Abstract
Natural killer (NK) cells of the innate immune system and natural killer T (NKT) cells, which have roles in both the innate and adaptive responses, are unique lymphocyte subsets that have similarities in their functions and phenotypes. Both cell types can rapidly respond to the presence of tumor cells and participate in immune surveillance and anti-tumor immune responses. This has incited interest in the development of novel cancer therapeutics based on NK and NKT cell manipulation. Chimeric antigen receptors (CARs), generated through fusion of an antigen-binding region of a monoclonal antibody or other ligand to intracellular signaling domains, can enhance lymphocyte targeting and activation toward diverse malignancies. The majority of CAR studies have focused on their expression in T cells, however, functional heterogeneity of CAR T cells limits their therapeutic potential and is associated with toxicity. CAR-modified NK and NKT cells are becoming more prevalent because they provide a method to direct these cells more specifically to target cancer cells, with less risk of adverse effects. This review will outline current NK and NKT cell CAR constructs and how they compare to conventional CAR T cells, and discuss future modifications that can be explored to advance adoptive cell transfer of NK and NKT cells.
INTRODUCTION
Adoptive cell transfer (ACT) refers to the ex vivo stimulation and expansion of autologous or allogeneic lymphocytes, followed by reinfusion of the expanded lymphocyte population back into the patient. ACT of tumor specific T cells has demonstrated great clinical success for the treatment of cancer; however, preexisting tumor reactive cells are difficult to identify in non-melanoma malignancies. Efforts to engineer T cells with enhanced tumor specificity is an area of intense research. One approach has been to engineer T cells to express chimeric antigen receptors (CARs), artificial receptors that can redirect T cells to tumor targets. CAR therapy has shown great promise in recent years for hematological malignancies and has an emerging role against solid tumors. In general, CARs are composed of an extracellular single chain variable fragment (scFv) of an antibody for antigen binding linked to one or more intracellular signaling domains. CARs have been classified by the differences in the intracellular signaling domains. First-generation CARs consisted of scFv and the T cell receptor CD3ζ chain without the presence of any co-stimulatory domains. Second generation CARs included a co-stimulatory molecule, such as CD28 and 4-1BB, in the intracellular domain (1, 2), which greatly enhanced expansion and persistence of T cell activation (3). The third generation included two co-stimulatory molecules which also enhanced activation, proliferation, and survival of T cells, thereby improving efficacy (4). Although CAR T cell-based therapies are revolutionizing adoptive cell immunotherapy, a significant obstacle with this approach is the need to isolate and use autologous cells. Moreover, T cells have been shown to persist for months up to years after infusion (5) which may result in chronic on-target-off-tumor effects such as B cell aplasia with the anti-CD19 CARs being used currently in clinical trials (6, 7). There are also significant toxicity-related safety concerns for the use of polyclonal T cells for CAR therapy (8). A common complication is the development of cytokine release syndrome (CRS) which refers to the production of several pro-inflammatory cytokines, such as IFN-γ, TNFα, and IL-6, resulting from the large number of activated lymphocytes mediating tumor cell death (9). Although several avenues are being explored to limit CAR T cell therapy toxicity, an alternative approach would be to use other cell populations, such as natural killer (NK) and natural killer T (NKT) cells, which have potent anti-tumor activity and documented roles in tumor immunosurveillance, as well as characteristics that could make them more effective than autologous T cells. In this review, we describe some of the most recent and promising advances in CAR-engineered NK and NKT cells as well as new technologies that may be applicable for NK and NKT cells in the future.
NK cell biology
NK cells are effector lymphocytes of the innate immune system that are part of the first line of defense that protects the body from pathogen invasion and malignant transformation. In contrast to T lymphocytes, NK cells do not express antigen specific receptors, rather their effector function is determined by signals received through germ-line-encoded receptors that can recognize ligands on their cellular targets. They are characterized by the lack of T cell receptor (TCR) and by expression of CD16 (FcγRIII) and CD56 surface antigens. The majority of NK cells in the circulation are CD56 dim, and are characterized by their ability to mediate cytotoxicity (10, 11). NK cells that reside in lymphoid organs are CD56 bright, are considered more immature, but have a greater capability to secrete and respond to cytokines (10, 12). NK cells are also distinguished by their differential expression of CD16, which binds the Fc portion of immunoglobulin G1 and mediates antibody-dependent cellular cytotoxicity (ADCC) by NK cells. CD16 is expressed highly in CD56 dim NK cells while CD56 bright NK cells are CD16 dim or negative (12). NK cell function, including cytotoxicity and cytokine release, is governed by a balance between signals received from inhibitory and activating receptors. NK cells express inhibitory receptors for molecules of major histocompatability complex (MHC) class I, namely Ly49 receptors in mice, killer immunoglobulin-like receptors (KIRs) in humans, and the CD94-heterodimeric C-type lectin receptor NKG2A heterodimer in both species. Binding of self MHC class I is proposed as a major mechanism for the tolerance of NK cells to self-tissue, and engagement of self MHC class I by developing NK cells allows their “licensing” (13). Activating receptors include the natural cytotoxicity receptors (NCRs) NKp46, NKp30, NKp44, and the C-type lectin-like activating immunoreceptor NKG2D (10). These receptors activate signaling adapter proteins which contain immunoreceptor tyrosine-based activation motifs that initiate the release of perforin and granzymes, and control production and release of cytokines and chemokines (14). NK cells also express several activating receptors that are potentially specific for self-molecules. For example, KIR2DS1 has been reported to interact with group 2 human leukocyte antigen (HLA)-C2 molecules, and KIR2DS2 was shown to recognize HLA-A*11 (15). Therefore, mechanisms to prevent activation against normal healthy tissues is required. When engaged by HLA class 1 molecules, KIR receptor signaling blocks NK effector responses, resulting in NK cell “tolerance to self”. The presence of these receptors suggest that NK cells are constantly surveying tissues for normal levels of the ubiquitously expressed MHC class I molecules (16). In transformed or infected cells, surface expression of MHC class I is often reduced or lost to evade anti-tumor T cell recognition. Therefore, when a mature NK cell encounters cells lacking MHC class I, their inhibitory receptors are not engaged, allowing the activating signals to prompt cytokine secretion and target cell death (11). Cellular stress and DNA damage can also upregulate “stress ligands”, such as MHC class I chain-related gene MICA/B, which can be recognized by activating NK receptors (17). Figure 1 outlines the role of activating and inhibitory receptor expression on target cells in NK cell recognition and activation.
Figure 1.
