Abstract
Excoriation (skin-picking) disorder (SPD) shares symptomology with other obsessive-compulsive and related disorders. Few studies, however, have examined the neurological profile of patients with SPD. This study examined differences in cortical thickness and basal ganglia structural volumes between 20 individuals with SPD and 16 healthy controls using magnetic resonance imaging (MRI). There were no significant differences in demographic variables (age, gender, education and race) between groups. All subjects completed a structural MRI scan and completed a battery of clinical assessments focusing on SPD symptom severity, depression and anxiety symptoms, and quality of life. No statistically significant differences in basal ganglia (caudate, putamen, and nucleus accumbens) structural volumes were found between groups. In individuals with SPD, increasing impulsiveness correlated positively with increased cortical thickness in the left insula, and skin picking severity correlated negatively with cortical thickness in the left supramarginal gyrus and a region encompassing the right inferior parietal, right temporal and right supramarginal gyrus. This study suggests similarities and differences exist in symptomology between SPD and the other obsessive-compulsive and related disorders. Additional neuroimaging research is needed to better delineate the underlying neurobiology of SPD.
Keywords: Skin picking disorder, obsessive-compulsive, cortical thickness, structural volume, brain imaging
1. Introduction
Excoriation (skin-picking) disorder (SPD), also known as dermotillomania, was added to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) in the category of ‘Obsessive-Compulsive and Related Disorders’ in 2013 (American Psychiatric Association, 2013). SPD is regarded as a type of body focused repetitive behavior (BFRB), along with hair pulling (trichotillomania) and nail biting, and is characterized by excessive, repeated scratching or picking of the skin leading to tissue damage (Niemeir et al., 2015; Grant et. al., 2012; Arnold et. al., 1998; Odlaug and Grant, 2008). SPD has an estimated prevalence of 1.2-5.4% in the general population and appears to be more common in women (Monzani et. al., 2012; Hayes et. al., 2009; Keuthen et. al., 2010; Wilhelm et. al., 1999). Those with SPD report that the time spent picking can lead to social and work related problems (Flessner et. al., 2006).
Preliminary studies have explored the role of genetic and environmental influences in SPD pathophysiology. SPD is frequently comorbid with obsessive-compulsive disorder (OCD) (Grant & Stein, 2014; Torres et. al., 2016), body dysmorphic disorder (BDD) (Arnold et. al., 1998; Grant et. al., 2015; Phillips, 2005) and trichotillomania (Grant et. al., 2012), and one model proposes that these disorders share a latent liability factor conferring genetic risk to all obsessive-compulsive spectrum disorders (Monzani et. al., 2014). SPD has been found to be present across familial generations in case studies (Khumalo, et. al., 2016). Furthermore, a twin study found genetic factors to account for approximately 40% of variance in skin picking, supporting the hypothesis that genetic vulnerability may lead to more severe symptoms in some patients with SPD (Monzani, et. al., 2012).
A few studies have sought to examine possible brain abnormalities in people with SPD, in an effort to understand its neurobiology. In one study, Roos and colleagues used structural MRI imaging and found subjects with SPD to have increased ventral striatum volume, increased accumbens volume, reduced right hemisphere cortical thickness in the frontal areas, and increased bilateral thickness of the cuneus when compared to a control population and a trichotillomania group (Roos, et. al., 2015). A different study using diffusion tensor imaging found that SPD was associated with significantly reduced fractional anisotropy in white matter tracts, including the anterior cingulate cortices (Grant et. al., 2013). In a recent functional MRI (fMRI) study examining 18 patients with SPD compared to a sample of 15 healthy controls, Odlaug and colleagues found functional abnormalities in striatal circuitry and right medial frontal regions when presented with an executive planning task and neural regions associated with habit generation and inhibitory processing (Odlaug et al., 2016). In a prior fMRI study examining those with concurrent daily skin picking and Prader-Willi syndrome, Klabunde and colleagues found activation of introceptive, motor, attention and somatosensory processing during skin-picking episodes (Klabunde, et. al., 2015). Additionally, some studies examining cognitive dysfunction in individuals with SPD have found impaired stop-signal reaction times (i.e. impaired inhibitory control) but intact cognitive flexibility compared to controls (Odlaug, et. al., 2010; Grant et. al., 2011; Snorrason et. al., 2011).
Due to the limited data regarding the neurobiology of SPD, this study sought to examine specific structural differences in neuroanatomy between those with SPD and a healthy control group. Based on previous studies, we hypothesized that subjects with SPD would have reduced cortical thickness in frontal cortical regions and increased volumes in basal ganglia structures. Furthermore, we predicted that worse disease severity in SPD would be associated with higher striatum volumes and lower frontal cortical thickness.
