Abstract
Background
The use of chemotherapy among patients with stage II colon cancer is controversial. We aimed to define the utilization and factors associated with the receipt of chemotherapy and the impact of chemotherapy on long-term prognosis among a large, multi-institutional cohort of patients.
Materials and Methods
We identified 876 patients who underwent resection for stage II colon cancer between 2004 and 2013 at one of 7 participating institutions. Overall (OS) and recurrence-free (RFS) survival time was calculated from the date of the index procedure to the date of death.
Results
163 patients (18.6%) received adjuvant chemotherapy and this utilization decreased over time (P=0.003). Younger age (P<0.001), margin positivity (OR 12.16, 95%CI 2.57–57.52; P=0.002) and the presence of perineural invasion (OR 1.24, 95%CI 1.07–1.44; P=0.005) increased the likelihood of receiving chemotherapy. Receipt of chemotherapy was associated with improved median OS and RFS. After controlling for all factors, the addition of oxaliplatin to 5-flurorouracil did not affect survival, and there was no difference in OS (HR: 0.74, 95%CI 0.27–2.06; P=0.57) or RFS (HR 0.71, 95%CI 0.32–1.58; P=0.88) with adjuvant treatment, including for patients with high-risk features (OS - HR: 0.63, 95%CI 0.33–1.19; P=0.15; RFS - HR: 0.77, 95%CI 0.32–1.86; P=0.56).
Conclusions
The utilization of chemotherapy has declined over time after resection for stage II colon cancer. Chemotherapy was not independently associated with improved OS or RFS in this study group, including in patients with high-risk features. Future prospective studies should strive to identify the subset of stage II colon cancer patients that will benefit the most from the addition of adjuvant chemotherapy.
Keywords: colon cancer, surgery, chemotherapy, outcomes, survival
Introduction
Colorectal cancer is the third most common cancer in the US and the second leading cause of cancer-related deaths.1 In 2015, there were an estimated 132,700 patients newly diagnosed with the disease resulting in nearly 50,000 deaths.1 As the use of surveillance colonoscopy and other diagnostic techniques have significantly increased over time, localized disease that has not spread to the regional lymph node basin is the most common presentation accounting for nearly 40% of all cases.1 Complete surgical resection along with analysis of at least 12 lymph nodes is the mainstay of treatment with curative intent for these resectable tumors.
For patients with advanced stage III and stage IV disease, the indications and survival advantage of adjuvant chemotherapy is well established. In stage III disease, adjuvant based chemotherapy with fluoropyrimides is recommended due to demonstrated improved overall survival (OS) of up to 33% after 5-years.2–4 However, the role of adjuvant chemotherapy among patients with stage II disease, where disease recurrence occurs in only approximately 25% of patients,5 is controversial6–9 and identifying patients with the highest risk of recurrence has been an ongoing challenge.10, 11 Based on current National Comprehensive Cancer Network (NCCN) clinical guidelines, the routine use of adjuvant chemotherapy is not recommended in the absence of definitive randomized controlled trials.12 However, along with the American Society of Clinical Oncology (ASCO), these guidelines recommend that patients with high-risk features including those with inadequately sampled nodes, T4 lesions, perforation, or poorly differentiated histology be considered for adjuvant chemotherapy.13 Recent studies have been mixed, however, in demonstrating a survival benefit with the addition of adjuvant chemotherapy in these high-risk patients.14, 15
We recently performed a hospital-based analysis of over 150,000 patients with stage II disease and found that improved OS was associated with adjuvant chemotherapy regardless of single vs. multi-agent regimen, patient age, or high-risk pathologic risk features.16 However, information on patient selection, specific chemotherapy agent, disease recurrence, and certain high-risk pathologic features was not available in this database. Therefore, we identified 7 participating large academic-affiliated and community-based institutions in the Advocate healthcare system, the largest fully integrated healthcare network in the state of Illinois that diagnoses and treats more cancer patients than any other system in Illinois, to address these limitations. The goal of the current study was to define the utilization and factors associated with the receipt of chemotherapy and the impact of chemotherapy on long-term prognosis among a large, multi-institutional cohort of patients undergoing curative-intent resection for colon cancer in a “real-world” setting.
Methods
Selection of Cohort
All patients who underwent resection for stage II colon cancer between 2004 and 2013 at one of 7 participating institutions were identified after IRB approval from each institution. Standard data on clinicopathologic characteristics were collected. All patients underwent resection with curative intent. Patients who were found to have a malignancy other than adenocarcinoma of the colon (ie. appendiceal tumor) were excluded. Furthermore, patients with rectal tumors were also excluded from analysis. Patients were classified according to whether or not they received chemotherapy. High-risk features included T4 tumors, the presence of lymphovascular invasion or perineural invasion, or those patients who had <12 nodes examined.12–14 Microsatellite instability was not distinguished as sporadic or germline, however the presence of microsatellite stability (MSS/absence of microsatellite instability) was considered higher risk than an instable tumor. In the instance that chemotherapy administration was related to surgical quality, hospitals with the lowest surgical quality were identified based on total number of nodes examined at the time of surgery as well as rates of margin positivity on final pathology. “Low performance” hospitals were labeled as those in the lower quartile based on the proportion of patients who had less than 12 nodes examined at the time of surgery as well as those with the lowest rate of R0 resections. All tumors are processed at a central pathology facility for the entire healthcare system. This controls for differences in processing between hospitals. Pathologists at the seven hospitals are part of the same group, and as per the systemwide policy, are held to the same standards, use the same synoptic reports, and must confirm all new diagnoses of malignancy by at least one other pathologist in their group. Patients who experienced a death within 30 days were excluded from analysis. The primary endpoint was death or time to first recurrence. Data on patient comorbidities and the incidence of perioperative complications was not collected.