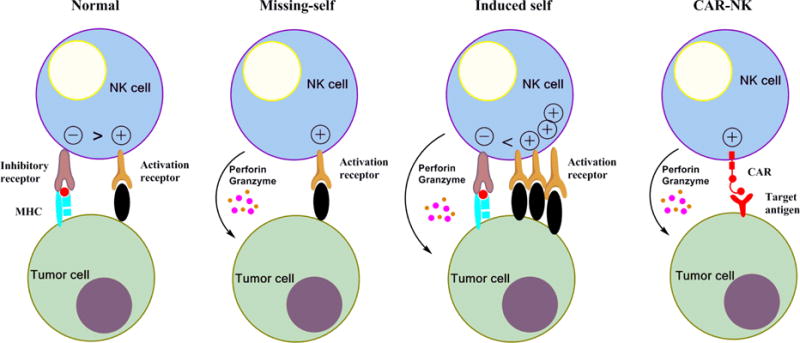
Enhancement of NK-cell antitumor activity by the expression of chimeric antigen receptors. The natural cytotoxicity of natural killer (NK) cells is regulated by signals from stimulatory and inhibitory receptors. Under normal conditions, signaling from inhibitory receptors for MHC-I overpower stimulation through activation receptors. When cells lack or downregulate MHC-1 expression, activation receptors signal NK cytoxicity. Activation against self can occur when the target cell is stressed and upregulates ligands for activation receptors, thereby overcoming MHC class I inhibitory signaling. The expression of a chimeric antigen receptor (CAR) specific for tumor-associated cell surface antigens efficiently redirects NK cells to malignant cells, and facilitates their cytolytic activity independently from the activation of endogenous stimulatory receptors.
NK cells for cancer therapy
Over the past decade, adoptive transfer of ex vivo-activated or -expanded allogeneic NK cells has emerged as a promising immunotherapeutic strategy for cancer. Autologous or allogeneic NK cells are typically obtained from donor peripheral blood but can also be derived from bone marrow or umbilical-cord blood. Human embryonic stem cells or induced pluripotent stem cells (iPSC) are also under investigation as alternative NK cell sources (18). Because naïve NK cells exhibit limited cytotoxic activity and only a small percentage of NK cells circulate in the blood, methods for clinical grade purification and expansion of donor NK cells from peripheral blood have been established to obtain large numbers of cells with full anti-tumor functions (19–21). In general, magnetic depletion of T cells is performed first to enrich for NK cells. T cell depletion can also be used in combination with positive selection of CD56 positive cells, however CD56 selection has been shown to result in fewer NK cells (22). NK cells are then expanded in culture for 1–3 weeks in the presence of IL-2 with or without feeder cells (such as K562) that are modified to express co-stimulatory molecules or cytokines. Unlike conventional T cells, NK cells are able to kill infected or malignant cells without prior sensitization and without the need for HLA matching (23). NK cells can directly kill tumor cells through several mechanisms, including ADCC (10), release of cytoplasmic granules containing perforin and granzyme (24), expression of tumor necrosis factor (TNF) family members, such as FasL or TNF-related apoptosis-inducing ligand (TRAIL), which induce tumor cell apoptosis by interacting with their respective receptors Fas and TRAIL receptor (25). For autologous transfer, NK cells isolated from the patient are activated and expanded in vitro in the presence of cytokines. The NK cells are then reinfused back into the patient, typically along with exogenous cytokine to help sustain expansion and function of the NK cells. Although autologous NK cells might recognize activating signals such as stress molecules on cancer cells, their anti-tumor activity is limited by the inhibitory signal transmitted by self-HLA molecules. For allogeneic transfer, NK cells are obtained from HLA-matched or haploidentical donors. Donor T cells are removed since they may cause graft versus host disease (GVHD) if infused (26). Allogeneic NK cells are advantageous because, unlike autologous cells, they are less likely to be inhibited as a result of NK cell recognition of self-MHC molecules. The most optimal responses are obtained when haploidentical donors do not express KIRs that recognize the patient’s HLA molecules, since tumor cells lack the appropriate MHC class I ligands to engage inhibitory KIRs and are thus eliminated by the alloreactive NK cells. Transfer of allogeneic NK cells are also advantageous for therapy because they can prevent GVHD through elimination of host antigen-presenting cells (27).
CAR NK primary cells
NK cells have gained significant interest as a potential “off the shelf”, allogeneic cell product for CAR therapy (28). Both primary human NK cells and NK cell lines have been investigated for CAR therapy, each with advantages and disadvantages. Activated primary human NK cells express a wider range of activating receptors and KIRs than NK cell lines, which are important for NK cell licensing (29). In contrast to NK cell lines which are transformed and must be irradiated prior to patient administration, primary NK cells do not require irradiation and are therefore able to expand in vivo, a quality which has been correlated with effectiveness in trials involving acute myeloid leukemia (AML) (30). Pre-clinical data has been reported for CAR-modified human primary NK cells re-directed against CD19 (31, 32), CD20(33), CD244 (34), and HER2 (35). Chang et al. constructed a CAR composed of the NK cell activating molecule, NKG2D, along with two key signaling molecules, DAP10 and CD3ζ, They found that expression of this CAR could significantly enhance the cytotoxicity of activated NK cells against leukemias and solid tumors, and that cytotoxicity was induced through direct engagement of NKG2D without off target effects, as increased cytotoxicity was not achieved when tested with non-transformed cells or cells with little or no NKG2D ligand expression (36).
Primary NK cells engineered to express CARs present several advantages over their T cell counterparts. While T lymphocytes only kill their targets by a CAR-specific mechanism, NK cells have spontaneous cytotoxic activity and can trigger target cell death independent of tumor antigen. Therefore, in the event of antigen downregulation by tumor cells attempting to evade immune detection, NK cells would still be effective against tumor cells, whereas CAR T cell function would be hindered. In addition, ex vivo expanded primary human NK cells produce cytokines, such as IFN-γ, IL-3, and granulocyte macrophage colony stimulating factor (GM-CSF), that differ from the proinflammatory cytokines produced by T cells and associated with the onset of CRS. (28, 37). It is also known that individual NK cells can survive after making contact with and killing multiple target cells (38), potentially reducing the number of cells that need to be adoptively transferred compared to T cells. Furthermore, whereas the long-term persistence of CAR T cells may maintain on-target off-tumor toxicity, such as B cell aplasia seen with anti-CD19 CAR T cells, mature NK cells are short lived, and are expected to disappear rapidly after mediating their anti-cancer effects (39). Therefore, suicide genes may not be required to attenuate toxicity related side effects (28). An exception to this would be NK cells isolated from cord blood or iPSC, which are more immature and would persist longer in the patient. While this may improve anti-tumor effects, there would be an increased risk of adverse effects that may require safety measures. Despite such advantages, primary cells are difficult to isolate and expand from donor peripheral blood and the yield is variable depending on the donor. Therefore, NK cell lines have become attractive for CAR NK cell therapy.
