Abstract
Precise identification of drinking and smoking patterns during pregnancy is crucial to better understand the risk to the fetus. The purpose of this manuscript is to describe the methodological approach used to define prenatal drinking and smoking trajectories from a large prospective pregnancy cohort, and to describe maternal characteristics associated with different exposure patterns. In the Safe Passage Study, detailed information regarding quantity, frequency, and timing of exposure was self-reported up to four times during pregnancy and at 1 month post-delivery. Exposure trajectories were developed using data from 11,692 pregnancies (9,912 women) where pregnancy outcome was known. Women were from three diverse populations: white (23%) and American Indian (17%) in the Northern Plains, US, and mixed ancestry (59%) in South Africa (other/not specified [1%]). Group-based trajectory modeling was used to identify 5 unique drinking trajectories (1 none/minimal, 2 quitting groups, 2 continuous groups) and 7 smoking trajectories (1 none/minimal, 2 quitting groups, 4 continuous groups). Women with pregnancies assigned to the low- or high-continuous drinking groups were less likely to have completed high school and were more likely to have enrolled in the study in the third trimester, be of mixed ancestry, or be depressed than those assigned to the none/minimal or quit-drinking groups. Results were similar when comparing continuous smokers to none/minimal and quit-smoking groups. Further, women classified as high- or low-continuous drinkers were more likely to smoke at moderate-, high-, and very high-continuous levels, as compared to women classified as non-drinkers and quitters. This is the first study of this size to utilize group-based trajectory modeling to identify unique prenatal drinking and smoking trajectories. These trajectories will be used in future analyses to determine which specific exposure patterns subsequently manifest as poor peri- and postnatal outcomes.
Keywords: pregnancy, ethanol, smoking, prenatal exposure, exposure patterns, group-based trajectory modeling
Introduction
In the US from 2002 to 2013, the most recent years for which data are available, 54% of women between the reproductive ages of 15 and 44 reported alcohol use and 23% reported binge drinking (five or more drinks per occasion, per the National Survey on Drug Use and Health; Slater, Haughwout, & Castle, 2015). Among the women reporting drinking in 2013, 29% also reported concurrent smoking (Slater et al., 2015). Despite public warnings and well-documented complications to the fetus due to alcohol and cigarette tobacco exposure, alone or in combination, in 2013, the proportions of women reporting drinking or smoking during pregnancy were 9.4% and 15.4%, respectively (Slater et al., 2015). It is likely that the proportion reporting drinking early in pregnancy is an underestimate, because many women do not change their drinking behavior until they learn they are pregnant and often do not include the period of time between conception and pregnancy confirmation when reflecting on drinking during pregnancy (Day, Wagener, & Taylor, 1985).
Alcohol and cigarette exposure information is generally obtained by either self-report or assessment of biomarkers, and both methods have limitations (Dawson, 2003; Day et al., 1985; Dukic, Niessner, Benowitz, Hans, & Wakschlag, 2007; Joya et al., 2012; Pickett, Rathouz, Kasza, Wakschlag, & Wright, 2005). Biomarkers are costly and are limited in their ability to provide quantity, frequency, and timing of exposure during specific time windows in pregnancy. Self-reported exposure measures may be summarized as indicators (e.g., binge drinking, counts such as number of days exposed or measures of quantity/intensity such as mean number of drinks or cigarettes per exposure day). Exposures assessed in pregnancy are often aggregated by trimester (Day et al., 1985) or even over the entire pregnancy. However, alcohol and cigarette tobacco exposure are time-varying, and more precise identification of quantity, frequency, and timing over the course of pregnancy (e.g., month, week) offers the opportunity for better understanding of the risk of exposure to the fetus (Bailey & Sokol, 2011; Day et al., 1985).