Statistical Analysis
Discrete variables were described as medians with interquartile range. Categorical variables were described as totals and frequencies. Univariable comparisons were assessed using the chi-squared, analysis of variance, or Mann-Whitney U test as appropriate. Univariable and multivariable logistic regression models were constructed to determine the association of relevant clinicopathological factors with receipt of chemotherapy. The most parsimonious models were created using a stepwise approach including factors that were of clinical importance or were statistically significant on univariable analysis. Overall (OS) and recurrence-free (RFS) survival time was calculated from the date of the index procedure to the date of death. Survival adjusted for censoring was calculated using the Kaplan-Meyer method and median values compared using the log-rank test. Variables were entered into the fully adjusted Cox proportional hazards model based on statistical or clinical significance. Utilizing a power of 80% and a two-sided log-rank test at the 5% significance level, the minimal detectable hazard ratio reduction between patients receiving and not receiving chemotherapy was calculated to be 0.18. All analyses were carried out using STATA version 13.0 (StataCorp, College Station, TX), and a P-value of <0.05 (two-tailed) was considered statistically significant.
Results
Clincopathologic and Operative Characteristics of Cohort
We identified 876 patients who underwent resection for stage II colon cancer and met the inclusion criteria. The median age of the cohort was 74 years with a nearly equal split between male (n=422, 48.2%) and female (n=454, 51.8%) patients. (Table 1) The majority of patients were of Caucasian race (n=705, 80.5%). At the time of surgery, most patients had tumors in the ascending colon (n=474, 54.1%) with the remaining tumors located in the transverse (n=100, 11.4%), descending (n=57, 6.5%) or rectosigmoid (n=245, 28.0%) colon. On pathology, an overwhelming majority of patients had negative margins (n=843, 96.2%) with an average of 17 total lymph nodes examined (IQR: 13, 23). 151 patients (n=17.2%) had less than 12 nodes examined. Interestingly, the proportion of patients with at least 12 nodes examined varied by hospital (range: 1.0% – 34.2%; P<0.001). Hospitals in the lower quartile based on the proportion of patients who had less than 12 nodes examined at the time of surgery as well as those with the lowest rate of R0 resections were labeled as “low performance”. According to the American Joint Committee on Cancer (AJCC) 7th edition staging system, most patients had either stage IIA (n=744, 84.9%) or stage IIB (n=116, 13.2%) disease. High-risk microscopic features were reported on patients following 2010. Among this subset of patients 81% (n=281) of tumors had evidence of microsatellite stability, 16.7% (n=53) were found to have lymphovascular invasion, and 3.9% (n=12) had perineural invasion.
Table 1.
Clinicopathologic and operative characteristics of the cohort stratified by receipt of chemotherapy
| Total (n=876) | No Chemotherapy (n=713) | Chemotherapy (CTx) (n=163) | P-value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 74 (63, 81.5) | 76 (66, 83) | 62 (53, 72) | <0.001 |