CAR expression on NK cell lines
The most commonly used human NK cell line is NK-92, a transformed cell line of activated NK cells that is easy to transduce and expand. NK-92 cells are cytotoxic against diverse malignances and infusions have been shown to be safe and well tolerated in cancer patients (40). Unlike primary NK cells, NK cell lines have a more homogenous, well-defined population that do not require isolation from donors. The NK-92 cell line is characterized by the expression of CD56 and CD2, and the absence of CD3, CD8, and CD16. NK-92 cells also lack expression of most KIRs (41). Other NK cell lines that have been explored for allogeneic NK cell therapy include NKG, YT, NK-YS, HANK-1, YTS cells, and NKL cells, which also lack CD16 expression (42, 43). Most of the studies for CAR-modified NK-92 cells have used first generation CARs that only have a CD3ζ intracellular signaling domain. Several antigens have been targeted by these first generation CAR-NK cells, including CD19 and CD20 for B cell lymphoma (44–46), ErbB2 for breast, ovarian, and squamous cell carcinoma (47–49), GD2 for neuroblastoma (50), and CD138 for multiple myeloma (51). Second generation CAR-NK cells from the NK-92 line have also been created for several antigens, including EpCAM for multiple carcinomas (52), HLA-A2 EBNA3 complex for Epstein–Barr virus (53), CS1 for multiple myeloma (54), and ErbB2 for HER2 positive epithelial cancers (48, 49). The most common intracellular costimulatory domain used alongside CD3ζ in second generation NK-92 CARs is CD28. However, the potential effect of the CD28 domain is unclear since NK cells do not naturally express CD28 (55). Additional second generation CARs have incorporated the 4-1BB intracellular signaling domain along with CD3ζ to improve NK cell persistence. Schonfeld et al. compared functionality of different intracellular domains using an ErbB2 scFv fused with CD3ζ alone, CD28 and CD3ζ, or 4-1BB and CD3ζ tested against breast cancer cells. They found that both of the second generation constructs improved killing compared to the first generation CARs and the CD28 and CD3ζ had 65% target lysis, the 4-1BB and CD3ζ lysed 62%, and CD3ζ alone killed 51% of targets (49). 4-1BB and CD28 intracellular domains were also compared in a recent study using anti-CD19 CARs expressed on NK-92 cells for B cell malignances. Oelsner et al. found that CD3ζ/4-1BB constructs were less effective than CD3ζ/CD28 in cell killing and cytokine production, highlighting differential effects of CD28 and 4-1BB costimulatory domains (56). A third generation NK-92 CAR comprised of anti-CD5 scFv with CD3ζ, CD28, and 4-1BB intracellular signaling domains demonstrated specific and potent anti-tumor activity against a variety of T-cell leukemia and lymphoma cell lines and primary tumor cells, and was able inhibit disease progression in xenograft mouse models of T cell Acute lymphoblastic leukemia (ALL) cell lines as well as primary tumor cells (57). A significant obstacle for the efficacy of CAR NK therapy is overcoming the immunosuppressive tumor microenvironment (TME). One of the major immunosuppressive cytokines present in the TME is transforming growth factor-β (TGF-β), which has been shown to inhibit NK cells, and plays a role in tumor initiation and progression (58, 59). One group has used TGF-β expression as an advantage for CAR NK cells by transducing NK-92 cells with a CAR that has the TGF-β type II receptor extracellular and transmembrane domains, and the intracellular domain of NKG2D, resulting in NK cell activation upon binding of TGF-β. These modified NK-92 cells displayed increased cytotoxicity against tumor cells, increased IFN-γ secretion, and enhanced migration to TGF-β producing cells in vitro. Moreover, when adoptively transferred, TGF-betaR II expression slightly enhanced the anti-tumor effects of NK-92 cells in a xenograft model of hepatocellular carcinoma (60).
CAR NK cells in clinical trials
In contrast to the numerous clinical studies using CAR T cells for cancer treatment, relatively few CAR NK cell clinical studies have received regulatory approval. One is a study being conducted at St. Jude Children’s Research Hospital using haploidentical NK cells modified with anti-CD19 CARs for the treatment of B lineage ALL (NCT00995137). For this study, NK cells from donors were expanded by co-culture with K562 feeder cells that have membrane-bound IL-15 or 4-1BB ligand. Another study for refractory ALL being conducted at the National University Hospital in Singapore (NCT01974479) is using haploidentical NK cells activated with IL-2 in culture followed by transduction with an anti-CD19 CAR (61). Four additional trials using CAR NK cells began recruiting in 2016. Three of the studies are being conducted by PersonGen BioTherapeutics in Suzhou, China and employ NK-92 cells engineered with CARs against either CD7, CD33, or CD19, attached to CD3ζ, CD28, and 4-1BB signaling domains for the treatment of patients with CD7+ relapsed or refractory leukemia and lymphoma (NCT02742727), relapsed CD33+ Acute myeloid leukemia (AML) (NCT02944162), and relapsed CD19+ leukemia and lymphoma, respectively. PersonGen BioTherapeutics is also testing an anti-MUC1 CAR-modified NK cells in patients with MUC1+ relapsed or refractory solid tumors (NCT02839954).
NKT cell biology
NKT cells constitute a small subset of lymphocytes which are characterized by the expression of NK cell lineage markers as well as αβ T-cell receptors (TCR). NKT cells develop in the thymus and arise from the same common lymphoid precursors as conventional T cells, but NKT possess phenotypic and functional characteristics that set them apart from conventional T cells. (62). After αβ T cell lineage commitment and the generation of double-positive thymocytes, the NKT cell development pathway diverges from that of conventional T cells. (63, 64). NKT cell precursors are selected following rearrangement of the TCR α-chain gene. In contrast to highly polymorphic TCRs, the TCR repertoire expressed by a major subset of NKT cells is highly invariant—a canonical Vα24- Jα18 chain rearrangement-associated with a single Vβ11 chain in humans (65). NKT cells are divided into 3 major subsets in human peripheral blood, namely CD4+, CD8+, and CD4/CD8 double negative (DN), which differ in their cytokine secretion profile and expression of chemokine receptors, integrins, and NK receptors (66,67). Unlike conventional T cells that recognize peptide antigens presented by MHC class I and II molecules, NKT cells recognize glycolipid antigens presented by CD1d molecules (68), MHC-like molecules that are constitutively expressed by antigen presenting cells such as dendritic cells (DC), B cells, and macrophages (69). To date, the most well-characterized activating glycolipid antigen recognized by NKT cells is α-galactosylceramide (α-GalCer) discovered initially in bacterial infected marine sponges (70) α-GalCer shows a strong affinity for CD1d molecules in both humans and mice. NKT cells are unique because they have the ability to respond both as innate cells, with minimal TCR involvement, and as memory-like cells through the engagement of their semi-invariant TCR. In this way, they are able to bridge the innate and adaptive immune responses. Activation of NKT cells is accompanied by the rapid and robust production of both T-helper 1 and T-helper 2 cytokines (71). Similar to conventional T cells, this requires engagement of costimulatory molecules such as CD40:CD40L and B7:CD28 pathways (72, 73). Both CD4+ and DN NKT cells can produce Th1 cytokines, but the production of Th2 cytokines, such as IL-13 and IL-4, is exclusive to CD4+ NKT cells (66). CD4+ NKT cells are therefore considered helper/regulatory cells while DN NKT cells are considered effector cells.