2. Experimental Proceedings
2.1. Participants
Individuals between the ages of 18 and 65 with a primary current diagnosis of SPD (using criteria that would later be the basis for the DSM-5 criteria) were recruited via newspaper advertisements and referrals. Exclusion criteria included: unstable medical illness, history of seizures, lifetime bipolar disorder, dementia or psychotic disorder, substance disorder within the last 3 months, current suicide risk, and current pregnancy or inadequate contraception in women of child-bearing potential. A board certified psychiatrist specializing in obsessive-compulsive disorders assessed patients to confirm the diagnosis. Healthy control participants were recruited via posters, newspaper advertisements and word of mouth. Healthy controls had no lifetime or current diagnosis of a psychiatric disorder.
This was a two-site study with the same principal investigator and identical procedures at each site. Data were collected from November 2010 through April 2012 at the University of Minnesota. Data were collected from November 2014 through February 2015 at the University of Chicago. The institutional review boards at both universities approved this study. Participants provided written informed consent and received financial compensation. This study was carried out according to the principles of the Declaration of Helsinki (World Medical Association, 2013).
2.2. Clinical Variables
Severity of skin-picking symptoms was evaluated using the Yale-Brown Obsessive Compulsive Scale modified for Neurotic Excoriation (NE-YBOCS), and the Skin Picking Self Assessment Scale (SP-SAS) (Grant et al., 2007). The NE-YBOCS is a clinician-administered scale of skin picking severity, whereas the SP-SAS is a self-report measure. Both instruments assess severity of SPD symptoms over the past seven days and have total possible scores of 40 and 24 respectively, with higher scores equating to worse symptom severity.
Subjects completed the Quality of Life Inventory (QoLI), a self-administered survey examining the importance and satisfaction of the subject with various parts of their life (Frisch, 2013). Additionally, anxiety and depressive symptoms were evaluated using the Hamilton Anxiety Rating Scale (HAM-A) and the Hamilton Depression Rating Scale (HAM-D), respectively (Hamilton, 1959; Hamilton, 1960).
2.3. Data Analysis
Demographic and clinical variables were tabulated and compared between groups using independent t-tests and chi-squared tests as appropriate. Where model assumptions were violated, alternative non-parametric tests were used, as indicated in the text. Statistical tests used IBM SPSS software version 22.
MRI scans were acquired at the University of Chicago using a 3T Philips Achieva Quasar Dual 16 Ch system. MRI scans from the University of Minnesota were also acquired using a 3T machine. Scans comprised T1-weighted images obtained using a spoiled-gradient recall sequence with slice thickness of 2 mm, temporal resolution of 33 ms, echo time of 3 ms, field view of 24 cm, flip angle of 40 degrees, and matrix size of 256 x 256. Brain imaging MRI scans were processed using FreeSurfer v5.3 (www.freesurfer.net). FreeSurfer has been previously validated and explained in detail (Dale, et. al., 1999; Fischl, et. al., 1999; Fischl, et. al., 2000). Using automated algorithms, brain data were transformed to standard space, segmented, normalized, and smoothed using a standard 10mm kernel. Differences in regional cortical thickness were compared between SPD and controls in FreeSurfer (Qdec software) using a voxel-wise significance threshold of p<0.001 uncorrected (main effect of group). Potential differences in volumes of a priori structures of interest (caudate, putamen, nucleus accumbens) between the groups were explored using t-tests. These regions of interest were selected because they have been implicated in previous neuroimaging work of SPD or the related disorder, trichotillomania. Relationships between symptom severity and brain structure were explored using (i) QDec for cortical regions (voxel-wise threshold of p<0.001 uncorrected); and (ii) t-tests for the same a priori subcortical regions (p<0.05 uncorrected). Only regions with a significant correlation at p<0.001 are described by name.
3. Results
No significant differences existed between individuals with SPD (n=20) and healthy controls (n=16) on any demographic variable (Table 1). As expected, we found significant differences in HAM-A (p<0.001), HAM-D (p<0.001) and QoLI (p=0.002) scores between the two groups (Table 2). SPD participant scores on HAM-A and HAM-D, however, were well beneath threshold for clinically significant mood and anxiety disorders. There were no significant differences in cortical thickness between SPD and control groups at p<0.001 uncorrected; nor did the two groups differ significantly in terms of striatal volumes at p<0.01 uncorrected (Table 3).
Table 1.