| Male sex | 422 (48.2) | 341 (47.8) | 81 (50.0) | 0.67 |
| Ethnicity | 0.78 | |||
| Caucasian | 705 (80.5) | 577 (80.9) | 128 (78.6) | |
| Black | 129 (14.7) | 104 (14.6) | 25 (15.3) | |
| Asian | 24 (2.7) | 16 (2.2) | 8 (4.9) | |
| Location of Tumor | 0.66 | |||
| Ascending Colon | 474 (54.1) | 393 (55.1) | 81 (49.7) | |
| Transverse Colon | 100 (11.4) | 80 (11.2) | 20 (12.3) | |
| Descending Colon | 57 (6.5) | 45 (6.3) | 12 (7.4) | |
| Rectosigmoid Colon | 245 (28.0) | 195 (27.4) | 50 (30.7) | |
| Year of Surgery | 0.01 | |||
| Before 2010 | 530 (60.5) | 417 (58.5) | 113 (69.3) | |
| After 2010 | 346 (39.5) | 296 (41.5) | 50 (30.7) | |
| Stage | <0.001 | |||
| IIA | 744 (84.9) | 623 (87.4) | 121 (74.2) | |
| IIB | 116 (13.2) | 80 (11.2) | 36 (22.1) | |
| IIC | 16 (1.8) | 10 (1.4) | 6 (3.7) | |
| Lymph nodes examined (IQR) | 17 (13, 23) | 16 (13, 22) | 18 (14, 26) | 0.002 |
| Margins | 0.002 | |||
| R0 | 843 (96.2) | 693 (97.2) | 150 (92.0) | |
| R1 | 33 (3.8) | 20 (2.8) | 13 (8.0) | |
| Lymphovascular Invasion (N=317) | 53 (16.7) | 42 (15.4) | 11 (24.2) | 0.13 |
| Perineural Invasion (N=312) | 12 (3.9) | 6 (2.3) | 6 (13.3) | <0.001 |
| Microsatellite Stability (N=347) | 281 (81.2) | 245 (82.8) | 36 (72.0) | 0.07 |
Receipt of Chemotherapy
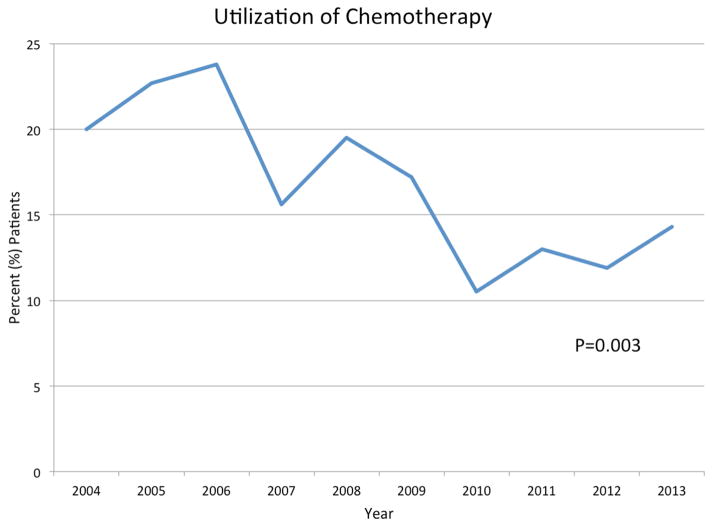
Following surgical resection, 163 patients (18.6%) received adjuvant chemotherapy. The most common form of chemotherapy received was FOLFOX (n=75, 46.0%) followed by 5-fluorouracil alone (n=49, 30.1%). The use of chemotherapy declined over time (P=0.003) (Figure 1). Several clinicopathologic differences were found among patients who did and did not receive chemotherapy. Younger patients (median age, chemotherapy: 62 years, IQR: 53, 72 vs. no chemotherapy: 76 years, IQR: 66, 83; P<0.001) and those who were treated prior to 2010 (P=0.003) more commonly received chemotherapy for their disease. However, nearly one-third of patients who received chemotherapy were over the age of 70 (n=49, 30.1%). Among pathologic features, patients with stage IIB or IIC disease (chemotherapy, stage IIA: n=121, 16.3% vs. stage IIB: n=36, 31.0% vs. stage IIC: n=6, 37.5%; P<0.001), those with greater number of lymph nodes examined (chemotherapy: 18, IQR: 14, 26 vs. no chemotherapy: 16, IQR: 13, 22; P=0.002), and those who had evidence of perineural invasion (chemotherapy: n=6, 13.3% vs. no chemotherapy: n=6, 2.3%; P=0.001) more commonly received chemotherapy. Among patients with no high-risk pathologic features, 85 patients (18.9%) received adjuvant chemotherapy. Chemotherapy usage did not differ based on hospital performance quality, regardless of the presence or absence of high-risk pathologic features (P>0.05).
Figure 1.
Utilization of adjuvant chemotherapy over time (P=0.003)
After controlling for all measurable factors, several clinicopathologic differences were found among patients who did and did not receive chemotherapy (Table 2). Among patient factors, each year of increasing age resulted in a decreased likelihood of receiving chemotherapy (OR 0.92, 95%CI 0.91–0.96; P<0.001). Pathologic features resulting in an increased likelihood of receiving chemotherapy included margin positivity (OR 12.16, 95%CI 2.57–57.52; P=0.002) and the presence of perineural invasion (OR 1.24, 95%CI 1.07–1.44; P=0.005).
Table 2.
