understand this heterogeneity and to develop new therapies, researchers have tried to uncover the basis of this diversity, using histological and molecular tools. Although it seems that no consensus has yet been made, some advances point to the same direction and patient segregation can be achieved: TNBC arising in patients with the BRCA1 germline mutation, TNBC associated with an immune phenotype with lymphocytic infiltration or TNBC arising in African-descent patients that is more chemoresistant.
So, to improve patient welfare, it is likely that several therapeutic strategies will be implemented according to the subtype of TNBC. These therapies can go from PARPi or platinum agents, for BRCA1 mutation carriers, to immune checkpoint inhibitors, that can modulate the tumour immune microenvironment to stimulate immune responses and control tumour progression.
Nevertheless, the findings in this area are still preliminary and more studies need to be performed to ascertain the true effect of these treatments in TNBC treatment.
Footnotes
Contributors: DPS, MGC and SB reviewed the literature, wrote the manuscript and AJ revised the text.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Hospital Prof Doutor Fernando da Fonseca.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. World Health Organization. Women and health: today’s evidence tomorrow’s agenda. 2009.
- 2. Howlader N, Krapcho M, Miller D, et al. . SEER Cancer Statistics Review, 1975-2012: National Cancer Institute, 2015. [Google Scholar]
- 3. Marques AR, Teixeira E, Diamond J, et al. . Detection of human mammaglobin mRNA in serial peripheral blood samples from patients with non-metastatic breast cancer is not predictive of disease recurrence. Breast Cancer Res Treat 2009;114:223–32. 10.1007/s10549-008-0002-9 [DOI] [PubMed] [Google Scholar]
- 4. Ravdin PM, Cronin KA, Howlader N, et al. . The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007;356:1670–4. 10.1056/NEJMsr070105 [DOI] [PubMed] [Google Scholar]
- 5. Slamon DJ, Clark GM, Wong SG, et al. . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 6. Engebraaten O, Vollan HK, Børresen-Dale AL. Triple-negative breast cancer and the need for new therapeutic targets. Am J Pathol 2013;183:1064–74. 10.1016/j.ajpath.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 7. Piccart-Gebhart M, Holmes E, Baselga J, et al. . Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol 2016;34:1034–42. 10.1200/JCO.2015.62.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet 2016;293:247–69. 10.1007/s00404-015-3859-y [DOI] [PubMed] [Google Scholar]
- 9. Dent R, Trudeau M, Pritchard KI, et al. . Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13(15 Pt 1):4429–34. 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 10. Onitilo AA, Engel JM, Greenlee RT, et al. . Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009;7(1-2):4–13. 10.3121/cmr.2008.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spitale A, Mazzola P, Soldini D, et al. . Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol 2009;20:628–35. 10.1093/annonc/mdn675 [DOI] [PubMed] [Google Scholar]
- 12. Curtis C, Shah SP, Chin SF, et al. . The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346–52. 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eralp Y, Derin D, Ozluk Y, et al. . MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol 2008;19:669–74. 10.1093/annonc/mdm522 [DOI] [PubMed] [Google Scholar]
- 14. Ivanov O, Chen F, Wiley EL, et al. . alphaB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2008;111:411–7. 10.1007/s10549-007-9796-0 [DOI] [PubMed] [Google Scholar]
- 15. Wysocki PJ, Korski K, Lamperska K, et al. . Primary resistance to docetaxel-based chemotherapy in metastatic breast cancer patients correlates with a high frequency of BRCA1 mutations. Med Sci Monit 2008;14:1079–10. 10.1200/jco.2008.26.15_suppl.1079 [DOI] [PubMed] [Google Scholar]