Demographic variables in skin picking disorder (SPD) subjects and control populations
| SPD
Subjects (n=20) |
Controls (n=16) |
Statistic | p-value | |
|---|---|---|---|---|
| Age (mean, SD) | 29.50 (9.42) | 34.00 (15.58) | t=-1.02 | 0.32 |
| Gender (n,
%) Male Female |
0 (0%) 20 (100%) |
4 (25.0%) 12 (75.0%) |
χ2=3.38 | 0.07^ |
| Education (n,
%) Some college or less College graduate or more |
8 (44.44%) - 10 (55.56%) - |
4 (25.0%) 12 (75.0%) |
χ2=1.40 | 0.24 |
| Race (n,
%) Caucasian Latino/Hispanic Asian |
19 (95.0%) 0 (0%) 1 (5.0%) |
13 (81.3%) 2 (12.5%) 1 (6.3%) |
χ2=2.71 | 0.26 |
p-value with Yates correction
Education data missing for 2 subjects in the SPD group
Table 2.
Clinical measures in skin picking disorder (SPD) subjects and control populations
| SPD Subjects | Controls | Statistic (t) | p-value | |
|---|---|---|---|---|
| HAM-A (mean, SD) | 4.84 (3.11) | 0.21 (0.58) | 6.33 | <0.001*** |
| HAM-D (mean, SD) | 4.84 (2.79) | 0.50 (1.32) | 6.03 | <0.001*** |
| QoLI (mean, SD) | 33.45 (16.11) | 50.36 (10.54) | -3.70 | 0.002** |
| NE-YBOCS (mean, SD) | 21.20 (5.08) | - | - | - |
| SP-SAS (mean, SD) | 30.80 (5.10) | - | - | - |
HAM-A = Hamilton Anxiety Rating Scale: subjects (n=19), controls (n=14)
HAM-D = Hamilton Depression Rating Scale: subjects (n=19), controls (n=16)
QoLI = Quality of Life Inventory: subjects (n=20), controls (n=14)
NE-YBOCS = Yale Brown Obsessive Compulsive Scale Modified for Neurotic Excoriation: subjects (n=20), controls (n=0)
SP-SAS = Skin Picking Self-Assessment Scale: subjects (n=20), controls (n=0)
p<0.001,
p<0.01
Table 3.
Volume differences between skin picking disorder (SPD) subjects and control populations in a priori structures of interest.
| SPD
Subjects (n=20) |
Controls (n=16) |
Statistic (t) |
p-value | |
|---|---|---|---|---|
| Caudate (mean,
SD) Left Right |
3690.9 (536.5) 4093.4 (574.3) |
3597.6 (518.2) 3887.4 (538.1) |
0.53 1.10 |
0.60 0.28 |
| Putamen (mean,
SD) Left Right |
6431.8 (980.0) 6201.5 (920.8) |
6421.7 (838.2) 6256.4 (779.8) |
0.03 -0.19 |
0.97 0.85 |
| Nucleus accumbens (mean,
SD) Left Right |
806.6 (151.9) 710.8 (171.2) |
775.9 (163.3) 729.1 (137.6) |
0.58 -0.35 |
0.56 0.73 |
Two subjects were currently taking psychotropic medications, both of whom were taking stable doses for over a year. One subject was taking bupropion (450 mg/day), venlafaxine (225 mg/day), and trazodone (100 mg/prn), whereas the other was taking lamotrigine (300 mg/day), venlafaxine (375 mg/day) and trazodone (100 mg/prn). No one was receiving cognitive behavior therapy for SPD.
