Abstract
Flexible spatial navigation depends on cognitive mapping, a function that declines with increasing age. In young adults, a brief period of post-navigation rest promotes the consolidation/integration of spatial memories into accurate cognitive maps. We examined (1) whether rest promotes spatial memory consolidation/integration in older adults and (2) whether the magnitude of the rest benefit changes with increasing age. Young and older adults learned a route through a virtual environment, followed by a 10min delay comprising either wakeful rest or a perceptual task, and a subsequent cognitive mapping task, requiring the pointing to landmarks from different locations. Pointing accuracy was lower in the older than younger adults. However, there was a comparable rest-related enhancement in pointing accuracy in the two age groups. Together our findings suggest that (i) the age-related decline in cognitive mapping cannot be explained by increased consolidation interference in older adults, and (ii) as we grow older rest continues to support the consolidation/integration of spatial memories.
Keywords: Spatial navigation, cognitive map, wakeful rest, memory consolidation, long-term memory, spatial memory
1. Introduction
We are often faced with challenges when navigating in the real world. For example, while driving home from work you may find that your usual route is blocked. In such a case you would be required to find an alternative route home, possibly via a path that you have never experienced directly, but that you know travels in the general direction of your destination. As we grow older our ability to navigate flexibly is reduced. In this paper we report a study that examines the cognitive basis of the age-related deficit in flexible navigation, and the extent to which wakeful rest after route learning can ease this deficit.
In order to navigate flexibly we must acquire and store configural knowledge during initial navigation of a spatial environment. Configural knowledge refers to the directions and distances between objects and locations in the spatial environment. When this knowledge is obtained during navigation, it can be integrated via the automatic formation (Ishikawa and Montello, 2006; Montello, 1998) of ‘cognitive maps’ (Tolman, 1948). A cognitive map is a flexible mental representation of a spatial environment that can be accessed from any perspective and vantage point (Wolbers and Hegarty, 2010). This mental representation does not represent a specific experience, but an overarching relational memory built via the integration of a number of memories pertaining to a spatial experience.
The hippocampal-entorhinal circuit plays a critical role in supporting cognitive maps. Research in rodents demonstrates that during spatial navigation, hippocampal place cells and entorhinal grid cells fire, coding the animal’s location as it travels (for review, see Moser et al., 2008; O’Keefe et al., 1998). Following navigation, place cell firing patterns are reactivated (i.e. replayed) in a forwards and reverse direction (Foster and Wilson, 2006). In addition, firing patterns relating to trajectories that were never directly experienced during navigation are observed, i.e. possible future routes are preplayed (Dragoi and Tonegawa, 2011; Gupta et al., 2010). These patterns of neural reactivation, which occur especially during post-navigation periods of relative immobility and reduced sensory input (i.e. wakeful rest and sleep) (Davidson et al., 2009; Jackson et al., 2006; Karlsson and Frank, 2009), are hypothesised to support the consolidation of spatial memories for specific experiences (e.g. a travelled route) and their wider integration (e.g. into accurate cognitive maps) (Carr et al., 2011; Csicsvari and Dupret, 2014; Gupta et al., 2010; Karlsson and Frank, 2009).
The reactivation-consolidation hypothesis is supported by work in rodents demonstrating that (i) the disruption of neural reactivation impairs subsequent spatial memory (Ego-Stengel and Wilson, 2011; Girardeau et al., 2009), and (ii) the quantity of neural reactivation during post-learning rest positively predicts performance in subsequent spatial memory tests (Carr et al., 2011; Foster and Wilson, 2006). The latter finding resonates with human neuroimaging work which shows an association between the reactivation of stimulus-specific neural activity during post-learning rest and performance in a subsequent memory task (Staresina et al., 2013).
Further human evidence for the reactivation-consolidation hypothesis comes from recent behavioural work in young adults. A short period of post-navigation rest promotes the consolidation and wider integration of memories into accurate cognitive maps (Craig et al., 2016). This finding resonates with previous work demonstrating that rest promotes the consolidation of specific memories (e.g. lists of words and short stories) in young and older adults (Craig et al., 2015, 2014; Dewar et al., 2014, 2012a). It is hypothesised that post-learning rest promotes the consolidation of specific memories and the wider integration of memories by providing conditions of reduced sensory input (and associated encoding) that would otherwise interfere with consolidation-related processes such as neural reactivation (Dewar et al., 2014, 2012a).
However, it remained unknown whether the beneficial effect of post-navigation rest in the integration of spatial memories into accurate cognitive maps extends from young adults to older adults. This is important as older adults demonstrate an age-related decline in cognitive mapping ability (Iaria et al., 2009; Liu et al., 2011; Moffat and Resnick, 2002). Hitherto, this decline has been attributed mainly to age-related changes in the ability to encode and/or retrieve spatial memories (Iaria et al., 2009; Meulenbroek et al., 2004; Moffat, 2009; Rodgers et al., 2012). Little consideration has been given to the possibility that age-related changes in consolidation could account, at least in part, for these cognitive mapping deficit in older adults. However, recent work in rodents has revealed that older rodents demonstrate impairments in hippocampal reactivation of recent spatial memories during post-navigation rest, i.e. reduced reactivation (Gerrard et al., 2008). Moreover, work in humans has shown that increasing age impairs memory consolidation (during sleep) in humans, although findings have been mixed (Harand et al., 2012; Spencer et al., 2007).
If spatial memory consolidation/integration does decline with increasing age, then one possible mechanism is an increase in consolidation interference from ongoing sensory input and associated encoding in older adults. If this is the case, older adults should benefit more from post-learning rest than young adults. This hypothesis is supported by robust findings in patients with amnestic Mild Cognitive Impairment (aMCI) and Alzheimer’s disease (AD). Relative to healthy older adults, these patients demonstrate a severe consolidation interference effect in tests of delayed verbal recall (e.g. wordlists and short stories), such that retention is profoundly impaired if learning is followed by cognitive tasks, e.g. a spot-the-difference game or a picture naming task (Alber et al., 2014; Dewar et al., 2012b, 2009). Importantly, their retention deficit can be reduced substantially if learning is followed by a short rest period (Alber et al., 2014; Dewar et al., 2012b, 2009). As a result, the rest-related memory benefit is much larger in aMCI and AD patients than in healthy older adults (Alber et al., 2014; Dewar et al., 2012b, 2009).
It is also possible that ageing reduces memory consolidation/integration more generally, e.g. due to reduced quantity and/or quality of neural reactivation. Such a shortfall in consolidation would be predicted to result in poorer overall spatial memory performance in older than young adults when probed at a delayed stage. Moreover, such a consolidation deficit could decrease the beneficial effect of rest, paralleling the accounts of a reduction in sleep-related memory benefits in older adults (Harand et al., 2012).
