Abstract
In the last decade, extracellular vesicles (EVs) have emerged as a key cell-free strategy for the treatment of a range of pathologies, including cancer, myocardial infarction and inflammatory diseases. Indeed, the field is rapidly transitioning from promising in vitro reports towards in vivo animal models and early clinical studies. These investigations exploit the high physicochemical stability and biocompatibility of EVs, as well as their innate capacity to communicate with cells over long distances via signal transduction and membrane fusion. This review will focus on methods in which EVs can be chemically or biologically modified to broaden, alter or enhance their therapeutic capability. We will examine two broad strategies, which have been used to introduce a wide range of nanoparticles, reporter systems, targeting peptides, pharmaceutics and functional RNA molecules. First, we will explore how EVs can be modified by manipulating their parent cells; either through genetic or metabolic engineering, or by introducing exogenous material that is subsequently incorporated into secreted EVs. Second, we consider how EVs can be directly functionalized using strategies such as hydrophobic insertion, covalent surface chemistry and membrane permeabilization. We will discuss the historical context of each specific technology, present prominent examples and evaluate the complexities, potential pitfalls and opportunities presented by different re-engineering strategies.
Keywords: Extracellular Vesicles, Exosomes, Microvesicles, Functionalization, Genetic Manipulation, Drug Loading, Membrane Modification, Cell-Free Therapy
Extracellular Vesicles: Cell-Derived Nanovectors
Extracellular vesicles (EVs) are a collective of small, naturally-derived particles, which, until recently, represented an overlooked and underappreciated component of the cellular secretome. Three major categories of EV have been defined, predominantly based upon vesicle biogenesis, but with notable differences in size and composition.1,2 Exosomes are formed when the peripheral membrane of multivesicular bodies (MVBs) undergo reverse budding to form small nanovesicles (30-100 nm in diameter) that are released when MVBs fuse with the cytoplasmic membrane.3 Microvesicles are larger in size (c.f. 100-1000 nm) and are produced during shedding or budding of the cytoplasmic membrane.4 Exosomes and microvesicles are produced by healthy cells as part of regular membrane turnover and exocytosis. In contrast, apoptotic bodies (c.f. 500-2000 nm) are generated from outward membrane blebbing in cells undergoing apoptosis.5 Apoptotic bodies, microvesicles and exosomes are each enclosed by a phospholipid membrane bilayer, comparable to the cytoplasmic membrane. The EV membrane contains ligand receptors, major histocompatibility complex molecules6 as well as vesicle-specific markers, such as G-proteins (Rab5, Rab7) and tetraspanins (CD9, CD63, CD81, CD82) that are characteristic of exosomes.3 The EV lumen also contains many soluble proteins, including active enzymes.7–9 In addition, EVs possess an array of oligonucleotides, specifically mitochondrial DNA,10,11 messenger RNA (mRNA),12,13 microRNA (miRNA)12–15 and many other non-coding RNA sequences.16
EVs are secreted from all cell types and have been isolated from tissues and a wide range of bodily fluids, including plasma,17 breast milk,18 urine,19 saliva,20 synovial fluid,21 bile,22 amniotic fluid,23 semen,24 and ascites fluid.25 For many years, it was believed that they had a single function; the packaging and release of unwanted cellular material.26 EVs have now been shown to play an integral role as intercellular communication vectors, interacting with recipient cells by various means. Firstly, EVs can bind to surface receptors and trigger signal cascades across the cytoplasmic membrane, a process that complements classical paracrine signaling of secreted soluble factors.27 Secondly, surface-bound EVs can be internalized, a process that can occur via clathrin-dependent endocytosis, caveolin-mediated uptake, lipid-raft mediated endocytosis, phagocytosis or macropinocytosis.28–32 Thirdly, EVs can fuse with the recipient cell, which allows delivery of material directly to the cytoplasmic membrane and the cytosol.29,33–37 The capacity to deliver large quantities of functional biomaterials to neighboring cells, something that cannot be achieved with simple soluble factors, is exploited by cells in the horizontal transfer of proteins 8,13,34–38 and genetic material.12,13,33,39 While EVs play an essential role in normal physiological processes, such as inflammation, homeostasis, coagulation and calcification,27 they are also heavily implicated in pathological processes, notably autoimmune diseases and cancer.40–42 This has led to two burgeoning fields of research; the identification of pathological EVs as diagnostic biomarkers and therapeutic targets, and the administration of therapeutic EVs in the treatment of diseases.5,43
Exploiting Extracellular Vesicles for Applications in Nanomedicine
EV-based therapy represents a logical progression from stem cell therapy, which was once heralded as the miracle cure for in vivo regeneration. Indeed, many of the beneficial effects once attributed to stem cell engraftment and differentiation are now believed to be mediated by paracrine factors packaged within EVs.44 This realization sparked intensive investigation into whether the administration of EVs alone could offer comparable pharmacological benefits, or even present alternative therapeutic opportunities.5 The results of this research effort have been outstanding; EVs have been shown to inhibit apoptosis and improve cell proliferation,45,46 induce angiogenesis,47–55 alter inflammation and immune response,56–62 initiate coagulation,63 influence differentiation pathways64,65 and enhance cellular engraftment.66 EVs derived from a range of sources, most commonly mesenchymal stem cells (MSCs), have demonstrated great regenerative and protective potential in animal models of myocardial infarction,67–72 kidney ischemia,73–78 pancreatic islet transplantation,79 liver fibrosis,80 pulmonary hypertension,81 osteochondral defects,82 arthritis,83–85 burn injuries,86 graft-versus-host disease,87 and inflammation.88 Alongside these native effects, EVs have been used as vectors to deliver drugs and oligonucleotides in vivo,89–99 while EVs derived from antigen-pulsed dendritic cells (DCs) or macrophages have been used as vaccines against infectious diseases and cancer.100–105 Translation of this basic science has recently begun, with early clinical studies showing EVs to be safe and effective as a vaccine for meningitis,106 as well as a therapy for cancer107,108 and graft-versus-host disease109 (Table 1 & Figure 1).
Table 1. Selected clinical and pre-clinical examples of EVs in therapy.
| Target | Stage | EV Source | Reported Outcomes |
|---|---|---|---|
| Myocardial Infarction | Mouse | MSCs | Reduced infarct size.67 |
| Mouse | MSCs | Reduced infarct size; enhanced myocardial viability; preserved left ventricular geometry and contraction; reduced local and systemic inflammation.68 | |
| Rat | CPCs | Inhibited cardiomyocyte apoptosis; reduced scarring; enhanced angiogenesis; improved left ventricular ejection fraction.69 | |
| Rat | MSCs | Reduced infarct size, preserved cardiac systolic and diastolic performance; enhanced blood flow recovery.70 | |
| Pig | MSCs | Increased angiogenesis; reduced infarct size; preserved systolic and diastolic cardiac performance.71 | |
| Pig | MSCs | Reduced infarct size; decreased myocardial nuclear oxidative stress; improved systolic and diastolic cardiac performance.72 | |
| Kidney Injury | Mouse | MSCs | Decreased levels of creatine, urea, proteinuria; reduced fibrosis, decreased number of interstitial lymphocyte infiltrates; reduced tubular atrophy.73 |
| Rat | MSCs | Inhibited apoptosis; stimulated tubular endothelial cell proliferation; reduced acute kidney injury and chronic kidney disease.74 | |
| Rat | Liver Stem Cells | Increased hepatocyte proliferation; improved morphology and function.75 | |
| Rat | MSCs | Inhibited the increase of creatine, urea, fractional sodium extraction; slowed apoptosis and necrosis; increased cell proliferation.76 | |
| Rat | MSCs | Reduced apoptosis; decreased mortality.77 | |
| Rat | Endothelial Progenitor Cells | Enhanced tubular cell proliferation; reduced apoptosis; inhibited capillary rarefaction, glomerulosclerosis and tubulointerstitial fibrosis.78 | |
| Pancreatic Islet Transplantation | Mouse | Endothelial Progenitor Cells | Enhanced vascularization; increased inulin section; increased survival of islets; reduced apoptosis; induced cellular organization.79 |
| Liver Fibrosis | Mouse | MSCs | Reduced fibrous capsules; decreased inflammation and collagen deposition.80 |
| Pulmonary Hypertension | Mouse | MSCs | Suppressed macrophage influx; induced pro-inflammatory and proliferative factors; inhibited vascular remodeling; reduced pulmonary hypertension.81 |
| Osteochondral Defect | Rat | MSCs | Restored damaged tissue; improved gross appearance and histological score.82 |
| Arthritis | Mouse | DCs | Reduced inflammation; prevented or alleviated collagen-induced arthritis.83,84 |
| Mouse | Neutrophils | Stimulated TGF-β production; enhanced matrix deposition.85 | |
| Skin Wound | Rat | MSCs | Accelerated re-epithelization; increased CK19, PCNA and collagen I.86 |
| Inflammation | Mouse | Dental Pulp | Reduced edema; suppressed capthesin B, matrix metalloproteinase activity.88 |
| Mouse | Cancer Cells (+ drug) | Inhibited inflammation; reduced autoimmune response.89,90 | |
| Cerebral Occlusion | Rat | MSCs (+ miRNA) | Functional improvement; increased axonal plasticity and neurite remodeling.91 |
| Neurodegenerative Diseases | Mouse | DCs (+ siRNA) | siRNA delivered to the brain; reduced α-synuclein mRNA and aggregates.92 |
| Mouse | DCs (+ siRNA) | siRNA delivered to the brain; BACE1 knocked down; decreased β-amyloid.93 | |
| Cancer | Mouse | Cancer Cells (+ drug) | Reduced tumour growth.89 |
| Mouse | Cancer Cells (+ drug) | Reduced tumour growth.94 | |
| Mouse | Blood (+ drug) | Reduced tumour growth.95 | |
| Mouse | Macrophages (+ drug) | Reduced tumour growth.96 | |
| Mouse | Kidney Cells (+ miRNA) | Reduced tumour growth.97 | |
| Mouse | Kidney Cells (+ miRNA) | Reduced tumour growth.98 | |
| Mouse | DCs (+ drug) | Reduced tumour growth.99 | |
| Mouse | Pulsed DCs | Stimulated T-cell response; suppressed growth or eradicated tumours.100 | |
| Mouse | Pulsed DCs | Stimulated natural killer cell proliferation/activity; anti-metastatic response.101 | |
| Human | Pulsed DCs | Stimulated T-cell response; increased natural killer cell activity.107 | |
| Human | Pulsed DCs | In progress: https://clinicaltrials.gov/show/NCT01159288 (Phase II) | |
| Human | Plant (+ drug) | In progress: https://clinicaltrials.gov/show/NCT01294072 (Phase I) | |
| Human | Ascites Fluid | Anti-tumor cytotoxic T-lymphocyte response observed when used in combination with granulocyte-macrophage colony stimulating factor.108 | |
| Infectious Diseases | Mouse | Pulsed Macrophages | Primed protective immunity; boosted prior tuberculosis immunization.102 |
| Mouse | Pulsed DCs | Reduced number of cysts; induced immunity against toxoplasmosis.103 | |
| Mouse | Pulsed DCs | Induced immunity against diphtheria toxoid.104 | |
| Chicken | Pulsed DCs | Increased body weight; decreased feed conversion ratios; reduced fecal oocyst shedding; diminished intestinal lesions; reduced mortality from coccidiosis.105 | |
| Human | Neisseria meningitidis | Increased bactericidal activity against group B meningococcus.106 | |
| Graft-versus-Host Disease | Mouse | MSCs | Reduced CD3+CD8+ T-cell number; decreased level of pro-inflammatory cytokines; reduced mortality.87 |
| Human | MSCs | Reduced diarrhea volume; improved cutaneous and mucosal symptoms.109 |
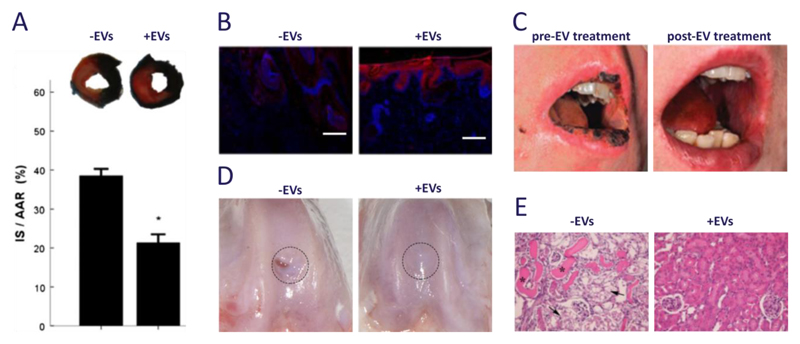
Figure 1. Clinical and pre-clinical studies using EVs derived from mesenchymal stem cells.