Univariable and multivariable analysis of factors associated with receipt of chemotherapy
| Univariable Analysis
|
Multivariable Analysis
|
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.93 | 0.92–0.95 | <0.001 | 0.92 | 0.91–0.96 | <0.001 |
| Male Sex | 1.08 | 0.77–1.51 | 0.67 | |||
| Race | ||||||
| Caucasian | Ref | - | ||||
| Black | 1.08 | 0.68–1.75 | 0.73 | |||
| Asian | 1.86 | 0.76–4.58 | 0.18 | |||
| Tumor Location | ||||||
| Right Colon | Ref | - | ||||
| Transverse Colon | 1.21 | 0.70–2.09 | 0.49 | |||
| Descending Colon | 1.29 | 0.66–2.55 | 0.46 | |||
| Rectosigmoid Colon | 1.24 | 0.84–1.84 | 0.28 | |||
| <12 Nodes Examined | 1.99 | 1.16–3.40 | 0.01 | 7.09 | 0.81–61.99 | 0.08 |
| R1 Margin | 3.00 | 1.46–6.17 | 0.003 | 12.16 | 2.57–57.52 | 0.002 |
| Stage | ||||||
| IIA | Ref | - | Ref | - | ||
| IIB | 2.32 | 1.50–3.59 | <0.001 | 2.55 | 0.88–7.34 | 0.08 |
| IIC | 3.09 | 1.10–8.66 | 0.03 | 1.75 | 0.44–7.00 | 0.43 |
| Lymphovascular Invasion | 1.77 | 0.83–3.77 | 0.14 | |||
| Perineural Invasion | 6.69 | 2.06–21.79 | 0.002 | 1.24 | 1.07–1.44 | 0.005 |
| Microsatellite Stability | 0.54 | 0.27–1.06 | 0.08 | |||
Factors Associated with Overall and Recurrence-Free Survival
At a median follow-up of 50.56 months, mortality occurred in 295 (33.7%) patients. Median OS among the entire cohort was 106 months (95% CI 93.89 months-Not reached) with 1-, 3-, and 5-year OS being 93.8%, 82.1%, and 70.2%, respectively. Several factors were associated with shorter median OS (Table 3). Older patients >65 years of age had shorter median OS as compared to patients <65 years of age (>65 years: 80.21 months vs. <65 years: not reached: P<0.001). Similarly, patients with <12 lymph nodes examined (<12 lymph nodes: 67.65 months vs. =12 lymph nodes: 108.09 months; P=0.001), stage IIB or IIC disease (stage IIA disease: 121.63 months vs. stage IIB disease: 76.96 months vs. stage IIC disease: 42.63; P<0.001), and evidence of PNI (no PNI: 70.68 months vs. PNI: 36.98 months; P=0.008) had shorter OS. In the proportional hazards cox regression model, increasing age (HR: 1.06, 95%CI 1.02–1.09; P<0.001) and patients with stage IIB or IIC disease (stage IIB: HR: 2.42, 95%CI 1.17–5.03; stage IIC: HR: 3.84, 95%CI 1.03–14.35; both P<0.05) were found to be at independently higher risk of death.
Table 3.
Hazard regression analysis of factors associated with overall survival
| Survival Analysis | |||||
|---|---|---|---|---|---|
|
|
|
||||
| Variables | Median Survival (Months) | P value | Hazard Ratio | 95% CI | P value |
| Age | - | - | 1.06 | 1.02–1.09 | <0.001 |
| Race | |||||
| Caucasian | 108.1 | Ref | - | ||
| Black | 86.52 | 0.27 | 1.32 | 0.70–2.51 | 0.39 |
| Other | Not Reached | 0.68 | 0.94 | 0.18–4.89 | 0.94 |
| Tumor Location | |||||
| Right Colon | 94.64 | ||||
| Transverse Colon | 121.63 | 0.17 | |||
| Descending Colon | 99.28 | 0.60 | |||
| Rectosigmoid Colon | 120.35 | 0.52 | |||
| Total Nodes Examined | |||||
| <12 | 67.65 | ||||
| >12 | 108.09 | 0.001 | 0.62 | 0.30–1.26 | 0.18 |
| Margin Status | |||||
| R0 | 108.09 | Ref | - | ||
| R1 | 67.65 | 0.18 | 0.52 | 0.09–2.98 | 0.46 |
| Stage | |||||
| IIA | 121.63 | Ref | - | ||
| IIB | 76.96 | <0.001 | 2.42 | 1.17–5.03 | 0.02 |
| IIC | 42.63 | 3.84 | 1.03–14.35 | 0.04 | |
| Lymphovascular Invasion (LVI) | |||||
| No LVI | 67.36 | Ref | - | ||
| LVI | Not Reached | 0.67 | 0.75 | 0.34–1.63 | 0.47 |
| Perineural Invasion (PNI) | |||||
| No PNI | 70.68 | ||||
| PNI | 36.98 | 0.008 | 1.72 | 0.57–5.17 | 0.34 |
| Microsatellite Stability (MSS) | |||||
| MSS | 67.36 | Ref | - | ||
| Microsatellite Instability | Not Reached | 0.81 | 1.18 | 0.55–2.52 | 0.67 |
| Perioperative Therapy | |||||
| No Chemotherapy | 90.73 | Ref | - | ||
| Chemotherapy | Not Reached | <0.001 | 0.74 | 0.27–2.06 | 0.57 |
Recurrence occurred in 95 (10.8%) patients, most commonly at a distant site (n=52, 54.7%) consistent with previously reported data.17 Median RFS among the entire cohort was 94.48 months (95% CI 84.88–113.35 months) with 1-, 3-, and 5-year RFS being 90.6%, 74.6%, and 64.9%, respectively. Older patients >65 years of age had shorter RFS as compared to patients <65 years of age (>65 years: 76.53 months vs. <65 years: not reached: P<0.001). Similarly, patients with stage IIB disease (stage IIA disease: 104.71 months vs. stage IIB disease: 53.06 months; P<0.001) and those who had <12 nodes examined (<12 nodes: 63.12 months vs. >12 nodes: 100.20 months; P=0.003) had shorter RFS. In the proportional hazards cox regression model, increasing age (HR: 1.03, 95%CI 1.01–1.05; P=0.04) and patients with stage IIB disease (HR: 2.76, 95%CI 1.50–5.10; P=0.001) were found to be at independently higher risk of recurrence.