- 16. Lehmann BD, Jovanović B, Chen X, et al. . Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One 2016;11:e0157368 10.1371/journal.pone.0157368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehmann BD, Bauer JA, Chen X, et al. . Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–67. 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burstein MD, Tsimelzon A, Poage GM, et al. . Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21:1688–98. 10.1158/1078-0432.CCR-14-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Honorio M, Guerra Pereira N. Decreased survival in African patients with triple negative breast cancer. J Palliat Care Med 2016;06:2 10.4172/2165-7386.1000270 [DOI] [Google Scholar]
- 20. Anderson WF, Chatterjee N, Ershler WB, et al. . Estrogen receptor breast cancer phenotypes in the surveillance, epidemiology, and end results database. Breast Cancer Res Treat 2002;76:27–36. 10.1023/A:1020299707510 [DOI] [PubMed] [Google Scholar]
- 21. Lund MJ, Trivers KF, Porter PL, et al. . Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 2009;113:357–70. 10.1007/s10549-008-9926-3 [DOI] [PubMed] [Google Scholar]
- 22. Muguti GI. Experience with breast cancer in Zimbabwe. J R Coll Surg Edinb 1993;38:75–8. [PubMed] [Google Scholar]
- 23. Yin WJ, Lu JS, Di GH, et al. . Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat 2009;115:325–33. 10.1007/s10549-008-0096-0 [DOI] [PubMed] [Google Scholar]
- 24. Curtin JA, Fridlyand J, Kageshita T, et al. . Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47. 10.1056/NEJMoa050092 [DOI] [PubMed] [Google Scholar]
- 25. Stephens PJ, McBride DJ, Lin ML, et al. . Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 2009;462:1005–10. 10.1038/nature08645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collett K, Stefansson IM, Eide J, et al. . A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol Biomarkers Prev 2005;14:1108–12. 10.1158/1055-9965.EPI-04-0394 [DOI] [PubMed] [Google Scholar]
- 27. Seewaldt VL, Scott V. Images in clinical medicine. Rapid progression of basal-type breast cancer. N Engl J Med 2007;356:e12 10.1056/NEJMicm063760 [DOI] [PubMed] [Google Scholar]
- 28. Vona-Davis L, Rose DP, Hazard H, et al. . Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev 2008;17:3319–24. 10.1158/1055-9965.EPI-08-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gadiyaram V, Kurian S, Abraham J, et al. . Predominance of brain and lung metastases in triple-negative breast cancer patients. Cancer Res 2009;69(24 Supplement):6159 10.1158/0008-5472.SABCS-09-6159 [DOI] [Google Scholar]
- 30. Pinilla SM, Honrado E, Hardisson D, et al. . Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat 2006;99:85–90. 10.1007/s10549-006-9184-1 [DOI] [PubMed] [Google Scholar]
- 31. Rodríguez-Pinilla SM, Sarrió D, Honrado E, et al. . Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res 2006;12:1533–9. 10.1158/1078-0432.CCR-05-2281 [DOI] [PubMed] [Google Scholar]
- 32. Tsang J, Li V, Lai E, et al. . Triple negative breast cancer patients with brain metastasis are associated with more concurrent lung metastases. Cancer Res 2009;69(24 Supplement):3072 10.1158/0008-5472.SABCS-09-3072 [DOI] [Google Scholar]
- 33. Wei B, Wang J, Bourne P, et al. . Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol 2008;39:1809–15. 10.1016/j.humpath.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 34. Lee Y, Kwon S, Ko B, et al. . Triple negative breast cancer has a worse prognosis within 3 years after treatment compared to non-triple negative breast cancer. Cancer Res 2009;69(24 Supplement):4044 10.1158/0008-5472.SABCS-09-4044 [DOI] [Google Scholar]
- 35. Tse GM, Tan PH, Lau KM, et al. . Breast cancer in the elderly: a histological assessment. Histopathology 2009;55:441–51. 10.1111/j.1365-2559.2009.03400.x [DOI] [PubMed] [Google Scholar]