To date, no study has directly compared the magnitude of the rest effect in younger and older adults within the same paradigm. In the study reported here we examined if cognitive mapping deficits in older adults can be accounted for, at least in part, by changes in the consolidation and integration of spatial memories. Specifically, we examined whether, and how much, post-navigation rest promotes the consolidation/integration of spatial memories into an accurate cognitive map in older adults as compared to young adults. To this end, we used an established virtual reality cognitive mapping paradigm that has been found to be sensitive to the effect of post-navigation cognitive activity (rest vs. perceptual task) in younger adults (Craig et al., 2016).
2. Method
Since our study used a modified version of already published methodology (Craig et al., 2016) only core methodological details and relevant modifications are detailed below.
2.2. Participants
Twenty healthy young adults (11 males, 9 females; mean age = 26.25 years, SD = 2.75, age range: 21-33 years) and twenty healthy older adults (9 males, 11 females; mean age = 67.95 years, SD = 3.95, age range: 61-74 years) were recruited as participants by the German Center for Neurodegenerative Diseases (DZNE), Magdeburg, where the study was conducted. Older adults did not have any known premorbid psychiatric or neurological disorders and performed within the normal range on the Mini-mental State Examination (mean score = 28.5, SD = 1.37) (Folstein et al., 1975; Petersen et al., 1999). All participants had normal or corrected-to-normal vision and received compensation of €6.50 per hour for their time.
2.3. Design
A between-subjects design was employed with two factors: 10min delay condition (wakeful rest vs. perceptual task) and age group (young vs. older adults). The experiment comprised a learning phase, a delay phase, and a testing phase. The critical between-subjects manipulation occurred during the 10min delay phase, where participants either: (i) rested wakefully, or (ii) performed an unrelated perceptual task – a spot-the-difference game. During the subsequent testing phase, participants performed (i) a free recall test for landmarks from the virtual environment, and (ii) a cognitive map test assessing the accuracy of a newly formed cognitive map of the navigated virtual environment.
2.4. Materials
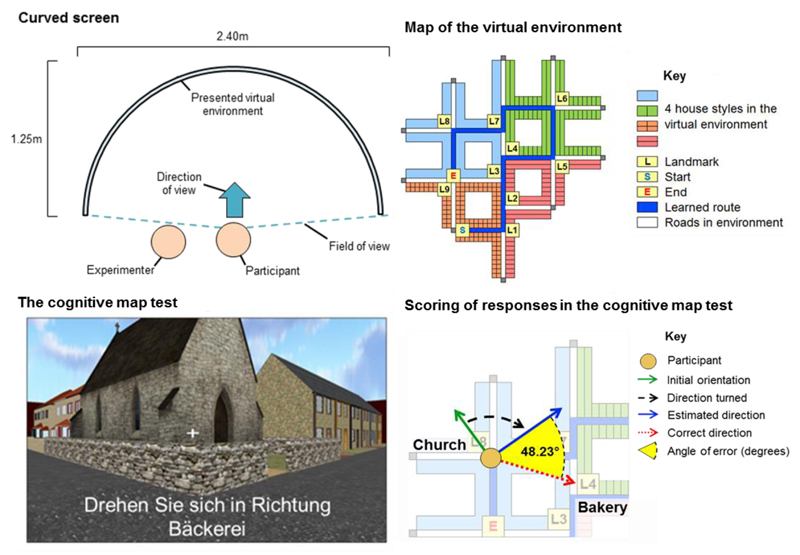
The virtual environment was a modified version of an existing environment (Craig et al., 2016; Harris and Wolbers, 2014). All landmarks within the virtual environment were common real-world buildings that would be found in a German town-like environment, e.g. a bakery (Bäckerei), a supermarket (Supermarkt) and a church (Kirche) (see Fig 1). The virtual environment was presented to participants on a large curved (180 degrees) projector screen (height = 2.00m, width = 2.40m, depth = 1.25m; see Fig 1). Participants were seated in a central position where the screen curved around them. The curvature of the screen meant that participants’ field of view was mostly filled by the presented virtual environment, and thus provided a more immersive and ecological virtual navigation experience relative to flat-screen computer monitors often used in virtual reality spatial navigation studies. A computer gaming steering wheel was used to input responses during learning and testing.
Fig 1.
Top left: Illustration of the curved screen (bird’s eye view). Participants sat in a central position where their field of view was mostly filled by the presented virtual environment. The experimenter (the ‘driver’) sat to the left of the participant (the ‘passenger’). Top right: Bird’s eye map of the virtual environment. The route learned by participants is shown via the blue line; ‘S’ = start of route, ‘E’ = end of route. Landmarks (L) were all common real-world buildings: L1 = petrol station, L2 = bank, L3 = convenience store, L4 = bakery, L5 = hotel, L6 = hardware store, L7 = bar, L8 = church, L9 = supermarket. Bottom left: A screenshot from one of the 16 cognitive map test trials. The text onscreen reads: “Turn to face the direction of the bakery”. Participants were required to provide verbal instructions (that the experimenter input) to rotate left or right until they believed the crosshair in the centre of the screen was directly facing the direction of the target landmark (e.g. the bakery). Bottom right: An illustration of how the pointing error measure was calculated in the cognitive map test. The illustration reflects the trial shown in the image on the bottom left where the participant’s initial orientation (green arrow) was directly facing the church (L8). The participant was asked to turn (black dashed arrow) to face towards the bakery (L4). The target landmark (e.g. the bakery) was never visible from any orientation during the cognitive map trials. The pointing error (degrees; yellow cone) between the correct direction (red arrow) and estimated direction (blue arrow) was calculated for each trial. Figure modified from Craig et al. (2016).
2.5. Procedure
2.5.1. Learning phase
As in our previous work (Craig et al., 2016), participants were informed that they would be a ‘passenger’ in a car travelling through a virtual town. They were informed that they would be required to learn a long, indirect route through the environment and would be asked to provide directions to the experimenter (the ‘driver’) during a subsequent trial. Route learning was broken into learning cycles; each learning cycle included two learning trials followed by one probe trial. In a learning trial, the participant travelled the trained route (total distance = 480.00 metres) via automated movement (linear movement = 3.20 metres per second, rotation movement = 12 degrees per second, total travel time = 153 seconds). In a probe trial, the participant again travelled the trained route. However, on this occasion automated movement temporarily paused at each junction (i.e. decision point) and the participant was asked to verbally state to the experimenter the direction (i.e. “left”, “right”, or “straight on”) that the trained route continued from that point. If the participant responded correctly (e.g. they responded “left”, where the correct direction was ‘left’), automated movement then continued to the next junction along the trained route. If the participant provided an incorrect response (e.g. they responded “right”, where the correct direction was ‘left’), a message (“incorrect”) appeared onscreen informing them of their error. This was shortly followed by a large arrow that appeared on the ground of the virtual environment and indicated the correct direction. Automated movement then continued to the next junction along the trained route. Participant’s verbal responses were input by the experimenter via a gaming steering wheel. Route learning was scored by calculating, for each participant, the total number of errors made during each route learning probe trial (minimum = 2 trials, maximum = 10 trials), until the participant was able to recall the route without making a single error. The total number of learning cycles required to learn the route was also recorded. Group means were computed subsequently.
