Abstract
Studies from our laboratory showed that upregulation of glutamate transporter 1 (GLT-1) and cystine-glutamate exchanger (xCT) expression with ceftriaxone, β-lactam antibiotic, in the brain was associated with attenuation of ethanol consumption. In this study, we tested clavulanic acid, which is another β-lactam compound with negligible antimicrobial activity, on ethanol consumption and expression of GLT-1, xCT and glutamate aspartate transporter (GLAST) in male alcohol-preferring (P) rats. Clavulanic acid has the central β-lactam pharmacophore that is critical for the upregulation of GLT-1 and xCT expression. We found that clavulanic acid, at 5 mg/kg (i.p.) dose, significantly attenuated ethanol consumption and ethanol preference in P rats as compared to vehicle-treated group. This effect was associated with a significant increase in water intake in clavulanic acid treated group. Importantly, we found that clavulanic acid increased the expression of GLT-1 and xCT in nucleus accumbens. However, there was no effect of clavulanic acid on GLAST expression in the nucleus accumbens. Clavulanic acid treatment did not upregulate the expression of GLT-1, xCT and GLAST in prefrontal cortex. These findings revealed that clavulanic acid at 20–40 fold lower dose than ceftriaxone can attenuate ethanol consumption, in part through upregulation of GLT-1 and xCT expression in the nucleus accumbens. Thus, we suggest that clavulanic acid might be used as an alternative option to ceftriaxone to attenuate ethanol drinking behavior.
Keywords: GLT-1, xCT, GLAST, Glutamate, Clavulanic acid, ethanol dependence
Introduction
Ethanol dependence is a complex social and psychiatric problem that involves several neurotransmitter systems, including glutamate (1–3). Ethanol dependence induced a dysregulation of glutamatergic neurotransmission in brain regions involved in drug reward and reinforcement (4–6). This includes elevation of extracellular glutamate concentration and reduction in the expression of glial glutamate transporters in the mesocorticolimbic brain regions, including the nucleus accumbens (NAc) (4, 7–10). Glutamatergic projections from the prefrontal cortex (PFC) to the NAc are critical in the reinstatement to several drugs of abuse, including cocaine and heroin (11, 12). Extracellular glutamate concentration is regulated by several glutamate transporters (13, 14). Glutamate transporter 1 (GLT-1) is responsible for the uptake of majority of extracellular glutamate concentration in the brain (13, 15–17). Several studies from our laboratory demonstrated that chronic ethanol consumption decreased the expression of GLT-1 and its isoforms (GLT-1a and GLT-1b) (4, 7, 18–20).
Cystine/glutamate exchanger (xCT) is another glial glutamate transporter, which also regulates the extracellular glutamate concentration in the brain (21, 22). Reduction in xCT expression can lead to an impairment in glutamate uptake and increase in the extracellular glutamate concentration (22). xCT was downregulated following chronic ethanol exposure in alcohol-preferring (P) rats (7, 23). In addition, glutamate aspartate transporter (GLAST) is another glial glutamate transporter that is less expressed in the forebrain but highly expressed in the cerebellum (24).
Previous studies demonstrated that treatment with β-lactam antibiotic, ceftriaxone, reduced ethanol intake and attenuated the reinstatement of cocaine seeking behavior in rat animal model (20, 23, 25, 26). This effect was due to the fact that ceftriaxone restores the extracellular glutamate concentration in the NAc (4), and possibly in other brain regions. Ceftriaxone’s upregulatory effect on GLT-1 is mainly attributed to the β-lactam ring found in β-lactam compounds (27). A ceftriaxone alternative is clavulanic acid (CA), which is normally administered in combination with amoxicillin to overcome resistance to β-lactamase enzyme-producing bacteria. CA is another β-lactam compound that has a negligible therapeutic value as antimicrobial, orally active and stable with high bioavailability (64–75%) (28). CA structure has β-lactam core, which is required for the upregulation of GLT-1 (27). The drug was found to readily cross the blood brain barrier in patients with intact meninges, permitting its effects in the brain (29). These advantages suggest that CA can serve as potential safe compound to attenuate ethanol intake in rat animal model. In this study, we examined the effects of CA on ethanol consumption, ethanol preference, water intake, and body weight in P rats as it has been performed in previous studies from our laboratory with other β-lactam compounds (7, 23, 30). Furthermore, we investigated the effects of CA on the expression of the glial glutamate transporters such as GLT-1, xCT and GLAST.