- 36. Gong Y, Yan K, Lin F, et al. . Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol 2007;8:203–11. 10.1016/S1470-2045(07)70042-6 [DOI] [PubMed] [Google Scholar]
- 37. Crowe JP, Gordon NH, Hubay CA, et al. . The interaction of estrogen receptor status and race in predicting prognosis for stage I breast cancer patients. Surgery 1986;100:599–605. [PubMed] [Google Scholar]
- 38. Natarajan N, Nemoto T, Mettlin C, et al. . Race-related differences in breast cancer patients. Results of the 1982 national survey of breast cancer by the American College of Surgeons. Cancer 1985;56:1704–9. [DOI] [PubMed] [Google Scholar]
- 39. Swanson GM, Lin CS. Survival patterns among younger women with breast cancer: the effects of age, race, stage, and treatment. J Natl Cancer Inst Monogr 1994;16:69–77. [PubMed] [Google Scholar]
- 40. Carey LA, Perou CM, Livasy CA, et al. . Race, breast cancer subtypes, and survival in the Carolina breast cancer Study. JAMA 2006;295:2492–502. 10.1001/jama.295.21.2492 [DOI] [PubMed] [Google Scholar]
- 41. Huo D, Ikpatt F, Khramtsov A, et al. . Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 2009;27:4515–21. 10.1200/JCO.2008.19.6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaky S, Lund M, May K, et al. . The triple threat of recurrence after breast conserving therapy: race, receptor status and age. Cancer Res 2009;69(24 Supplement):6045 10.1158/0008-5472.SABCS-09-6045 [DOI] [Google Scholar]
- 43. Stead LA, Lash TL, Sobieraj JE, et al. . Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Research 2009;11:11 1 10.1186/bcr2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim E, Vaillant F, Wu D, et al. . Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 2009;15:907–13. 10.1038/nm.2000 [DOI] [PubMed] [Google Scholar]
- 45. Kim K, Lee E, Lee J, et al. . Clinicopathologic Signature of TNBC Patients with Good Prognosis. Cancer Res 2009;69(24 Supplement):4065 10.1158/0008-5472.SABCS-09-4065 [DOI] [Google Scholar]
- 46. Kwong A, Wong K, Wong C, et al. . The Epidemiology, Clinico-Pathologic and Prognostic Characteristics of Triple-Negative Breast Cancer Compared with Non Triple-Negative Breast Cancer. Cancer Res 2009;69(24 Supplement):3071 10.1158/0008-5472.SABCS-09-3071 [DOI] [Google Scholar]
- 47. Nishimura R, Arima N. Is triple negative a prognostic factor in breast cancer? Breast Cancer 2008;15:303–8. 10.1007/s12282-008-0042-3 [DOI] [PubMed] [Google Scholar]
- 48. Frasci G, Comella P, Rinaldo M, et al. . Preoperative weekly cisplatin-epirubicin-paclitaxel with G-CSF support in triple-negative large operable breast cancer. Ann Oncol 2009;20:1185–92. 10.1093/annonc/mdn748 [DOI] [PubMed] [Google Scholar]
- 49. Linn SC, Van ’t Veer LJ. Clinical relevance of the triple-negative breast cancer concept: genetic basis and clinical utility of the concept. Eur J Cancer 2009;45 Suppl 1:11–26. 10.1016/S0959-8049(09)70012-7 [DOI] [PubMed] [Google Scholar]
- 50. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol 2007;8:235–44. 10.1016/S1470-2045(07)70074-8 [DOI] [PubMed] [Google Scholar]
- 51. Boyd NF, Li Q, Melnichouk O, et al. . Evidence that breast tissue stiffness is associated with risk of breast cancer. PLoS One 2014;9:e100937 10.1371/journal.pone.0100937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCormack VA, dos Santos Silva I, Silva dosS I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159–69. 10.1158/1055-9965.EPI-06-0034 [DOI] [PubMed] [Google Scholar]
- 53. Barlow WE, White E, Ballard-Barbash R, et al. . Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst 2006;98:1204–14. 10.1093/jnci/djj331 [DOI] [PubMed] [Google Scholar]
- 54. Albain KS, Unger JM, Crowley JJ, et al. . Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009;101:984–92. 10.1093/jnci/djp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chlebowski RT, Chen Z, Anderson GL, et al. . Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 2005;97:439–48. 10.1093/jnci/dji064 [DOI] [PubMed] [Google Scholar]