The Safe Passage Study, conducted by the Prenatal Alcohol in Sudden Infant Death Syndrome (SIDS) and Stillbirth (PASS) Network, was designed to investigate the role of prenatal drinking, modified by prenatal smoking, on poor peri- and postnatal outcomes, particularly SIDS and stillbirth, in populations at high risk for drinking and smoking during pregnancy (Dukes et al., 2014). The Safe Passage Study was a multicenter, prospective study that followed ~12,000 pregnancies and offspring for one postnatal year, the period of risk for SIDS. The high-risk maternal populations included the American Indians in the Northern Plains (NP), US, and the mixed ancestry women in Cape Town, South Africa (SA); white women and women of other racial backgrounds also participated. The research was overseen by the Network’s Steering Committee, an external Advisory and Safety Monitoring Board and by local institutional review boards and Tribal research councils (Angal, Petersen, Tobacco, & Elliott, 2016; Dukes et al., 2014). The purpose of this report is to describe the methodological approach used to define prenatal drinking and smoking trajectories from longitudinal data collected in the Safe Passage Study regarding quantity and frequency of alcohol and cigarette tobacco exposure, and to describe maternal demographic features associated with the different exposure patterns.
Material and methods
Study Design/Sample
Consenting and eligible women were enrolled during pregnancy and followed through 1 year post-delivery. Gestational age at enrollment was determined during the first prenatal visit using standard clinical practices at each study center – ultrasound in SA, and a combination of clinical examination, ultrasound, and last menstrual period in the NP. Maternal self-report was used to obtain drinking and smoking exposure information. Maternal characteristics collected include age, race, education, partner status, receiving government assistance, depression (based on Edinburgh Depression Scale scores ≥13), and gestational age at time of recruitment.
Self-reported Exposure Measures
Prenatal alcohol exposure information was obtained using the Timeline Follow-back (TLFB) interview (Sobell, Maisto, Sobell, & Cooper, 1979; Sobell & Sobell, 1979, 1992, 1995). Our study population required modification to the TLFB to include collection of additional details on sharing, type/brand of beverages consumed (to assign specific alcohol by volume), and container sizes (Dukes et al., this issue). Prenatal smoking exposure information was obtained by querying how often the participant smoked a tobacco cigarette using graduated frequency response options (i.e., none, monthly or less, 2–4 days per month, 2–3 days per week, 4–6 days per week, and 7 days per week) and the number of cigarettes smoked on a typical day. Collection of drinking and smoking exposure was through interviews administered by trained research staff (Dukes et al., this issue). Depending on the timing of participant enrollment, exposure information was collected up to four times during pregnancy: at recruitment (6+ gestational weeks), 20–24 weeks, 28–32 weeks (random subset only), and 34+ weeks, and also at 1 month post-delivery, each covering the 30-day reference period prior to the last reported drinking or smoking day. The first prenatal interview included information regarding alcohol exposure 1 year prior to pregnancy and around the last menstrual period (15 days before and 15 days after). Collection of exposure at multiple time points minimized the length of recall and captured data across pregnancy for use in identifying patterns of drinking and smoking.
Mean drinks per drinking day were calculated by summing standardized beverages (defined as 14 g of ethanol) consumed on each drinking day (Brick, 2006; ICAP, 1998). Binge drinking was defined as four or more drinks per occasion (NIAAA, 2004). Mean cigarettes per day were computed from the two smoking questions described above (see Appendix A for more details). Heavy smoking was defined as 10 or more cigarettes per day (Lubin et al., 2010, 2011, 2012; Odendaal, Steyn, Elliott, & Burd, 2009). Drinking and smoking days defined per month were derived by summing the numbers of days in a month for which the corresponding exposure status was known. Mean drinking and smoking measures were defined for each month in pregnancy; Month 1 was defined as 0 weeks, 1 day through 3 weeks, 6 days gestation; Month 2 as 4 weeks, 0 days through 7 weeks, 6 days gestation, etc., and Month 11 as 40 weeks, 0 days through 43 weeks 6 days gestation. Exposure validation metrics include pregnancy months defined, pregnancy months exposed, drinks per drinking day or cigarettes per day by pregnancy month, year prior to pregnancy and post-pregnancy.