(a) Exosomes are well known to be effective in myocardial tissue repair after ischemia-reperfusion injury; in this mouse model, infarct size (IS, stained white) as a proportion of area-at-risk (AAR, stained red) was reduced from 39 ± 2% to 21 ± 2% (t = 1 day). Images were adapted from Arslan et al.68 and reproduced with permission from Elsevier. (b) Exosomes were used to promote wound healing in a rat model of skin deep second degree burn injury. Immunostaining of CK19 expression (red) along with Hoechst stain (blue) showed re-epithelization at the wound area for rats treated with exosomes (t = 2 weeks, scale bars = 200 μm). Images were reproduced under creative commons licence from Zhang B et al. (2015) Stem Cells doi:10.1002/stem.1771.86 (c) Exosomes were used in a clinical study to reduce pro-inflammatory cytokine response and alleviate the symptoms of therapy-refractive graft-versus-host disease. Images were adapted from Kordelas et al.109 and reproduced with permission from Nature Publishing Group. (d) Exosomes have also been used to enhance in vivo cartilage repair in 1 mm deep osteochondral defects created on the trochlear grooves of distal femurs of adult rats (t = 6 weeks). Images reproduced under creative commons licence from Zhang S et al. (2016) Osteoarthritis and Cartilage doi:10.1016/j.joca.2016.06.022.82 (e) Microvesicles have been shown to provide protection against tubular injury in an acute kidney injury mouse model. Here, cisplatin was used to induce intra-tubular casts (asterisks) and tubular necrosis (arrows), which was alleviated with multiple injections of microvesicles (t = 4 days, magnification = 200X). Images were reproduced under open access from Bruno S et al. (2012) PLoS ONE 7(3) doi:10.1371/journal.pone.0033115.77
Using EV-based therapeutics circumvents biological issues associated with cell-based strategies, such as stress-induced necrosis or aberrant differentiation.5 The small size of EVs, compared to whole cells, also offers therapeutic benefits, including reduced macrophage phagocytosis110 and vascular occlusion,5 easier injection and improved extravasation through tumor vasculature.110 Although small synthetic vectors (e.g. liposomes, nanoparticles) offer similar size benefits over cell-based systems,111 the biological structure and function of EVs afford a host of therapeutic advantages. For instance, EVs offer innate biocompatibility, high physicochemical stability,112 long-distance communication,113,114 and the inherent ability to interact with cells through signaling, fusion and delivery.115 Certain studies have also demonstrated that EVs exhibit cell-selective fusion116 and tissue-specific tropism,117 as well as an ability to transverse the blood-brain barrier118 and penetrate dense structural tissue.85 On the other hand, liposome and nanoparticle systems offer a high degree of methodological flexibility, from the choice of reagents, preparation route and surface functionalization. This affords synthetic systems a toolkit of biomimetic components, such as grafted antibodies or targeting ligands, as well as non-biological units, such as contrast agents or photothermal materials.
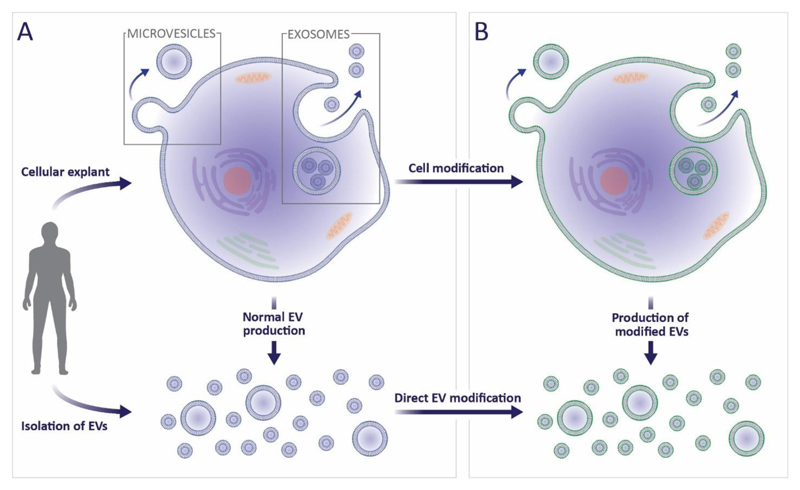
The remainder of this review will explore how many of these synthetic strategies are now being applied to EVs, to complement or enhance their therapeutic applicability. Many of the approaches discussed for EV modification have already been used for cell functionalization, a far more established field. Cell modification is generally achieved either by hijacking biosynthesis to favour the production of specific endogenous material or by delivering exogenous species to the cytoplasmic membrane.119 Both approaches can be used to manipulate cells to secrete modified EVs, while the latter approach can also be used to directly functionalize purified EVs (Figure 2). This review will discuss the challenges and prospects in translating these cell-based technologies to EVs, as well as considering examples of EV modification strategies that could not be applied to living cells. These studies are limited in number (<100 at the time of writing), of which most are proof-of-concept reports. As a result, this field offers the exciting opportunity to investigate new functionalization technologies and advance towards highly-effective therapeutic applications. Here we will highlight these gaps and suggest future opportunities in the re-engineering of EVs for cell-free therapy.
Figure 2. The isolation, secretion and modification of EVs.
(a) EVs can either be isolated directly from bodily fluids or indirectly from in vitro cultured cells. As part of normal exocytosis, cells will shed microvesicles from the cytoplasmic membrane, and release exosomes from multivesicular bodies. (b) Cell manipulation can indirectly lead to modified exosomes and microvesicles, alternatively, the EVs themselves can be directly functionalized or loaded.
Engineering Extracellular Vesicles via Cell Modification
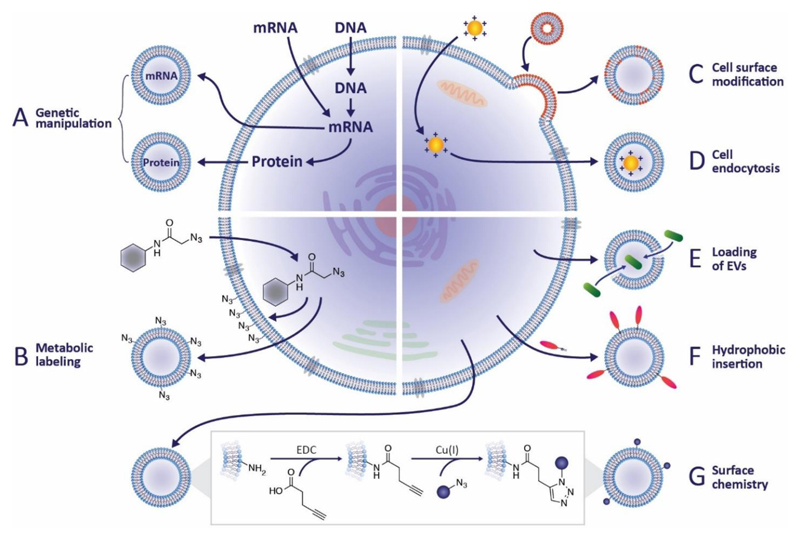
For several decades, researchers have introduced non-native materials to cells to augment therapeutic function.120,121 It is highly likely that many of these species will have unwittingly ended up within EVs. For instance, materials delivered to the membrane will naturally be incorporated into microvesicles while internalized material may be secreted in exosomes. Taking advantage of these scenarios allows cellular processes and cell engineering techniques to be specifically adapted to EV functionalization. In this section, we will discuss how techniques such as genetic engineering, metabolic labeling and exogenous delivery, can be exploited for the modification of EVs (Figure 3A-D).