Effect of Chemotherapy on Overall and Recurrence-Free Survival
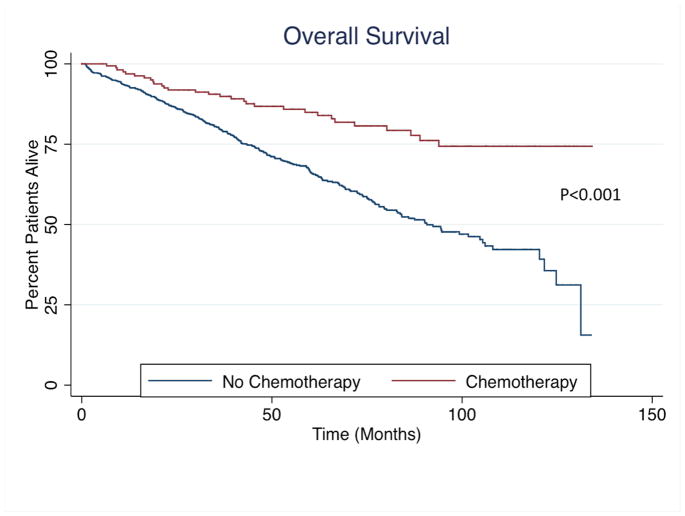
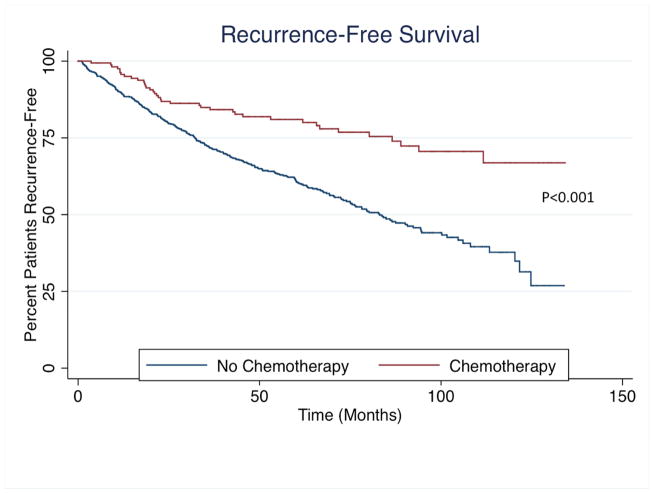
Patients receiving chemotherapy had longer median OS (chemotherapy: not reached vs. no chemotherapy: 90.73 months; P<0.001) and RFS (chemotherapy: not reached vs. no chemotherapy: 83.04 months; P<0.001) as compared to patients who did not receive adjuvant chemotherapy (Figure 2). After controlling for all factors, OS (HR: 0.74, 95%CI 0.27–2.06; P=0.57) and RFS (HR 0.71, 95%CI 0.32–1.58; P=0.88) were not affected by receipt of chemotherapy. Furthermore, among patients who received chemotherapy, the addition of oxaliplatin to 5-flurorouracil did not affect OS or RFS (both P>0.05).
Figure 2.
(A) Overall and (B) Recurrence-free survival among entire cohort stratified by receipt of chemotherapy
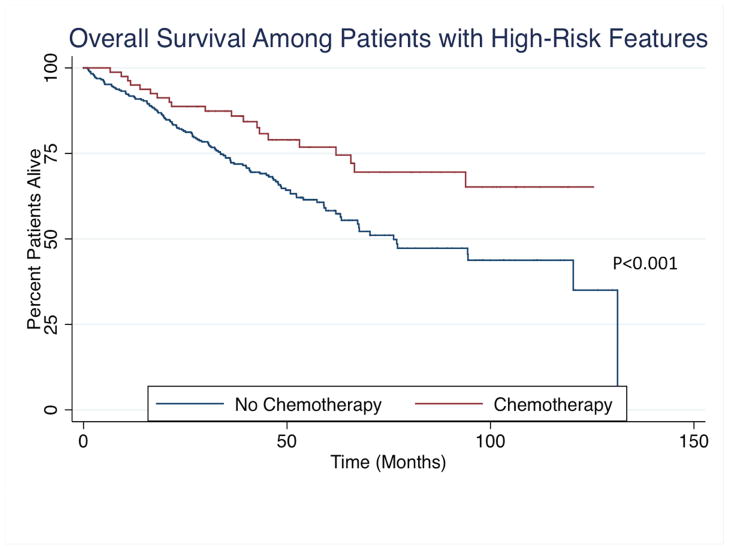
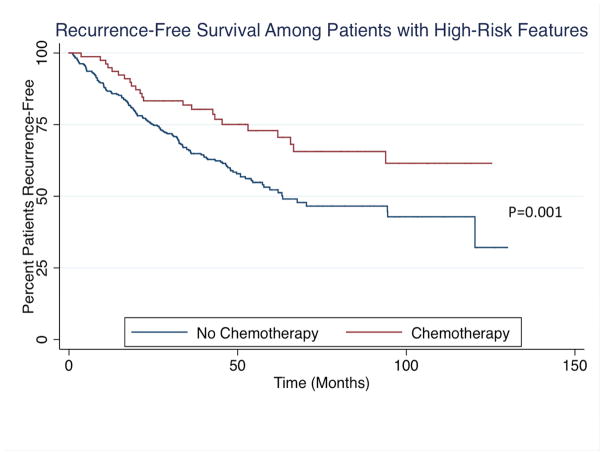
In a planned subset analysis of patients with high-risk features, chemotherapy on univariable analysis resulted in longer median OS (chemotherapy: not reached vs. no chemotherapy: 76.20 months) and RFS (chemotherapy: not reached vs. no chemotherapy: 63.35 months) as compared to patients who did not receive chemotherapy (Figure 3). After controlling for all factors, chemotherapy was not associated with improved OS (HR: 0.63, 95%CI 0.33–1.19; P=0.15) or RFS (HR: 0.77, 95%CI 0.32–1.86; P=0.56). In the subset of patients with microsatellite stable disease, chemotherapy again was not associated with improved OS (P=0.26) or RFS (P=0.38).
