Abstract
Offspring from multiplex, alcohol-dependent families are at heightened risk for substance use disorders (SUDs) in adolescence and young adulthood. These high-risk offspring have also been shown to have atypical structure and function of brain regions implicated in emotion regulation, social cognition, and reward processing. This study assessed the relationship between amygdala and orbitofrontal cortex (OFC) volumes obtained in adolescence and SUD outcomes in young adulthood among high-risk offspring and low-risk controls. A total of 78 participants (40 high-risk; 38 low-risk) from a longitudinal family study, ages 8–19, underwent magnetic resonance imaging; volumes of the amygdala and OFC were obtained with manual tracing. SUD outcomes were assessed at approximately yearly intervals. Cox regression survival analyses were used to assess the effect of regional brain volumes on SUD outcomes. The ratio of OFC to amygdala volume significantly predicted SUD survival time across the sample; reduction in survival time was seen in those with smaller ratios for both high-risk and low-risk groups. Morphology of prefrontal relative to limbic regions in adolescence prospectively predicts age of onset for substance use disorders.
Keywords: amygdala, orbitofrontal cortex, family history, alcohol use disorders, adolescence, structural magnetic resonance imaging
1. Introduction
Individuals with a family history of alcohol use disorders (AUDs) are at increased risk for developing substance use disorders (SUDs) (Cloninger et al., 1981; Verhulst et al., 2015), and offspring from multiplex, alcohol-dependent families are at especially high risk for early onset SUDs (Hill et al., 2008). Determining the specific genetic mechanisms of familial transmission has been challenging given the multiple clinical subtypes of SUD and variable expression across the lifespan (Hill, 2010). Accordingly, increased attention has been focused on finding biological variation associated with familial risk that predisposes individuals to increased risk for SUD. Longitudinal studies that follow individuals with a family history of AUD from childhood and adolescence into young adulthood may allow for identification of potential biomarkers that contribute to risk and resilience within at-risk populations (Hill and O’Brien, 2015).
High-risk offspring with a family history of AUD have been shown to demonstrate atypical structure and function of brain regions involved in executive control, affective regulation, decision making, and social cognition (Cservenka, 2016; Hill and O’Brien, 2015). Previous research indicates that compared to healthy controls from low-risk families, adolescent and young adult offspring with a family history of AUD show volumetric reductions in the right hemisphere of the orbitofrontal cortex (OFC) (Hill et al., 2010; Hill et al., 2009b) and the amygdala (Benegal et al., 2007; Dager et al., 2015; Hill et al., 2001; Hill et al., 2013). These results have been observed in samples where either the majority of cases had not yet developed a substance use disorder (Dager et al., 2015; Hill et al., 2001), were alcohol-naïve (Benegal et al., 2007), or the reduction in volume was seen even when cases with substance use disorder were removed (Hill et al., 2013; Hill et al., 2009b). Adults with AUD also show volumetric reductions of the OFC and amygdala compared to healthy controls (Durazzo et al., 2011; Makris et al., 2008), and atypical structure and function of these regions may be one biological mechanism that confers risk for SUDs.
Importantly, atypical morphologies of the orbitofrontal cortex and amygdala during childhood and adolescence have been shown to relate to established risk factors for substance use disorders. Longitudinal family studies, as well as cross-sectional research on healthy adults and children, have demonstrated that reductions in orbitofrontal cortex volume and cortical thickness are associated with higher rates of externalizing behaviors (Ameis et al., 2014), greater impulsivity (Hill et al., 2009b; Schilling et al., 2013) and impaired decision-making (Hill and O’Brien, 2015), deficits that are independently associated with increased risk for substance use, abuse, and dependence (Bechara et al., 2001; O’Brien et al., 2014; Verdejo-Garcia et al., 2008). Amygdala volume has been shown to be significantly correlated with P300 amplitude (Hill et al., 2001), a well-established endophenotype of risk for substance use and externalizing behavioral disorders (Hill et al., 2009a; Iacono et al., 2002). In addition, the volume of both amygdala and OFC seen in adolescence have been associated with variation in the tendency to be behaviorally inhibited in early childhood (Hill et al., 2010). Importantly, other longitudinal studies have demonstrated that the degree of behavioral inhibition seen in early childhood is related to subsequent SUD outcomes (Caspi et al., 1996; Williams et al., 2010). These results suggest that atypical structure and function of the OFC and amygdala during adolescence, when alcohol and drug use behaviors first emerge, may confer particular risk for substance use disorders.