NKT cells and cancer
NKT cells are of particular interest for ACT because NKT cell infiltration of primary tumors is associated with better outcomes in diverse tumors (74, 75). Several studies have reported that donor-derived NKT cells may suppress GVHD while still maintaining anti-tumor function (76, 77). In agreement with these reports, recent studies demonstrated that reconstitution of NKT cells in peripheral blood is associated with long-term remission of pediatric leukemia patients receiving haploidentical transplantation (78–80). Moreover, NKT cells have been shown to co-localize with tumor-associated macrophages (TAMs) and can kill or inhibit these growth-promoting cells in a CD1d-dependent manner (81). TAMs are known to be major producers of IL-6 that promotes proliferation of many solid tumors, including neuroblastoma, breast, and prostate carcinomas (82). Based on the initial successes in preclinical studies that demonstrate the potent antitumor activity of NKT cells, intense efforts have been made in the last decade to initiate NKT-based immunotherapeutic approaches for the treatment of cancer. Some of the broad strategies used to manipulate NKT cells in vivo include the direct injection of α-GalCer and the reinfusion of autologous DC loaded ex vivo with α-GalCer (83). Numerous studies, however, have shown that cancer patients have a deficiency in both NKT cell number and function (84–86), suggesting that in vivo NKT cell modulation may be ineffective in patients. Adoptive immunotherapy using ex vivo expanded NKT cells may be a more productive strategy, therefore methods have been developed to isolate and expand donor-derived NKT cells (87, 88). Motohashi et al. demonstrated that adoptive transfer of ex vivo expanded autologous NKT cells was tolerated well in a small cohort of non-small cell lung cancer patients. Although subsequent expansion of NKT cells was observed in a few patients, none showed partial or complete remission (89). Upon antigen stimulation, expansion of NKT cells from human peripheral blood produces similar numbers of CD4+ and DN NKT cells (90). An area of consideration for adoptive transfer of NKT cells is which subset to use since it has been reported that CD4+ and DN NKT cell subsets have differential anti-tumor immunity. In a mouse model of methylcholanthrene-induced sarcoma as well as B16F10 melanoma metastases, it was found that CD4-NKT cells (liver-derived) were more potent anti-tumor mediators than the CD4+ population (91). Bricard et al. reported that in an ex vivo analysis of NKT cells from patients with hepatocellular carcinoma, the proportion of CD4+ NKT cells gradually increased from the blood, to liver, to tumor. These CD4+ NKT cells had reduced cytolytic activity and increased Th2 cytokine secretion (92), which may be detrimental in the TME as CD4+ NKT cells have been shown to inhibit the expansion of antigen specific cytotoxic T cells (93). However, it is unclear at this time how CD4 distribution in adoptively transferred NKT cell populations may impact therapeutic outcomes.
Although there are numerous studies documenting a protective role of NKT cells in tumor immunity, it has been reported that in some cases NKT cells can prevent effective anti-tumor responses. In a CD1d knockout (KO) mouse model, it was reported that CD1d-restricted CD4+ NKT cells prevented effective cytotoxic T cell mediated tumor eradication in an IL-13-dependent manner (94). In addition, CD1d KO mice developed fewer tumor nodules in the lungs than wild type mice when tumor cells were injected intravenously (95), and in another study were shown to be more resistant to the development of spontaneous mammary carcinoma metastases that wild type mice (96).
CAR NKT cells
As shown in Figure 2, CAR NKT cells have distinct mechanistic advantages over CARs generated from bulk T cells. Upon activation, NKT cells exhibit direct NK-like MHC-independent cytotoxic activity against tumor cells through several mechanisms, including perforin and granzyme secretion, Fas ligand, or tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (84, 97). In addition, NKT cells indirectly contribute to tumor cell death through the induction of dendritic cell maturation in a CD40-CD40L dependent manner and through the production of large amounts of cytokines that can act on other immune cells (98). For example, upon activation NKT cells secrete IFN-γ, which can act on NK cells and CD8+ T cells to promote tumor cell killing. Moreover, in contrast to the genetic polymorphism and ubiquitous expression of HLA molecules, the CD1d gene is monomorphic and expressed by only a few cell types (99), limiting the potential toxicity of NKT cells and allowing them to be adoptively transferred to patients regardless of HLA allele expression.
Figure 2.

Activated NKT cells can mount both direct and indirect anti-tumor responses. Following activation, NKT cells rapidly secrete cytokines, such as IFN-γ, which can promote the activation of NK cells and CD8+ T cells, and in combination with CD40/CD40L interactions can also lead to the maturation of dendritic cells which can secrete IL-12 and further enhance NK and CD8+ T cell activation. NKT cells can also directly mediate cytotoxicity through FAS/FASL, perforin, and granzyme. NKT cells may be directed towards tumor cells expressing specific antigen through the transduction and expression of chimeric antigen receptors (CAR). CARs have an extracellular antigen-targeting domain capable of binding their target antigen in an MHC-independent manner. Current research efforts are focused on harnessing the adaptability of CARs to enhance NKT cell targeting of tumors.
Studies using CAR-modified NKT cells, however, have been relatively scant. Using a model of neuroblastoma, Heczey et al. demonstrated that the ex vivo expansion of human primary NKT modified with CARs specific for the ganglioside GD2 exhibited potent yet specific cytotoxicity against both GD2-positive tumor cells as well as CD1d-positive M2 macrophages in vitro (100). Because the presence of TAMs with M2-like phenotype is associated with poor patient prognosis in neuroblastoma (101), the elimination or inhibition of TAMs by anti-GD2 CAR NKT cells could sensitize tumor cells to CAR-mediated cytotoxicity and decrease the possibility of tumor immune escape. Interestingly, when the anti-GD2 CAR constructs contained a 4-1BB endodomain either alone or in combination with CD28, the NKT cells were Th1-polarized, releasing increased levels of IFN-γ and GM-CSF and reduced levels of IL-4 and IL-10, compared to CAR constructs without any costimulatory domains, highlighting the importance of costimulatory domain selection in CAR construction (100). Because CAR expression can alter the cytokine expression profiles of transduced NKT cells, it is unclear how critical the starting subset of NKT cells (CD4+/-) is prior to transduction. Anti-GD2 CAR NKT cells also effectively localized to the tumor site and had potent anti-tumor activity in a metastatic model of neuroblastoma without the induction of GVHD, successfully demonstrating the potential of NKT cells to serve as a safe and effective platform for CAR-redirected cancer immunotherapy (100). A third generation anti-GD2 CAR NKT containing the CD3 chain along with the signaling domains of the co-stimulatory molecules CD28 and OX-40, and the suicide gene inducible caspase 9, is currently in phase 1 clinical trials for patients with relapsed or refractory neuroblastoma (NCT02439788). NKT cells have also been modified with a CD19-specific CAR for the treatment of B cell lymphomas (102). In 2016, Tian et al. (102) investigated the functional significance of CD62L expression on NKT cells, since reports in T cells have demonstrated that CD62L+ central memory T cells have stem cell properties and superior therapeutic activity in cell therapy products (103–105). They found that upon ex vivo stimulation, the CD62L positive subset of NKT cells from peripheral blood is responsible for cell expansion, as only CD62L positive NKT cells survive and proliferate in response to repeated TCR-stimulation, while CD62L negative cells undergo early exhaustion and cell death. In addition, when engineered to express CD19-specific CARs, CD62L positive, but not CD62L negative CAR NKT cells produced sustained tumor regression in a B cell lymphoma model in NOD/SCID/IL-2Rγ (NSG) mice. To determine optimal ex vivo expansion conditions of CD62L+ NKT cells, they created artificial antigen-presenting cells (aAPCs) with varying co-stimulatory molecules and found that the combination of CD86, 4-1BBL, and OX40L molecules enabled highly efficient clinical-scale NKT expansion with maximal preservation of CD62L expression. CAR NKT cells generated using the aAPCs demonstrated prolonged in vivo persistence and superior therapeutic activity in models of lymphoma and neuroblastoma (102), establishing the potential of NKT cells to serve as a safe and effective platform for CAR cancer immunotherapy. Of note, both before and after expansion, CD62L was more frequently expressed on CD4+ NKT cells and CAR NKT cells contained a mixed population of CD4+ and CD4− cells, suggesting that expression of CD62L, regardless of the subset of NKT cells infused, is an important factor in therapeutic success.