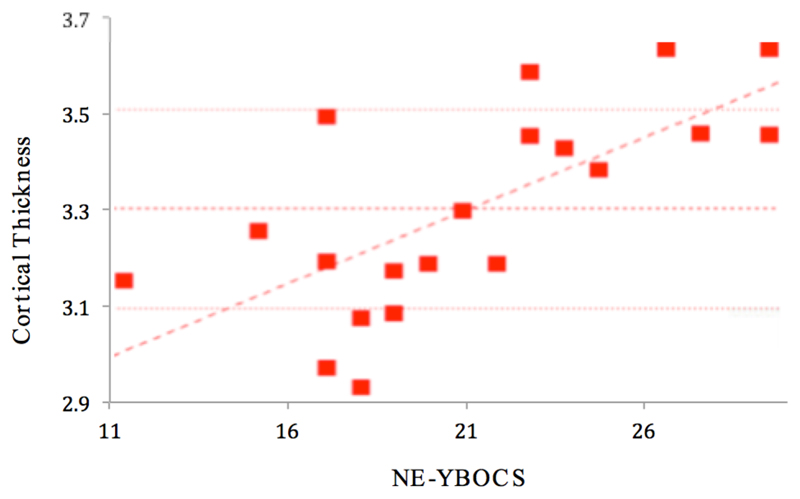
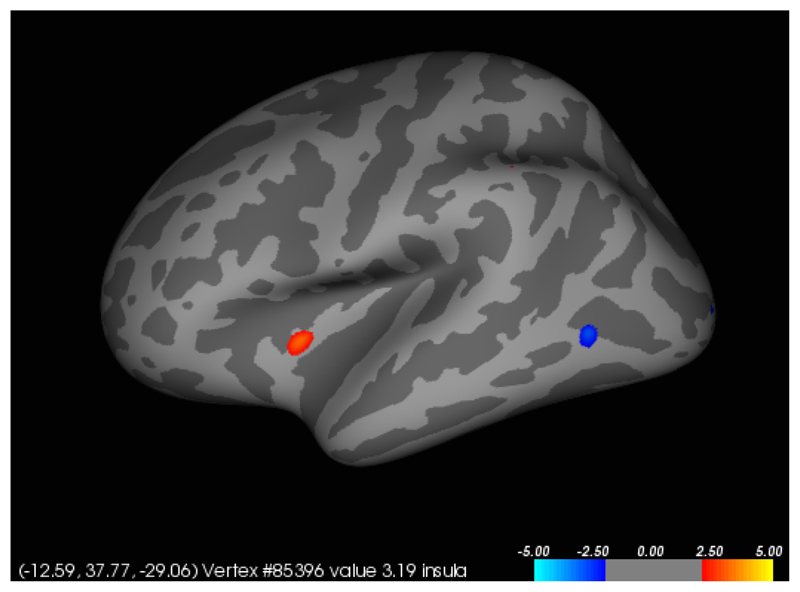
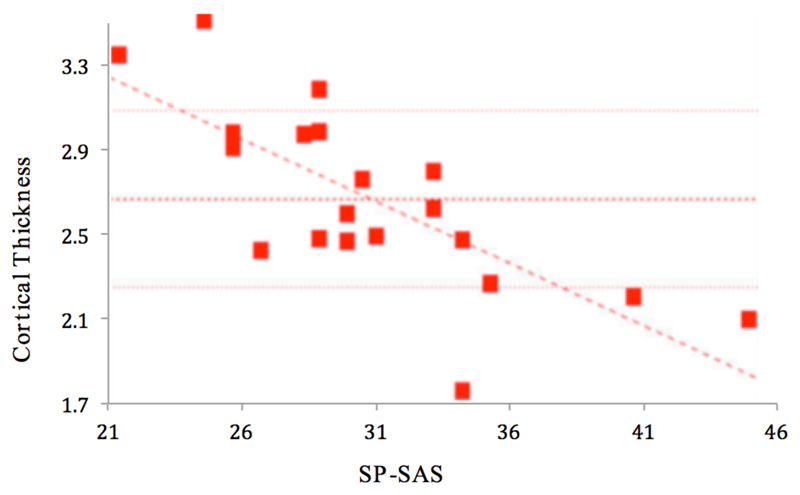
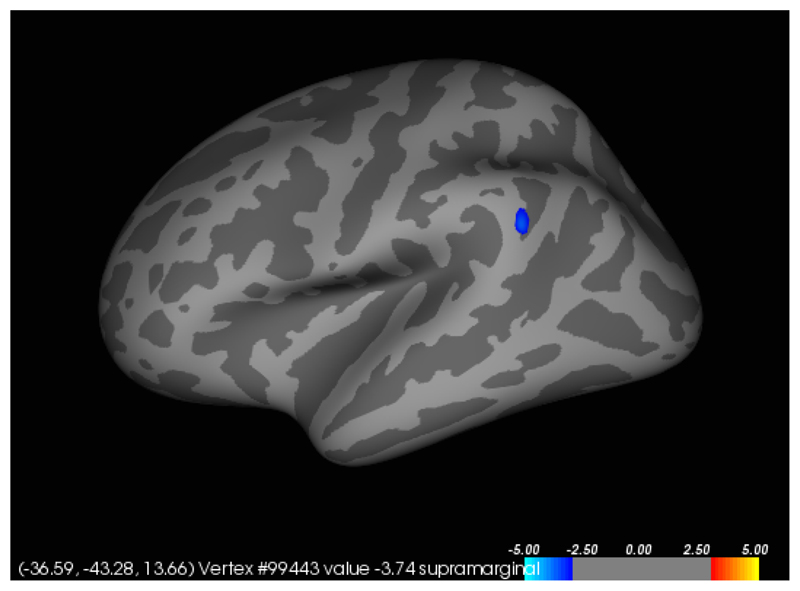
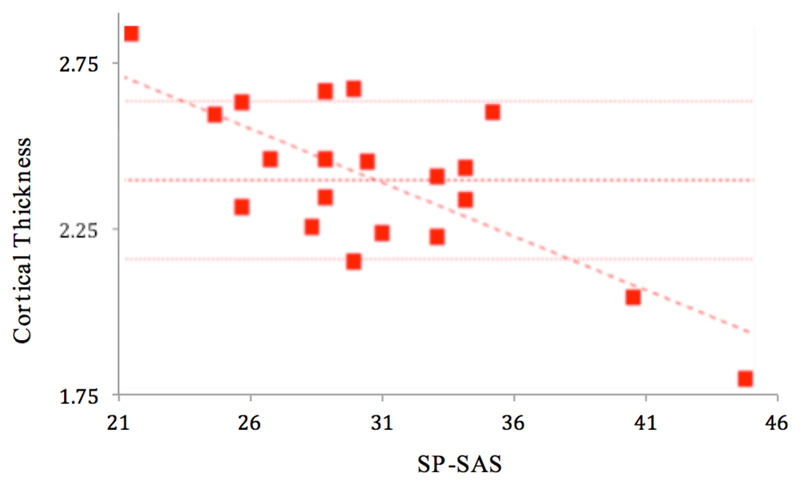
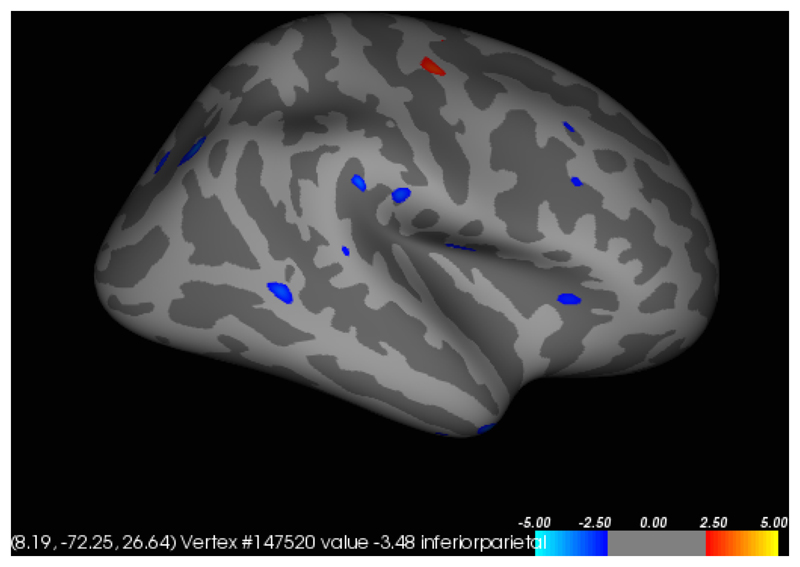
In participants with SPD, a significant (p<0.001) positive correlation was found between total NE-YBOCS score and cortical thickness in the left insula (Figures 1 and 2). A significant (p<0.001) negative correlation was found between total SP-SAS score and left supramarginal gyrus cortical thickness (Figures 3 and 4) and a significant (p<0.001) negative correlation was found between inferior parietal, right temporal and right supramarginal gyrus cortical thickness and SP-SAS score (Figures 5 and 6).
Figure 1.
Cortical thickness (mm) in left insula correlates significantly and positively with NE-YBOCS total scores.
Figure 2.
Liberal map at p<0.01 uncorrected for regions in which cortical thickness correlated with NE-YBOCS scores, in the left hemisphere. Higher NE-YBOCS symptom severity correlated significantly with greater left insula thickness at p<0.001 uncorrected (red). The posterior region of negative correlation (blue) was not significant at the p<0.001 threshold.
Figure 3.
Cortical thickness (mm) in left supramarginal gyrus correlates significantly and negatively with SP-SAS.
Figure 4.
Liberal map at p<0.01 uncorrected for regions in which cortical thickness correlated with SP-SAS scores, in the left hemisphere. Higher SP-SAS symptom severity correlated significantly with lower left supramarginal gyrus thickness at p<0.001 uncorrected (blue).
Figure 5.
Significant regions of negative correlation between right inferior parietal, right temporal and right supramarginal gyrus cortical thickness (in mm) and SP-SAS.
Figure 6.