MATERIALS AND METHODS
Animals
Male P rats were procured from Indiana University, School of Medicine (Indianapolis, IN, USA) at the age of 21–30 days, and housed in the Department of Laboratory Animal Resources, University of Toledo, Health Science Campus. At the age of 90 days, rats were individually housed in a plastic cage and divided into two groups: (a) Control group, which received intraperitoneal (i.p.) injections of saline for five consecutive days (n=7); and (b) CA group, which received i.p. injections of CA at dose of 5 mg/kg/day for five consecutive days (n=7). All animal procedures were in compliance and approved by the Institutional Animal Care and Use committee of The University of Toledo in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
Behavioral drinking paradigms
At the age of 90 days, rats had a free choice of two ethanol concentrations (15% and 30% v/v), water and food for a period of five weeks. After the third week, ethanol and water consumptions were measured three times a week for two weeks and considered as baseline intake. The P rats line displays excessive binge-like ethanol drinking with average ethanol intake of more than 5 g/kg/day, attaining blood ethanol concentration of 200 mg% (31, 32). Therefore, animals with a baseline ethanol consumption of less than 4 g/kg/day were not included in the study as performed in previous studies (20, 33, 34). During Week 6, vehicle or CA was administered for five consecutive days. Ethanol (15% and 30%) consumption, water intake and animal body weight were measured every day through the last day of the experiment.
Brain tissue harvesting
All rats were euthanized using carbon dioxide inhalation 24 hours after the last CA or vehicle i.p. injections and decapitated using guillotine. Brains were then removed and immediately stored at −80°C. Brain regions (NAc and PFC) were microdissected according to the Rat Brain Atlas (35) and stored at −80°C for Western blot analysis.
Western blot analysis for detection of GLT-1, xCT and GLAST
Isolated brain regions were examined for changes in GLT-1, xCT and GLAST expression relative to the total β-tubulin using Western blotting procedure as previously described (7, 26). Guinea pig anti-GLT1 (1:5,000), rabbit anti-xCT antibody (1:1,000), or rabbit anti-EAAT1 (GLAST) antibody (1:5,000) was used as primary antibody. The selection of secondary antibody was according to the primary antibody used; these secondary antibodies were guinea pig anti-GLT-1 secondary antibody (1:5,000) and rabbit anti-xCT secondary antibody (1:5000). β-tubulin was used as loading control (1:5,000). The detected bands were quantified using MCID system, and the results were expressed as a percentage of the ratio of tested protein/β-tubulin relative to control group (100% control-value) as performed in recent studies from our laboratory (7, 36).
Statistical analyses
Statistical analysis using two-way repeated measure ANOVA was conducted to determine the main effect of Days and Treatment x Day interaction on ethanol intake, ethanol preference, water intake, and body weight between control and CA groups. Bonferroni multiple comparisons post-hoc test was used whenever a significant main effect or interaction was found. An unpaired t-test was used to compare Western blot results (GLT-1/β-tubulin, xCT/β-tubulin and GLAST/β-tubulin) between control and CA groups. All statistical analyses were based on statistical significance value of p < 0.05.