FUTURE DIRECTIONS
BiKes and TriKes
An innovative immunoglobulin-based strategy to redirect lymphocyte cytotoxicity towards tumor cells is to create either bispecific or trispecific antibodies (113). The concept of bispecific antibodies was first introduced as a method to target multiple antigens by a single antibody (106). They are composed of fragments of two different monoclonal antibodies that can bind two different antigens and can be generated with or without an Fc region (107). Bispecific antibodies with anti-CD3 and anti-CD19 components have been utilized in numerous preclinical studies, resulting in rapid and effective cytotoxicity specific for CD19+ B cells (108, 109). Blinatumomab, a bi-specific T cell engager (BiTE) antibody construct made by fusing an anti-CD3 scFv to an anti-CD19 scFv via a short five residue peptide linker has performed impressively in multiple clinical trials as a single agent for relapsed/refractory B cell ALL (110, 111). Because the BiTEs were specific for CD3 on one arm and a tumor antigen on the second, they can bring T cells and tumor cells into close proximity and do not need conventional MHC recognition to induce T-cell activation (112). However, Stone et al compared the in vitro sensitivity of these two strategies and found that CAR-expressing T cells were more sensitive than BiTE-modified T cells to low numbers of antigens per cell (113), indicating that when epitope densities are low, CAR-expressing T cells may be considered preferential. To allow for even more targeting specificities, tri-specific antibodies have also been developed to drive re-directed lysis of tumor cells.
Bi- and tri-specific killer engagers (BiKEs and TriKEs) are smaller molecules composed of 2–3 variable portions of antibodies with different specificities, and represent a novel and more versatile strategy compared to traditional bi- and tri-specific antibody platforms. In NK cells, bi- and tri-specific antibodies work through binding of a tumor antigen and direct binding and crosslinking of the CD16 receptor, thus bypassing the need for binding of the Fc portion of mono-specific antibodies. Gleason et al. demonstrated the ability of a CD16/CD19 BiKE and a CD16/CD19/CD22 TriKE to trigger NK cell activation through direct signaling of CD16 and induce target cell death through lytic granule secretion. BiKEs and TriKEs have been shown to effectively mediate NK cytotoxicity of lymphoma targets at high and low effector-to-target ratios. Vallera et al previously generated an NK-BiKE containing a scFv against CD16 and CD33 to create an immunologic synapse between NK cells and CD33+ myeloid targets. More recently, this BiKE has been modified to incorporate a novel human IL-15 crosslinker, producing a TriKE, which effectively promotes in vivo persistence, activation, and survival of NK cells (114). Although not as common, bi-specific antibodies have also been used for NKT cells. Using CD8+ NKT cells redirected with a bi-specific antibody against HER2 and CD3, Scheffold et al. demonstrated that administration of these NKT cells resulted in rapid, and in most instances sustained, eradication of HER2-expressing tumor cells in a SCID mouse model, highlighting a promising strategy for NKT-based adoptive immunotherapy of neoplastic diseases (115).
TRUCKS
In order to combat the tumor immunosuppressive microenvironment, CAR T cells redirected for universal cytokine killing (TRUCKs) are equipped with an inducible cytokine expression cassette, such as IL-12 or proliferative T cell–costimulatory ligands (1). Upon antigen engagement, these armored CAR T cells secrete IL-12 in a locally restricted manner, and recruit both primary adaptive and innate immune cells, such as cytotoxic T cells and NK cells, to the tumor site (116, 117) and can impact local suppressive cells, such as regulatory T cells within the tumor stroma that are aimed at recruiting a second wave of immune cells in a locally restricted fashion to initiate the recognition of cancer cells that have lost the expression of the CAR target antigen (118). Pegram et al. demonstrated that CAR-T cells modified to secrete IL-12 can effectively eradicate systemic tumors in their thymoma mouse model without the need for conditioning chemotherapy, unlike CAR T cells without IL-12 release (119). Although pre-clinical data has shown that TRUCKs can enhance anti-tumor activity and modify tumor microenvironment, clinical experience is limited. To date, no studies employing an NK or NKT TRUCK have been published, but it may be an avenue to explore for future modifications to enhance efficacy.
CONCLUSION
Recent advances in the understanding of NK and NKT cell immunobiology have paved the way for novel and innovative anti-cancer therapies. The ability to engineer NK and NKT cells with CARs holds great promise as a novel cellular immunotherapy against refractory malignancies. Because both NK and NKT are not HLA-restricted, they can potentially provide an off- the-shelf, standardized allogeneic treatment that would eliminate the need for patient-specific cellular therapy that is currently required for CAR T cell-based therapies. The use of NK or NKT cells is also less likely to have the same severe toxicity issues as CAR T cell therapy using bulk T cells, since mature NK cells have a shorter lifespan than conventional T cells and NKT cells are CD1d-restricted. Moreover, both cell types secrete a cytokine profile that differs from the pro-inflammatory panel released by T cells and associated with toxicity. With increasing focus on genetically modifying NK and NKT cells to redirect their specificity or engager-modified cells, it is likely that NK and NKT cells will move to the forefront of cancer therapy over the next few years.
Acknowledgments
All authors have read the journal’s policy on disclosure of potential conflicts of interest and have no conflicts to declare. All authors have read the journal’s authorship agreement and have read and approved of the manuscript.
Funding: This research was supported by grants R21CA184469 and R21CA199544 from the National Cancer Institute of the National Institutes of Health to TJW.
Abbreviations
- aAPC
artificial antigen presenting cells
- αGalCer
α-galactosylceramide
- ACT
adoptive cell transfer
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ALL
Acute Lymphoblastic Leukemia
- AML
acute lymphoid leukemia
- CAR
chimeric antigen receptor
- CRS
cytokine release syndrome
- DC
dendritic cell
- GM-CSF
granulocyte macrophage colony stimulating factor
- GVHD
graft versus host disease
- HLA
human leukocyte antigen
- iPSC
induced pluripotent stem cells
- KIR
killer immunoglobulin-like receptors
- MHC
major histocompatibility complex
- NCR
natural cytotoxicity receptors
- NK
Natural Killer
- NKT
Natural Killer T
- scFv
single chain variable fragment
- TAM
tumor associated macrophage
- TCR
T cell receptor
- TGF-β
transforming growth factor-β
- TME
tumor microenvironment
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Financial Disclosure Statement: The authors declare that they have no competing interests.