Liberal map at p<0.01 uncorrected for regions in which cortical thickness correlated with SP-SAS, in the right hemisphere. Higher SP-SAS symptom severity correlated significantly and negatively (p<0.001 uncorrected) in regions including the right inferior parietal, right temporal and right supramarginal gyri (blue). The region of positive correlation was not significant at p<0.001.
4. Discussion
Although SPD has a long history in the medical literature, the neurobiology of the behavior has received relatively scant research attention. To our knowledge, this is one of only a few neuroimaging studies of individuals with SPD. Our study sought to expand upon the previously published Roos study in a larger sample size and across a broader range of outcome measures (Roos et al., 2015). Although we found no significant abnormalities in cortical thickness or volumes of select sub-cortical structures between people with SPD and healthy controls, we identified several significant relationships between cortical thickness and SPD symptom severity; results which suggest a possible correlation between cortical architecture and symptom manifestation.
This study found a negative correlation between cortical thickness in the supramarginal gyrus, the right inferior parietal cortex and the right temporal cortex and a self-report measure of skin picking severity (i.e. total SP-SAS scores). Decreased cortical thickness in each region was associated with higher SP-SAS scores, and this coincides with a diffusion tensor imaging study that found individuals with SPD to have dysfunction of the white matter tracts at the temporoparietal junction (Grant et al., 2013). The inferior parietal lobe has been shown to be involved in both simple and complex finger movements, suggesting it may play a role in the planning and initiation of motor action of the hand (Seitz, et. al.,1997; Jäncke, et al, 2001). Gross and colleagues found the inferior parietal cortex to be involved in both shape and object recognition (Gross et al., 1972). This suggests that a decrease in inferior parietal cortex thickness may alter the normal neuronal exchange between tactile perception and responding motor action, resulting in an abnormal feeling of satisfaction from the act of picking, or could also result in an abnormal response to pick one’s skin whenever a bump is felt. Additional research showing the parietal lobe’s role in connecting tactile identification and manipulation supports such an interpretation (Pause, et. al., 1989; Binkofski et. al., 1999). The posterior temporal sulcus activates in response to affective touch (Gordon et al., 2013). These findings suggest that altered structure and integrity of white matter tracts that connect temporal and parietal areas, which have each been shown to activate in response to various types of tactile stimulation, may contribute to SPD symptom severity.
Additionally, in individuals with SPD, this study found a positive correlation in NE-YBOCS score with increased cortical thickness in the left insula. This finding correlates with previous research that has shown insula activity to correlate directly with risk aversion in individuals with OCD (Luigjes et al., 2016). The finding of this study thus lends partial support for the inclusion of SPD as an ‘obsessive-compulsive related disorder’ in the DSM-5. However, another study found reduced insular activity to correlate with increased skin picking severity in a group of patients with Prader Willi Syndrome (Klabunde et al., 2015). Thus, despite their similarities relating to obsessive-compulsivity, the act of picking in SPD may have disparate pathophysiology compared to OCD. Likewise, other studies of OCD have reported different findings to this study including reduced cortical thickness and reduced caudate volume (Nakamae, et. al., 2012; Robinson, et. al., 1995). The lack of any significant group associations in this study between the SPD and control groups in NE-YBOCS scores and basal ganglia volume structures also may lend support to the conclusion that basal ganglia dysfunction does not play a role in SPD, contrary to the existing literature on OCD.
This study has several strengths, notably that it is one of only a few imaging studies in SPD and included participants with a range of SPD symptom severity. A few limitations, however, should be considered. First, these results come from a relatively small and homogeneous sample; patients were of average mild-to-moderate disease severity. Even though the SPD subjects and controls were both relatively evenly split between the two sites, the small sample size prevented an analysis of site effect. Future imaging studies of SPD should consider multi-site collaborations to increase sample size and demographic diversity. Further, as we recruited from advertisements, there is the possibility of selection bias. We do not yet know therefore whether these findings generalize to the SPD population more broadly. Although SPD participants were free from anxiety and depressive disorders, and scored well beneath threshold for clinically significant mood and anxiety symptoms, their scores on the HAM-A and HAM-D were significantly higher than controls. In Figure 2 we recognize that the insula is a deep structure, but was presented superimposed onto a cortical template as this is standard procedure for the software used. Finally, the conclusions made from imaging data in this study are correlative. Longitudinal imaging studies would be beneficial to the field and allow for a better establishment of the causal relationship between the neural findings and observed subject behavior.