Statistical Methods
Group-based trajectory modeling (GBTM) was used to identify distinct latent trajectories of prenatal alcohol exposure and distinct latent trajectories of prenatal cigarette exposure over the course of pregnancy. Drinks per drinking day was aggregated to month of pregnancy, and 4, 5, 6, and 7 group models were examined for both exposures. Because many women were unexposed during pregnancy and both drinks per drinking day and cigarettes smoked per day are discrete, zero-inflated Poisson was chosen as the probability distribution function to model exposures. Each exposure model, considered separately, was adjusted for number of days defined (number of days in the month where exposure status was known) in order to assign appropriate weight to the quantity variable based on how many days of data were available for a given month.
Both substantive and statistical criteria were used to determine the most appropriate and parsimonious models to describe drinking and smoking trajectory groups. We hypothesized between 4 and 7 trajectory groups for each exposure, based on review of the literature and substantive knowledge of exposure patterns in pregnancy. Statistical criteria included examination of: 1) overall model fit using the Bayesian Information Criterion (BIC) or Bayes Factor to determine the number of trajectory groups, although these are sensitive to large sample size and the number of parameters being estimated; 2) the model standard errors with concern for large values which imply over-parameterization; 3) review of individual posterior probabilities, which assess discrimination, aiming for mean probabilities >0.7 for each trajectory group; 4) the percentage of participants assigned to each trajectory group to ensure sufficient representation (recommended >5%); and 5) visual review of trajectory plots and inspection of 95% confidence intervals for overlap (Arrandale, Koehoorn, MacNab, & Kennedy, 2006; Jones, Nagin, & Roeder, 2001; Nagin, 1999). Graphical analysis in the form of spaghetti plots was also reviewed in a random sample of participants for each trajectory group to aid in the determination of order (e.g., linear, quadratic) for each trajectory.
To examine the effect of missing data, sensitivity analyses were performed on two subsets using GBTM as described above. The first subset included pregnancies that resulted in a miscarriage, stillbirth, or delivery prior to 28 weeks or were enrolled in the third trimester. The second subset included those with drinking and smoking status defined for all three trimesters of pregnancy.
After determination of the optimal number of drinking and smoking trajectories using GBTM methods, descriptive statistics of alcohol and cigarette exposure metrics and participant demographic characteristics were summarized using proportions for categorical measures and means and standard deviations for continuous measures. Where appropriate, information is presented by month, trimester, or drinking and smoking trajectory groups.
Chi-square, Mantel-Haenszel, and Analysis of Variance were used to test for associations between trajectory groups with respect to maternal characteristics and exposure validation metrics. Statistical significance was determined assuming two-sided tests with an α level of 0.05. No formal statistical testing was performed for subsequent pairwise comparisons between specific trajectory groups; results are presented descriptively. Analyses were performed using SAS/STAT® software, Version 9.3, Copyright© 2011 (Cary, NC, US) and SAS Proc Traj, a custom SAS procedure available for free download (Jones et al., 2001).
Results
Subjects
Enrollment for the Safe Passage Study began on August 1, 2007, and 1-year post-delivery follow-up was completed on October 4, 2016. A total of 10,088 women were enrolled from three diverse populations: white (23%) and American Indian (17%) in the NP, and mixed ancestry (59%) in SA (other/not specified [1%]). There were 11,892 pregnancies and 12,029 fetuses (1.2% twins). Exposure trajectories were developed where pregnancy outcome was known (98.3%), representing 11,692 pregnancies from 9,912 women; 1,581 women enrolled more than once (16%, range 2 to 6).