Figure 3. Strategies for EV modification.
(a) Genetic engineering can be used to introduce coding and non-coding oligonucleotides into cells. There it can be packaged into EVs to promote gene expression or regulate transcription in recipient cells. Alternatively, transgenic proteins can be incorporated into EVs, for instance, as fluorescent reporters or targeting moieties. (b) Metabolic labeling, in which metabolite analogues are incorporated into cell biosynthesis, has been widely used to introduce non-native moieties into cells. This approach can be used to introduce functional groups, such as azides, to EVs, which allows subsequent bio-orthogonal reactions to be performed. (c) Exogenous material may be introduced to EVs via liposomes or micelles that fuse with cytoplasmic membranes. (d) Alternatively, the process of packaging endocytosed material into EVs as part of normal membrane turnover and exocytosis can be hijacked to introduce exogenous species to EVs. (e) A direct EV modification strategy is to permeabilize the vesicle membrane to allow the active loading of molecules into the EV interior, an approach that has been exploited for drug delivery. (f) A similar approach uses lipophilic or amphiphilic molecules that can insert into the EV membrane via hydrophobic interactions with the phospholipid bilayer. (g) Chemical reactions may also be performed directly on the vesicle membrane, for instance, carbodiimides can be used to modify native amines in order to present azide groups for click chemistry reactions.
Genetic Manipulation of Cells for EV Modification
Undoubtedly the most well-established of all cell manipulation strategies, it was inevitable that genetic engineering would be used to modify EVs for therapeutic applications. mRNA introduced to a cell may be packaged into EVs, which can then fuse with a target cell to induce transgene protein expression.13,98,113,114,122 Similarly, gene regulation can be mediated by delivery of EVs enriched in non-coding RNA sequences, such as miRNA91,95,97,123–129 or small interfering RNA (siRNA).92,93,97,124,130,131 This biomimetic approach elegantly exploits EVs for their innate cell-binding capacity and protection against degradative RNAases.12,13,129 There are, however, several issues that need to be addressed and carefully controlled for during these experiments. For instance, it has been suggested that observed changes attributed to the EV-encapsulated RNA could actually be due other vesicle components stimulating upregulation of endogenous miRNA.132 Moreover, miRNA can also be encapsulated and transferred to target cells by large protein complexes, lipoproteins or protein-oligonucleotide conjugates, which can co-elute with EVs during purification.132 Therefore, care should be taken to use thorough EV purification steps,133 and to select suitable models in which the target miRNA is not naturally expressed.61,128,129
Some extremely interesting insights into EV delivery were made by Kanada et al., who used differential centrifugation to isolate separate populations of exosomes and microvesicles from HEK293FT cells that were transiently transfected with either plasmid DNA or mRNA.134 Reporter protein expression in recipient HEK293FT or 4T1 cells was observed after microvesicle delivery of plasmid DNA, but not mRNA, while exosomes were incapable of transferring any functional oligonucleotides. There may be several interesting mechanisms underpinning these observed differences. First, the authors show that EV-delivered mRNA can undergo rapid degradation in the lysosome of recipient cells, a process that prevents translation and functional protein expression. Second, the loading of oligonucleotides into EVs was shown to be much higher for microvesicles, compared to exosomes, with the latter showing undetectable levels of encapsulated plasmid DNA. This may be partially explained by recent work from Skog et al., who showed that different oligonucleotide sequences are packaged into EVs with varying efficiency.13 Intriguingly, this difference is thought to be due to certain RNA molecules possessing “zip code” sequences that lead to selective enrichment within EVs.135,136 Considering these factors, it would be great to see how different sequences would affect the outcome of the work by Kanada and co-workers. Indeed, if the studies by Skog et al. and Kanada et al. are shown to have universal applicability across different cells, transfection systems and isolation protocols, then they will prove extremely valuable guides in defining the parameters and design criteria for genetic modification of EVs.
The examples above all use oligonucleotides to induce or regulate gene expression in a target cell. An alternative approach is to induce transgene expression in the parent cell, and use the protein product that is incorporated into EVs. A sensible approach will encode for genetic fusions of proteins that are enriched in EVs to ensure optimum localization of the expressed product. This approach requires careful design and a solid understanding of the molecular biology of vesicle proteins. Tetraspanins, for instance, have a complex tertiary structure with four transmembrane domains, three intra-vesicle segments and a pair of extra-vesicle loops.137 Lu et al. used this structural information to identify candidate sites on the tetraspanin CD63 that allowed stable integration of fluorescent fusion proteins on either the inner or outer leaflet of the exosomal membrane.138 Similar approaches have been used to express fluorescent protein or luciferase reporters of CD63 and other tetraspanins,125,138,139 Rab5a138 and lactadherin,140 all of which were identified in the daughter EVs. These systems have been used to study vesicle biogenesis, image exosome transfer between cells and visualize in vivo distribution after systemic EV therapy. A more advanced visualization system was recently reported by Lai et al. who used a palmitoylation signal fused with an RNA binding sequence. First, a post-transcriptional S-palmitoylation targeted the mRNA binding sequence to the membrane of human embryonic kidney (HEK) cells, where it was packaged into EVs. Here, the RNA binding sequence, in combination with a co-expressed eGFP-tagged bacteriophage coat protein, allowed direct visualization of mRNA packaged within EVs.141
A key challenge of vesicle engineering is to advance beyond simple receptor systems and towards modifications that can enhance the therapeutic function of EVs. For example, Alvarez-Erviti et al. used the RVG peptide to target exosomes to neurons, oligodendrocytes and microglia in an in vivo mouse model.93 In this work, RVG was fused with lysosome-associated membrane glycoprotein 2b (Lamp-2b), which has also been used as a base for integrin-binding99 or cell penetrating peptide tags.142 However, concerns have been raised over the long term stability of Lamp-2b hybrids.143 This has led to suggestions of more stable Lamp-2b alternatives, such as glycosylphosphatidylinositol (GPI), which was proposed by Kooijimans et al.144 Here, cancer cells expressing high levels of epidermal growth factor (EGF) were targeted by EVs expressing GPI-anchored, anti-EGF receptor nanobodies. In a similar report, Ohno et al. generated a HEK cell line that stably expressed the transmembrane receptor of platelet-derived growth factor (PDGF) fused with an EGF-binding peptide, and used the secreted EVs in targeted tumor therapy.97 The C1C2 domain of lactadherin is another commonly used base for fusion protein display, which has been used to generate antibodies against tumor biomarkers,145 and to increase the immunogenicity of cells to tumor-associated antigens.146,147 Finally, a fascinating approach was introduced by Maguire et al., in which adeno-associated viruses introduced to a parent cell were incorporated into daughter EVs, termed vexosomes.148 The encapsulated capsids were used to deliver genetic material to target cells, with higher transfection efficiency than observed for naked viruses. Overall, despite some concerns over how transfection agents may affect gene expression in the donor cells,149 genetic manipulation represents a highly accessible strategy for the presentation of functional oligonucleotides, non-native proteins and virus particles within EVs.
Metabolic Labeling of Cells for EV Modification
Metabolic labeling is a well-established cell functionalization strategy that circumvents many of the issues of genetic manipulation. This approach involves hijacking cellular biosynthesis by supplementing cell culture medium with non-native metabolites, such as amino acids, lipids, oligonucleotides or glycans. These metabolites are taken up by cells and integrate into the proteome, lipidome, genome and glycome, respectively. While cell functionalization strategies often target substitutions on the cytoplasmic membrane,150–152 metabolic labeling is an indiscriminate technique that modifies biomolecules throughout the entirety of the cell. Therefore, both exosomes and microvesicles would be expected to contain metabolically-labelled sites, for instance, on endosomal proteins or cytoplasmic membrane lipids. Metabolic labeling was recently explored by Wang et al., who used the non-native amino acid L-azidohomoalanine as a methionine substitute to incorporate azide groups into the proteome of melanoma cell EVs.153 In the same study, a synthetic sugar precursor tetraacetylated N-azidoacetyl-D-mannosamine was used to generate EVs presenting azide-modified sialic acid.153
The study by Wang et al. is a rare example of metabolic EV labeling. In contrast, metabolic cell labeling has been used extensively to endow cells with rare or unnatural functional groups, such as azides, alkynes, thiols, methacryloyls and ketones. These moieties are reactive and can be modified at the cell surface with little or no side reactions using bio-orthogonal chemistry.154 This approach has been used to modify cells for drug conjugation,155 selective killing of cells,154 surface-induced gelation156 and artificial adhesion to 2D substrates151 or 3D scaffolds.152 To date, bio-orthogonal chemistry has only been used in proof-of-principle experiments to introduce simple proteins and fluorophores to azide-modified EVs.153 In practice, the principles of bio-orthogonal chemistry should be readily transferred from cells to EVs. Indeed, performing this secondary labeling step upon purified EVs, rather than on live cells that are more sensitive to their chemical environment, should allow the range of reagents and reaction conditions to be significantly expanded (this is discussed further below in the “Direct Modification of Extracellular Vesicles”).