Adolescence is characterized by dynamic brain changes that occur in the context of major physiological, psychological, and social transitions. This developmental period is also associated with increased emotional reactivity, sensation seeking, and risky behavior, along with dramatic increases in rates of alcohol and drug use during the teenage years (Bava and Tapert, 2010; Casey et al., 2008). Developmental neuroimaging studies indicate that during adolescence, the brain undergoes regionally-specific trajectories of neurogenesis and pruning in subcortical, limbic brain regions, including the amygdala, reaching peak volume in early adolescence, whereas prefrontal regions, including the OFC, undergo a protracted period of development extending into adulthood (Giedd et al., 2015; Ostby et al., 2009). In fact, amygdala volume is inversely correlated with cortical thickness of prefrontal brain regions, including the OFC, in typically developing youth (Albaugh et al., 2013). Accordingly, the increased incidence of risk-taking and impulsive behavior that characterize adolescence likely relates to the unique imbalance of functionally mature limbic regions and immature prefrontal regions during this period of development (Casey et al., 2008; Galvan et al., 2007). Although adolescents, as a group, are more likely to engage in risky behaviors, some adolescents are more prone to engage in risky behaviors than others, putting these individuals at potentially greater risk for negative outcomes (Casey et al., 2008; Galvan et al., 2007). Individuals who demonstrate an exacerbated discrepancy in development of prefrontal versus limbic brain regions in adolescence may be at especially high risk for poor outcomes, including substance use disorders.
Converging evidence indicates that structural abnormalities in the OFC and amygdala may increase risk for SUDs. To the best of our knowledge, no studies to date have provided evidence for a direct association between premorbid volumetric reductions in the OFC and/or amygdala and subsequent substance use disorder outcomes. Accordingly, the current study sought to determine whether volumetric differences in the amygdala and OFC observed between high-risk adolescents and low-risk controls would relate to early-onset SUD outcomes in young adulthood. Utilizing a developmental perspective informed by neurobiological models of risk taking in adolescence (Casey et al., 2008), we hypothesized that volume of the OFC, relative to volume of the amygdala, would be a stronger predictor of SUD outcome than either regional volume considered independently.
2. Methods
2.1. Subjects
The present report is based on analysis of data for 78 third-generation offspring who are part of an ongoing family study that selected families through their parents’ generation. The offspring were evaluated during childhood, adolescence, and young adulthood at approximately yearly intervals in childhood and biennially in young adulthood. A total of 40 high-risk (HR) offspring from paternal multiplex families were studied (17 females and 23 males) along with 38 low-risk (LR) offspring (19 females and 19 males). MRI data was collected when participants were between the ages of 8 and 19. Mean age at last follow-up for the present sub-sample is 20.6 years. This study has ongoing approval from the University of Pittsburgh Institutional Review Board. All participants provided consent at each visit. Children provided assent with parental consent.
High-risk offspring were drawn from families selected to be part of a larger family study of alcohol dependence in which the presence of two adult alcohol dependent (AD) brothers were required for entry into the study. These brothers are the fathers or uncles of the HR subjects in the present analyses. In-person structured interviews using the Diagnostic Interview Schedule (DIS) (Robins et al., 1981) had been performed for the majority of all living and available relatives of the proband by risk-status-blind interviewers, with two family history reports used for deceased or unavailable relatives. Families had not been included if primary, recurrent major depression, bipolar disorder, schizophrenia, or a primary SUD other than AD was present, for either the proband pair of AD brothers or their first-degree relatives.