Exposure ascertainment
For the 11,692 pregnancies, drinking and smoking exposure information was successfully obtained at nearly 100% of eligible prenatal visits. Drinking status was known for all months of pregnancy for over 76% of pregnancies (Table 1) and for at least 6 of the months for 93.9% of pregnancies; the mean number of drinking days defined during pregnancy was 232.5 ± 58.7. Smoking status was known for all months of pregnancy for over 56% of pregnancies (Table 1) and for at least 6 of the months for 80.4% of pregnancies; the mean number of smoking days defined during pregnancy was 196.3 ± 80.2.
Table 1.
Drinking and Smoking Descriptives by Month
| Pregnancy Month | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 z | Summary y | |
|
| |||||||||||
| Drinking | |||||||||||
| Status known | 11153 (95.4%) | 9842 (84.2%) | 10094 (86.3%) | 10412 (89.1%) | 10896 (93.2%) | 11124 (95.1%) | 10472 (89.6%) | 10846 (92.8%) | 10682 (91.4%) | 9248 (79.1%) | 8914 (76.2%) |
| Days defined | 11153 | 9842 | 10094 | 10412 | 10896 | 11124 | 10472 | 10846 | 10682 | 9248 | 11692 |
| 24.9 (6.2) | 26.4 (5.2) | 26.5 (5.1) | 26.6 (4.9) | 27.0 (3.9) | 26.4 (4.8) | 27.0 (4.1) | 26.9 (4.0) | 26.1 (4.4) | 21.2 (10.3) | 232.5 (58.7) | |
| 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 22.0 | 257.0 | |
| 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 28.0 | 1.0 : 56.0 | 28.0 : 308.0 | |
| Drinkers | 3978 (35.7%) | 1782 (18.1%) | 916 (9.1%) | 935 (9.0%) | 1184 (10.9%) | 1353 (12.2%) | 751 (7.2%) | 869 (8.0%) | 1056 (9.9%) | 287 (3.1%) | 7003 (61.2%) |
| Drinks per drinking day | 3978 | 1782 | 916 | 935 | 1184 | 1353 | 751 | 869 | 1056 | 287 | |
| 5.3 (5.0) | 5.1 (5.0) | 4.7 (4.4) | 4.1 (3.8) | 4.0 (3.4) | 3.8 (3.7) | 3.8 (3.7) | 3.7 (3.3) | 3.3 (2.9) | 3.7 (4.3) | ||
| 4.0 | 3.8 | 3.6 | 3.5 | 3.5 | 2.9 | 3.1 | 3.1 | 2.7 | 2.8 | ||
| 0.0 : 38.0 | 0.0 : 38.0 | 0.0 : 38.0 | 0.0 : 38.0 | 0.0 : 30.2 | 0.0 : 35.5 | 0.0 : 38.0 | 0.0 : 28.2 | 0.0 : 23.3 | 0.0 : 47.8 | ||
| Smoking | |||||||||||
| Status known | 11677 (99.9%) | 7388 (63.2%) | 8162 (69.8%) | 8643 (73.9%) | 10022 (85.7%) | 10628 (90.9%) | 8922 (76.3%) | 10550 (90.2%) | 10510 (89.9%) | 6345 (54.3%) | 6613 (56.6%) |
| Days defined | 11677 | 7388 | 8162 | 8643 | 10022 | 10628 | 8922 | 10550 | 10510 | 6345 | 11692 |
| 27.0 (4.1) | 26.3 (5.3) | 25.4 (6.4) | 24.8 (7.1) | 24.7 (6.6) | 23.9 (6.9) | 25.5 (6.0) | 23.1 (7.5) | 24.0 (5.4) | 21.8 (10.5) | 196.3 (80.2) | |