- 56. Lindner R, Sullivan C, Offor O, et al. . Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One 2013;8:e71915 10.1371/journal.pone.0071915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. King TD, Suto MJ, Li Y. The Wnt/β-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem 2012;113:13–18. 10.1002/jcb.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wend P, Runke S, Wend K, et al. . WNT10B/β-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med 2013;5:264–79. 10.1002/emmm.201201320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nalwoga H, Arnes JB, Wabinga H, et al. . Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer 2010;102:369–75. 10.1038/sj.bjc.6605488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carey LA, Dees EC, Sawyer L, et al. . The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–34. 10.1158/1078-0432.CCR-06-1109 [DOI] [PubMed] [Google Scholar]
- 61. Esserman LJ, Perou C, Cheang M, et al. , 2009. Breast cancer molecular profiles and tumor response of neoadjuvant doxorubicin and paclitaxel: the I-SPY TRIAL (CALGB 150007/150012, ACRIN 6657). ASCO Annual Meeting Proceedings LBA515 [Google Scholar]
- 62. Liedtke C, Mazouni C, Hess KR, et al. . Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–81. 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 63. von Minckwitz G, Kümmel S, Vogel P, et al. . Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 2008;100:552–62. 10.1093/jnci/djn089 [DOI] [PubMed] [Google Scholar]
- 64. von Minckwitz G, Kümmel S, Vogel P, et al. . Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst 2008;100:542–51. 10.1093/jnci/djn085 [DOI] [PubMed] [Google Scholar]
- 65. Wang S, Yang H, Tong F, et al. . Response to neoadjuvant therapy and disease free survival in patients with triple-negative breast cancer. Gan To Kagaku Ryoho 2009;36:255–8. [PubMed] [Google Scholar]
- 66. Kassam F, Enright K, Dent R, et al. . Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009;9:29–33. 10.3816/CBC.2009.n.005 [DOI] [PubMed] [Google Scholar]
- 67. Lin NU, Claus E, Sohl J, et al. . Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer. Cancer 2008;113:2638–45. 10.1002/cncr.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bear HD, Anderson S, Smith RE, et al. . Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24:2019–27. 10.1200/JCO.2005.04.1665 [DOI] [PubMed] [Google Scholar]
- 69. Hüsemann Y, Geigl JB, Schubert F, et al. . Systemic spread is an early step in breast cancer. Cancer Cell 2008;13:58–68. 10.1016/j.ccr.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 70. Weigelt B, Geyer FC, Natrajan R, et al. . The molecular underpinning of lobular histological growth pattern: a genome-wide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special type. J Pathol 2010;220:45–57. 10.1002/path.2629 [DOI] [PubMed] [Google Scholar]
- 71. Cameron D, Brown J, Dent R, et al. . Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol 2013;14:933–42. 10.1016/S1470-2045(13)70335-8 [DOI] [PubMed] [Google Scholar]
- 72. ClinicalTrials.gov. Bevacizumab + paclitaxel + capecitabine in triple negative metastatic breast cancer. https://clinicaltrials.gov/ct2/show/NCT01069796.
- 73. Yardley DA, Bosserman LD, Keaton MR, et al. . TITAN: Phase III study of doxorubicin/cyclophosphamide (AC) followed by ixabepilone (Ixa) or paclitaxel (Pac) in early-stage, triple-negative breast cancer (TNBC). J Clin Oncol 2015;33. [Google Scholar]
- 74. ClinicalTrials.gov. Randomized phase II study of ixabepilone alone and ixabepilone plus cetuximab as first-line treatment for female subjects with triple negative locally advanced non-resectable and/or metastatic breast cancer. https://clinicaltrials.gov/ct2/show/NCT00633464.