Low-risk community control families consisting of two brothers and their parents were identified through an index case who responded to a newspaper advertisement requesting participants who were interested in a study of heritable aspects of personality. Families were chosen on the basis of having the same structural characteristics as the HR families (at least two adult brothers) and an absence of axis I psychopathology based on the outcome of a DIS interview that provided Diagnostic and Statistical Manual (DSM) and Feighner criteria for alcoholism (Feighner et al., 1972). Low-risk families were included if all first- and second-degree relatives of the index case were free of alcohol and other drug dependence.
2.2. Procedures
2.2.1. Substance Use Outcome Data
SUD outcome was determined using age-appropriate clinical diagnoses obtained during childhood/adolescence (yearly before age 19) with the Schedule for Affective Disorders and Schizophrenia (K-SADS) (Chambers et al., 1985) and with the Composite International Diagnostic Interview (CIDI) (Janca et al., 1992) and CIDI-Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989) biennially during young adulthood.
2.2.2. Socioeconomic status
Socioeconomic status (SES) was assessed using the Hollingshead Four-Factor Index of Socioeconomic Status (Hollingshead, 1975) at each yearly visit. The SES status closest to the time of MRI acquisition was selected for use in analyses.
2.2.3. MRI Structural Acquisition Methods
Subjects were scanned during childhood and adolescence using a GE 1.5 Tesla scanner located at the University of Pittsburgh Medical Center Magnetic Resonance Research Center. After a localizer scan to ensure optimal head placement, T1 weighted axial images with a slice thickness of 1.5 mm were obtained using a 3 dimensional spoiled gradient recalled echo in the steady state (3D SPGR) (TE = 5 ms, TR = 24 ms, flip angle = 45 degrees, acquisition matrix = 192 × 256, NEX = 1, FOV = 24 cm). Slices were resliced in the coronal plane through the anterior commissures to provide a reproducible guide for image orientation. In addition, axial proton density and T2 weighted images were obtained covering the whole brain at a slice thickness of 5 mm, slice gap = 0 mm ([double spin echo, TE = 17 ms and 102 ms, TR = 3000ms], acquisition matrix = 256 × 192, NEX = 1, FOV = 24 cm). All scans were reviewed by a neuroradiologist when suspected structural abnormalities were present.
2.2.4. Region of Interest Analysis
Region of interest (ROI) volumes were obtained by reliable raters using manual tracing techniques with BRAINS2 software (Magnotta et al., 2002). Semi-automated segmentation of grey, white, and cerebrospinal fluid volumes was completed by the raters using successive iterations to maximize the kappa value. After aligning T1, T2, and proton density images, two raters blind to group membership traced the volumes of the OFC, amygdala, and intracranial volume (ICV) according to boundaries described previously (Hill et al., 2001; Hill et al., 2009b). ROI manual tracing was performed in the coronal plane; inter-rater reliability exceeded .90 for all measurements. The orbitofrontal cortex to amygdala ratio was calculated by dividing the total OFC volume by the total amygdala volume. Ratios were not corrected for ICV.
2.2.5. Statistical Analyses
Demographic data were analyzed with Pearson’s chi-square (categorical variables) or linear mixed models (continuous variables). Risk-group differences in brain volumes were assessed with linear mixed models, controlling for the effects of scan age, ICV, and sex and familial relatedness. Participants who met DSM-IV criteria for alcohol abuse, alcohol dependence, drug abuse, or drug dependence by their age at last follow-up visit were classified as SUD positive. In order to account for variation in age at last clinical follow-up, Cox regression survival analyses were conducted to assess the effect of brain volumes on SUD outcome. Survival analyses controlled for relevant covariates of risk status, scan age, sex, and total intracranial volume (ICV). All analyses were performed in SPSS version 20.