| 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 23.0 | 228.0 | |
| 1.0 : 28.0 | 1.0 : 29.0 | 1.0 : 29.0 | 1.0 : 29.0 | 1.0 : 29.0 | 1.0 : 30.0 | 1.0 : 29.0 | 1.0 : 29.0 | 1.0 : 30.0 | 1.0 : 56.0 | 29.0 : 308.0 | |
| Smokers | 6225 (53.3%) | 1671 (22.6%) | 1848 (22.6%) | 2169 (25.1%) | 3535 (35.3%) | 4054 (38.1%) | 2425 (27.2%) | 4022 (38.1%) | 3973 (37.8%) | 679 (10.7%) | 6466 (56.3%) |
| Cigarettes per day | 6221 | 1670 | 1847 | 2169 | 3533 | 4045 | 2425 | 4021 | 3969 | 676 | |
| 6.3 (5.8) | 3.3 (3.9) | 3.8 (4.2) | 4.3 (4.8) | 4.3 (4.2) | 4.4 (4.0) | 4.1 (3.9) | 4.2 (3.9) | 4.3 (4.0) | 4.2 (4.0) | ||
| 5.0 | 2.0 | 3.0 | 3.0 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | ||
| 0.0 : 80.0 | 0.0 : 40.0 | 0.0 : 60.0 | 0.0 : 80.0 | 0.0 : 69.7 | 0.0 : 40.0 | 0.0 : 40.0 | 0.0 : 66.8 | 0.0 : 80.0 | 0.0 : 25.0 | ||
NOTE: Statistics reported are n, mean (S.D.), median, minimum, and maximum for continuous variables and n (%) for categorical variables.
Month 10 includes 11-month data for 22.3% of participants for drinking data and 15.1% of participants for smoking data.
- Days defined = total days defined in pregnancy
- Status known = status is known for all months of pregnancy
- Drinkers = ever drank in pregnancy
- Smokers = ever smoked in pregnancy
Extent of exposure
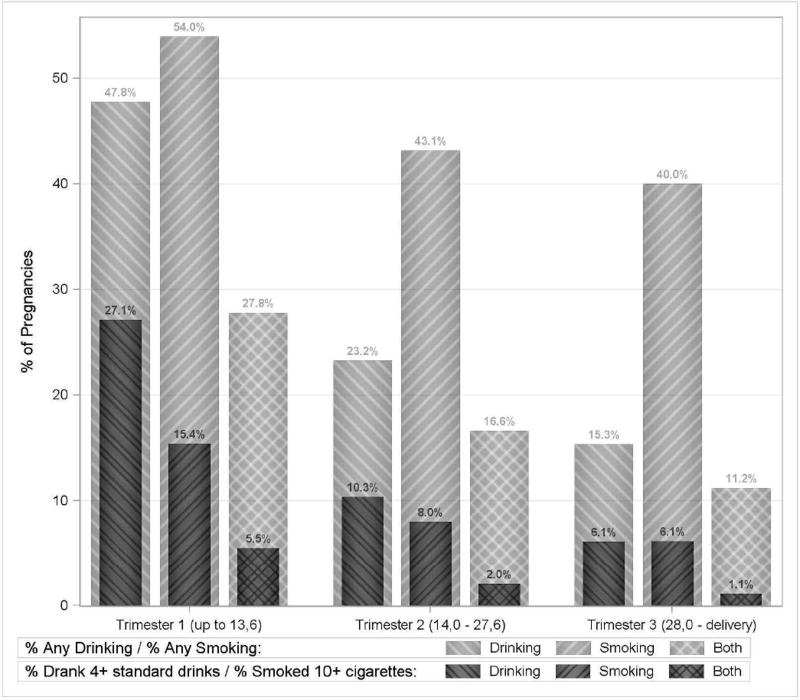
Women reported drinking during pregnancy in 61.2% of the 11,692 pregnancies, smoking in 56.3% (Table 1), and dual exposure in 37.4%. The proportion of pregnancies exposed, as well as the quantity of exposure, decreased over the course of pregnancy (Table 1, Fig. 1). In the first trimester of pregnancy, women reported drinking in 47.8% of pregnancies (27.1% binge-drank), smoking in 54.0% (15.4% smoked ≥10 cigarettes/day) and dual exposure in 27.8% (5.5% binge-drank and smoked ≥10 cigarettes/day) (Fig. 1). On average, consumption was 5.0 ± 4.6 drinks per drinking day among drinkers, and 5.4 ± 5.2 cigarettes per day among smokers. In the third trimester of pregnancy, 15.3% of pregnancies reported drinking (6.1% binge-drank), 40.0% reported smoking (6.1% smoked ≥10 cigarettes/day), and 11.2% reported both (1.1% binge-drank and smoked ≥10 cigarettes/day). On average, consumption was 3.3 ± 2.9 drinks per drinking day among drinkers, and 4.1 ± 3.9 cigarettes per day among smokers.