While we measured participants’ route memory performance immediately prior to the delay phase we did not measure their cognitive map performance at this juncture. A baseline cognitive map test could have resulted in participants suspecting a further cognitive map test, which could have motivated specific rehearsal of landmark-to-landmark relationships, especially during rest, and perhaps more so in the young than the older adults, thus adding confounds to the study. Moreover, given the dimensions of the virtual environment and the fixed number of landmarks within it, there was a limited number of possible trials that could be used to ensure that the cognitive map test did not probe memory for target landmarks that were visible or close in temporal order to the presented landmark.
2.5.2. Delay phase
During the 10min delay phase, participants either (i) rested wakefully under conditions of minimal sensory input, or (ii) performed an unrelated perceptual task (a spot-the-difference game) involving continuous sensory input. Twenty participants (10 young and 10 older adults) were allocated pseudo-randomly to each delay condition. See Craig et al. (2016) for more specific details regarding the two delay conditions.
2.5.3. Testing phase
Following the delay phase, participants performed a free recall test for landmarks from the earlier navigated virtual environment. Participants were asked to verbally recall as many landmarks as possible. Once the participant had recalled all nine landmarks, or was unable to recall any further landmarks, they proceeded to the cognitive map test. The landmark free recall test (and subsequent landmark recall/recognition check built into the cognitive map test – see next paragraph) were included to verify whether any potential substantial errors in the cognitive map test were associated with poor memory for landmarks per se, rather than with poor memory for the spatial relationships (i.e. directions) between landmarks.
The cognitive map test comprised 16 trials that were presented via the curved screen, as during route learning. In each trial, the participant was positioned facing one of the nine landmarks within the environment. They were then instructed on-screen to rotate to face towards a second, specified target landmark within the environment (see Fig 1). Prior to rotating, participants were asked “can you recall the visual appearance of the target landmark?” If yes, they proceeded to perform the cognitive map trial (i.e. began rotating to face the target landmark). If they were unable to recall the visual appearance of the target landmark, they were presented with a colour photo of the target landmark and asked a follow-up question “do you recognise this landmark?” If yes, they proceeded to perform the cognitive map trial. If, however, participants were unable to recall and recognise the target landmark, the cognitive map trial was abandoned, and the participant proceeded to the next cognitive map trial. The colour photos of target landmarks contained no contextual information from the virtual environment that could aid performance in the cognitive map test, e.g. surrounding roads and buildings.
For each cognitive map trial, pointing responses were input by the experimenter on behalf of the participant via the same steering wheel used during route learning. This was done to minimise the effects of possible individual differences in computer use and familiarity with gaming controls. Participants were required to provide verbal instructions to rotate within the environment, which the experimenter input via the ‘left’ and ‘right’ directional keys on the same gaming steering wheel. Care was taken to ensure that the participant was satisfied with the exact direction that they were facing before the experimenter input each response.
Upon completion of the cognitive map test, participants completed the Freiburg (German) version of the Santa Barbara Sense of Direction (F-SBSOD) questionnaire (Hegarty et al., 2002; Montello and Xiao, 2011), as well as a detailed post-experimental questionnaire that probed participants’ past experience using computers (Moffat et al., 2001) and whether they actively rehearsed task-relevant information during their allocated delay condition (see Craig et al., 2016).
2.6. Scoring
The landmark free recall test was scored by extracting the total number of landmarks correctly recalled for each participant. Group means were computed subsequently. For the cognitive map test, we extracted the accuracy of responses for each trial, i.e. the number of degrees of absolute error between (i) the correct direction of the target landmark within the environment and (ii) the estimated direction of the target landmark (see Fig. 1). Group means were computed subsequently (see Craig et al., 2016 for full scoring details).
2.7. Statistical analyses
ANOVAs with between-subject factors delay condition (wakeful rest vs. perceptual task) and age (young vs. older) were performed to examine group differences in learning and cognitive map test performance. ANCOVAs were run with covariates gender, self-reported sense of direction (F-SBSOD) and past experience using computers, in order to examine group differences in learning and cognitive map performance after controlling for the effects of these variables. In order to examine whether the cognitive map test might have been solved via mental travel along the route, rather than direct access to landmark-to-landmark relationships, Pearson correlations were calculated for each participant between absolute pointing error (degrees) and the distance travelled previously between the presented landmark and the target landmark. These correlation coefficients were normalized using Fisher’s r-to-z transformation and subsequently compared to 0 (no correlation) via Bayesian one-sample t-tests, for each of the four delay/age groups separately. The default Cauchy(0,1) prior for effect size (σ = 0.707) was used for Bayesian analyses (Rouder et al., 2009).
3. Results
3.1. Background measures
There was no difference between the ratio of males and females in the wakeful rest and perceptual task groups in younger adults (wakeful rest: 5 females, 5 males; perceptual task: 4 females, 6 males; p = 1.000, Fisher’s exact test) or older adults (wakeful rest: 6 females, 4 males; perceptual task: 5 females, 5 males; p = 1.000, Fisher’s exact test). As expected, age differed significantly between the two age groups (F1,36 = 164.429, p < .001), but not between delay conditions (F1,36 = 0.370, p = .547). Thus, other than for the expected age group manipulation, our delay condition groups were matched in terms of gender and age.
For self-reported sense of direction (F-SBSOD) scores, we found a significant main effect of age group (F1,36 = 4.215, p = .047) due to older adults (mean = 3.49/7, SD = 0.78) reporting having a better sense of direction than younger adults (mean = 2.95/7, SD = 0.85). No main effect of delay condition (F1,36 = 0.540, p = .467), or interaction between age group and delay condition was observed (F1,36 = 0.208, p = .651).
For self-reported past-experience using computers, a significant main effect of age was again observed (F1,36 = 18.807, p < .001), but on this occasion it was due to younger adults reporting having more experience (mean = 13.9/21, SD = 3.19) than older adults (mean = 8.70/21, SD = 4.19). No significant main effect of delay condition (F1,36 = 0.696, p = .410), or significant interaction between delay condition and age group (F1,36 = 0.028, p = .868) was observed.
3.2. Route learning
3.2.1. Learning cycles
All but one participant (older adult – perceptual task group) were able to learn the route to a 100% criterion within the fixed minimum of two learning cycles (four learning trials and two probe trials). The participant who did not learn the route within two learning cycles was able to do so within three learning cycles. We found no significant main effect of age group (F1,36 = 1.000, p = .324) or delay condition (F1,36 = 1.000, p = .324) in the number of learning cycles required to learn the route, nor did we observe a significant interaction between age group and delay condition (F1,36 = 1.000, p = .324). These findings did not change after controlling for the effects of gender, self-reported sense of direction, and past experience with computers (age group: F1,33 = 0.114, p = .738; delay condition: F1,33 = 0.998, p = .325; delay condition*age group interaction: F1,33 = 0.866, p = .359). In addition, no covariates were significantly related to the number of learning trials required (all p > .340).