3. Results
3.1. Offspring Demographics
The high-risk and low-risk offspring groups were similar in gender composition, age at study entry, scan age, and age at last clinical follow-up (Table 1). The groups differed significantly in the number of individuals developing a substance use disorder with the high-risk offspring having twice the rate of SUD by the time of the last follow up than did the low-risk offspring (Table 1). The groups differed in socioeconomic status (SES) with the low-risk group having statistically higher SES t=2.06, df =75, p=0.042 (Table 1), though the means of each group are within the same Hollingshead group (minor professional and technical occupations). Across the sample, the mean age at the time of MRI acquisition was 14.64 years (range=8–19 years), and mean age at last clinical follow-up was 20.61 years (range=12–27 years). High-risk offspring had significantly greater total intracranial volume (ICV) than low-risk offspring (F (1,74)=5.20, p=0.025). ICV also differed by sex (F (1,74)=42.49, p< 0.0001.
Table 1.
Demographic Characteristics of High-Risk (HR) and Low-Risk (LR) Subjects
| HR (n=40) | LR (n=38) | p | ||
|---|---|---|---|---|
| Male/Female [n] | 23/17 | 19/19 | NS | |
| Socioeconomic Statusa,b | 42.87 (10.27) | 47.32 (8.52) | 0.04 | |
| Age at Study Entry | 11.78 (2.95) | 11.61 (2.64) | NS | |
| Scan Age | 15.08 (2.75) | 14.13 (3.19) | NS | |
| Age at Last Follow-Up | 21.13 (3.24) | 20.08 (2.94) | NS | |
| Substance Use Disorder Lifetime,C | 19 (47.5%) | 9 (23.7%) | 0.03 | |
| Age at SUD Onset | 18.21 (2.27) | 19.22 (2.59) | NS | |
| SUD prior to scan [n]d | 1 | 2 | NS | |
Data are presented with the mean (standard deviation) unless otherwise noted.
Socioeconomic status was assessed with the Hollingshead Four-Factor Index (Hollingshead, 1975).
X2 = 4.80,df =1, p=0.028
The high risk individual with an SUD diagnosis before the scan met criteria for alcohol dependence and drug abuse. The two low risk individuals with an SUD diagnosis before the scan consisted of one with both an alcohol abuse diagnosis and alcohol dependence, and the other with alcohol dependence only.
3.2. Risk Status and SUD Outcomes
To assess the effect of familial risk on SUD outcome beyond the time at which the scan occurred, a Cox regression survival analysis with risk as the independent variable and SUD outcome as the dependent variable was performed. This confirmed that HR offspring were significantly more likely to meet criteria for SUD (Wald=4.80, p=0.028), with 47.5% of HR offspring affected, compared to 23.7% of LR subjects.
3.3. Brain Volumes and SUD Outcomes
Cox regression survival analysis controlling for familial risk status, sex, and scan age indicates that the median split for the OFC/amygdala ratio is a significant predictor of SUD outcome along with familial risk, and age at the time of the MRI scan (Table 2). These results appear to be minimally influenced by alcohol and drug use prior to the scan because participants with an SUD before the scan were removed. Moreover, even those without an SUD included in the analysis show minimal exposure to drugs, alcohol and cigarettes due to their age (Table 3). Individuals with OFC to amygdala ratios below the median of the sample were significantly more likely to meet criteria for SUDs (Figure 1). A Cox survival analysis conducted for the OFC using familial risk, sex and scan age showed marginal significance for volume of the OFC (Wald =3.12, df=1, p=0.077). In a separate survival analysis of total amygdala volume and controlling for the same variables (risk, scan age and sex), volume of the amygdala was not found to be a significant predictor of SUD survival time (Wald =0.256, df=1, p=0.613)
Table 2.