Fig. 1.
Self-reported drinking and smoking exposure by trimester of pregnancy (n = 11,692 pregnancies).
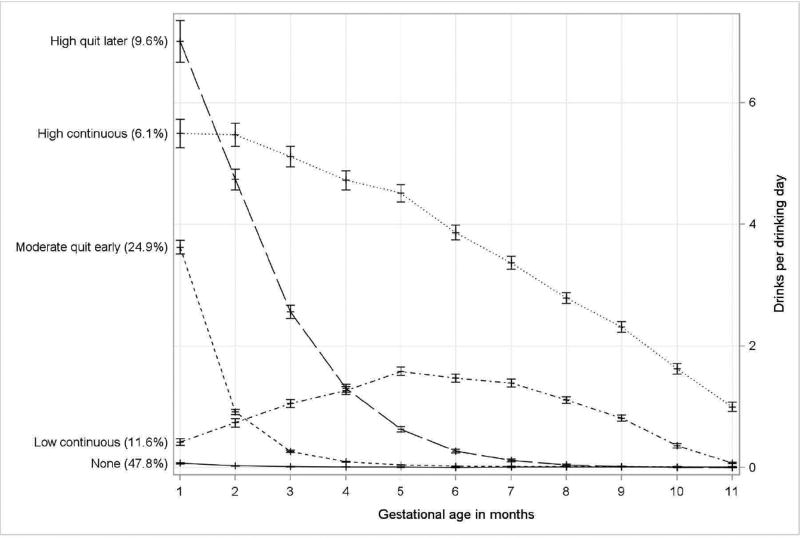
Drinking Trajectory Groups
The 5-group trajectory model (Fig. 2), with quadratic order for each group, was selected to best characterize prenatal alcohol exposure patterns during pregnancy. The 5 trajectories include: 1) non-drinkers/minimal exposure (none, 47.8% of pregnancies); 2) early moderate exposure with cessation around month 3 (moderate quit-early, 24.9% of pregnancies); 3) early high exposure with cessation around month 5 (high quit-later, 9.6% of pregnancies); 4) low continuous exposure (low-continuous, 11.6% of pregnancies); and 5) high continuous exposure (high-continuous, 6.1% of pregnancies). The average individual posterior probability for each trajectory group was greater than 0.92. Only 797 (6.8%) and 83 (0.7%) pregnancies had posterior probabilities less than 0.7 and less than 0.5, respectively. Upon visual inspection, there was no overlap in 95% confidence intervals for the drinking trajectory groups. Among women of mixed ancestry, 10.0% were classified as high- and 18.4% as low-continuous drinkers as compared to American Indians and white women, where <1.3% were classified as high-and <1.8% as low-continuous drinkers (Appendix B, Fig. 1).
Fig. 2.
Drinking trajectory groups based on drinks per drinking day in each month of pregnancy (n = 11,692 pregnancies).