Two older adults suffered from nausea during route learning and exited route learning after one learning cycle (two learning trials and one probe trial). These two participants made zero errors during the probe trial of learning cycle one (i.e. they appeared to have learned the route), and no results changed when these participants were removed from analyses.
3.2.2. Learning errors
Table 1 shows (i) the mean number of errors made by younger and older adults in the rest and perceptual task delay conditions during each route learning probe trial individually, as well as during learning overall, and (ii) the number of participants who performed at criterion (i.e. zero errors) in each probe trial relative to the number of participants who completed each probe trial. We found no significant main effect of delay condition in the number of errors made during route learning (F1,36 = 0.027, p = .871). There was however a significant effect of age group on the number of errors made (F1,36 = 4.540, p = .040), due to older adults (mean = 1.00, SD = 1.26) making a greater number of errors than younger adults (mean = 0.20, SD = 0.41) across both delay conditions, i.e. no significant interaction between delay condition and age group was observed (F1,36 = 0.242, p = .626). When controlling for the effects of gender, self-reported sense of direction, and past experience with computers, the significant main effect of age group was no longer observed, though it was approaching significance (F1,33 = 2.821, p = .098). No change was observed in other results (delay condition: F1,33 = 0.099, p = .755; delay condition*age group interaction: F1,33 = 0.098, p = .756), and as before, no covariates were significantly related to the number of errors made during learning (all p > .466).
Table 1.
For younger and older adults in the wakeful rest and perceptual task delay conditions, the table shows (i) mean number of errors (wrong turns) made during route learning probe trials (standard deviations are shown in parentheses), and (ii) number of participants who performed at criterion (i.e. zero errors) in each probe trial relative to the number of participants who completed each probe trial. *One older adult in the rest group and one older adult in the task group exited the learning phase after Probe trial 1 due to nausea associated with the virtual reality equipment. These participants made zero errors in Probe trial 1.
| Delay condition | Probe 1 errors | Probe 1 criterion | Probe 2 errors | Probe 2 criterion | Probe 3 errors | Probe 3 criterion | Overall |
|---|---|---|---|---|---|---|---|
| Young adults | |||||||
| Wakeful rest | 0.30 (0.48) | 7/10 | 0.00 | 10/10 | - | - | 0.30 (0.48) |
| Perceptual task | 0.10 (0.32) | 9/10 | 0.00 | 10/10 | - | - | 0.10 (0.32) |
| Older adults | |||||||
| Wakeful rest | 1.00 (1.05) | 4/10 | 0.00 | 9/9* | - | - | 1.00 (1.05) |
| Perceptual task | 0.70 (0.67) | 4/10 | 0.30 (0.95) | 8/9* | 0.00 | 1/1 | 1.00 (1.49) |
Together, these analyses indicate that delay conditions (wakeful rest vs. perceptual task) did not differ significantly in terms of route memory performance prior to the critical delay. While an effect of age was observed in route learning, all participants learned the route to criterion, and thus pre-delay route memory performance was matched across all groups.
3.3. Free recall of landmarks
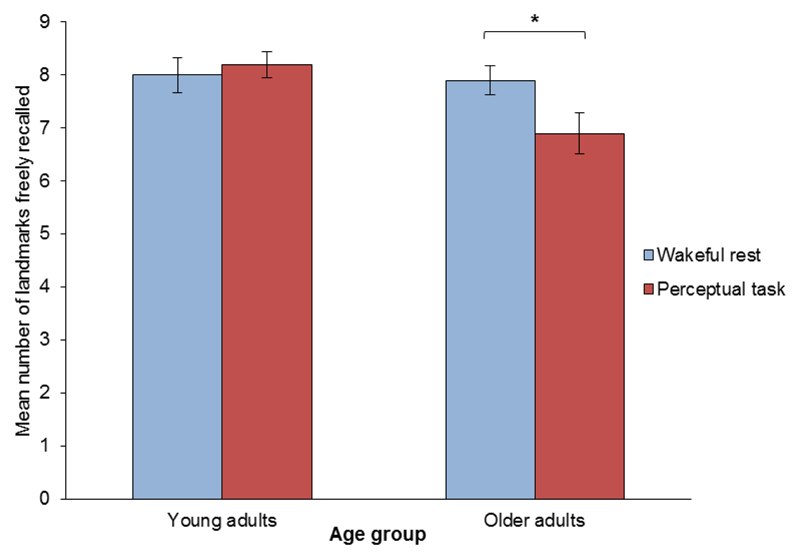
Performance across both delay conditions and age groups was near ceiling (see Fig 2). As a result, there was no significant main effect of delay condition on the number of landmarks recalled (F1,36 = 1.627, p = .210). However, we did observe a significant main effect of age group (F1,36 = 4.983, p = .032), such that younger adults recalled more landmarks than older adults. We also found a near significant interaction between delay condition and age group (F1,36 = 3.661, p = .064), indicating possible differing effects of the two delay conditions in the two age groups. Planned comparisons revealed no significant difference in the number of landmarks recalled between delay conditions in young adults (t18 = -0.480, p = .637). However, among older participants, those who rested after learning recalled a significantly greater number of landmarks than those who performed the perceptual task (t18 = 2.132, p = .047).
Fig 2. Landmark free recall test.
Mean number of landmarks recalled as a function of delay condition (wakeful rest vs. perceptual task) and age group (young vs. older adults). There were nine landmarks in total, and thus the maximum possible score in this test was /9. A significant benefit of rest was observed in the number of landmarks recalled by older adults. Error bars show the standard error of the mean. Significance thresholds: * = p <.05, ** = p < .01, and *** = p < .001.
After controlling for the effects of gender, self-reported sense of direction, and past experience with computers, the previously significant effect of age was non-significant (age group: F1,33 = 0.978, p = .330). No other results were found to change (delay condition: F1,33 = 2.152, p = .152; delay condition*age group interaction: F1,33 = 3.124, p = .086). No covariates were significantly related to the mean number of landmarks recalled (all p > .279).
In addition, after controlling for the number of learning cycles required to learn the route, none of our results changed (delay condition: F1,35 = 1.209, p = .279; age group: F1,35 = 4.170, p = .049; delay condition*age group interaction: F1,35 = 2.986, p = .093). The number of learning cycles was not significantly related to the number of landmarks recalled (F1,35 = 0.913, p = .346). Similarly, after controlling for the number of errors made during route learning, none of our results changed (delay condition: F1,35 = 1.506, p = .228; age group: F1,35 = 5.261, p = .028; delay condition*age group interaction: F1,35 = 3.732, p = .062). The number of errors made during learning was not significantly related to the number of landmarks recalled (F1,35 = 0.438, p = .512).Taken together, these findings demonstrate that post-delay memory for landmarks was unrelated to pre-delay route learning performance.