Loading EVs using Cellular Uptake of Exogenous Material
Genetic modification and metabolic labeling strategies hijack cellular biosynthesis to generate in situ products that are incorporated into EVs. An alternative approach is to introduce exogenous material to the cell, which can subsequently be packaged into EVs. The EV loading is typically dependent on the amount of material delivered to the cell, which is in turn governed by the strength of the material-cell interaction. For instance, a nanoparticle with little or no cell binding capability will rely upon weak, non-specific interactions with the cytoplasmic membrane. In this situation, high nanoparticle concentrations and prolonged incubation times are needed to maximize the number of binding events and generate sufficient cell loading.121 For instance, Neubert & Glumm required a 24 hour incubation with 0.5 mM superparamagnetic iron oxide nanoparticles (SPIONs) to generate loaded EVs from primary neuronal cell cultures.157 This situation can be partially avoided by using macrophages, which actively engulf and internalize large amounts of exogenous material through phagocytosis. To this end, Silva et al. used macrophages incubated with iron oxide nanoparticles and small molecule photosensitizers to generate magnetically and optically responsive EVs.96,158 These EVs, termed theranosomes, offer potential for in vivo magnetic targeting, magnetic resonance imaging and photodynamic therapy. However, a key limitation of this approach is that it relies upon phagocytosis as an uptake mechanism. As a result, it will be challenging to achieve comparable loadings with other, non-phagocytic cells.
One approach to increase cell binding uses hydrophobic interactions between the exogenous material and the cytoplasmic membrane. For instance, Tatischeff et al. demonstrated that the drug hypericin can be readily taken up by cells and packaged into EVs.159 To advance beyond small, hydrophobic molecules, a common approach is to use liposomal systems as delivery vectors that can directly fuse with cell membranes.160,161 Amphiphilic materials can insert into the liposome membrane bilayer, while hydrophilic moieties can be encapsulated within the aqueous cavity. This was aptly demonstrated by Lee et al., who used membrane fusogenic liposomes to deliver hydrophobic payloads to the cytoplasmic membrane and hydrophilic species to the cytosol.162 Direct delivery to the cytosol is highly desirable as it circumvents endosomal entrapment and lysosomal degradation, which increases the amount of material available for packaging into EVs. Interestingly, the authors observed that the amount of lipid delivered to the cell was not proportional to the amount of lipid incorporated into the EVs. This apparent lack of control over the degree of EV packaging represents a key limitation of this technique. A further limitation with liposome-based strategies is the inefficiencies in loading, which is a particular issue with large or bulky payloads.163
Direct Modification of Extracellular Vesicles
Cell-based EV functionalization strategies typically package only a small fraction of total modified content into the secretome. Such inefficient incorporation offers an extremely poor return on reagents and costs. In contrast, direct functionalization of purified EVs ensures that all modified sites or encapsulated species are localized at the vesicle. Here, we will discuss several different approaches for modifying the EV surface with membrane-binding species, as well as active and passive methods for encapsulating material into the vesicle interior (Figure 3E-G).
Covalent Modification of the EV Membrane
EVs have one major advantage over cells when it comes to surface modification; they are non-living entities. As such, it is possible to use reagents and reaction conditions that could not be used for live cell functionalization. Nevertheless, there are still constraints to be considered. For instance, excessive temperatures, pressures or solvent exposure can cause membrane disruption and surface protein denaturation, while introducing low or high salt concentrations will lead to osmotic stress. Moreover, many of these reaction conditions, as well as certain chemical modifications, can induce vesicle aggregation. Accordingly, common bioconjugation and “click chemistry” reactions that rapidly form chemical bonds under ambient conditions have become natural candidates for covalent EV modification. This approach was used by Smyth et al., who performed sequential chemical reactions at the EV surface.164 First, carbodiimide coupling was used to graft the alkyne-containing 4-pentynoic acid onto EV membrane amines. Introducing a reactive alkyne base allowed a second, click chemistry reaction with an azide-tagged fluorophore. Amines are a reactive functional group naturally expressed on biological membranes, and therefore represent a reasonably straightforward target. However, it is possible that such bioconjugation strategies could impair function by altering or obscuring the active site of modified surface proteins. To circumvent this issue, covalent reactions could be performed upon EVs previously modified to express additional or bio-orthogonal moieties, either through transfection or metabolic labeling (opportunities for this were discussed above in “Engineering Extracellular Vesicles via Cell Modification”). As a proof-of-principle, however, this example aptly demonstrates that common chemical reactions can be applied to EV surface modification, without any observed effects on vesicle structure or cell fusion.
Non-Covalent Modification of the EV Membrane
The stability of the surface modifications are highly dependent on the strength of the bond that links the exogenous species to the EV. Covalent bonds typically have bond energies in the region of 200-900 kJ mol-1,165 which is much greater than the values for non-covalent interactions (c.f. 2-13 kJ mol-1).166 Accordingly, covalently-bound species are less prone to dissociation by chemical displacement or changes in ionic strength, temperature or solvent. There are, however, three non-covalent strategies that are commonly used to provide stable modification of biological membranes; multivalent electrostatic interactions, receptor-ligand binding and hydrophobic insertion. Multivalent electrostatic approaches rely on the cumulative action of multiple charge interactions, which typically involves a highly cationic species adhering to negatively-charged functional groups present on biological membranes.121 For instance, Nakase and Futaki used electrostatic interactions to bind cationic lipids to the surface of exosomes.167 In turn, this produced EVs with a positively-charged surface potential that enhanced binding and uptake into recipient cells. However, there are concerns that certain cationic nanomaterials can cause cytotoxicity through membrane thinning and hole formation.168 Perhaps a more pertinent issue is that cationic nanomaterials are typically taken up into the cell via endocytosis, leading to lysosomal degradation and poor EV loading.162 This may be less of an issue for the modification of microvesicles, which bud directly from the labelled cytoplasmic membrane, but would limit exosome modification to degradation-resistant materials.
The second non-covalent strategy involves receptor-ligand binding. A notable example was reported by Qi et al., who used transferrin-conjugated superparamagnetic nanoparticle clusters that effectively bound to the surface of exosomes isolated from blood.169 This approach targeted transferrin receptors already present on the EV membrane, however, an alternative strategy is to target non-native binding groups introduced through transgene expression. This strategy was employed by Maguire et al. in the streptavidin-mediated binding of biotinylated magnetic nanoparticles to transgenic biotin-acceptor peptides on the surface of EVs.148 The major downside to this approach is the synthetic challenge and cost of presenting functional ligands (e.g. transferrin, biotin) on the exogenous material, rather than simple chemical species (e.g. azides, alkynes). If this can be achieved, receptor binding strategies offer an effective, bio-inspired approach that can be readily transferred from cell modification to EV functionalization. Indeed, the specificity of this approach may offer some interesting in vivo opportunities, particularly if the receptor is enriched on the EV surface. For instance, targeting EV surface receptors could be used as a strategy to bind and eliminate vesicles implicated in pathological processes, such as cancer metastasis.
In contrast, it can often be more challenging to adapt hydrophobic insertion strategies from cells to EVs. The high level of cholesterol, sphingomyelin and ganglioside in the membrane of EVs creates a more rigid bilayer structure than observed in the parent cells.170 This prevents the facile fusion of lipid-based particles, such as liposomes or micelles. For example, while cells will take up micellar structures under ambient conditions, EVs require aggressive freeze-thaw (T = -196°C) or high temperature (T = 40°C) cycling to disrupt the vesicular membrane and promote fusion.171,172 Hydrophobic interactions are, however, highly effective at driving the spontaneous integration of small lipophilic species into the EV membrane. This can be achieved using a simple co-incubation under ambient conditions (25-37°C), with loading efficiencies that positively correlate with the hydrophobicity of the exogenous species.173 This approach is used in most commercial EV membrane stains, including the commonly-used dyes BODIPY TR ceramide,174 DiI,175 and PKH-67.176 Furthermore, hydrophobic sequestration is used to load EVs with small lipophilic drugs, such as anti-inflammatory curcumin,89,90 common porphyrin photosensitizers,173 and chemotherapeutic agents curcubitacin,89 doxorubicin,177,178 paclitaxel178 and methotrexate.94
Active Loading of EVs
Passive loading strategies that rely on spontaneous interactions are often limited by poor loading efficiency. In an effort to address this, membrane permeabilization strategies have been adapted from the fields of bacterial transformation and liposome modification, and re-purposed for the active loading of EVs. For example, electroporation is commonly used to transiently permeabilize the EV membrane to enhance the uptake of siRNA,92,93,142,179 small molecule drugs 99,173 and SPIONs.180 Membrane stabilizers can be employed to improve colloidal stability of the vesicles,180 while care should be taken not to mistake precipitation and micellar aggregation for loaded EVs.149 For instance, Fuhrmann et al. diligently monitored precipitation levels while quantitatively comparing active EV loading methods of different porphyrins.173
Interestingly, this study showed that while electroporation can increase the loading efficiency of certain porphyrins, an even higher degree of internalization can be achieved using saponin treatment and hypotonic dialysis (Figure 4A). Saponin permeabilizes biological membranes by complexing with cholesterol,181 while hypotonic dialysis, commonly used to load erythrocyte “ghost” cells, uses osmotic pressure to enhance drug internalization efficiency.182 Such strategies are inherently more disruptive than passive approaches, and thus careful handling and characterization should be employed to ensure the integrity and functionality of the EV is retained, post modification.
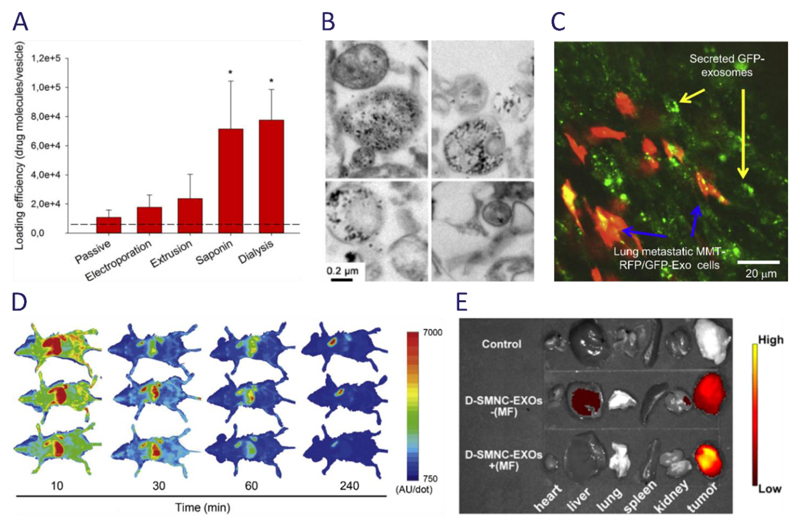
Figure 4. Examples and applications of re-engineered EVs.