References
- 1.Brentjens RJ, Curran KJ. Novel cellular therapies for leukemia: CAR-modified T cells targeted to the CD19 antigen. Hematology Am Soc Hematol Educ Program. 2012;2012:143–51. doi: 10.1182/asheducation-2012.1.143. Epub 2012/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MG, Kim D, Suh SK, Park Z, Choi MJ, Oh YK. Current status and regulatory perspective of chimeric antigen receptor-modified T cell therapeutics. Arch Pharm Res. 2016;39(4):437–52. doi: 10.1007/s12272-016-0719-7. Epub 2016/02/20. [DOI] [PubMed] [Google Scholar]
- 3.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121(5):1822–6. doi: 10.1172/JCI46110. Epub 2011/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(2):413–20. doi: 10.1038/mt.2009.210. Epub 2009/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science translational medicine. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. Epub 2015/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–20. doi: 10.1182/blood-2011-10-384388. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–28. doi: 10.1182/blood-2011-04-348540. Epub 2011/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Molecular therapy oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. Epub 2016/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magee MS, Snook AE. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov Med. 2014;18(100):265–71. Epub 2014/11/27. [PMC free article] [PubMed] [Google Scholar]
- 10.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood reviews. 2006;20(3):123–37. doi: 10.1016/j.blre.2005.10.001. Epub 2005/12/21. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL. NK cell recognition. Annual review of immunology. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. Epub 2005/03/18. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–51. doi: 10.1182/blood.v97.10.3146. Epub 2001/05/09. [DOI] [PubMed] [Google Scholar]
- 13.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nature reviews Immunology. 2006;6(7):520–31. doi: 10.1038/nri1863. Epub 2006/06/27. [DOI] [PubMed] [Google Scholar]
- 14.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature immunology. 2008;9(5):495–502. doi: 10.1038/ni1581. Epub 2008/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Xiao Z, Ko HL, Shen M, Ren EC. Activating killer cell immunoglobulin-like receptor 2DS2 binds to HLA-A*11. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(7):2662–7. doi: 10.1073/pnas.1322052111. Epub 2014/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–76. doi: 10.1182/blood-2009-08-238469. Epub 2009/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nature reviews Immunology. 2003;3(10):781–90. doi: 10.1038/nri1199. Epub 2003/10/03. [DOI] [PubMed] [Google Scholar]
- 18.Becker PS, Suck G, Nowakowska P, Ullrich E, Seifried E, Bader P, et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer immunology, immunotherapy : CII. 2016;65(4):477–84. doi: 10.1007/s00262-016-1792-y. Epub 2016/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyengar R, Handgretinger R, Babarin-Dorner A, Leimig T, Otto M, Geiger TL, et al. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy. 2003;5(6):479–84. doi: 10.1080/14653240310003558. Epub 2003/12/09. [DOI] [PubMed] [Google Scholar]
- 20.Koehl U, Brehm C, Huenecke S, Zimmermann SY, Kloess S, Bremm M, et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Frontiers in oncology. 2013;3:118. doi: 10.3389/fonc.2013.00118. Epub 2013/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy. 2010;12(8):1044–55. doi: 10.3109/14653249.2010.504770. Epub 2010/08/28. [DOI] [PubMed] [Google Scholar]
- 22.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. doi: 10.1182/blood-2013-10-532531. Epub 2014/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–9. doi: 10.1126/science.1198687. Epub 2011/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nature reviews Immunology. 2006;6(12):940–52. doi: 10.1038/nri1983. Epub 2006/11/25. [DOI] [PubMed] [Google Scholar]
- 25.Screpanti V, Wallin RP, Ljunggren HG, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. Journal of immunology. 2001;167(4):2068–73. doi: 10.4049/jimmunol.167.4.2068. Epub 2001/08/08. [DOI] [PubMed] [Google Scholar]
- 26.Bishara A, De Santis D, Witt CC, Brautbar C, Christiansen FT, Or R, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue antigens. 2004;63(3):204–11. doi: 10.1111/j.0001-2815.2004.00182.x. Epub 2004/03/03. [DOI] [PubMed] [Google Scholar]
- 27.Ruggeri L, Mancusi A, Burchielli E, Capanni M, Carotti A, Aloisi T, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood cells, molecules & diseases. 2008;40(1):84–90. doi: 10.1016/j.bcmd.2007.06.029. Epub 2007/10/30. [DOI] [PubMed] [Google Scholar]
- 28.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. Epub 2014/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. Epub 2005/08/05. [DOI] [PubMed] [Google Scholar]
- 30.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. Epub 2005/01/06. [DOI] [PubMed] [Google Scholar]
- 31.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–83. doi: 10.1182/blood-2004-12-4797. Epub 2005/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimasaki N, Fujisaki H, Cho D, Masselli M, Lockey T, Eldridge P, et al. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy. 2012;14(7):830–40. doi: 10.3109/14653249.2012.671519. Epub 2012/03/31. [DOI] [PubMed] [Google Scholar]
- 33.Chu Y, Hochberg J, Yahr A, Ayello J, van de Ven C, Barth M, et al. Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-Modified Expanded Natural Killer Cells In Vitro and in NSG Mice. Cancer immunology research. 2015;3(4):333–44. doi: 10.1158/2326-6066.CIR-14-0114. Epub 2014/12/11. [DOI] [PubMed] [Google Scholar]
- 34.Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(15):4857–66. doi: 10.1158/1078-0432.CCR-08-2810. Epub 2009/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17481–6. doi: 10.1073/pnas.0804788105. Epub 2008/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73(6):1777–86. doi: 10.1158/0008-5472.CAN-12-3558. Epub 2013/01/11. [DOI] [PubMed] [Google Scholar]
- 37.Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L, et al. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16+ and CD16− subpopulations and in vivo influence after haploidentical NK cell infusion. Journal of immunotherapy. 2010;33(2):200–10. doi: 10.1097/CJI.0b013e3181bb46f7. Epub 2010/02/11. [DOI] [PubMed] [Google Scholar]
- 38.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells–enhancement by therapeutic antibodies. PloS one. 2007;2(3):e326. doi: 10.1371/journal.pone.0000326. Epub 2007/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brehm C, Huenecke S, Quaiser A, Esser R, Bremm M, Kloess S, et al. IL-2 stimulated but not unstimulated NK cells induce selective disappearance of peripheral blood cells: concomitant results to a phase I/II study. PloS one. 2011;6(11):e27351. doi: 10.1371/journal.pone.0027351. Epub 2011/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–70. doi: 10.1016/j.jcyt.2013.06.017. Epub 2013/10/08. [DOI] [PubMed] [Google Scholar]
- 41.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10(3):369–83. doi: 10.1089/152581601750288975. Epub 2001/07/17. [DOI] [PubMed] [Google Scholar]