3.4. Cognitive map test
3.4.1. Landmark recall/recognition
There were only a small number of trials (/160 per group) during which participants reported being unable to recall the visual appearance of one or more target landmarks in the cognitive map test (young wakeful rest: 2/160 trials; young perceptual task: 2/160 trials; older wakeful rest: 6/160 trials; older perceptual task: 2/160 trials). Furthermore, when presented with a photo cue of the target landmark, no participants reported being unable to recognise target landmarks. Thus, this substantially reduced the possibility of cognitive map errors being driven by a failure to remember landmarks and thus by guessing.
3.4.2. Accuracy of responses
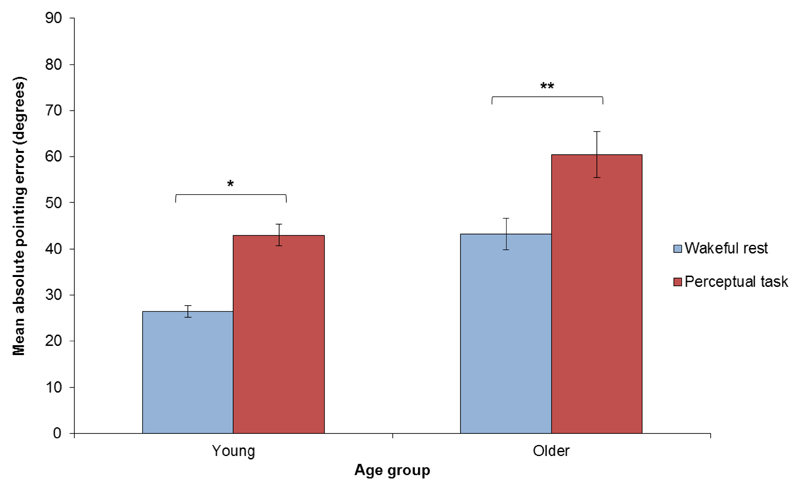
Across all participants, six cognitive map test trials (0.94% of all trials) were abandoned due to experimenter error and were thus removed from analyses. Data from the remaining trials revealed a significant main effect of delay condition on the accuracy of responses in the cognitive map test (F1,36 = 20.866, p < .001), such that those who experienced wakeful rest after learning outperformed those who performed the perceptual task after learning (Fig 3). In addition, a significant main effect of age group was observed (F1,36 = 21.531, p < .001), such that younger adults provided more accurate responses than older adults. Our results indicate that this was the case across both delay conditions (wakeful rest: t18 = -3.801, p = 001; perceptual task: t18 = -2.951, p = 009; planned comparisons), and that there was no significant interaction between delay condition and age group (F1,36 = 0.008, p = .931), thus suggesting that the rest-related enhancement in pointing accuracy was comparable in younger and older adults. Planned comparisons confirmed that young participants in the wakeful rest delay condition outperformed young participants in the perceptual task delay condition in the cognitive map test (t18 = -2.769, p = .013). This was also the case for older participants (t18 = -3.960, p = .001).
Fig 3. The cognitive map test.
Mean angle of absolute error (degrees) in the cognitive map test as a function of delay condition (wakeful rest vs. perceptual task) and age group (young vs. older adults). Younger and older adults who experienced wakeful rest after learning performed significantly better than those who performed a perceptual task. Error bars show the standard error of the mean. Significance thresholds: * = p <.05, ** = p < .01, and *** = p < .001.
The benefit of wakeful rest in cognitive map test performance remained after controlling for the effects of gender, self-reported sense of direction, and past experience with computers (F1,33 = 25.674, p < .001). The significant effect of age group also remained when controlling for these covariates (F1,33 = 13.036, p = .001). As before, no significant interaction between delay condition and age group was observed (F1,33 = 0.023, p = .881). No covariates were significantly related to mean pointing error (all p > .348).
When the number of landmarks recalled prior to the cognitive map test was included as a covariate, no results changed (delay condition: F1,35 = 19.098, p = .001; age group: F1,35 = 17.869, p < .001; interaction: F1,35 = 0.301, p = .587). Furthermore, there was no significant main effect of the number of landmarks recalled in predicting pointing error throughout the cognitive map test (F1,35 = 0.001, p = .979), suggesting that cognitive map test performance differences could not be accounted for by differences in landmark free recall but by memory for spatial relationships (i.e. directions and distances) between landmarks in the spatial environment.
In addition, after controlling for the number of learning cycles required to learn the route, no results changed (delay condition: F1,35 = 21.161, p < .001; age group: F1,35 = 21.817, p < .001; delay condition*age group interaction: F1,35 = 0.043, p = .837). The number of learning cycles was not significantly related to cognitive map test pointing error (F1,35 = 0.554, p = .462). Similarly, after controlling for the number of errors made during route learning, no results changed (delay condition: F1,35 = 20.354, p < .001; age group: F1,35 = 16.656, p < .001; delay condition*age group interaction: F1,35 = 0.006, p = .941). Furthermore, the number of errors made during route learning was not significantly related to cognitive map test pointing error (F1,35 = 0.044, p = .836). Taken together, this demonstrates that post-delay cognitive map test performance was unrelated to pre-delay route learning performance.
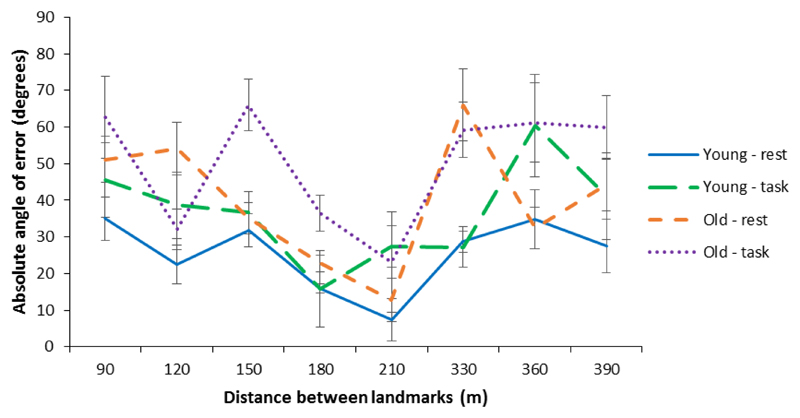
Instead of directly accessing landmark-to-landmark relationships, participants could have solved the cognitive map pointing task by mentally travelling along the route from the presented landmark to the target landmark. This strategy would lead to larger pointing errors on trials for which the distance between the two landmarks, as travelled during learning, was longer (Wolbers et al., 2004). However, Bayesian one-sample t-tests on Fisher z-transformed r values provided some (BF01 = odds greater than 3) evidence (Jeffreys, 1961) in favour of the null hypothesis, i.e. our data suggest there was no correlation between the distance between landmarks and pointing errors (young adults - wakeful rest: BF01 = 3.137, perceptual task: BF01 = 2.429; older adults - wakeful rest: BF01 = 3.209, perceptual task: BF01 = 2.378). This finding is reflective of our recent work (Craig et al., 2016) and suggests that participants did not solve the cognitive map test via mentally travelling along the route. Figure 4 shows mean pointing error scores, for each group, as function of the distance between the presented and target landmark.