- 75. Robson ME, Im S-A, Senkus E, et al. . OlympiAD: Phase III trial of olaparib monotherapy versus chemotherapy for patients (pts) with HER2-negative metastatic breast cancer (mBC) and a germline BRCA mutation (gBRCAm). Journal of Clinical Oncology 2017;35:LBA4 10.1200/JCO.2017.35.18_suppl.LBA4 [DOI] [Google Scholar]
- 76. Zitvogel L, Apetoh L, Ghiringhelli F, et al. . The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008;118:1991–2001. 10.1172/JCI35180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maehle BO, Thoresen S, Skjaerven R, et al. . Mean nuclear area and histological grade of axillary-node tumour in breast cancer, related to prognosis. Br J Cancer 1982;46:95–100. 10.1038/bjc.1982.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. O’Reilly SM, Camplejohn RS, Barnes DM, et al. . DNA index, S-phase fraction, histological grade and prognosis in breast cancer. Br J Cancer 1990;61:671–4. 10.1038/bjc.1990.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hance KW, Anderson WF, Devesa SS, et al. . Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 2005;97:966–75. 10.1093/jnci/dji172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jung SY, Kim HY, Nam BH, et al. . Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat 2010;120:627–37. 10.1007/s10549-010-0780-8 [DOI] [PubMed] [Google Scholar]
- 81. Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology 2008;52:108–18. 10.1111/j.1365-2559.2007.02889.x [DOI] [PubMed] [Google Scholar]
- 82. Rizzo M, Lund MJ, Mosunjac M, et al. . Characteristics and treatment modalities for African American women diagnosed with stage III breast cancer. Cancer 2009;115:3009–15. 10.1002/cncr.24334 [DOI] [PubMed] [Google Scholar]
- 83. Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer 2007;7:791–9. 10.1038/nrc2212 [DOI] [PubMed] [Google Scholar]
- 84. Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer 2007;7:823–33. 10.1038/nrc2253 [DOI] [PubMed] [Google Scholar]
- 85. Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab 2004;15:193–7. 10.1016/j.tem.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 86. Liu S, Ginestier C, Charafe-Jauffret E, et al. . BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA 2008;105:1680–5. 10.1073/pnas.0711613105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ben-Porath I, Thomson MW, Carey VJ, et al. . An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008;40:499–507. 10.1038/ng.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ginestier C, Liu S, Wicha MS. Getting to the root of BRCA1-deficient breast cancer. Cell Stem Cell 2009;5:229–30. 10.1016/j.stem.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 89. Pece S, Tosoni D, Confalonieri S, et al. . Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010;140:62–73. 10.1016/j.cell.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 90. Eiermann W, Bergh J, Cardoso F, et al. . Triple negative breast cancer: proposals for a pragmatic definition and implications for patient management and trial design. Breast 2012;21:20–6. 10.1016/j.breast.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 91. Adams S, Gray RJ, Demaria S, et al. . Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959–66. 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kandel MJ, Stadler Z, Masciari S, et al. , 2006. Prevalence of BRCA1 mutations in triple negative breast cancer (BC). ASCO Annual Meeting Proceedings 508. [Google Scholar]
- 93. Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. . Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med 2007;357:115–23. 10.1056/NEJMoa070608 [DOI] [PubMed] [Google Scholar]
- 94. Venkitaraman AR. Targeting the molecular defect in BRCA-deficient tumors for cancer therapy. Cancer Cell 2009;16:89–90. 10.1016/j.ccr.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 95. Fong PC, Boss DS, Yap TA, et al. . Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–34. 10.1056/NEJMoa0900212 [DOI] [PubMed] [Google Scholar]
- 96. O’Shaughnessy J, Osborne C, Pippen JE, et al. . Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 2011;364:205–14. 10.1056/NEJMoa1011418 [DOI] [PubMed] [Google Scholar]
- 97. Gelmon KA, Tischkowitz M, Mackay H, et al. . Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852–61. 10.1016/S1470-2045(11)70214-5 [DOI] [PubMed] [Google Scholar]
- 98. O’Shaughnessy J, Schwartzberg L, Danso MA, et al. . Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 2014;32:3840–7. 10.1200/JCO.2014.55.2984 [DOI] [PubMed] [Google Scholar]