- 42.Matsuo Y, Drexler HG. Immunoprofiling of cell lines derived from natural killer-cell and natural killer-like T-cell leukemia-lymphoma. Leuk Res. 2003;27(10):935–45. doi: 10.1016/s0145-2126(03)00024-9. Epub 2003/07/16. [DOI] [PubMed] [Google Scholar]
- 43.Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Frontiers of medicine. 2012;6(1):56–66. doi: 10.1007/s11684-012-0177-7. Epub 2012/03/31. [DOI] [PubMed] [Google Scholar]
- 44.Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer immunology, immunotherapy : CII. 2008;57(3):411–23. doi: 10.1007/s00262-007-0383-3. Epub 2007/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boissel L, Betancur M, Lu W, Wels WS, Marino T, Van Etten RA, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma. 2012;53(5):958–65. doi: 10.3109/10428194.2011.634048. Epub 2011/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS, et al. Retargeting NK-92 cells by means of CD19− and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. 2013;2(10):e26527. doi: 10.4161/onci.26527. Epub 2014/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100(4):1265–73. Epub 2002/08/01. [PubMed] [Google Scholar]
- 48.Liu H, Yang B, Sun T, Lin L, Hu Y, Deng M, et al. Specific growth inhibition of ErbB2expressing human breast cancer cells by genetically modified NK92 cells. Oncol Rep. 2015;33(1):95–102. doi: 10.3892/or.2014.3548. Epub 2014/10/22. [DOI] [PubMed] [Google Scholar]
- 49.Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(2):330–8. doi: 10.1038/mt.2014.219. Epub 2014/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16(3):569–81. doi: 10.1111/j.1582-4934.2011.01343.x. Epub 2011/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8(2):297–310. doi: 10.1016/j.molonc.2013.12.001. Epub 2014/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahm C, Schonfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer immunology, immunotherapy : CII. 2012;61(9):1451–61. doi: 10.1007/s00262-012-1212-x. Epub 2012/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tassev DV, Cheng M, Cheung NK. Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer gene therapy. 2012;19(2):84–100. doi: 10.1038/cgt.2011.66. Epub 2011/10/08. [DOI] [PubMed] [Google Scholar]
- 54.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–27. doi: 10.1038/leu.2013.279. Epub 2013/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang S, Vujanovic NL, Wollenberg B, Whiteside TL. Absence of B7.1-CD28/CTLA-4-mediated co-stimulation in human NK cells. European journal of immunology. 1998;28(3):780–6. doi: 10.1002/(SICI)1521-4141(199803)28:03<780::AID-IMMU780>3.0.CO;2-8. Epub 1998/04/29. [DOI] [PubMed] [Google Scholar]
- 56.Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy. 2017;19(2):235–49. doi: 10.1016/j.jcyt.2016.10.009. Epub 2016/11/27. [DOI] [PubMed] [Google Scholar]
- 57.Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, et al. Preclinical targeting of aggressive T cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017 doi: 10.1038/leu.2017.8. Epub 2017/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–30. doi: 10.1016/j.cell.2008.07.001. Epub 2008/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cellular & molecular immunology. 2016;13(5):628–39. doi: 10.1038/cmi.2015.42. Epub 2015/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Guo L, Song Y, Zhang Y, Lin D, Hu B, et al. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-betaR II and NKG2D. Cancer immunology, immunotherapy : CII. 2017 doi: 10.1007/s00262-017-1959-1. Epub 2017/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimasaki N, Campana D. Natural killer cell reprogramming with chimeric immune receptors. Methods Mol Biol. 2013;969:203–20. doi: 10.1007/978-1-62703-260-5_13. Epub 2013/01/09. [DOI] [PubMed] [Google Scholar]
- 62.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296(5567):553–5. doi: 10.1126/science.1069017. Epub 2002/04/24. [DOI] [PubMed] [Google Scholar]
- 63.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nature immunology. 2001;2(10):971–8. doi: 10.1038/ni710. Epub 2001/09/11. [DOI] [PubMed] [Google Scholar]
- 64.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22(6):705–16. doi: 10.1016/j.immuni.2005.03.011. Epub 2005/06/21. [DOI] [PubMed] [Google Scholar]
- 65.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8− T cells in mice and humans. The Journal of experimental medicine. 1994;180(3):1097–106. doi: 10.1084/jem.180.3.1097. Epub 1994/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. The Journal of experimental medicine. 2002;195(5):637–41. doi: 10.1084/jem.20011908. Epub 2002/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. The Journal of experimental medicine. 2002;195(5):625–36. doi: 10.1084/jem.20011786. Epub 2002/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995;269(5221):185–6. doi: 10.1126/science.7542402. Epub 1995/07/14. [DOI] [PubMed] [Google Scholar]
- 69.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. Journal of immunology. 1998;160(7):3121–7. Epub 1998/04/08. [PubMed] [Google Scholar]
- 70.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. Epub 1997/12/31. [DOI] [PubMed] [Google Scholar]
- 71.Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, et al. NKT cells are phenotypically and functionally diverse. European journal of immunology. 1999;29(11):3768–81. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. Epub 1999/11/11. [DOI] [PubMed] [Google Scholar]
- 72.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. Journal of immunology. 2005;175(5):3092–101. doi: 10.4049/jimmunol.175.5.3092. Epub 2005/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. Journal of immunology. 2001;166(10):6012–8. doi: 10.4049/jimmunol.166.10.6012. Epub 2001/05/09. [DOI] [PubMed] [Google Scholar]
- 74.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. The Journal of experimental medicine. 2004;199(9):1213–21. doi: 10.1084/jem.20031462. Epub 2004/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(20):7322–7. doi: 10.1158/1078-0432.CCR-05-0877. Epub 2005/10/26. [DOI] [PubMed] [Google Scholar]
- 76.Morris ES, MacDonald KP, Rowe V, Banovic T, Kuns RD, Don AL, et al. NKT cell-dependent leukemia eradication following stem cell mobilization with potent G-CSF analogs. The Journal of clinical investigation. 2005;115(11):3093–103. doi: 10.1172/JCI25249. Epub 2005/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. Journal of immunology. 2007;178(10):6242–51. doi: 10.4049/jimmunol.178.10.6242. Epub 2007/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casorati G, de Lalla C, Dellabona P. Invariant natural killer T cells reconstitution and the control of leukemia relapse in pediatric haploidentical hematopoietic stem cell transplantation. Oncoimmunology. 2012;1(3):355–7. doi: 10.4161/onci.18399. Epub 2012/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM, et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4− subset dynamics and correlates with remission state. Journal of immunology. 2011;186(7):4490–9. doi: 10.4049/jimmunol.1003748. Epub 2011/03/02. [DOI] [PubMed] [Google Scholar]
- 80.Dellabona P, Casorati G, de Lalla C, Montagna D, Locatelli F. On the use of donor-derived iNKT cells for adoptive immunotherapy to prevent leukemia recurrence in pediatric recipients of HLA haploidentical HSCT for hematological malignancies. Clin Immunol. 2011;140(2):152–9. doi: 10.1016/j.clim.2010.11.015. Epub 2010/12/28. [DOI] [PubMed] [Google Scholar]