RESULTS
Effect of CA on ethanol intake
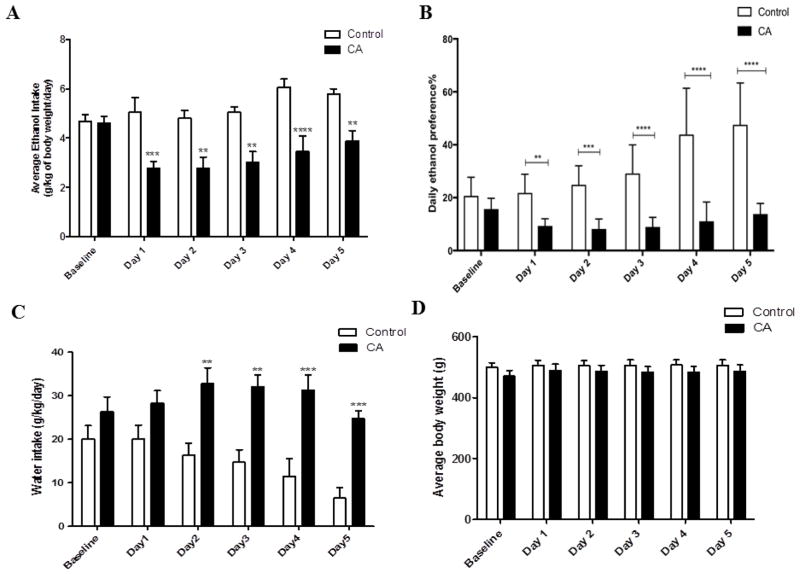
Statistical analysis using two-way repeated measure ANOVA revealed a significant main effect of Days [F (1, 5) = 4.605, p < 0.01] and significant Treatment x Day interaction [F (1, 5) = 4.241, p < 0.01]. Bonferroni multiple comparisons post-hoc test revealed a significant reduction in ethanol consumption in CA treated group as compared to control group starting on Day 1 (24 hrs after the first day of treatment) through Day 5 (p < 0.01) (Fig. 1A).
Figure 1.
Daily average ethanol intake, ethanol preference, water intake and body weight following CA treatment in P rats exposed to free choice of ethanol and water for five weeks. A) Effect of CA on average ethanol intake as compared to control group (g/kg/day). CA treatment induced significant reduction in ethanol intake as compared to control group starting 24 hours after the first day of treatment. B) Effect of CA on percent of ethanol preference as compared to control group. CA treatment induced significant decrease in ethanol preference as compared to control group. C) Effect of CA on water intake as compared to control group (g/kg/day). CA treatment induced a significant increase in water intake as compared to control group. D) Effect of CA on average body weight of P rats. There was no significant difference in the average body weight between the CA and control groups. Data are represented as mean ± SEM (**p < 0.01, ***p < 0.001, ****p < 0.0001), (n=7 for each group).
Effect of CA on ethanol preference
The percentage of ethanol preference was calculated daily by the following equation: [total ethanol consumption/total fluid consumption x 100] using the daily ethanol and water consumption for each rat as described previously (37). Two-way repeated measure ANOVA revealed a significant main effect of Day [F (1, 5) = 14.03, p < 0.0001] and significant Treatment x Day interaction [F (1, 5) = 10.2, p < 0.0001]. Bonferroni multiple comparisons post-hoc test revealed a significant reduction in ethanol preference from Day 1 through the last day of the experiment in CA group (p < 0.01) as compared to the control group (Fig. 1B).
Effect of CA on water intake
Two-way repeated measure ANOVA demonstrated a significant effect of Days [F (1, 5) = 4.470, p < 0.01] and significant Treatment x Day interaction [F (1, 5) = 3.225, p < 0.05]. Bonferroni multiple comparisons post-hoc test revealed a significant increase in water intake from Day 2 through Day 5 in rats treated with CA as compared to the control group (p < 0.01) (Fig. 1C).
Effect of CA on body weight
Two-way repeated measure ANOVA revealed a significant main effect of Days [F (1, 5) = 7.276, p < 0.0001]. However, there was no significant change in Treatment x Day interaction effect [F (1, 5) = 1.377, p > 0.05]. Bonferroni multiple comparisons post-hoc test revealed no significant difference in the body weight during the five days of treatment between CA-treated and control groups (Fig. 1D).