The results of this study indicate that worse symptom severity in SPD is significantly related with decreased cortical thickness in the left supramarginal gyrus, increased cortical thickness in the left insula, and decreased cortical thickness in the right inferior parietal, right temporal and right supramarginal gyrus. However, this study found no differences in striatal or accumbens volumes between the SPD group and controls. Therefore, these data offer partial support for the view that sub-cortical structures contribute to the pathophysiology of SPD, both supporting its categorization as an OC-Related Disorder in DSM-5 and suggesting that there exists a pathophysiological difference between SPD and OCD. A better understanding of the neurological underpinnings of SPD will help guide the development of additional treatment options, especially in regard to pharmacological targets for intervention.
Footnotes
Conflicts of Interest:
Mr. Michael Harries, Ms. Sarah Redden and Mr. Austin Blum report no conflicts of interest. Dr. Samuel Chamberlain consults for Cambridge Cognition and Shire. He also receives funding from the Wellcome Trust Clinical Fellowship. Dr. Brian Odlaug has received research funding from the TLC Foundation for Body Focused Repetitive Behaviors and has received royalties from Oxford University Press and Johns Hopkins Press. He has consulted for and is currently employed by H. Lundbeck A/S. His contribution to this project concluded prior to his employment with H. Lundbeck A/S. Dr. Jon Grant currently has research grants from the National Center for Responsible Gaming, Brainsway, the American Foundation for Suicide Prevention, the TLC Foundation for Body Focused Repetitive Behaviors, Forest Takeda and Psyadon Pharmaceuticals. He receives yearly compensation from Springer Publishing for acting as Editor-in-Chief of the Journal of Gambling Studies and has received royalties from Oxford University Press, Johns Hopkins Press, American Psychiatric Publishing, Inc., Norton Press, and McGraw Hill.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed) 2013 [Google Scholar]
- Arnold LM, McElroy SL, Mutasim DF, Dwight MM, Lamerson CL, Morris EM. Characteristics of 34 adults with psychogenic excoriation. J Clin Psychiatry. 1998;59:509–514. doi: 10.4088/jcp.v59n1003. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Dohle C, Seitz RJ, Freund HJ. Mirror agnosia and mirror ataxia constitute different parietal lobe disorders. Ann Neurol. 1999;46:51–61. doi: 10.1002/1531-8249(199907)46:1<51::aid-ana9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999:79–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-basedanalysis. II. Inflation, flattening and a surface-based coordinate system. Neuroimage. 1999:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flessner CA, Woods DW. Phenomenological characteristics, social problems, and the economic impact associated with chronic skin picking. Behav Modif. 2006;30:944–63. doi: 10.1177/0145445506294083. [DOI] [PubMed] [Google Scholar]
- Frisch MB. Evidence-based well-being/positive psychology assessment and intervention with quality of life therapy and coaching and the quality of life inventory (QOLI) Soc Indic Res. 2013;114:193–227. [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD. Brain mechanisms for processing affective touch. Hum Brain Mapp. 2013;34(4):914–22. doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. Lamotrigine treatment of pathological skin picking: an open-label study. J Clin Psychiatry. 2007;68(9):1384–91. doi: 10.4088/jcp.v68n0909. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR. A cognitive comparison of pathological skin picking and trichotillomania. J Psychiatr Res. 2011;45(12):1634–8. doi: 10.1016/j.jpsychires.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR, Keuthen NJ, Lochner C, Stein DJ. Skin picking disorder. Am J Psychiatry. 2012;169(11):1143–9. doi: 10.1176/appi.ajp.2012.12040508. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Hampshire A, Schreiber LR, Chamberlain SR. White matter abnormalities in skin picking disorder; a diffusion tensor imaging study. Neuropsychopharmacology. 2013;38(5):763–9. doi: 10.1038/npp.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Stein DJ. Body-focused repetitive behavior disorders in ICD-11. Revista Brasileira de Psiquiatria. 2014;36(Suppl. 1):59–64. doi: 10.1590/1516-4446-2013-1228. [DOI] [PubMed] [Google Scholar]
- Grant JE, Redden SA, Leppink EW, Odlaug BL. Skin picking disorder with co-occurring body dysmorphic disorder. Body Image. 2015;15:44–8. doi: 10.1016/j.bodyim.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Brit J Med Psycho. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SL, Storch EA, Berlanga L. Skin picking behaviors: an examination of the prevalence and severity in a community sample. J Anxiety Disord. 2009;23:314–319. doi: 10.1016/j.janxdis.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ. The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex. 2001;11(2):114–21. doi: 10.1093/cercor/11.2.114. [DOI] [PubMed] [Google Scholar]