- 81.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. The Journal of clinical investigation. 2009;119(6):1524–36. doi: 10.1172/JCI37869. Epub 2009/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110(9):1911–28. doi: 10.1002/cncr.22999. Epub 2007/09/13. [DOI] [PubMed] [Google Scholar]
- 83.Pilones KA, Aryankalayil J, Demaria S. Invariant NKT cells as novel targets for immunotherapy in solid tumors. Clinical & developmental immunology. 2012;2012:720803. doi: 10.1155/2012/720803. Epub 2012/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59(20):5102–5. Epub 1999/10/28. [PubMed] [Google Scholar]
- 85.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. Journal of immunology. 2001;167(7):4046–50. doi: 10.4049/jimmunol.167.7.4046. Epub 2001/09/21. [DOI] [PubMed] [Google Scholar]
- 86.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. British journal of haematology. 2003;122(4):617–22. doi: 10.1046/j.1365-2141.2003.04465.x. Epub 2003/08/06. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi T, Nieda M, Koezuka Y, Nicol A, Porcelli SA, Ishikawa Y, et al. Analysis of human V alpha 24+ CD4+ NKT cells activated by alpha-glycosylceramide-pulsed monocyte-derived dendritic cells. Journal of immunology. 2000;164(9):4458–64. doi: 10.4049/jimmunol.164.9.4458. Epub 2000/04/26. [DOI] [PubMed] [Google Scholar]
- 88.Rogers PR, Matsumoto A, Naidenko O, Kronenberg M, Mikayama T, Kato S. Expansion of human Valpha24+ NKT cells by repeated stimulation with KRN7000. Journal of immunological methods. 2004;285(2):197–214. doi: 10.1016/j.jim.2003.12.003. Epub 2004/02/26. [DOI] [PubMed] [Google Scholar]
- 89.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(20 Pt 1):6079–86. doi: 10.1158/1078-0432.CCR-06-0114. Epub 2006/10/10. [DOI] [PubMed] [Google Scholar]
- 90.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. Journal of immunology. 2001;167(6):3114–22. doi: 10.4049/jimmunol.167.6.3114. Epub 2001/09/07. [DOI] [PubMed] [Google Scholar]
- 91.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. The Journal of experimental medicine. 2005;202(9):1279–88. doi: 10.1084/jem.20050953. Epub 2005/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. Journal of immunology. 2009;182(8):5140–51. doi: 10.4049/jimmunol.0711086. Epub 2009/04/04. [DOI] [PubMed] [Google Scholar]
- 93.Osada T, Morse MA, Lyerly HK, Clay TM. Ex vivo expanded human CD4+ regulatory NKT cells suppress expansion of tumor antigen-specific CTLs. International immunology. 2005;17(9):1143–55. doi: 10.1093/intimm/dxh292. Epub 2005/07/20. [DOI] [PubMed] [Google Scholar]
- 94.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature immunology. 2000;1(6):515–20. doi: 10.1038/82771. Epub 2001/03/23. [DOI] [PubMed] [Google Scholar]
- 95.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. International journal of cancer. 2005;114(1):80–7. doi: 10.1002/ijc.20669. Epub 2004/11/04. [DOI] [PubMed] [Google Scholar]
- 96.Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. Journal of immunology. 2002;169(10):5796–804. doi: 10.4049/jimmunol.169.10.5796. Epub 2002/11/08. [DOI] [PubMed] [Google Scholar]
- 97.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383–9. doi: 10.1182/blood-2003-04-1155. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 98.Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nature immunology. 2003;4(12):1164–5. doi: 10.1038/ni1203-1164. Epub 2003/11/26. [DOI] [PubMed] [Google Scholar]
- 99.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, et al. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. The Journal of experimental medicine. 1998;188(8):1521–8. doi: 10.1084/jem.188.8.1521. Epub 1998/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124(18):2824–33. doi: 10.1182/blood-2013-11-541235. Epub 2014/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Asgharzadeh S, Salo JA, Ji L, Oberthuer A, Fischer M, Berthold F, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30(28):3525–32. doi: 10.1200/JCO.2011.40.9169. Epub 2012/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. The Journal of clinical investigation. 2016;126(6):2341–55. doi: 10.1172/JCI83476. Epub 2016/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graef P, Buchholz VR, Stemberger C, Flossdorf M, Henkel L, Schiemann M, et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity. 2014;41(1):116–26. doi: 10.1016/j.immuni.2014.05.018. Epub 2014/07/19. [DOI] [PubMed] [Google Scholar]
- 104.Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, Chang WC, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. Journal of immunotherapy. 2012;35(9):689–701. doi: 10.1097/CJI.0b013e318270dec7. Epub 2012/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492–500. doi: 10.1038/leu.2015.247. Epub 2015/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raso V, Griffin T. Hybrid antibodies with dual specificity for the delivery of ricin to immunoglobulin-bearing target cells. Cancer Res. 1981;41(6):2073–8. Epub 1981/06/01. [PubMed] [Google Scholar]
- 107.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838–47. doi: 10.1016/j.drudis.2015.02.008. Epub 2015/03/03. [DOI] [PubMed] [Google Scholar]
- 108.Loffler A, Kufer P, Lutterbuse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–103. Epub 2000/03/09. [PubMed] [Google Scholar]
- 109.Schlereth B, Quadt C, Dreier T, Kufer P, Lorenczewski G, Prang N, et al. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer immunology, immunotherapy : CII. 2006;55(5):503–14. doi: 10.1007/s00262-005-0001-1. Epub 2005/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. Epub 2014/12/20. [DOI] [PubMed] [Google Scholar]
- 111.Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–40. doi: 10.1200/JCO.2014.56.3247. Epub 2014/11/12. [DOI] [PubMed] [Google Scholar]
- 112.Suryadevara CM, Gedeon PC, Sanchez-Perez L, Verla T, Alvarez-Breckenridge C, Choi BD, et al. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology. 2015;4(6):e1008339. doi: 10.1080/2162402X.2015.1008339. Epub 2015/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs) Oncoimmunology. 2012;1(6):863–73. doi: 10.4161/onci.20592. Epub 2012/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(14):3440–50. doi: 10.1158/1078-0432.CCR-15-2710. Epub 2016/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scheffold C, Kornacker M, Scheffold YC, Contag CH, Negrin RS. Visualization of effective tumor targeting by CD8+ natural killer T cells redirected with bispecific antibody F(ab’)(2)HER2xCD3. Cancer Res. 2002;62(20):5785–91. Epub 2002/10/18. [PubMed] [Google Scholar]
- 116.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–54. doi: 10.1517/14712598.2015.1046430. Epub 2015/05/20. [DOI] [PubMed] [Google Scholar]
- 117.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697–706. doi: 10.1158/0008-5472.CAN-11-0103. Epub 2011/07/12. [DOI] [PubMed] [Google Scholar]
- 118.Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. CAR T cells: driving the road from the laboratory to the clinic. Immunol Rev. 2014;257(1):91–106. doi: 10.1111/imr.12126. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 119.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–41. doi: 10.1182/blood-2011-12-400044. Epub 2012/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]