Effects of CA on the expression of GLT-1, xCT and GLAST in the NAc and PFC
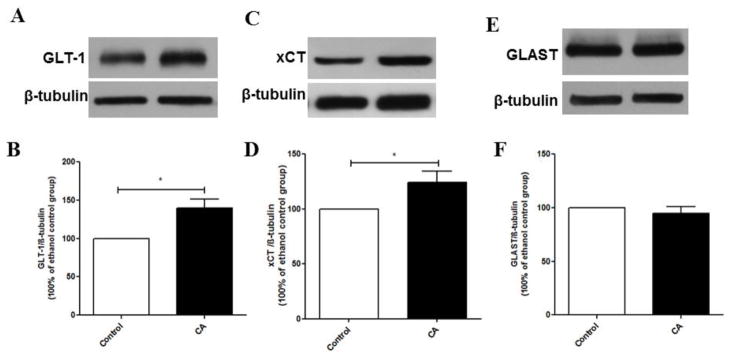
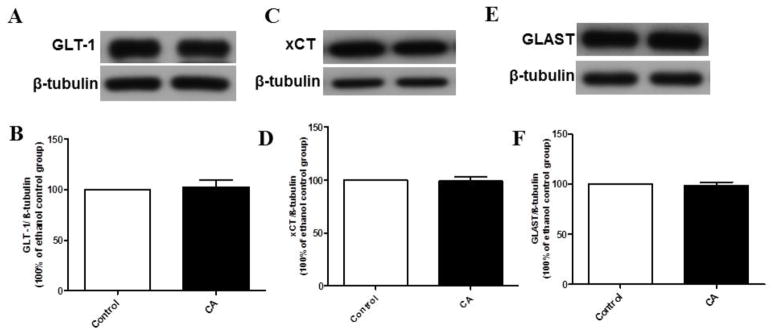
Statistical analysis of GLT-1 immunoblots in the NAc using unpaired t-test revealed a significant upregulation of GLT-1 expression in CA group as compared to the control group (p < 0.05), (Fig. 2A, B). In addition, unpaired t-test revealed a significant upregulation of xCT expression in CA group as compared to control group in the NAc (p < 0.05), (Fig. 2C, D). However, unpaired t-test did not reveal any significant difference in GLAST expression between CA and control groups in the NAc (p > 0.05), (Fig. 2E, F). Furthermore, we investigated the effect of CA treatment on the expression of GLT-1, xCT and GLAST in the PFC. CA treatment did not reveal any significant change in the expression of GLT-1, xCT and GLAST in the PFC as compared to control groups (Fig. 3 B, D, F).
Figure 2.
Effects of CA treatment on GLT-1, xCT and GLAST expression in the NAc. (A, C, E) Immunoblots for GLT-1/β-tubulin, xCT/β-tubulin, and GLAST/β-tubulin, respectively. (B) Quantitative analysis for the immunoblots revealed a significant upregulation of GLT-1 expression in the CA treated group as compared to control group in the NAc. (D) Quantitative analysis for the immunoblots revealed a significant upregulation of xCT expression in the CA treated group as compared to control group in the NAc. (F) Quantitative analysis for the immunoblots revealed no significant difference in GLAST expression between CA treated group and control group in the NAc. Data are represented as mean ± SEM, (*p < 0.05); (n=7 for each group).
Figure 3.
Effects of CA treatment on GLT-1, xCT and GLAST expression in the PFC. (A, C, E) Immunoblots for GLT-1/β-tubulin, xCT/β-tubulin and GLAST/β-tubulin, respectively. (B, D, F) Quantitative analysis for the immunoblots revealed no significant difference in GLT-1, xCT or GLAST expression in the CA treated group as compared to control group in the PFC. Data are represented as mean ± SEM; (n=7 for each group).
DISCUSSION
Several studies from our laboratory demonstrated that ceftriaxone can reduce ethanol consumption and upregulate GLT-1 expression in several brain regions (20, 23, 25). Due to the structural similarities between ceftriaxone and CA and the existence of the β-lactam core in both compounds, we proposed that CA might produce similar effect as ceftriaxone on ethanol consumption in P rats. Indeed, we revealed that treatment with CA at dose of 5 mg/kg for five consecutive days attenuated ethanol consumption starting from Day 1 throughout the treatment period. In addition, CA treatment significantly reduced ethanol preference starting from Day 1 throughout Day 5 as compared to control group. This is in accordance with previous studies from our laboratory that showed β-lactams treatment, including ceftriaxone and Augmentin, attenuated ethanol consumption starting from Day 1 (7, 25). This effect might be related to increase in the activity of GLT-1 or other unknown pharmacological effects. Furthermore, the reduction of ethanol consumption was associated with increase in water intake in CA treated group. This increase in water intake might be explained as compensatory mechanism for the total fluid intake in P rats as previously reported from our laboratory (7, 30, 33). It is important to note that CA i.p. injections did not change the body weight of P rats.