(a) A quantitative comparison of different strategies for loading porphyrin drugs into EVs showed that active loading strategies resulted in higher loading, with saponin treatment and hypotonic dialysis offering the greatest efficiency. Image reproduced from Fuhrmann et al.173 (b) Iron oxide nanoparticles exposed to macrophages can be passively packaged into EVs, as shown by these electron micrographs. Image reproduced from Silva et al.96 (c) Suetsugu et al. generated a mouse breast cancer cell line (MMT) expressing a CD63-GFP hybrid that was packaged into EVs and used to visualize intercellular vesicle transfer.139 Reproduced with permission from Elsevier. (d) Takahashi et al. used a truncated lactadherin fused with Gaussia luciferase to produce artificially chemiluminescent EVs. This allowed EVs to be traced after systemic administration into a mouse model using chemiluminescent imaging, an analysis that revealed rapid clearance from the blood circulation.140 Reproduced with kind permission from Elsevier. (e) Qi et al. used superparamagnetic nanoparticles functionalized with transferrin, which allowed them to bind to receptors present on the surface of blood-derived EVs. These responsive EVs were used in combination with an external magnetic field (MF) to enhance delivery to a tumor site, as shown in this ex vivo near infrared fluorescence image.169
Future Directions in Extracellular Vesicle Modification
The emergence of EVs as influential mediators of physiology and pathology has opened up exciting opportunities in nanomedicine.5 Modification strategies offer the tantalizing prospect of extending the therapeutic capability of EVs beyond their native function. Horizontal gene transfer, for instance, is an inherent function of EVs, but by genetic manipulation or direct loading we can now introduce non-native oligonucleotides that can alter the gene expression of target cells. When selecting a modification strategy, it is essential to understand the complexity of the system, in terms of the cargo, final application and other associated factors. For instance, can the cargo be conjugated without disruption of function? May the cargo be generated from within the cell, or must it be delivered directly to the EV? Does the final application require presentation of material on the outermost surface of the EV, or would encapsulation provide a more protective environment? Each application will pose different biological questions, which will in turn define the technical parameters of the modification strategy. Adopting a bespoke approach to EV modification offers the greatest chance of success, but also present major challenges to researchers in the field.
For instance, it is important to understand the biology of EVs and the physical basis of any interactions, particularly when translating a cell modification technique to vesicles. EVs are significantly smaller than cells, which provides a greater degree of membrane curvature, but they also possess a different lipid composition that creates a more rigid membrane.170 As non-living entities, harsher modification conditions can be employed for EVs compared to cells, while the lack of membrane turnover will benefit applications that require persistent membrane labeling. Clearly EVs do not possess full biosynthetic machinery, but they do possess functional enzymes that could be used, for example, in the post-translational modification of packaged proteins or the activation of encapsulated pro-drugs. Another major consideration is exactly how such modifications affect the structure and function of EVs. For example, direct binding or steric obstruction can impair the function of proteins and carbohydrates on the vesicle membrane, an altered surface charge potential can create colloidal instability, while the introduction of foreign species can generate unwanted immunogenicity. Modification may also change the membrane rigidity; this is likely to be a subtle effect, but one that could potentially modify the cell-binding capacity of the EV.170 To this end, many reports present cell uptake studies alone as evidence of EV function, neglecting to study how membrane modification can differentially disrupt different vesicle-cell interactions.28 For example, modified EVs may still be internalized via non-specific endocytosis, but if their surface ligands are inactivated, then they will be unable to trigger signal cascades. Similarly, if their ability to fuse with a recipient cells is impaired, then the fate of any delivered cargo will be significantly altered. These considerations are critical for therapeutic application and demand that, as a field in general, we place more thorough emphasis into characterizing and defining EV function.
RNA and drug encapsulation, along with fluorescent and magnetic labeling, represent the bulk of research on EV modification (Figure 4B-E). The reasons for this are twofold; the approaches often borrow from well-established cell manipulation technologies, and the materials used (oligonucleotides, small molecules and nanoparticles) exhibit function that are less dependent upon hierarchical structure. It is far more challenging, for instance, to immobilize proteins onto an EV membrane while retaining the tertiary structure required for active function. The EV field would benefit tremendously from technologies that allow the presentation of more complex structures capable of bestowing functions such as signaling, catalysis and adhesion. Progress in this area is being made; delivery of EVs to the brain with neuron-specific peptides by Alvarez-Erviti et al.93 is one of several emerging targeting therapies. Moreover, Yim et al. recently reported the optogenetic control of EVs using a reversible protein-protein interaction module, which allowed the controllable loading and release of cargo through exposure to blue light.183 These developments stand out among the plethora of proof-of-concept, in vitro studies into EVs modified with fluorescent reporters. While visualization is undoubtedly useful, particularly in vivo, the ability to augment EVs with targeting or stimuli-responsive capabilities affords control over a complex biological system. We believe that maintaining progress in these areas will truly advance the field and develop EVs from promising biological candidates into smart nanoscale therapeutics.
Vocabulary.
Extracellular Vesicles: An umbrella term for the range of membrane-bound particles secreted by cells, including exosomes, microvesicles and apoptotic bodies.
Exosomes: A major class of extracellular vesicles of endocytic origin that are released from multivesicular bodies, with a diameter of 30-100 nm.
Microvesicles: A major class of extracellular vesicles formed from the outward budding or shedding of the cytoplasmic membrane, with a diameter of 100-1000 nm.
Genetic Manipulation: The introduction of foreign nucleic acids into cells, usually used to bring about protein translation or regulate gene expression.
Metabolic Labeling: The hijacking of cellular biosynthetic machinery to introduce exogenous biomolecules that become incorporated into, for instance, the proteome, glycome or lipidome.
Bio-Orthogonal Chemistry: Chemical reactions that can occur within biological systems, without interfering with existing physiological processes.
Active Loading: The sequestration of material into a system using energetic input, for instance, heat.
Acknowledgments
J.P.K.A. acknowledges support from the Arthritis Research UK Foundation (21138). M.M.S. acknowledges the support of the Medical Research Council, the Engineering and Physical Sciences Research Council, and the Biotechnology and Biological Sciences Research Council UK Regenerative Medicine Platform Hub “Acellular Approaches for Therapeutic Delivery” (MR/K026682/1). The authors would like to thank T. Whittaker for his insight, suggestions and proofreading of the manuscript.
References
- (1).Raposo G, Stoorvogel W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gould SJ, Raposo G. As We Wait: Coping with an Imperfect Nomenclature for Extracellular Vesicles. J Extracell Vesicles. 2013;2:3–5. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, Biogenesis and Function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- (4).Gyorgy B, Szabo RG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- (6).Raposo G. B Lymphocytes Secrete Antigen-presentingVesicles. 1996;183 doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rosenthal AK, Gohr CM, Ninomiya J, Wakim BT. Proteomic Analysis of Articular Cartilage Vesicles from Normal and Osteoarthritic Cartilage. Arthritis Rheum. 2011;63:401–411. doi: 10.1002/art.30120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte Derived Microvesicles Deliver a Cell Death Message via Encapsulated Caspase-1. PLoS One. 2009;4:e7140. doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius A, Gabrielsson S, Radmark O. Exosomes from Human Macrophages and Dendritic Cells Contain Enzymes for Leukotriene Biosynthesis and Promote Granulocyte Migration. J Allergy Clin Immunol. 2010;126:1032–1040. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- (10).Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma Cells Release Exosomes Carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- (11).Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 Myoblasts Release Micro-Vesicles Containing mtDNA and Proteins Involved in Signal Transduction. Exp Cell Res. 2010;316:1977–1984. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- (12).Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-Mediated Transfer of mRNAs and microRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- (13).Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee MLT, Schmittgen TD, Nan-Sinkam SP, et al. Detection of microRNA Expression in Human Peripheral Blood Microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Taylor DD, Gercel-Taylor C. MicroRNA Signatures of Tumor-Derived Exosomes as Diagnostic Biomarkers of Ovarian Cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- (16).Ferguson SW, Nguyen J. Exosomes as Therapeutics: The Implications of Molecular Composition and Exosomal Heterogeneity. J Control Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- (17).Marie-Pierre C, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like Vesicles Are Present in Human Blood Plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- (18).Admyre C, Johansson SM, Qazi KR, Filén J-J, Lahesmaa R, Norman M, Neve EPA, Scheynius A, Gabrielsson S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- (19).Pisitkun T, Shen R-F, Knepper MA. Identification and Proteomic Profiling of Exosomes in Human Urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ogawa Y, Miura Y, Harazono A, Kanai-Azuma M, Akimoto Y, Kawakami H, Yamaguchi T, Toda T, Endo T, Tsubuki M, Yanoshita R. Proteomic Analysis of Two Types of Exosomes in Human Whole Saliva. Biol Pharm Bull. 2011;34:13–23. doi: 10.1248/bpb.34.13. [DOI] [PubMed] [Google Scholar]