- 99. Liu M, Mo QG, Wei CY, et al. . Platinum-based chemotherapy in triple-negative breast cancer: A meta-analysis. Oncol Lett 2013;5:983–91. 10.3892/ol.2012.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhou L, Xu S, Yin W, et al. . Weekly paclitaxel and cisplatin as neoadjuvant chemotherapy with locally advanced breast cancer: a prospective, single arm, phase II study. Oncotarget 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kiniwa Y, Miyahara Y, Wang HY, et al. . CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 2007;13:6947–58. 10.1158/1078-0432.CCR-07-0842 [DOI] [PubMed] [Google Scholar]
- 102. Liakou CI, Narayanan S, Ng Tang D, et al. . Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun 2007;7:10. [PMC free article] [PubMed] [Google Scholar]
- 103. Santoiemma PP, Powell DJ, Dj PJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 2015;16:807–20. 10.1080/15384047.2015.1040960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Teng F, Mu D, Meng X, et al. . Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res 2015;5:2064–74. [PMC free article] [PubMed] [Google Scholar]
- 105. Tsuyuguchi I, Shiratsuchi H, Fukuoka M. T-lymphocyte subsets in primary lung cancer. Jpn J Clin Oncol 1987;17:13–17. [PubMed] [Google Scholar]
- 106. Underwood JC. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer 1974;30:538–48. 10.1038/bjc.1974.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Woo JR, Liss MA, Muldong MT, et al. . Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med 2014;12:30 10.1186/1479-5876-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tuaillon E, Valea D, Becquart P, et al. . Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol 2009;182:7155–62. 10.4049/jimmunol.0803107 [DOI] [PubMed] [Google Scholar]
- 109. Willard-Gallo K, Buisseret L, Garaud S, et al. . Abstract PD1-3: The significance of tumor infiltrating lymphocyte density, subset composition and organization in breast cancer. Cancer Res 2015;75(9 Supplement):PD1-3 10.1158/1538-7445.SABCS14-PD1-3 [DOI] [Google Scholar]
- 110. Apetoh L, Ghiringhelli F, Tesniere A, et al. . The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007;220:47–59. 10.1111/j.1600-065X.2007.00573.x [DOI] [PubMed] [Google Scholar]
- 111. Casares N, Pequignot MO, Tesniere A, et al. . Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005;202:1691–701. 10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. . Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383–91. 10.1158/0008-5472.CAN-08-3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hornychova H, Melichar B, Tomsova M, et al. . Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest 2008;26:1024–31. 10.1080/07357900802098165 [DOI] [PubMed] [Google Scholar]
- 114. Mao Y, Qu Q, Zhang Y, et al. . The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One 2014;9:e115103 10.1371/journal.pone.0115103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, et al. . The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 2014;148:467–76. 10.1007/s10549-014-3185-2 [DOI] [PubMed] [Google Scholar]
- 116. Ali HR, Provenzano E, Dawson SJ, et al. . Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25:1536–43. 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]
- 117. Loi S, Sirtaine N, Piette F, et al. . Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;4. [DOI] [PubMed] [Google Scholar]
- 118. Yamaguchi R, Tanaka M, Yano A, et al. . Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 2012;43:1688–94. 10.1016/j.humpath.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 119. Mahmoud SM, Paish EC, Powe DG, et al. . Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949–55. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 120. Liu S, Lachapelle J, Leung S, et al. . CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 2012;14:R48 10.1186/bcr3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Baker K, Lachapelle J, Zlobec I, et al. . Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 2011;58:no–16. 10.1111/j.1365-2559.2011.03846.x [DOI] [PubMed] [Google Scholar]
- 122. Loi S, Michiels S, Salgado R, et al. . Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014;25:1544–50. 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 123. Issa-Nummer Y, Darb-Esfahani S, Loibl S, et al. . Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One 2013;8:e79775 10.1371/journal.pone.0079775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Teschendorff AE, Gomez S, Arenas A, et al. . Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer 2010;10:604 10.1186/1471-2407-10-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lee S, Cho EY, Park YH, et al. . Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol 2013;52:73–81. 10.3109/0284186X.2012.731520 [DOI] [PubMed] [Google Scholar]