Predictors of SUD outcome using a Cox Survival regression analysis – variables in the equation (N=78).
| Wald | df | Exp (B) | Significance | |
|---|---|---|---|---|
| OFC/Amygdala Ratioa | 5.45 | 1 | .389 | 0.020 |
| Familial Risk | 4.44 | 1 | .418 | 0.035 |
| Age at Scan | 7.28 | 1 | .786 | 0.007 |
| Sex | 2.07 | 1 | .552 | 0.15 |
Omnibus test of model coefficients χ2 = 18.12, df=4, p=0.001.
OFC/Amygdala ratios were run based on a median split of the sample.
Table 3.
Frequency of Alcohol and Drug Use Prior to Scan Based on K-SADS Interview.
| High-Risk (n=40) | Low-Risk (n=38) | Total (n=78) | ||
|---|---|---|---|---|
| Alcohola | Mean (SD) | 186.55 (468.20) | 105.88 (369.50) | 147.25 (422.23) |
| Min – Max | 0 – 2288 | 0 – 1846 | 0 – 2288 | |
| Cigarettesb | Mean (SD) | 125.69 (359.56) | 14.41 (88.82) | 71.48 (269.09) |
| Min – Max | 0 – 1788.50 | 0 – 547.50 | 0 – 1788.50 | |
| Cannabisc | Mean (SD) | 15.38 (44.22) | 0.87 (3.56) | 8.31 (32.40) |
| Min – Max | 0 – 228 | 0 – 20 | 0 – 228 | |
| Amphetamined | Mean (SD) | 1.70 (6.95) | 0.00 | 0.87 (5.02) |
| Min – Max | 0 – 39 | 0 – 39 | ||
| Cocained | Mean (SD) | 0.50 (3.16) | 0.00 | 0.26 (2.26) |
| Min – Max | 0 – 20 | 0 – 20 | ||
| Hallucinogensd | Mean (SD) | 0.50 (3.16) | 0.00 | 0.26 (2.26) |
| Min – Max | 0 – 20 | 0 – 20 | ||
| Opioidsd | Mean (SD) | 0.70 (3.25) | 0.00 | 0.36 (2.34) |
| Min – Max | 0 – 20 | 0 – 20 | ||
| Any Drug Used | Mean (SD) | 18.78 (49.26) | 0.87 (3.56) | 10.05 (36.28) |
| Min – Max | 0 – 232 | 0 – 20 | 0 – 232 |
Number of drinks in lifetime (1 drink = 1 12oz beer; 1 mixed drink of 1 1/2oz liquor; 1 6oz glass of wine).
Number of packs of cigarettes (20 cigarettes = pack) smoked in lifetime.
Number of times cannabis was smoked in lifetime.
Number of times the drug was used in participant’s lifetime.
Figure 1.
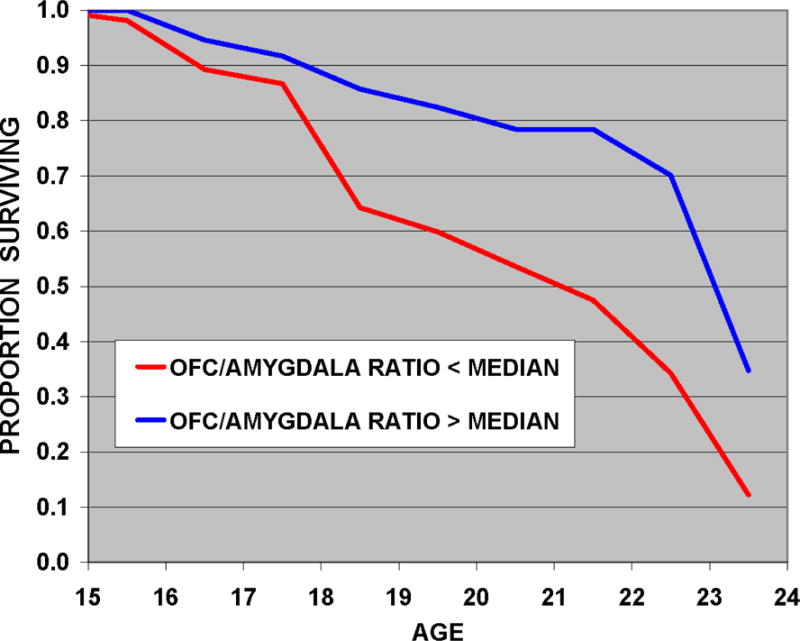
This Cox regression survival analysis of age at onset of substance use disorder (SUD) shows the influence of OFC/amygdala ratios on SUD outcome. The analysis included whether the individual was in a group with the OFC/amygdala ratio below or above the median of the sample, adjusted for risk group membership and age at scan. Individuals with volume ratios of the orbitofrontal cortex (OFC) to amygdala below the median had significantly earlier ages of substance use disorder onset than those with ratios above the median.