Glutamate transmission in central reward brain regions, particularly the NAc, plays critical role in dependence-related behavior, including drug-seeking behavior (38). The association between elevated extracellular glutamate concentration in the NAc and ethanol-seeking behavior has been previously determined (39, 40). Interestingly, upregulation of GLT-1 expression with ceftriaxone attenuated ethanol consumption and restored extracellular glutamate concentration in NAc (4). In this study, we revealed that CA treatment upregulated GLT-1 expression in the NAc but not in the PFC and attenuated ethanol consumption. A possibility for this differential effect of CA might be due to different levels of GLT-1 expression between NAc and PFC, which warranted further investigation. Previous reports from our laboratory have revealed that glial glutamate transporters, including GLT-1 and its isoforms were not altered in the PFC following chronic ethanol exposure in P rats (7, 18). To date, very little is known about the role of PFC glutamatergic neurotransmission in ethanol seeking behavior and other drugs of abuse.
While GLT-1 is responsible for regulating the majority of extracellular glutamate concentration, xCT plays a crucial role in controlling the extrasynaptic release of glutamate (41). xCT regulates the release of glutamate from astrocytes in exchange for cystine (42), and the impairment of xCT system has been involved in ethanol dependence in animal models (7, 23, 25, 43). Several studies have reported that β-lactam antibiotics induced a reduction in ethanol consumption, in part, by upregulating xCT expression in the NAc (7, 23, 25, 43). Similarly, CA treatment revealed a significant upregulation of xCT expression in the NAc but not in the PFC. CA administration did not change the expression of GLAST in the NAc and PFC. This is in accordance with previous studies from our laboratory that reported β-lactam treatment did not induce any significant difference in the GLAST expression as compared to the control group (43, 44).
Ceftriaxone treatment attenuated ethanol consumption and showed a potent upregulatory effect on GLT-1 expression in several brain regions (25, 33, 45) and increased the extracellular glutamate uptake in animal models with ethanol drinking behaviors (4). Ceftriaxone has poor brain penetrability, which requires higher doses to achieve desired efficacy. In contrast to ceftriaxone, CA shows favorable pharmacokinetic profile, including high brain penetration ability with cerebrospinal fluid/ plasma ratio of around 0.25 (29, 46). CA is a β-lactamase inhibitor with the existence of β-lactam core, which is considered as a critical site for the upregulation of GLT-1 expression in the brain (27). Importantly, the drug does not have any antibiotic activity as compared to ceftriaxone. CA has been reported to upregulate GLT-1 expression in mice (47). In this study, CA significantly attenuated ethanol consumption and upregulated GLT-1 expression in the NAc at 20-fold lower dose than ceftriaxone. We also showed, for the first time, that CA upregulated xCT expression in the NAc in P rats. These findings are in line with our previous reports, which demonstrated that β-lactam compounds attenuated ethanol consumption and upregulated GLT-1 and xCT expression in the NAc. Together, these findings provide ample evidence that CA can be a potential safe treatment for management of ethanol dependence and possibly other drugs of abuse.
Supplementary Material
Highlight.
Clavulanic acid (CA) attenuated ethanol consumption in P rats.
CA increased GLT-1 and xCT expression in the NAc.
CA did upregulate GLAST expression in the NAc.
CA did not upregulate GLT-1, xCT and GLAST expression in the PFC.