Fig 4.
Cognitive map test performance as a function of distance between presented and target landmarks. Mean absolute angle of error (degrees) in the cognitive map test for younger and older adults in the wakeful rest and perceptual task groups broken down by distance between the presented and target landmarks (metres). Error bars show the standard error of the mean.
3.5. Post-experimental reports
As found in our recent research (Craig et al., 2016, 2015, 2014; Dewar et al., 2012a), no participants reported sleep-related activity and the majority of participants in the wakeful rest delay condition (young adults: n = 7/10, 70%; older adults: n = 6/10, 60%) reported spontaneous task-unrelated thoughts, e.g. recalling the past and imagining the future. In addition, 10 (4/10 young adults, 40%; 6/10 older adults, 60%) participants in the wakeful rest delay condition and 9 participants (4/10 young adults, 40%; 5/10 older adults, 50%) in the perceptual task delay condition reported that they had expected a further memory test at the end of the experiment. Of these, five participants (1/10 young wakeful rest, 1/10 young perceptual task, 3/10 older wakeful rest) reported intentionally thinking about information pertaining to the learned route during the 10min delay condition. None of our results changed when these participants were removed from analyses. Furthermore, intentional thoughts relating to the route would have been limited to knowledge of specific experience (i.e. the learned route) rather than inter-landmark relationships, as required in solving the cognitive map test.
4. Discussion
We recently demonstrated that in healthy young adults the consolidation and integration of new spatial memories (into accurate cognitive maps) can be enhanced significantly via a short period of post-navigation rest (Craig et al., 2016). In the current study we replicate this finding in young adults and provide the first evidence that a comparable rest-related enhancement of cognitive map accuracy is observable in older adults. This novel finding suggests that rest continues to support spatial memory consolidation and integration as we grow older and that rest-related consolidation boosts do not decline with age.
4.1. The cognitive basis of the rest-related enhancement in cognitive map test accuracy
Our results suggest that the benefit of wakeful rest in cognitive map test pointing accuracy cannot be accounted for by group differences in pre-delay route memory, past experience with computers, or self-reported sense of direction (see also Craig et al., 2016). In young and older adults, our delay conditions did not differ in terms of route learning (i.e. the number of learning cycles required and errors made during route learning) prior to the critical delay condition phase (i.e. wakeful vs. perceptual task). Furthermore, participants’ ability to learn the route was unrelated to post-delay performance in the cognitive map test. Together, this suggests that the between-delay condition differences in cognitive map test performance in younger and older adults are likely to be a direct result of our critical delay condition manipulation (rest vs. task), although we note that, in the absence of a baseline cognitive map test, we cannot rule out the possibility of some pre-delay cognitive mapping differences between the delay conditions (i.e. "better pointers" in the wakeful rest condition and "worse pointers" in the perceptual task condition).
A small number of participants reported intentionally rehearsing information pertaining to the virtual environment during their allocated delay condition. This rehearsal could not account for the between-delay condition difference in cognitive map accuracy in younger and older adults, as this difference persisted following removal of these participants from the analysis - a finding reflective of previous work (Craig et al., 2016, 2015, 2014; Dewar et al., 2014, 2012a). Furthermore, given that participants were unaware that they would perform a post-delay cognitive map test, rehearsal in the current study is likely to have been focused on specific experiences, e.g. the travelled route. Rehearsal of such information would have been of limited use since the cognitive map test did not examine memory for specific experience, but rather examined knowledge of overarching (and never-directly experienced) spatial relations (i.e. directions) between landmarks. This notwithstanding, rehearsal could have aided cognitive map test performance if participants solved the test by mentally travelling the learned route between the presented and target landmarks. This is unlikely, though, as pointing errors did not increase as a function of increasing distance (between the presented and target landmarks) in cognitive map test trials, which would be predicted by this strategy (Ghaem et al., 1997; Wolbers et al., 2004).
When probing memory for landmarks experienced along the route via a free recall test, young adults’ performance was close to ceiling across both delay conditions. However, older adults’ performance was slightly better in the rest condition than in the perceptual task condition, and this difference reached significance. These results suggest that rest boosted landmark memory in the older adults. Based on previous research (Craig et al., 2016, 2015, 2014) we would also expect to observe a benefit of rest in landmark memory in young adults if it were not for the apparent ceiling effect within this age group. It could therefore be argued that between-delay condition differences in the cognitive map test were driven by a more basic difference in landmark memory, in as much as cognitive map test performance would be impeded by poorer landmark memory. However, free recall of landmarks was high across participants, even in the older adults in the perceptual task condition. Moreover, all participants were able to recall or recognise the visual appearance of all target landmarks in the cognitive map test, and the free recall of landmarks did not correlate with cognitive map test pointing error. Thus, while good landmark memory will have been essential for successful performance in the cognitive map test, our findings indicate that the benefit of rest in the cognitive map test was not driven primarily by superior memory of landmarks, but by enhanced configural knowledge of the spatial locations of landmarks and inter-landmark relationships (i.e. directions and distances).
This rest-related enhancement in configural knowledge can be explained by a consolidation account. Research suggests that post-learning wakeful rest provides conditions that are conducive to memory consolidation. Rest is hypothesised to support consolidation by reducing the amount of sensory input and associated encoding, which would otherwise interfere with consolidation-related processes (such as neural reactivation) during the critical minutes that immediately follow new learning when a memory trace is most labile (Alber et al., 2014; Dewar et al., 2012a; Mednick et al., 2011). In line with this hypothesis we put forward that the reduced sensory input provided by rest allowed for superior consolidation/integration of spatial memories into an accurate cognitive map of the navigated environment (see also Craig et al., 2016). Our proposal resonates with the hypothesis that memory consolidation is an opportunistic process (Mednick et al., 2011), and the quantity and/or quality of consolidation-related processes (e.g. hippocampal reactivation) may depend on the amount and/or regularity of sensory input and associated encoding in the time that follows learning (Craig et al., 2016).
We note that our wakeful rest condition – sitting in a dimly lit room in the absence of the experimenter under conditions of minimal sensory input for 10 minutes – might be conducive not only to wakeful resting but also to sleep. Research shows that many participants in resting state fMRI studies experience sleep after ~3 minutes (Tagliazucchi and Laufs, 2014). Sleep is known to play an important role in memory consolidation and integration. Thus, could sleep account for the benefit of ‘wakeful rest’ in our cognitive map test? When probed in detail after the experiment (post-experimental questionnaire), no participants reported any sleep-related activity during the rest delay. However, we cannot exclude the possibility that some participants entered brief local sleep or microsleeps. Further studies using electrophysiological methods are necessary to examine if local sleep and/or microsleep contribute to the benefit of wakeful rest.