4. Discussion
Utilizing a sample of high-risk offspring from multiplex, alcohol-dependent families as well as low-risk offspring from control families, the current study found that substance use disorder outcome status in young adulthood is significantly influenced by both familial risk status and orbitofrontal cortex to amygdala volume ratio in adolescence. Our finding that the volume of the OFC relative to the volume of the amygdala was a stronger predictor of SUD outcome than either regional volume alone is consistent with neurobiological models of adolescent risk taking. These models suggest that the increased emotional reactivity, sensation seeking, and risky behaviors that typically characterize adolescence relate to the unique imbalance of functionally mature limbic regions and immature prefrontal regions in the adolescent brain (Albaugh et al., 2013; Casey et al., 2008; Galvan et al., 2007). Furthermore, individuals who demonstrate an exacerbated discrepancy in development of prefrontal versus limbic brain regions in adolescence are believed to be at especially high risk for poor outcomes (Casey et al., 2008; Galvan et al., 2007). The current study provides evidence that adolescents with a smaller volume of the OFC relative to volume of the amygdala are at especially high risk for substance use disorders in young adulthood.
In addition, this study provides further evidence of increased rates of substance use disorders in offspring from multiplex, alcohol-dependent families, as well as volumetric reductions in the right OFC. These results converge with previous findings of atypical morphology of the orbitofrontal cortex and amygdala among adolescent offspring from multiplex, alcohol-dependent families (Benegal et al., 2007; Dager et al., 2015; Hill et al., 2001; Hill et al., 2010; Hill et al., 2013; Hill et al., 2009b), as well as adults with AUD (Durazzo et al., 2011; Makris et al., 2008). Taken together, these results suggest that inherited abnormalities in amygdala and OFC may contribute to the high prevalence of SUDs in individuals with a family history of alcoholism (Cloninger et al., 1981; Hill et al., 2008; Hill et al., 2011; Verhulst et al., 2015).
The volumetric ratio of the orbitofrontal cortex to amygdala was related to substance use disorder outcomes in the full sample of high- and low-risk participants. Structure and function of the OFC and amygdala may contribute to relative risk and resilience within both high- and low-risk populations given the putative role of these regions in a broad range of cognitive and psychological functions. Evidence from prospective family studies of high-risk offspring, as well as cross-sectional research with healthy children and adults, indicates that variation in volume of the OFC and amygdala relate to several established risk factors for SUD, including impulsivity (Hill et al., 2009b; Verdejo-Garcia et al., 2008), decision making (Hill and O’Brien, 2015; O’Brien et al., 2014), externalizing behaviors (Ameis et al., 2014), and early temperament (Caspi et al., 1996; Hill et al., 2010; Williams et al., 2010). The morphology of the OFC and amygdala also relate to genetic variation in 5-HTTLPR and BDNF (Hill et al., 2013; Hill et al., 2009b), with these candidate genes also associated with SUD (Feinn et al., 2005; Janak et al., 2006). Thus, morphology of both prefrontal and subcortical regions may confer risk for SUD in adolescents with and without a family history of substance use disorders.