- (21).Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of Citrullinated Proteins with Synovial Exosomes. Arthritis Rheum. 2006;54:3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- (22).Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, LaRusso NF. Biliary Exosomes Influence Cholangiocyte Regulatory Mechanisms and Proliferation through Interaction with Primary Cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–G999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, Witkin SS. Heat Shock Protein-Containing Exosomes in Mid-Trimester Amniotic Fluids. J Reprod Immunol. 2008;79:12–17. doi: 10.1016/j.jri.2008.06.001. [DOI] [PubMed] [Google Scholar]
- (24).Ronquist G, Brody I. The Prostasome: Its Secretion and Function in Man. Biochim Biophys Acta. 1985;822:203–218. doi: 10.1016/0304-4157(85)90008-5. [DOI] [PubMed] [Google Scholar]
- (25).Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, et al. Malignant Effusions and Immunogenic Tumour-Derived Exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- (26).Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of Small Molecules in Vesicles Shed by Cancer Cells: Association with Gene Expression and Chemosensitivity Profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- (27).Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Mulcahy LA, Pink RC, Carter DRF. Routes and Mechanisms of Extracellular Vesicle Uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE. Exosomes as a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J Immunol. 2008;180:3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- (30).Morelli AE, Larregina AT, Shufesky WJ, Sullivan MLG, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, et al. Endocytosis, Intracellular Sorting, and Processing of Exosomes by Dendritic Cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- (31).Fitzner D, Schnaars M, Van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch U-K, Simons M. Selective Transfer of Exosomes from Oligodendrocytes to Microglia by Macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- (32).Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular Internalization of Exosomes Occurs through Phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- (33).Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, et al. Mechanism of Transfer of Functional microRNAs between Mouse Dendritic Cells via Exosomes. Immunobiology. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial Expression of Autocrine VEGF upon the Uptake of Tumor-Derived Microvesicles Containing Oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-Derived Microparticles Stimulate Proliferation, Survival, Adhesion, and Chemotaxis of Hematopoietic Cells. Exp Hematol. 2002;30:450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- (36).Baj-Krzyworzeka M, Szatanek R, Wȩglarczyk K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ, Zembala M. Tumour-Derived Microvesicles Carry Several Surface Determinants and mRNA of Tumour Cells and Transfer Some of These Determinants to Monocytes. Cancer Immunol Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of mRNA and Protein Delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- (38).Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet- and Megakaryocyte-Derived Microparticles Transfer CXCR4 Receptor to CXCR4-Null Cells and Make Them Susceptible to Infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- (39).Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-Derived Microvesicles: Important and Underappreciated Mediators of Cell-to-Cell Communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- (40).Clarke Anderson H. Role of Extracellular Membrane Vesicles in the Pathogenesis of Various Diseases, Including Cancer, Renal Diseases, Atherosclerosis, and Arthritis. Lab Invest. 2010;90:1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- (41).Buzas EI, Gy"orgy B, Nagy G, Falus A, György B, Nagy G, Falus A, Gay S. Emerging Role of Extracellular Vesicles in Inflammatory Diseases. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- (42).Aharon A, Brenner B. Microparticles, Thrombosis and Cancer. Best Pract Res Clin Haematol. 2009;22:61–69. doi: 10.1016/j.beha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- (43).Fuhrmann G, Herrmann IK, Stevens MM. Cell-Derived Vesicles for Drug Therapy and Diagnostics: Opportunities and Challenges. Nano Today. 2015;10:397–409. doi: 10.1016/j.nantod.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine Action Accounts for Marked Protection of Ischemic Heart by Akt-Modified Mesenchymal Stem Cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- (45).Yang L, Wu X-H, Wang D, Luo C-L, Chen L-X. Bladder Cancer Cell-Derived Exosomes Inhibit Tumor Cell Apoptosis and Induce Cell Proliferation in Vitro. Mol Med Rep. 2013;8:1272–1278. doi: 10.3892/mmr.2013.1634. [DOI] [PubMed] [Google Scholar]
- (46).Inder KL, Ruelcke JE, Petelin L, Moon H, Choi E, Rae J, Blumenthal A, Hutmacher D, Saunders NA, Stow JL, Parton RG, et al. Cavin-1/PTRF Alters Prostate Cancer Cell-Derived Extracellular Vesicle Content and Internalization to Attenuate Extracellular Vesicle-Mediated Osteoclastogenesis and Osteoblast Proliferation. J Extracell Vesicles. 2014;3:23784. doi: 10.3402/jev.v3.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial Progenitor Cell-Derived Microvesicles Activate an Angiogenic Program in Endothelial Cells by a Horizontal Transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- (48).Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles Derived from Activated Platelets Induce Metastasis and Angiogenesis in Lung Cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- (49).Zhang H-C, Liu X-B, Huang S, Bi X-Y, Wang H-X, Xie L-X, Wang Y-Q, Cao X-F, Lv J, Xiao F-J, Yang Y, et al. Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Stimulated by Hypoxia Promote Angiogenesis Both in Vitro and in Vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Van Balkom BWM, De Jong OG, Smits M, Brummelman J, Den Ouden K, De Bree PM, Van Eijndhoven MAJ, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC. Endothelial Cells Require miR-214 to Secrete Exosomes That Suppress Senescence and Induce Angiogenes in Human and Mouse Endothelial Cells. Blood. 2013;121:3997–4007. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- (51).Hong BS, Cho J-H, Kim H, Choi E-J, Rho S, Kim J, Kim JH, Choi D-S, Kim Y-K, Hwang D, Gho YS. Colorectal Cancer Cell-Derived Microvesicles Are Enriched in Cell Cycle-Related mRNAs That Promote Proliferation of Endothelial Cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes Reflect the Hypoxic Status of Glioma Cells and Mediate Hypoxia-Dependent Activation of Vascular Cells during Tumor Development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-Derived Growth Factor Regulates the Secretion of Extracellular Vesicles by Adipose Mesenchymal Stem Cells and Enhances Their Angiogenic Potential. Cell Commun Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RCA, Sargent I, et al. New Mechanism for Notch Signaling to Endothelium at a Distance by Delta-like 4 Incorporation into Exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- (55).Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, et al. Exosomes from Human CD34+ Stem Cells Mediate Their Proangiogenic Paracrine Activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect Activation of Naïve CD4+ T Cells by Dendritic Cell-Derived Exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- (57).Lu C-W, Hung Y, Hsiao J-K, Yao M, Chung T-H, Lin Y-S, Wu S-H, Hsu S-C, Liu H-M, Mou C-Y, Yang C-S, et al. Bifunctional Magnetic Silica Nanoparticles for Highly Efficient Human Stem Cell Labeling. Nano Lett. 2007;7:149–154. doi: 10.1021/nl0624263. [DOI] [PubMed] [Google Scholar]
- (58).Bhatnagar S, Schorey JS. Exosomes Released from Infected Macrophages Contain Mycobacterium Avium Glycopeptidolipids and Are Proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 Translocates into the Plasma Membrane after Stress and Is Released into the Extracellular Environment in a Membrane-Associated Form That Activates Macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- (60).Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-Derived Microvesicles Induce, Expand and up-Regulate Biological Activities of Human Regulatory T Cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM. Exosome-Delivered microRNAs Modulate the Inflammatory Response to Endotoxin. Nat Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human Tumor-Derived Exosomes Selectively Impair Lymphocyte Responses to Interleukin-2. Cancer Res. 2007 doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- (63).Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-Factor-Bearing Microvesicles Arise from Lipid Rafts and Fuse with Activated Platelets to Initiate Coagulation. Blood. 2005;106:1604–1612. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- (64).Ekstrom K, Omar O, Graneli C, Wang X, Vazirisani F, Thomsen P. Monocyte Exosomes Stimulate the Osteogenic Gene Expression of Mesenchymal Stem Cells. PLoS One. 2013;8:e75227. doi: 10.1371/journal.pone.0075227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Nair R, Santos L, Awasthi S, von Erlach T, Chow LW, Bertazzo S, Stevens MM. Extracellular Vesicles Derived from Preosteoblasts Influence Embryonic Stem Cell Differentiation. Stem Cells Dev. 2014;23:1625–1635. doi: 10.1089/scd.2013.0633. [DOI] [PubMed] [Google Scholar]
- (66).Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-Derived Microparticles Bind to Hematopoietic Stem/progenitor Cells and Enhance Their Engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- (67).Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, et al. Exosome Secreted by MSC Reduces Myocardial Ischemia/reperfusion Injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- (68).Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, Van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, et al. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K / Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia / Reperfusion Injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- (69).Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular Vesicles from Human Cardiac Progenitor Cells Inhibit Cardiomyocyte Apoptosis and Improve Cardiac Function after Myocardial Infarction. Cardiovasc Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- (70).Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J Mol Med. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- (71).Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, Van Oorschot AAM, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, et al. Human Mesenchymal Stem Cell-Conditioned Medium Improves Cardiac Function Following Myocardial Infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- (72).Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, et al. Reduction of Myocardial Infarct Size by Human Mesenchymal Stem Cell Conditioned Medium. Stem Cell Res. 2008;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- (73).He J, Wang Y, Sun S, Yu M, Wang C, Pei X, Zhu B, Wu J, Zhao W. Bone Marrow Stem Cells-Derived Microvesicles Protect against Renal Injury in the Mouse Remnant Kidney Model. Nephrology. 2012;17:493–500. doi: 10.1111/j.1440-1797.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- (74).Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- (75).Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi a, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human Liver Stem Cell-Derived Microvesicles Accelerate Hepatic Regeneration in Hepatectomized Rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone Marrow-Derived Mesenchymal Stem Cells Repaired but Did Not Prevent Gentamicin-Induced Acute Kidney Injury through Paracrine Effects in Rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles Derived from Endothelial Progenitor Cells Protect the Kidney from Ischemia-Reperfusion Injury by microRNA-Dependent Reprogramming of Resident Renal Cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- (79).Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C, Segoloni GP, et al. Microvesicles Derived from Endothelial Progenitor Cells Enhance Neoangiogenesis of Human Pancreatic Islets. Cell Transplant. 2012;21:1305–1320. doi: 10.3727/096368911X627534. [DOI] [PubMed] [Google Scholar]