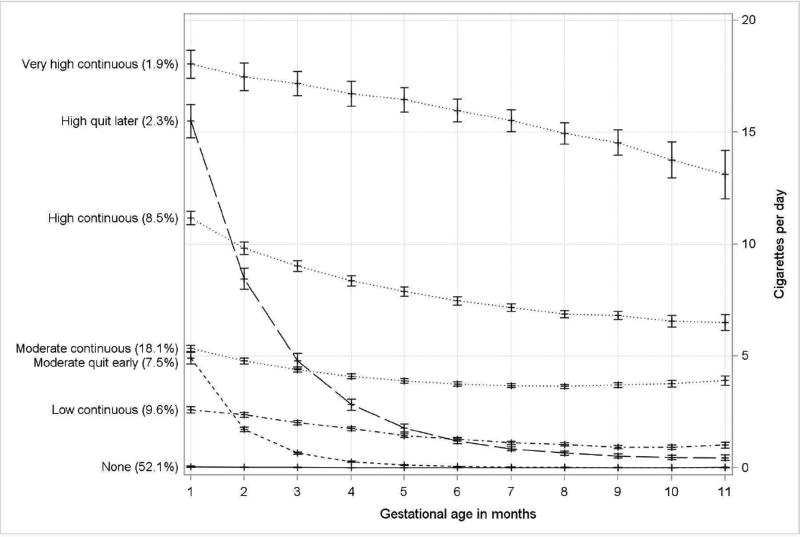
Smoking-Trajectory Groups
The 7-group trajectory model (Fig. 3), with quadratic order for each group, was selected to best characterize smoking patterns over pregnancy. The 7 trajectories include: 1) non-drinkers/minimal exposure (none, 52.1% of pregnancies); 2) early moderate exposure with cessation around month 3 (moderate quit-early, 7.5% of pregnancies); 3) early high exposure with cessation later in pregnancy (high quit-later, 2.3% of pregnancies); 4) low continuous exposure (low-continuous, 9.6% of pregnancies); 5) moderate continuous exposure (moderate-continuous, 18.1% of pregnancies); 6) high continuous exposure (high-continuous, 8.5% of pregnancies); and 7) very high continuous exposure (very high-continuous, 1.9% of pregnancies). While we aimed to develop groups with at least 5% of pregnancies, the high quit-later (n = 264) and very high-continuous (n = 217) smoking groups were retained as distinct and meaningful. The average individual posterior probability for each trajectory group was greater than 0.89. Only 683 (5.5%) and 33 (0.3%) pregnancies had posterior probabilities less than 0.7 and less than 0.5, respectively. Upon visual inspection, there was no overlap in the 95% confidence intervals for the smoking trajectory groups. Among women of mixed ancestry, 2.6% were classified as very high-continuous, 12.2% high-continuous, 28.2% moderate-continuous, and 12.8% low-continuous smokers as compared to American Indians and white women, where <1.3% were classified as very high-continuous, <3.5% as high-continuous, <7.0% as moderate-continuous, and <9.2% as low-continuous smokers (Appendix B, Fig. 2).
Fig. 3.
Smoking trajectory groups based on cigarettes per day in each month of pregnancy (n = 11,692 pregnancies).
Pregnancy Characteristics by Trajectory Groups
Overall, the mean maternal age was 25.6 ± 5.8 years and gestational age was 18.5 ± 6.7 weeks at the time of enrollment. Maternal characteristics were statistically significantly different across the 5 drinking-trajectory groups and the 7 smoking-trajectory groups (all p values <0.001; Tables 2 and 3). Pregnancies assigned to the low- or high-continuous drinking trajectory groups based on self-reported exposure were more likely to have enrolled in the third trimester (≥17.5%) than those assigned to the no-drinking or to either of the quit-drinking trajectory groups (≤10.5%). Continuous drinkers were less likely to have completed high school (≤18.7%) and more likely to be of mixed ancestry (≥95.0%), or depressed as defined by Edinburgh score ≥13 (≥59.0%), as compared to those reporting no drinking or quitting during pregnancy (Table 2). Maternal characteristics with respect to smoking-trajectory group assignment were similar to those of drinking-trajectory group assignments. Specifically, pregnancies assigned to the continuous-smoking groups (low, moderate, high, or very high) were from women who were less educated (≤24.8% completed high school), more likely to be of mixed ancestry (≥79.8%) or depressed (≥49.3%), and enrolled later in pregnancy (≥13.3% third trimester) as compared to the no-smoking or quit-smoking groups (Table 3).