Although this is the first study to demonstrate a direct association between premorbid volumes of the OFC and amygdala and substance use disorder outcomes, one prospective study on a community sample of adolescents found that smaller orbitofrontal cortex volume at age 12 years predicted initiation of cannabis use by age 16 (Cheetham et al., 2012). Several studies have also shown that heavy drinking in adolescence is associated with atypical structure and function of prefrontal and limbic regions (Squeglia et al., 2014). However, because the neurotoxic effects of drugs and alcohol interact with genetically-mediated developmental processes during adolescence, it is unclear to what extent these findings reflect pre-existing vulnerability for SUD. The current study’s use of data from a longitudinal, prospective study in which imaging occurred an average of four years before SUD onset provides unique evidence of the joint role of the OFC and amygdala in premorbid risk for SUD.
One limitation of the current study is the relatively wide age range (ages 8–19 years) of the participants at the time of MRI scanning. However, all analyses included scan age as a statistical covariate. The current study also provides data on only early-onset substance use disorders, as the average age of participants’ last clinical follow-up was 21 years old at the time of data analyses. Although it is possible that some individuals free from SUD at the age of last follow-up will go on to develop alcohol or drug use disorders, early onset SUDs are associated with particularly poor long-term outcomes (Cloninger et al., 1981). Nevertheless, the present results suggest that neurobiological concomitants of familial risk status are important determinants of early onset substance use disorder.
Similarly, use of ultra-high-risk AD families can be viewed as either a strength or weakness of our findings. On the positive side, families with increased transmission for AD are ideal for finding endophenotypic characteristics associated with familial risk. However, these families are not representative of AD families in the general population; follow-up of offspring from these multiplex families indicates an exceptionally high rate of AUD and substance use by young adulthood (Hill et al., 2008; Hill et al., 2011). Although these families may not be representative of AUD families in the general population, the study of multiplex families provides an efficient means for identifying risk factors and genetic variation that can then be taken to population samples for replication.
In conclusion, the current study provides evidence that volumetric ratio of the orbitofrontal cortex to amygdala in adolescence is a prospective risk factor for substance use disorder outcome in young adulthood in both high-risk offspring from multiplex, alcohol-dependent families and low-risk controls. The OFC and amygdala are associated with a myriad range of cognitive, social, and behavioral functions, and future research assessing the role of these structures in risk for SUD is needed to elucidate the specific mechanisms by which they confer risk for alcohol and drug use disorders.
Highlights.
High-risk offspring from multiplex, alcohol dependent families, and low-risk control offspring underwent magnetic resonance imaging during adolescence
The ratio of orbitofrontal cortex to amygdala volume obtained during adolescence predicted substance use disorder outcomes in young adulthood
Both high- and low-risk offspring with smaller ratios of orbitofrontal cortex to amygdala volume had reduced survival time to substance use disorder onset
Acknowledgments
The authors wish to acknowledge the contribution of Shuhui Wang, M.D. and Howard Carter M.D. for manual tracing of brain volumes. Also, the contributions of numerous staff members current and past who assessed research participants, maintained databases, and assisted with clinical evaluations are greatly appreciated. We also acknowledge the contribution of the family members who have continued to be involved in clinical follow-up.
Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [grant numbers AA018289, AA05909, AA08082, and AA015168].