While the data reported in this paper replicate our previous finding that rest enhances cognitive map accuracy in younger adults (Craig et al., 2016), the error scores observed in the current study are larger than those seen in our previous work. It is likely that this increase in error score is the result of differences in the method of presentation. In the current study we presented the virtual environment and cognitive map test on a large curved projector screen (h = 2.00m, w = 2.40m, d = 1.25m), while a much smaller (h = 0.34m, w = 0.45m) flat-screen computer monitor was used in our previous study (Craig et al., 2016). On the large curved display, the extraction of spatial information from rich visual input required much larger/more eye and/or head movements. This could have introduced additional noise in the ensuing spatial knowledge that increased error scores in younger and older adults across both the rest and task delay conditions. As a result, the error scores observed in the current study may be more reflective of those during real-world navigation. Indeed, similar research (Ishikawa and Montello, 2006) probing cognitive mapping in the real-world has demonstrated comparable error scores (~40 degrees of absolute error) to those found in our study.
4.2. Poorer cognitive map accuracy in older adults than young adults – cognitive basis
Older adults performed more poorly on the cognitive map test than did younger adults, i.e. they demonstrated larger pointing errors. It is unlikely that this group difference can be accounted for simply by differences between young and older adults’ experience using computers and virtual reality technology. We found no effect of past-experience using computers in cognitive map test performance, and participants provided verbal responses during learning and testing.
Thus, the age difference observed in our cognitive map test appears to reflect a genuine difference in spatial memory performance between younger and older adults. Previous research has focused on encoding and retrieval accounts of such age differences. For example, research demonstrates that older adults require more time exploring a spatial environment in order to encode/form a cognitive map (Iaria et al., 2009). In our study, we aimed to reduce the possible influence of an age-related encoding deficit by training all participants to criterion during route learning prior to entering the delay phase. This notwithstanding, we note that the older participants did make more errors during learning, and that comparable performance on the final trial of the route learning test need not imply comparable baseline memory. Age-related encoding deficits may have been detected less easily in the route memory test than in the subsequent cognitive map test due to differences in test resolution (error number vs degrees respectively) and/or the relative ease of the route memory test compared to the cognitive map test. Therefore, in the absence of a baseline cognitive map test, we cannot rule out the possibility that age-related encoding deficits contributed to the age effect in our cognitive map test, i.e. that older adults entered the delay phase with spatial memories that were already less specific, thus resulting in less accurate cognitive maps.
Previous cognitive map research in humans suggests that older adults present with a reduced ability to retrieve (i.e. access) a recently encoded cognitive map during subsequent navigation (Iaria et al., 2009). In keeping with this finding, research in rodents shows that older rodents present with deficits in the retrieval of recently encoded spatial memories (Neumeister et al., 2013), which can manifest as the retrieval of incorrect cognitive maps (i.e. competition for retrieval) (Barnes et al., 1997). Although our virtual environment was reflective of a town-like environment, it is unlikely that it was sufficiently similar to previously experienced real-world environments to lead to competition for retrieval. Furthermore, our between-subjects design rules out the possibility of competition for retrieval of experimental stimuli (i.e. between two similar virtual environments). It is therefore unlikely that increased retrieval interference in older adults can account for the negative effect of ageing in cognitive map test performance in our study. It is of course possible that a more general decline in retrieval ability was present in older adults and can account for the age effect. However, all participants performed near ceiling in the landmark free recall test and above chance in the cognitive map test, thus suggesting that older adults did not have any obvious deficit in retrieval that influenced cognitive map test performance, though a more subtle deficit may have contributed somewhat.
A hypothesis rarely considered is that the spatial navigation deficit in older adults, including cognitive mapping, may be explained, at least in part, by an age-related decline in the ability to consolidate and integrate new (spatial) memories. It is possible that as a consequence of increasing age, consolidation may change quantitatively, i.e. less consolidation-related activity (e.g. neural reactivation) may occur in older adults. Alternatively (or in addition), consolidation may change qualitatively, i.e. consolidation (e.g. neural reactivation) might be of a lower quality and/or is less efficient in older adults. This hypothesis is supported by sleep research in humans that demonstrates age-related changes in the quantity and quality of consolidation-related neural activity (e.g. sleep spindles and sharp wave ripples) (Harand et al., 2012). As a result, it is possible that a shortcoming in spatial memory consolidation/integration in older adults is responsible, at least in part, for the negative effect of age in our study.
4.3. Comparable rest-related benefits on cognitive map accuracy in young and older adults - cognitive basis
Interestingly, the magnitude of the rest benefit in cognitive map accuracy was comparable in young and older adults. This finding suggests that the consolidation/integration process in older adults does not become more susceptible to consolidation interference from ongoing sensory input (and associated encoding). If this were the case, a greater benefit of wakeful rest should have been observed in older than younger adults, as found in aMCI and AD patients who, relative to young/older adults, demonstrate a striking consolidation interference effect that can be reduced substantially by post-learning rest (Alber et al., 2014; Dewar et al., 2012b, 2009). Moreover, this finding suggests that rest-related consolidation boosts do not decline substantially in older age. If this were the case, a sizeable reduction in the benefit of wakeful rest should have been observed in older than in younger adults. Our finding of a comparable rest-related memory enhancement in young and older adults contrasts with the finding of age-related declines in neural activity associated with sleep-dependent memory consolidation (Harand et al., 2012). Further research should examine the effect of increasing age on consolidation at a behavioural and neural level, both under conditions of rest and sleep. We acknowledge that when the rest effect is viewed as a percentage (e.g. if the ‘task group’ mean = 45 degrees of error, and ‘rest group’ mean = 30 degrees of error, this would equate to a 33% ‘improvement’), the benefit of rest is slightly lower in older adults. Thus, our data suggest that wakeful rest benefits both older and younger subjects compared to a delay condition involving a perceptual task, but the relative magnitude of the wakeful rest effect could be somewhat different between the age groups.
5. Conclusion
In summary, we provide the first evidence that post-navigation rest promotes the integration of spatial memories into accurate cognitive maps in older adults. We also reveal that the magnitude of this rest-related enhancement does not change substantially as we grow older. However, older people performed more poorly than younger people on the cognitive map test overall. Although this effect could not be explained by increased consolidation interference in older adults, it is possible that a more general decline in the ability to consolidate and integrate new memories contributes to impoverished cognitive maps in older adults. Alternatively, it is possible that age-related reductions in cognitive mapping are the result of a deficit in initial encoding, or of a combination of age-related encoding and consolidation deficits. These hypotheses should be teased apart via future research.
Highlights.