- Keuthen NJ, Koran LM, Aboujaoude E, Large MD, Serpe RT. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183–186. doi: 10.1016/j.comppsych.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Khumalo NP, Shaboodien G, Hemmings SMJ, Moolman-Smook JC, Stein DJ. Pathologic grooming (acne excoriee, trichotillomania, and nail biting) in 4 generations of a single family. JAAD Case Rep. 2016;2(1):51–53. doi: 10.1016/j.jdcr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde M, Saggar M, Hustyi KM, Hammond JL, Reiss AL, Hall SS. Neural correlates of self-injurious behavior in Prader-Willi syndrome. Hum Brain Mapp. 2015;36(10):4135–43. doi: 10.1002/hbm.22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, Figee M, Tobler PN, van den Brink W, de Kwaasteniet B, van Wingen G, Denys D. Doubt in the Insula: Risk Processing in Obsessive-Compulsive Disorder. Front Hum Neurosci. 2016;10:283. doi: 10.3389/fnhum.2016.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani B, Rijsdijk F, Cherkas L, Harris J, Keuthen N, Mataix-Cols D. Prevalence and heritability of skin picking in an adult community sample: A twin study. Am J Med Genet. 2012;159B:605–610. doi: 10.1002/ajmg.b.32067. [DOI] [PubMed] [Google Scholar]
- Monzani B, Rijsdijk F, Harris J, Mataix-Cols D. The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry. 2014;71(2):182–9. doi: 10.1001/jamapsychiatry.2013.3524. [DOI] [PubMed] [Google Scholar]
- Nakamae T, Narumoto J, Sakai Y, Nishida S, Yamada K, Kubota M, Miyata J, Fukui K. Reduced cortical thickness in non-medicated patients with obsessive-compulsive disorder. Prog Neuropsychomaracol Biol Psychiatry. 2012;37(1):90–5. doi: 10.1016/j.pnpbp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Niemeier V, Peters E, Gieler U. Hautarzt. 2015;66:781. doi: 10.1007/s00105-015-3685-y. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Grant JE. Clinical characteristics and medical complications of pathologic skin picking. Gen Hosp Psychiatry. 2008;30(1):61–6. doi: 10.1016/j.genhosppsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Chamberlain SR, Grant JE. Motor inhibition and cognitive flexibility in pathological skin picking. Prog Neuropsychophamarcol Biol Psychiatry. 2010;34(1):208–11. doi: 10.1016/j.pnpbp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Hampshire A, Chamberlain SR, Grant JE. Abnormal brain activation in excoriation (skin-picking) disorder: evidence from an executive planning fMRI study. Br J Psychiatry. 2016;34(1):208–11. doi: 10.1192/bjp.bp.114.155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause M, Kunesch E, Binkofski F, Freund HJ. Sensorimotor disturbances in patients with lesions of the parietal cortex. Brain. 1989;112:1599–1625. doi: 10.1093/brain/112.6.1599. [DOI] [PubMed] [Google Scholar]
- Phillips KA. The Broken Mirror: Understanding and Treating Body Dysmorphic Disorder. New York: Oxford University Press; 2005. [Google Scholar]
- Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, Koreen A, Cole K, Bogerts B. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- Roos A, Grant JE, Fouche JP, Stein DJ, Lochner C. A comparison of brain volume and cortical thickness in excoriation (skin picking) disorder and trichotillomania (hair pulling disorder) in women. Behav Brain Res. 2015;279:255–8. doi: 10.1016/j.bbr.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Canavan AG, Yaguez L, Herzog H, Tellmann L, Knorr U, Huang Y, Homberg V. Representations of graphomotor trajectories in the human parietal cortex: evidence for controlled processing and automatic performance. Eur J Neurosci. 1997;9:378–389. doi: 10.1111/j.1460-9568.1997.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Snorrason Í, Smári J, Ólafsson RP. Motor inhibition, reflection impulsivity, and trait impulsivity in pathological skin picking. Behav Ther. 2011;42(3):521–32. doi: 10.1016/j.beth.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Torres AR, Fontenelle LF, Shavitt RG, Ferrão YA, de Rosário MC, Storch EA, Miguel EC. Comorbidity variation in patients with obsessive–compulsive disorder according to symptom dimensions: Results from a large multicentre clinical sample. J Affect Disord. 2016;190:508–516. doi: 10.1016/j.jad.2015.10.051. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Keuthen NJ, Deckersbach T, Engelhard IM, Forker AE, Baer L, O’Sullivan R, Jenike MA. Self-injurious skin picking: Clinical characteristics and comorbidity. J Clin Psychiatry. 1999;60:454. doi: 10.4088/jcp.v60n0707. [DOI] [PubMed] [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]