- 126. Cimino-Mathews A, Ye X, Meeker A, et al. . Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol 2013;44:2055–63. 10.1016/j.humpath.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol 2015;33:445–74. 10.1146/annurev-immunol-032414-112043 [DOI] [PubMed] [Google Scholar]
- 128. Wei B, Yao M, Xing C, et al. . The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. Onco Targets Ther 2016;9:5567–75. 10.2147/OTT.S108419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Markowitz J, Wesolowski R, Papenfuss T, et al. . Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat 2013;140:13–21. 10.1007/s10549-013-2618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nanda R, Chow LQ, Dees EC, et al. . Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460–7. 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Inozume T, Hanada K, Wang QJ, et al. . Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother 2010;33:956–64. 10.1097/CJI.0b013e3181fad2b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol 2015;33:23–35. 10.1016/j.coi.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 133. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Monney L, Sabatos CA, Gaglia JL, et al. . Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002;415:536–41. 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- 135. Allard B, Longhi MS, Robson SC, et al. . The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev 2017;276:121–44. 10.1111/imr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dougall WC, Kurtulus S, Smyth MJ, et al. . TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev 2017;276:112–20. 10.1111/imr.12518 [DOI] [PubMed] [Google Scholar]
- 137. Matlung HL, Szilagyi K, Barclay NA, et al. . The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev 2017;276:145–64. 10.1111/imr.12527 [DOI] [PubMed] [Google Scholar]
- 138. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561–84. 10.1038/nrd4591 [DOI] [PubMed] [Google Scholar]
- 139. García-Teijido P, Cabal ML, Fernández IP, et al. . Tumor-infiltrating lymphocytes in triple negative breast cancer: the future of immune targeting. Clin Med Insights Oncol 2016;10(Suppl 1):31 10.4137/CMO.S34540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schmid P, Park YE, Muñoz-Couselo E, et al. . Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): Preliminary results from KEYNOTE-173. J Clin Oncol 2017;35. [Google Scholar]
- 141. Nanda R, Liu MC, Yau C, et al. . Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol 2017;35. [Google Scholar]
- 142. Adams S, Schmid P, Rugo HS. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J Clin Oncol 2017;35. [DOI] [PubMed] [Google Scholar]
- 143. Turner NC, Telli ML, Rugo HS, et al. . Final results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO). J Clin Oncol 2017;35. [Google Scholar]
- 144. Litton JK, Blum JL, Im Y, et al. . A phase 3, open-label, randomized, parallel, 2-arm international study of the oral PARP inhibitor talazoparib (BMN 673) in BRCA mutation subjects with locally advanced and/or metastatic breast cancer (EMBRACA. J Clin Oncol 2015;33:15. [Google Scholar]
- 145. Earl HM, Vallier A, Qian W, et al. . PARTNER: Randomised, phase II/III trial to evaluate the safety and efficacy of the addition of olaparib to platinum-based neoadjuvant chemotherapy in triple negative and/or germline BRCA mutated breast cancer patients. J Clin Oncol 2017;35:abstr TPS591. [Google Scholar]
- 146. Litton JK, Scoggins M, Whitman GJ, et al. . A feasibility study of neoadjuvant talazoparib for early-stage breast cancer patients with a germline BRCA pathogenic variant: NCT02282345. J Clin Oncol 2017;35:abstr TPS595. [Google Scholar]
- 147. Silver DP, Richardson AL, Eklund AC, et al. . Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145–53. 10.1200/JCO.2009.22.4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Isakoff SJ, Mayer EL, He L, et al. . TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 2015;33:1902–9. 10.1200/JCO.2014.57.6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vernieri C, Milano M, Mennitto A, et al. . Antitumor activity and safety profile of weekly carboplatin plus paclitaxel in metastatic breast cancer: a ten-year, monocentric, retrospective study. Breast Cancer Res Treat 2017;165:365–73. 10.1007/s10549-017-4336-z [DOI] [PubMed] [Google Scholar]