- (80).Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes Mediate the Cytoprotective Action of Mesenchymal Stromal Cells on Hypoxia-Induced Pulmonary Hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes Derived from Human Embryonic Mesenchymal Stem Cells Promote Osteochondral Regeneration. Osteoarthr Cartil. 2016:6–11. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- (83).Kim S-H, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes Derived from IL-10-Treated Dendritic Cells Can Suppress Inflammation and Collagen-Induced Arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- (84).Kim S-H. Effective Treatment of Inflammatory Disease Models with Exosomes Derived from Dendritic Cells Genetically Modified to Express IL-4. J Immunol. 2007 doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- (85).Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell’Accio F, Pitzalis C, Oliani SM, et al. Neutrophil-Derived Microvesicles Enter Cartilage and Protect the Joint in Inflammatory Arthritis. Sci Transl Med. 2015;7:190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- (87).Wang L, Gu Z, Zhao X, Yang N, Wang F, Deng A, Zhao S, Luo L, Wei H, Guan L, Gao Z, et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016 doi: 10.1089/scd.2016.0107. [DOI] [PubMed] [Google Scholar]
- (88).Pivoraitė U, Jarmalavičiūte A, Tunaitis V, Ramanauskaitė G, Vaitkuvienė A, Kašėta V, Biziulevičienė G, Venalis A, Pivoriūnas A. Exosomes from Human Dental Pulp Stem Cells Suppress Carrageenan-Induced Acute Inflammation in Mice. Inflammation. 2015;38:1933–1941. doi: 10.1007/s10753-015-0173-6. [DOI] [PubMed] [Google Scholar]
- (89).Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs From the Nasal Region to the Brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G. A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol Ther. 2009;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b Promotes Neural Plasticity and Functional Recovery after Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Cooper JM, Wiklander PBO, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AHV, Simons JP, El-Andaloussi S, Alvarez-Erviti L. Systemic Exosomal siRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov Disord. 2014;29:1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat Biotechnol. 2011;29:341–347. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- (94).Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen G-X, Zhang G, et al. Delivery of Chemotherapeutic Drugs in Tumour Cell-Derived Microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- (95).Mocharla P, Briand S, Giannotti G, Do C, Jakob P, Paneni F, Luscher T, Landmesser U. AngiomiR-126 Expression and Secretion from Circulating CD34+ and CD14+ PBMCs: Role for Proangiogenic Effects and Alterations in Type 2 Diabetics. Blood. 2016;121:226–237. doi: 10.1182/blood-2012-01-407106. [DOI] [PubMed] [Google Scholar]
- (96).Silva AKA, Kolosnjaj-Tabi J, Bonneau S, Marangon I, Boggetto N, Aubertin K, Clement O, Bureau MF, Luciani N, Gazeau F, Wilhelm C. Magnetic and Photoresponsive Theranosomes: Translating Cell-Released Vesicles into Smart Nanovectors for Cancer Therapy. ACS Nano. 2013;7:4954–4966. doi: 10.1021/nn400269x. [DOI] [PubMed] [Google Scholar]
- (97).Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor microRNA to Breast Cancer Cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Ströbel T, Breakefield XO, Saydam O. Genetically Engineered Microvesicles Carrying Suicide mRNA/protein Inhibit Schwannoma Tumor Growth. Mol Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A Doxorubicin Delivery Platform Using Engineered Natural Membrane Vesicle Exosomes for Targeted Tumor Therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- (100).Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell-Derived Exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- (101).Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, et al. Dendritic Cell-Derived Exosomes Promote Natural Killer Cell Activation and Proliferation: A Role for NKG2D Ligands and IL-15Ra. PLoS One. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Cheng Y, Schorey JS. Exosomes Carrying Mycobacterial Antigens Can Protect Mice against Mycobacterium Tuberculosis Infection. Eur J Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes Are an Effective Vaccine against Congenital Toxoplasmosis in Mice. Vaccine. 2009;27:1750–1757. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- (104).Colino J, Snapper CM. Exosomes from Bone Marrow Dendritic Cells Pulsed with Diphtheria Toxoid Preferentially Induce Type 1 Antigen-Specific IgG Responses in Naive Recipients in the Absence of Free Antigen. J Immunol. 2006;177:3757–3762. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- (105).Del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C. Induction of Protective Immunity against Eimeria Tenella Infection Using Antigen-Loaded Dendritic Cells (DC) and DC-Derived Exosomes. Vaccine. 2011;29:3818–3825. doi: 10.1016/j.vaccine.2011.03.022. [DOI] [PubMed] [Google Scholar]
- (106).Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, Miller LB, Moon JE, Bowden RA, Cummings JF, Zollinger WD. A Phase 1 Study of a Meningococcal Native Outer Membrane Vesicle Vaccine Made from a Group B Strain with Deleted lpxL1 and synX, over-Expressed Factor H Binding Protein, Two PorAs and Stabilized OpcA Expression. Vaccine. 2011;29:1413–1420. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- (107).Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu D-H, et al. A Phase I Study of Dexosome Immunotherapy in Patients with Advanced Non-Small Cell Lung Cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I Clinical Trial of Autologous Ascites-Derived Exosomes Combined with GM-CSF for Colorectal Cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Kordelas L, Rebmann V, Ludwig A-K, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- (110).Van Den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA Delivery with Exosome Nanoparticles. Nat Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- (111).LaVan DA, McGuire T, Langer R. Small-Scale Systems for in Vivo Drug Delivery. Nat Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- (112).Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-Presenting Cell Exosomes Are Protected from Complement-Mediated Lysis by Expression of CD55 and CD59. Eur J Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- (113).Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, De Wit E, Berenguer J, Ellenbroek SIJ, Wurdinger T, et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behaviour. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Ridder K, Keller S, Dams M, Rupp A-K, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, et al. Extracellular Vesicle-Mediated Transfer of Genetic Information between the Hematopoietic System and the Brain in Response to Inflammation. PloS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Robbins PDP, Morelli AEA. Regulation of Immune Responses by Extracellular Vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Lösche W, Scholz T, Temmler U, Oberle V, Claus RA. Platelet-Derived Microvesicles Transfer Tissue Factor to Monocytes but Not to Neutrophils. Platelets. 2004;15:109–115. doi: 10.1080/09537100310001649885. [DOI] [PubMed] [Google Scholar]
- (117).Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Wood MJA, O’Loughlin AJ, Lakhal S. Exosomes and the Blood-Brain Barrier: Implications for Neurological Diseases. Ther Deliv. 2011;2:1095–1099. doi: 10.4155/tde.11.83. [DOI] [PubMed] [Google Scholar]
- (119).Armstrong JPK, Perriman AW. Strategies for Cell Membrane Functionalization. Exp Biol Med. 2016;241:1098–1106. doi: 10.1177/1535370216650291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Armstrong JPK, Shakur R, Horne JP, Dickinson SC, Armstrong CT, Lau K, Kadiwala J, Lowe R, Seddon AM, Mann S, Anderson JLR, et al. Artificial Membrane Binding Proteins Stimulate Oxygenation of Stem Cells during Tissue Engineering of Large Cartilage Constructs. Nat Commun. 2015;6:7405. doi: 10.1038/ncomms8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Correia Carreira S, Armstrong JPK, Seddon AM, Perriman AW, Hartley-Davies R, Schwarzacher W. Ultra-Fast Stem Cell Labelling Using Cationised Magnetoferritin. Nanoscale. 2016;8:7474–7483. doi: 10.1039/c5nr07144e. [DOI] [PubMed] [Google Scholar]
- (122).Ridder K, Sevko A, Heide J, Dams M, Rupp A-K, Macas J, Starmann J, Tjwa M, Plate KH, Sultmann H, Altevogt P, et al. Extracellular Vesicle-Mediated Transfer of Functional RNA in the Tumor Microenvironment. Oncoimmunology. 2015;4:e1008371. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y, Naoe T. Microvesicle-Mediated RNA Molecule Delivery System Using Monocytes / Macrophages. Mol Ther. 2011;19:395–399. doi: 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory Mechanisms and Intercellular Transfer of microRNAs in Living Cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Luo S-S, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, et al. Human Villous Trophoblasts Express and Secrete Placenta-Specific microRNAs into Maternal Circulation via Exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- (126).Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang J-D, Song E. Microvesicles Secreted by Macrophages Shuttle Invasion-Potentiating microRNAs into Breast Cancer Cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Qi S, et al. Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- (128).Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y, Ceroni A, et al. Exosomes Secreted by Nematode Parasites Transfer Small RNAs to Mammalian Cells and Modulate Innate Immunity. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, Van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, De Gruijl TD, Würdinger T, Middeldorp JM. Functional Delivery of Viral miRNAs via Exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Olson SD, Kambal A, Pollock K, Mitchell G-M, Stewart H, Kalomoiris S, Cary W, Nacey C, Pepper K, Nolta JA. Examination of Mesenchymal Stem Cell-Mediated RNAi Transfer to Huntington’s Disease Affected Neuronal Cells for Reduction of Huntingtin. Mol Cell Neurosci. 2012;49:271–281. doi: 10.1016/j.mcn.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, De Ruiter PE, Kwekkeboom J, Tilanus HW, Janssen HLA, Van Der Laan LJW. Hepatic Cell-to-Cell Transmission of Small Silencing RNA Can Extend the Therapeutic Reach of RNA Interference (RNAi) Gut. 2011;10:1136. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- (132).Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where to Go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- (133).Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Kanada M, Bachmann MH, Hardy JW, Omar D, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, et al. Differential Fates of Biomolecules Delivered to Target Cells via Extracellular Vesicles. Proc Natl Acad Sci USA. 2015:1433–1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of Nucleotide Patterns Enriched in Secreted RNAs as Putative Cis-Acting Elements Targeting Them to Exosome Nano-Vesicles. BMC Genomics. 2011;12:S18. doi: 10.1186/1471-2164-12-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, Fan JB, Breakefield XO, Saydam O. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Hassuna N, Monk PN, Moseley GW, Partridge LJ. Strategies for Targeting Tetraspanin Proteins Potential Therapeutic Applications in Microbial Infections. Biodrugs. 2009;23:341–359. doi: 10.2165/11315650-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B. Development of Exosome Surface Display Technology in Living Human Cells. Biochem Biophys Res Commun. 2016;472:53–59. doi: 10.1016/j.bbrc.2016.02.058. [DOI] [PubMed] [Google Scholar]