Acknowledgments
Funding
This work was supported by the National Institutes on Alcohol Abuse and Alcoholism (Award Number R01AA019458 to Y.S.). Alqassem Hakami was supported by a scholarship from King Saud bin Abdulaziz University for Health Sciences College of Medicine, Jeddah, Saudi Arabia.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman PL. Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis. 1995;10(1):73–9. doi: 10.1007/BF01991784. [DOI] [PubMed] [Google Scholar]
- 2.Rao P, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Frontiers in neuroscience. 2015:9. doi: 10.3389/fnins.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sari Y. Potential therapeutic role of glutamate transporter 1 for the treatment of alcohol dependence. OA Alcohol. 2013;1(1):6. doi: 10.13172/2053-0285-1-1-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33(11):1924–34. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakami AY, Hammad AM, Sari Y. Effects of Amoxicillin and Augmentin on Cystine-Glutamate Exchanger and Glutamate Transporter 1 Isoforms as well as Ethanol Intake in Alcohol-Preferring Rats. Front Neurosci. 2016;10:171. doi: 10.3389/fnins.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2005;29(3):326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- 9.Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology. 2007;190(4):415–31. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- 10.Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neuroscience letters. 1994;178(1):99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 11.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Journal of neuroscience. 2003;23(8):3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. Journal of Neuroscience. 2008;28(12):3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- 15.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 17.Danbolt N, Storm-Mathisen J, Kanner B. An [Na++ K+] coupledl-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience. 1992;51(2):295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- 18.Goodwani S, Rao PS, Bell RL, Sari Y. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res. 2015;1622:397–408. doi: 10.1016/j.brainres.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Frontiers in behavioral neuroscience. 2014;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci. 2013;51(3):779–87. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker D, Shen H, Kalivas P. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino acids. 2002;23(1–3):161–2. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- 22.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of neuroscience. 2005;25(27):6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231(20):4049–57. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. The Journal of neuroscience. 1998;18(21):8751–7. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao PS, Saternos H, Goodwani S, Sari Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29(29):9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 28.Davies B, Coates P, Clarke J, Thawley A, Sutton J. Bioavailability and pharmacokinetics of clavulanic acid in healthy subjects. International journal of clinical pharmacology, therapy, and toxicology. 1985;23(2):70–3. [PubMed] [Google Scholar]
- 29.Nakagawa H, Yamada M, Tokiyoshi K, Miyawaki Y, Kanayama T. Penetration of potassium clavulanate/ticarcillin sodium into cerebrospinal fluid in neurosurgical patients. The Japanese journal of antibiotics. 1994;47(1):93–101. [PubMed] [Google Scholar]
- 30.Alasmari F, Abuhamdah S, Sari Y. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neurosci Lett. 2015;600:148–52. doi: 10.1016/j.neulet.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction biology. 2006;11(3–4):270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 32.McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li T-K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats–animal models of alcoholism. Alcohol. 2014;48(3):209–15. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol and alcoholism. 2011;46(3):239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–35. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Compact. Vol. 1. San Diego: Academic Press Figure; 1997. pp. 25–30. [Google Scholar]
- 36.Althobaiti YS, Alshehri FS, Almalki AH, Sari Y. Effects of ceftriaxone on glial glutamate transporters in Wistar rats administered sequential ethanol and methamphetamine. Frontiers in Neuroscience. 2016:10. doi: 10.3389/fnins.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MR, Ruby CL, Hinton DJ, Choi S, Adams CA, Kang NY, et al. Striatal adenosine signaling regulates EAAT2 and astrocytic AQP4 expression and alcohol drinking in mice. Neuropsychopharmacology. 2013;38(3):437–45. doi: 10.1038/npp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalivas PW, LaLumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56:169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39(3):707–17. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapasova Z, Szumlinski KK. Strain Differences in Alcohol-Induced Neurochemical Plasticity: A Role for Accumbens Glutamate in Alcohol Intake. Alcoholism: Clinical and Experimental Research. 2008;32(4):617–31. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 41.Bridges R, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (system xc–) to normal and pathological glutamatergic signaling. Pharmacological reviews. 2012;64(3):780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. The Journal of physiology. 1999;514(3):783–93. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alasmari F, Rao P, Sari Y. Effects of cefazolin and cefoperazone on glutamate transporter 1 isoforms and cystine/glutamate exchanger as well as alcohol drinking behavior in male alcohol-preferring rats. Brain Research. 2016 doi: 10.1016/j.brainres.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, et al. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153(1):329–37. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolton G, Allen G, Davies B, Filer C, Jeffery D. The disposition of clavulanic acid in man. Xenobiotica. 1986;16(9):853–63. doi: 10.3109/00498258609038967. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, John J, Langford D, Walker E, Ward S, Rawls SM. Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino acids. 2015:1–8. doi: 10.1007/s00726-015-2117-8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.