Figure 3.
(A) Overall and (B) Recurrence-free survival among patients with high-risk features stratified by receipt of chemotherapy
Discussion
Nearly 25% of patients who undergo surgical resection for localized colon cancer will experience disease recurrence.5 As such, adjuvant chemotherapy has been suggested as a potential modality to improve both overall and recurrence-free survival. Clinical trials addressing the use of chemotherapy in this patient , however, have been mixed.8, 18–21 Current NCCN and ASCO guidelines recommend practitioners to consider the use of chemotherapy only among stage II colon cancer patients with high-risk features.12, 13 The impact of national clinical guidelines on the use of adjuvant chemotherapy among stage II colon cancer patients in the US community-based setting is not well-known.
Referral patterns for adjuvant therapies in cancer patients following surgical resection are often nonrandom and involve a variety of demographic, clinical and pathologic factors. Previous studies have shown several patient-specific factors associated with receipt of adjuvant therapy in patients with other abdominal malignancies.22–24 Among patients with stage II colon cancer, Schrag et al. found that overall utilization among Medicare beneficiaries was 27%. The authors also found that race and comorbidity status were associated with receipt of chemotherapy. Similarly, Kirkpatrick et al. reported the utilization of chemotherapy to be 28% among 287 stage II colon cancer patients. Our data describes the utilization of chemotherapy among stage II colon cancer patients in one of the largest cohort of patients treated in a community-based setting. We found that the overall utilization of chemotherapy was 18.6% among our patients. Interestingly, the use of chemotherapy significantly decreased over time (Figure 1). Along with current practice guidelines, patients who had tumors exhibiting perineural invasion, as well as younger patients, were independently more likely to receive chemotherapy. Interestingly, nearly one-third of patients who received chemotherapy were over the age of 70 (n=49, 30.1%). Thus, there was an association between current guidelines and chemotherapy administration, and as a result, the number of patients receiving chemotherapy for stage II disease also decreased over time. Our data reflects that the studied patient cohort is representative of the utilization of chemotherapy based on current guidelines. Chemotherapy utilization was also consistent with the 20% of stage II colon cancer patients that received adjuvant treatment in a study of nearly 25,000 patients using the SEER-Medicare database.14 Therefore, the practice patterns identified in this study appear consistent with those across the nation. It is Interesting, that in the “real-world” setting, approximately 20% of oncologists are also treating low-risk stage 2 colon cancer with adjuvant chemotherapy.
After controlling for all measurable confounders, our data revealed that the addition of chemotherapy was not associated with improved overall or recurrence-free survival among patients with stage II colon cancer. In a recent meta-analysis of 12 randomized controlled trials, the authors found an improvement in both OS (HR 0.8; P<0.001) and disease-free survival (HR 0.86; P=0.03) among patients who received adjuvant chemotherapy.25 This study, however, did note publication bias and heterogeneity of trials as significant limitations of their analysis.25 Furthermore, the overall majority of trials included in this study did not show an OS or RFS benefit for patients with stage II colon cancer. Based on our power calculations, the impact of adjuvant chemotherapy among stage II colon cancer patients was likely limited in the current analysis in part due to sample size, with previous trials estimating 5,000 patients needed per arm to show a difference in survival with chemotherapy.26 Though limited by follow-up and sample size, in this cohort of patients with stage II colon cancer there was not a clear association of chemotherapy and survival, whereas in our analysis of over 150,000 patients in the National Cancer Database (NCDB), adjuvant chemotherapy was found to be associated with improved OS.16 Reasons for this difference may include improved quality of surgery in the studied cohort and less stage migration, as evidence by only 17.2% of patients who had <12 nodes evaluated in the current study as compared to 45% in the study utilizing the National Cancer Database.27, 28 In addition, despite propensity matching, unmeasured selection bias was acknowledged as a limitation of the NCDB study which may have affected the association of survival with chemotherapy use. Furthermore, and possibly related, 5-year OS in the current patient cohort (chemotherapy: 84.9%; no chemotherapy: 66.5%) was improved compared to patients in the National Cancer Database (chemotherapy: 81.2%; no chemotherapy 65.3%). Taken together, these results indicate that there is heterogeneous use of adjuvant chemotherapy among patients with stage II colon cancer, and the impact of chemotherapy among patients with high-risk features may be limited by the sample size of the current study.