- (139).Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging Exosome Transfer from Breast Cancer Cells to Stroma at Metastatic Sites in Orthotopic Nude-Mouse Models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- (140).Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in Vivo Tracking of the Exosomes of Murine Melanoma B16-BL6 Cells in Mice after Intravenous Injection. J Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- (141).Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous Ba, Breakefield XO. Visualization and Tracking of Tumour Extracellular Vesicle Delivery and RNA Translation Using Multiplexed Reporters. Nat Commun. 2015;6:7029. doi: 10.1038/ncomms8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJA. Exosome-Mediated Delivery of siRNA in Vitro and in Vivo. Nat Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- (143).Hung ME, Leonard JN. Stabilization of Exosome-Targeting Peptides via Engineered Glycosylation. J Biol Chem. 2015;290:8166–8172. doi: 10.1074/jbc.M114.621383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Kooijmans SAA, Aleza CG, Roffler SR, Van Solinge WW, Vader P, Schiffelers RM. Display of GPI-Anchored Anti-EGFR Nanobodies on Extracellular Vesicles Promotes Tumour Cell Targeting. J Extracell Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, Villanueva J, Khine SS, Le Pecq JB. Exosome Display Technology: Applications to the Development of New Diagnostics and Therapeutics. Blood Cells, Mol Dis. 2005;35:158–168. doi: 10.1016/j.bcmd.2005.07.003. [DOI] [PubMed] [Google Scholar]
- (146).Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang XY, Osada T, Hobeika A, Delcayre A, Le Pecq JB, Morse MA, et al. Increasing Vaccine Potency through Exosome Antigen Targeting. Vaccine. 2011;29:9361–9367. doi: 10.1016/j.vaccine.2011.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Brinkmann K, Dirmeier U, Laus R, Delcayre A. Exosome Targeting of Tumor Antigens Expressed by Cancer Vaccines Can Improve Antigen Immunogenicity and Therapeutic Efficacy. Cancer Res. 2011;71:5235–5244. doi: 10.1158/0008-5472.CAN-10-4076. [DOI] [PubMed] [Google Scholar]
- (148).Maguire CA, Balaj L, Sivaraman S, Crommentuijn MHW, Ericsson M, Mincheva-Nilsson L, Baranov V, Gianni D, Tannous BA, Sena-Esteves M, Breakefield XO, et al. Microvesicle-Associated AAV Vector as a Novel Gene Delivery System. Mol Ther. 2012;10:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Kooijmans SAA, Stremersch S, Braeckmans K, De Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P. Electroporation-Induced siRNA Precipitation Obscures the Efficiency of siRNA Loading into Extracellular Vesicles. J Control Release. 2013;172:229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- (150).Keppler OT, Stehling P, Herrmann M, Kayser H, Grunow D, Reutter W, Pawlita M. Biosynthetic Modulation of Sialic Acid-Dependent Virus-Receptor Interactions of Two Primate Polyoma Viruses. J Biol Chem. 1995;270:1308–1314. doi: 10.1074/jbc.270.3.1308. [DOI] [PubMed] [Google Scholar]
- (151).Du J, Che P-L, Wang Z-Y, Aich U, Yarema KJ. Designing a Binding Interface for Control of Cancer Cell Adhesion via 3D Topography and Metabolic Oligosaccharide Engineering. Biomaterials. 2011;32:5427–5437. doi: 10.1016/j.biomaterials.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Juillerat FK, Borcard F, Staedler D, Scaletta C, Applegate LA, Comas H, Gauckler LJ, Gerber-Lemaire S, Juillerat-Jeanneret L, Gonzenbach UT. Functionalization of Microstructured Open-Porous Bioceramic Scaffolds with Human Fetal Bone Cells. Bioconjugate Chem. 2012;23:2278–2290. doi: 10.1021/bc300407x. [DOI] [PubMed] [Google Scholar]
- (153).Wang M, Altinoglu S, Takeda YS, Xu Q. Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery. PLoS One. 2015;10:e0141860. doi: 10.1371/journal.pone.0141860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Mahal LK, Yarema KJ, Bertozzi CR. Engineering Chemical Reactivity on Cell Surfaces through Oligosaccharide Biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- (155).Okeley NM, Toki BE, Zhang X, Je SC, Burke PJ, Alley SC, Senter PD. Metabolic Engineering of Monoclonal Antibody Carbohydrates for Antibody-Drug Conjugation. 2013 doi: 10.1021/bc4002695. [DOI] [PubMed] [Google Scholar]
- (156).Iwasaki Y, Matsunaga A, Fujii S. Preparation of Biointeractive Glycoprotein-Conjugated Hydrogels through Metabolic Oligosacchalide Engineering. Bioconjugate Chem. 2014;25:1626–1631. doi: 10.1021/bc5003295. [DOI] [PubMed] [Google Scholar]
- (157).Neubert J, Glumm J. Promoting Neuronal Regeneration Using Extracellular Vesicles Loaded with Superparamagnetic Iron Oxide Nanoparticles. Neural Regen Res. 2016;11:61–63. doi: 10.4103/1673-5374.175043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Silva AKA, Luciani N, Gazeau F, Aubertin K, Bonneau S, Chauvierre C, Letourneur D, Wilhelm C. Combining Magnetic Nanoparticles with Cell Derived Microvesicles for Drug Loading and Targeting. Nanomedicine Nanotechnology, Biol Med. 2015;11:645–655. doi: 10.1016/j.nano.2014.11.009. [DOI] [PubMed] [Google Scholar]
- (159).Tatischeff I, Alfsen A. A New Biological Strategy for Drug Delivery: Eucaryotic Cell-Derived Nanovesicles. J Biomater Nanobiotechnol. 2011;2:494–499. [Google Scholar]
- (160).Peterson BR. Synthetic Mimics of Mammalian Cell Surface Receptors: Prosthetic Molecules That Augment Living Cells. Org Biomol Chem. 2005;3:3607–3612. doi: 10.1039/b509866a. [DOI] [PubMed] [Google Scholar]
- (161).Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM. Engineered Mesenchymal Stem Cells with Self-Assembled Vesicles for Systemic Cell Targeting. Biomaterials. 2010;31:5266–5274. doi: 10.1016/j.biomaterials.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, Park J-H. Cellular Engineering with Membrane Fusogenic Liposomes to Produce Functionalized Extracellular Vesicles. ACS Appl Mater Interfaces. 2016;8:6790–6795. doi: 10.1021/acsami.6b01315. [DOI] [PubMed] [Google Scholar]
- (163).Barenholz Y. Liposome Application: Problems and Prospects. Curr Opin Colloid Interface Sci. 2001;6:66–77. [Google Scholar]
- (164).Smyth TJ, Petrova K, Payton NM, Persaud I, Redzic JS, Graner M, Smith-Jones P, Anchordoquy TJ. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjugate Chem. 2014;25:1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).AK D. A Text Book of Inorganic Chemistry. New Age International Limited; New Delhi: 2003. Chemical Bonding and Structure. [Google Scholar]
- (166).Berg J, Tymoczko J, Stryer L. Biochemistry. W H Freeman; New York: 2002. Chemical Bonds in Biochemistry. [Google Scholar]
- (167).Nakase I, Futaki S. Combined Treatment with a pH-Sensitive Fusogenic Peptide and Cationic Lipids Achieves Enhanced Cytosolic Delivery of Exosomes. Sci Rep. 2015;5:10112. doi: 10.1038/srep10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- (169).Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, Qian X, Jia H, Zhao J, Sun J, Hou X, et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano. 2016;10:3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- (170).Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J Biol. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Sato YT, Umezaki K, Sawada S, Mukai S-A, Sasaki Y, Harada N, Shiku H, Akiyoshi K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Kooijmans SA, Fliervoet LAL, Van Der Meel R, Fens MHAM, Heijnen HFG, Van Bergen En Henegouwen PMP, Vader P, Schiffelers RM. PEGylated and Targeted Extracellular Vesicles Display Enhanced Cell Specificity and Circulation Time. J Control Release. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- (173).Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active Loading into Extracellular Vesicles Significantly Improves the Cellular Uptake and Photodynamic Effect of Porphyrins. J Control Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- (174).Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, Garcia-Barrio M, Taylor RN, Gold B, Jefferson S, Sidell N, et al. Exosomes Derived from Endometriotic Stromal Cells Have Enhanced Angiogenic Effects in Vitro. Cell Tissue Res. 2016;365:187–196. doi: 10.1007/s00441-016-2358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Nicola AM, Frases S, Casadevall A. Lipophilic Dye Staining of Cryptococcus Neoformans Extracellular Vesicles and Capsule. Eukaryot Cell. 2009;8:1373–1380. doi: 10.1128/EC.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Maas SLN, De Vrij J, Van Der Vlist EJ, Geragousian B, Van Bloois L, Mastrobattista E, Schiffelers RM, Wauben MHM, Broekman MLD, Nolte-’T Hoen ENM. Possibilities and Limitations of Current Technologies for Quantification of Biological Extracellular Vesicles and Synthetic Mimics. J Control Release. 2015;200:87–96. doi: 10.1016/j.jconrel.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and Delivery Efficiency of Unmodified Tumor-Derived Exosomes. J Control Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Wahlgren J, Karlson TDL, Brisslert M, Sani FV, Telemo E, Sunnerhagen P, Valadi H. Plasma Exosomes Can Deliver Exogenous Short Interfering RNA to Monocytes and Lymphocytes. Nucleic Acids Res. 2012:1–12. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Hood JL, Scott MJ, Wickline SA. Maximizing Exosome Colloidal Stability Following Electroporation. Anal Biochem. 2014;448:41–49. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Wassler M, Jonasson I, Persson R, Fries E. Differential Permeabilization of Membranes by Saponin Treatment of Isolated Rat Hepatocytes Release of Secretory Proteins. Biochem J. 1987;247:407–415. doi: 10.1042/bj2470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Gutiérrez Millán C, Castañeda AZ, Sayalero Marinero ML, Lanao JM. Factors Associated with the Performance of Carrier Erythrocytes Obtained by Hypotonic Dialysis. Blood Cells, Mol Dis. 2004;33:132–140. doi: 10.1016/j.bcmd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- (183).Yim N, Ryu S, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park J, Kim D, et al. Exosome Engineering for Efficient Intracellular Delivery of Soluble Proteins Using Optically Reversible Protein-Protein Interaction Module. Nat Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]