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ. Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: implications for the neural substrates of emotion regulation. Neuroimage. 2013;71:42–49. doi: 10.1016/j.neuroimage.2012.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, Zhao L, Khundrakpam B, Collins DL, Lerch JP, Wheeler A, Schachar R, Evans AC, Karama S. Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biol Psychiatry. 2014;75:65–72. doi: 10.1016/j.biopsych.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Chambers W, Puig-Antich J, Hirsch M, Paez P, Ambrosini P, Tabrizi M, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview: test–retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cottler L, Robins L, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, McKay DR, Kent JW, Jr, Curran JE, Knowles E, Sprooten E, Goring HH, Dyer TD, Pearlson GD, Olvera RL, Fox PT, Lovallo WR, Duggirala R, Almasy L, Blangero J, Glahn DC. Shared Genetic Factors Influence Amygdala Volumes and Risk for Alcoholism. Neuropsychopharmacology. 2015;40:412–420. doi: 10.1038/npp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the national institute of mental health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2015;40:43–49. doi: 10.1038/npp.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY. Neural plasticity, human genetics, and risk for alcohol dependence. Int Rev Neurobiol. 2010;91:53–94. doi: 10.1016/S0074-7742(10)91003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, O’Brien JW. Psychological and neurobiological precursors of alcohol use disorders in high-risk youth. Current Addiction Reports. 2015;2:104–113. doi: 10.1007/s40429-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Res. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: a prospective study. Biol Psychiatry. 2009a;66:750–757. doi: 10.1016/j.biopsych.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Tessner K, Wang S, Carter H, McDermott M. Temperament at 5 years of age predicts amygdala and orbitofrontal volume in the right hemisphere in adolescence. Psychiatry Res. 2010;182:14–21. doi: 10.1016/j.pscychresns.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Tessner KD, McDermott MD. Psychopathology in offspring from families of alcohol dependent female probands: a prospective study. J Psychiatr Res. 2011;45:285–294. doi: 10.1016/j.jpsychires.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, McDermott MD, Zezza N, Stiffler S. Amygdala Volume in Offspring from Multiplex for Alcohol Dependence Families: The Moderating Influence of Childhood Environment and 5-HTTLPR Variation. Journal of alcoholism and drug dependence Suppl. 2013;(Suppl 1) doi: 10.4172/2329-6488.S1-001. 001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2009b;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Janak PH, Wolf FW, Heberlein U, Pandey SC, Logrip ML, Ron D. BIG news in alcohol addiction: new findings on growth factor pathways BDNF, insulin, and GDNF. Alcohol Clin Exp Res. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Janca A, Robins L, Cottler L, Early T. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI field trials B wave II at the St. Louis site. Br J Psychiatry. 1992;160:815–818. doi: 10.1192/bjp.160.6.815. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JW, Lichenstein SD, Hill SY. Maladaptive decision making and substance use outcomes in high-risk individuals: preliminary evidence for the role of 5-HTTLPR variation. J Stud Alcohol Drugs. 2014;75:643–652. doi: 10.15288/jsad.2014.75.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Romanowski A, Banaschewski T, Barbot A, Barker GJ, Bruhl R, Buchel C, Charlet K, Conrod PJ, Czech K, Dalley JW, Flor H, Hake I, Ittermann B, Ivanov N, Mann K, Ludemann K, Martinot JL, Palafox C, Paus T, Poline JB, Reuter J, Rietschel M, Robbins TW, Smolka MN, Strohle A, Walaszek B, Kathmann N, Schumann G, Heinz A, Garavan H, Gallinat J, consortium, I. Common structural correlates of trait impulsiveness and perceptual reasoning in adolescence. Hum Brain Mapp. 2013;34:374–383. doi: 10.1002/hbm.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handb Clin Neurol. 2014;125:501–510. doi: 10.1016/B978-0-444-62619-6.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45:1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Fox NA, Lejuez CW, Reynolds EK, Henderson HA, Perez-Edgar KE, Steinberg L, Pine DS. Early temperament, propensity for risk-taking and adolescent substance-related problems: a prospective multi-method investigation. Addict Behav. 2010;35:1148–1151. doi: 10.1016/j.addbeh.2010.07.005. [DOI] [PubMed] [Google Scholar]