Given the heterogeneity of conclusions regarding the impact of adjuvant chemotherapy in stage II disease, the NCCN and ASCO recommend the consideration of chemotherapy among patients with high-risk features.12, 13 In a study of 43,000 Medicare patients with stage II or III disease, adjuvant chemotherapy was not found to have a survival advantage, regardless of whether any high-risk features were present.29 Contrastingly, Kumar et al. reported adjuvant chemotherapy to improve outcomes in stage II colon cancer patients with high-risk features, particularly those with T4 tumors. Our data shows that patients T4 tumors were associated with over a 3-time higher risk of both death and recurrence. However, in our planned subset analysis of patients with high-risk features and among patients with only T4 tumors, there was not an overall or recurrence-free survival benefit with the addition of chemotherapy. Though not statistically significant, this may have been limited due to the large sample size required to identify a difference. Thus, the addition of chemotherapy may have a possible survival advantage in a certain subset of patients. Some authors suggest the evaluation of molecular tumor biomarkers in all stage II colon cancer patients in order to define which patients may benefit from adjuvant treatment.30 Our survival analysis using the current accepted definition of “high-risk” tumors, though would be stronger with greater patients numbers, adds information to the current available literature on the topic, and furthermore, helps us highlight a critical issue, which is that current definitions of risk are likely incomplete. Further prospective trials should focus on identifying stage II colon cancer patients with high-risk features and/or high-risk gene expression profiles in order to better select patients for adjuvant treatment.31
There are several limitations to be considered in this analysis. As with all retrospective studies, selection bias is always a possibility. We found that younger patients and those with certain high-risk features were more likely to receive chemotherapy. Therefore, this selection bias may underestimate the true impact of chemotherapy found among these patients, particularly among patients with high-risk features. This limitation, however, adds to the generalizability of the study as it reflects current practice patterns. Additionally, socioeconomic or insurance status was not obtained and thus was not able to be controlled for in our analysis. Finally, our analysis was limited by the lack of data on comorbidities and the incidence of perioperative complications that were not collected in the database utilized for this study. The goal of this study, however, was long-term overall and recurrence-free survival, though evaluation of potential selection bias secondary to patient performance status was unable to be performed.
In conclusion, the utilization of chemotherapy has declined over time with currently nearly 1 out of every 5 patients receiving adjuvant chemotherapy after resection for stage II colon cancer. We found that in the setting of high-quality curative intent surgery, chemotherapy was not independently associated with improved overall or recurrence-free survival, even among patients with high-risk features. Future studies should strive to identify the subset of stage II colon cancer patients that will benefit the most from the addition of adjuvant chemotherapy in a prospective fashion.
Acknowledgments
The authors would like to acknowledge Mary Kobialka, Dana Villenes and the cancer registrars for their assistance in compiling the dataset.
Footnotes
Authors’ Contributions – AE performed statistical analyses, interpretation of results, writing and critical review of the manuscript. LC performed data collection. AM was responsible for the design, oversight of the study, data interpretation, and critical review of the manuscript. All authors reviewed the report.