Rest enhances spatial memory consolidation/integration in older adults
Rest-related enhancement in consolidation is comparable in young and older adults
Susceptibility to consolidation interference is not increased in older adults
Acknowledgements
Thanks go to all participants who gave their time to take part in our research. This research was funded (1) by the Alzheimer’s Society as part of a Ph.D. studentship awarded to Michaela Dewar and Sergio Della Sala and held by Michael Craig; Grant number 139, (2) by a Personal Research Fellowship awarded to Michaela Dewar by the Royal Society of Edinburgh and Lloyds/TSB Foundation for Scotland; Grant number 29400 R41255, and (3) an ERC Starting Grant awarded by the European Research Council to Thomas Wolbers; Grant number: 335090 (AGESPACE).
Footnotes
Disclosure statement
This research was approved by the Ethics Committee of the University of Magdeburg. All participants provided their informed consent in writing prior to taking part. The authors have no conflicts of interest to declare.
References
- Alber J, Della Sala S, Dewar M. Minimising interference with early consolidation boosts 7-day retention in amnesic patients. Neuropsychology. 2014;28:667–675. doi: 10.1037/neu0000091. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M, Della Sala S, Dewar M. Autobiographical thinking interferes with episodic memory consolidation. PLoS One. 2014;9:e93915. doi: 10.1371/journal.pone.0093915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M, Dewar M, Della Sala S, Wolbers T. Rest Boosts the Long-term Retention of Spatial Associative and Temporal Order Information. Hippocampus. 2015;25:1017–1027. doi: 10.1002/hipo.22424. [DOI] [PubMed] [Google Scholar]
- Craig M, Dewar M, Harris Ma, Della Sala S, Wolbers T. Wakeful rest promotes the integration of spatial memories into accurate cognitive maps. Hippocampus. 2016;26:185–193. doi: 10.1002/hipo.22502. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Dupret D. Sharp wave/ripple network oscillations and learning-associated hippocampal maps. Philos Trans R Soc Lond B Biol Sci. 2014;369:20120528. doi: 10.1098/rstb.2012.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M, Alber J, Butler C, Cowan N, Della Sala S. Brief Wakeful Resting Boosts New Memories Over the Long Term. Psychol Sci. 2012a;23:955–960. doi: 10.1177/0956797612441220. [DOI] [PubMed] [Google Scholar]
- Dewar M, Alber J, Cowan N, Della Sala S. Boosting Long-Term Memory via Wakeful Rest: Intentional Rehearsal Is Not Necessary, Consolidation Is Sufficient. PLoS One. 2014;9:e109542. doi: 10.1371/journal.pone.0109542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M, Garcia YF, Cowan N, Della Sala S. Delaying interference enhances memory consolidation in amnesic patients. Neuropsychology. 2009;23:627–634. doi: 10.1037/a0015568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M, Pesallaccia M, Cowan N, Provinciali L, Della Sala S. Insights into spared memory capacity in amnestic MCI and Alzheimer’s Disease via minimal interference. Brain Cogn. 2012b;78:189–199. doi: 10.1016/j.bandc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2011;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes Ca. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 1997;8:739–44. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- Girardeau M, Benchenane K, Wiener S, Buzsáki G, Zugaro M. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harand C, Bertran F, Doidy F, Guénolé F, Desgranges B, Eustache F, Rauchs G. How aging affects sleep-dependent memory consolidation? Front Neurol. 2012 Feb;:1–6. doi: 10.3389/fneur.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Wolbers T. How age-related strategy switching deficits affect wayfinding in complex environments. Neurobiol Aging. 2014;35:1095–102. doi: 10.1016/j.neurobiolaging.2013.10.086. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Richardson AE, Montello DR, Lovelace K, Subbiah I. Development of a self-report measure of environmental spatial ability. Intelligence. 2002;30:425–447. [Google Scholar]
- Iaria G, Palermo L, Committeri G, Barton JJS. Age differences in the formation and use of cognitive maps. Behav Brain Res. 2009;196:187–191. doi: 10.1016/j.bbr.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Montello DR. Spatial knowledge acquisition from direct experience in the environment: Individual differences in the development of metric knowledge and the integration of separately learned places. Cogn Psychol. 2006;52:93–129. doi: 10.1016/j.cogpsych.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Jackson JC, Johnson A, Redish AD. Hippocampal sharp waves and reactivation during awake states depend on repeated sequential experience. J Neurosci. 2006;26:12415–12426. doi: 10.1523/JNEUROSCI.4118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys H. Theory of probability. 3rd ed. Oxford University Press, Clarendon Press; Oxford: 1961. [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu I, Levy RM, Barton JJS, Iaria G. Age and gender differences in various topographical orientation strategies. Brain Res. 2011;1410:112–119. doi: 10.1016/j.brainres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34:504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbroek O, Petersson KM, Voermans N, Weber B, Fernández G. Age differences in neural correlates of route encoding and route recognition. Neuroimage. 2004;22:1503–1514. doi: 10.1016/j.neuroimage.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Moffat SD. Aging and spatial navigation: What do we know and where do we go? Neuropsychol Rev. 2009;19:478–489. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Effects of Age on Virtual Environment Place Navigation and Allocentric Cognitive Mapping. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiol Aging. 2001;22:787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Montello DR. A new framework for understanding the acquisition of spatial knowledge in large-scale environments. In: Egenhoger MJ, Golledge RG, editors. Spatial and Temporal Reasoning in Geographic Information Systems. Oxford University Press; New York: 1998. pp. 143–154. [Google Scholar]
- Montello DR, Xiao D. Linguistic and cultural universality of the concept of sense-of-direction. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics) LNCS. 2011;6899:264–282. [Google Scholar]
- Moser EI, Kropff E, Moser M-B. Place cells, grid cells, and the brain’s spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Neumeister KL, Lohmann P, Riepe MW. Dissociative decline of spatial learning and recall with aging in male CD-1 mice. 2013 Oct;:351–357. [Google Scholar]
- O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353:1333–40. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild Cognitive Impairment: Clinical Characterization and Outcome. JAMA Neurol. 1999;56:303–309. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rodgers MK, Sindone JA, Moffat SD. Effects of age on navigation strategy. Neurobiol Aging. 2012;33:202.e15–22. doi: 10.1016/j.neurobiolaging.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. 2007:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Alink A, Kriegeskorte N, Henson RN. Awake reactivation predicts memory in humans. Proc Natl Acad Sci. 2013;110:21159–21164. doi: 10.1073/pnas.1311989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding Wakefulness Levels from Typical fMRI Resting-State Data Reveals Reliable Drifts between Wakefulness and Sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M. What determines our navigational abilities? Trends Cogn Sci. 2010;14:138–46. doi: 10.1016/j.tics.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Weiller C, Büchel C. Neural foundations of emerging route knowledge in complex spatial environments. Brain Res Cogn Brain Res. 2004;21:401–11. doi: 10.1016/j.cogbrainres.2004.06.013. [DOI] [PubMed] [Google Scholar]