Conflict of interest statements – No conflicts of interest
Ethics committee approval – Approved by the IRB
Role of Funding Source: AVM was supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA190855. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. http://seer.cancer.gov/csr/1975_2013/. based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. The New England journal of medicine. 1990 Feb 8;322(6):352–8. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 3.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995 Dec;13(12):2936–43. doi: 10.1200/JCO.1995.13.12.2936. [DOI] [PubMed] [Google Scholar]
- 4.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995 Apr 15;345(8955):939–44. [PubMed] [Google Scholar]
- 5.Fang SH, Efron JE, Berho ME, Wexner SD. Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. Journal of the American College of Surgeons. 2014 Nov;219(5):1056–69. doi: 10.1016/j.jamcollsurg.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993 Oct;11(10):1879–87. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson NW, Yothers G, Lopa S, Costantino JP, Petrelli NJ, Wolmark N. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Annals of surgical oncology. 2010 Apr;17(4):959–66. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quasar Collaborative G. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007 Dec 15;370(9604):2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 9.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999 May;17(5):1356–63. [PubMed] [Google Scholar]
- 10.Mayer RJ. Oxaliplatin as part of adjuvant therapy for colon cancer: more complicated than once thought. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Sep 20;30(27):3325–7. doi: 10.1200/JCO.2012.44.1949. [DOI] [PubMed] [Google Scholar]
- 11.Meropol NJ. Ongoing challenge of stage II colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Sep 1;29(25):3346–8. doi: 10.1200/JCO.2011.35.4571. [DOI] [PubMed] [Google Scholar]
- 12.Benson AB, 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 3.2014. Journal of the National Comprehensive Cancer Network : JNCCN. 2014 Jul;12(7):1028–59. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 13.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Aug 15;22(16):3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Sep 1;29(25):3381–8. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015 Feb 15;121(4):527–34. doi: 10.1002/cncr.29072. [DOI] [PubMed] [Google Scholar]
- 16.Casadaban LRG, Aklilu M, Villines D, Freels S, Maker A. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer. 2016 doi: 10.1002/cncr.30181. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaniboni A, Labianca R Gruppo Italiano per lo Studio e la Cura dei Tumori del D. Adjuvant therapy for stage II colon cancer: an elephant in the living room? Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2004 Sep;15(9):1310–8. doi: 10.1093/annonc/mdh342. [DOI] [PubMed] [Google Scholar]
- 18.Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, et al. Adjuvant chemotherapy in colorectal cancer: a joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta oncologica. 2005;44(8):904–12. doi: 10.1080/02841860500355900. [DOI] [PubMed] [Google Scholar]
- 19.Hartung G, Hofheinz RD, Dencausse Y, Sturm J, Kopp-Schneider A, Dietrich G, et al. Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study. Onkologie. 2005 Jun;28(6–7):347–50. doi: 10.1159/000084595. [DOI] [PubMed] [Google Scholar]
- 20.Schippinger W, Samonigg H, Schaberl-Moser R, Greil R, Thodtmann R, Tschmelitsch J, et al. A prospective randomised phase III trial of adjuvant chemotherapy with 5-fluorouracil and leucovorin in patients with stage II colon cancer. British journal of cancer. 2007 Oct 22;97(8):1021–7. doi: 10.1038/sj.bjc.6604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe M, Kodaira S, Takahashi T, Tominaga T, Hojo K, Kato T, et al. Randomized trial of the efficacy of adjuvant chemotherapy for colon cancer with combination therapy incorporating the oral pyrimidine 1-hexylcarbamoyl-5-fluorouracil. Langenbeck's archives of surgery/Deutsche Gesellschaft fur Chirurgie. 2006 Aug;391(4):330–7. doi: 10.1007/s00423-006-0044-6. [DOI] [PubMed] [Google Scholar]
- 22.Hyder O, Dodson RM, Sachs T, Weiss M, Mayo SC, Choti MA, et al. Impact of adjuvant external beam radiotherapy on survival in surgically resected gallbladder adenocarcinoma: A propensity score-matched Surveillance, Epidemiology, and End Results analysis. Surgery. 2013 Jul 19; doi: 10.1016/j.surg.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejaz A, Spolverato G, Kim Y, Squires MH, Poultsides G, Fields R, et al. Impact of External-Beam Radiation Therapy on Outcomes Among Patients with Resected Gastric Cancer: A Multi-institutional Analysis. Annals of surgical oncology. 2014 Oct;21(11):3412–21. doi: 10.1245/s10434-014-3776-5. [DOI] [PubMed] [Google Scholar]
- 24.Davila JA, Chiao EY, Hasche JC, Petersen NJ, McGlynn KA, Shaib YH. Utilization and determinants of adjuvant therapy among older patients who receive curative surgery for pancreatic cancer. Pancreas. 2009 Jan;38(1):e18–25. doi: 10.1097/MPA.0b013e318187eb3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Zhang J, He X, Wang C, Lian L, Liu H, et al. Postoperative adjuvant chemotherapy for stage II colorectal cancer: a systematic review of 12 randomized controlled trials. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012 Mar;16(3):646–55. doi: 10.1007/s11605-011-1682-8. [DOI] [PubMed] [Google Scholar]
- 26.Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 May 15;22(10):1797–806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Stewart AK, Talamonti MS, Winchester DP, Russell TR, et al. Lymph node evaluation as a colon cancer quality measure: a national hospital report card. Journal of the National Cancer Institute. 2008 Sep 17;100(18):1310–7. doi: 10.1093/jnci/djn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilimoria KY, Stewart AK, Palis BE, Bentrem DJ, Talamonti MS, Ko CY. Adequacy and importance of lymph node evaluation for colon cancer in the elderly. Journal of the American College of Surgeons. 2008 Feb;206(2):247–54. doi: 10.1016/j.jamcollsurg.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 29.Neuman HB, Weiss JM, Schrag D, Ronk K, Havlena J, Loconte NK, et al. Patient Demographic and Tumor Characteristics Influencing Oncologist Follow-Up Frequency in Older Breast Cancer Survivors. Annals of surgical oncology. 2013 Aug 14; doi: 10.1245/s10434-013-3170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You YN, Rustin RB, Sullivan JD. Oncotype DX((R)) colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: A review of the evidence. Surgical oncology. 2015 Jun;24(2):61–6. doi: 10.1016/j.suronc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. The New England journal of medicine. 2016 Jan 21;374(3):211–22. doi: 10.1056/NEJMoa1506597. [DOI] [PMC free article] [PubMed] [Google Scholar]