Table 2.
Participant Characteristics by Drinking Trajectory Group
| Drinking Trajectory Group | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Statistic | None n=5585 |
Moderate quit early n=2932 |
High quit later n=1122 |
Low continuous n=1334 |
High continuous n=719 |
Overall n=11692 |
|
|
| |||||||
| Maternal age (years) | n | 5585 | 2932 | 1122 | 1334 | 719 | 11692 |
| mean (S.D.) | 26.0 (5.9) | 25.7 (5.5) | 24.6 (5.5) | 24.2 (5.8) | 26.3 (5.9) | 25.6 (5.8) | |
| median | 26.0 | 25.0 | 24.0 | 23.0 | 26.0 | 25.0 | |
| min : max | 15.0 : 45.0 | 16.0 : 44.0 | 15.0 : 43.0 | 16.0 : 43.0 | 16.0 : 42.0 | 15.0 : 45.0 | |
| Maternal race | |||||||
| American Indian or Alaska Native | n (%) | 1108 (19.8%) | 596 (20.3%) | 258 (23.0%) | 35 (2.6%) | 24 (3.3%) | 2021 (17.3%) |
| Mixed Ancestry | n (%) | 3004 (53.8%) | 1177 (40.1%) | 752 (67.0%) | 1267 (95.0%) | 688 (95.7%) | 6888 (58.9%) |
| White | n (%) | 1397 (25.0%) | 1138 (38.8%) | 105 (9.4%) | 29 (2.2%) | 3 (0.4%) | 2672 (22.9%) |
| Other/Unknown | n (%) | 76 (1.4%) | 21 (0.7%) | 7 (0.6%) | 3 (0.2%) | 4 (0.6%) | 111 (0.9%) |
| Trimester participant was recruited | |||||||
| First trimester | n (%) | 1550 (27.8%) | 784 (26.7%) | 236 (21.0%) | 245 (18.4%) | 124 (17.2%) | 2939 (25.1%) |
| Second trimester | n (%) | 3544 (63.5%) | 1978 (67.5%) | 768 (68.4%) | 855 (64.1%) | 441 (61.3%) | 7586 (64.9%) |
| Third trimester | n (%) | 491 (8.8%) | 170 (5.8%) | 118 (10.5%) | 234 (17.5%) | 154 (21.4%) | 1167 (10.0%) |
| Gestational age at recruitment (weeks) | n | 5585 | 2932 | 1122 | 1334 | 719 | 11692 |
| mean (S.D.) | 18.0 (6.6) | 17.3 (5.9) | 19.4 (6.5) | 21.1 (7.2) | 21.7 (7.5) | 18.5 (6.7) | |
| median | 17.0 | 16.4 | 18.9 | 20.7 | 21.9 | 17.4 | |
| min : max | 3.6 : 39.4 | 3.7 : 38.6 | 5.9 : 40.3 | 6.1 : 38.4 | 6.0 : 39.9 | 3.6 : 40.3 | |
| High school education | n (%) | 2837 (50.8%) | 1862 (63.5%) | 444 (39.6%) | 250 (18.7%) | 106 (14.7%) | 5499 (47.0%) |
| Living with spouse or partner | n (%) | 5065 (90.7%) | 2604 (88.8%) | 936 (83.4%) | 1223 (91.7%) | 639 (88.9%) | 10467 (89.5%) |
| Edinburgh Depression Scale ≥13 | n (%) | 1769 (31.7%) | 735 (25.1%) | 480 (42.8%) | 787 (59.0%) | 430 (59.8%) | 4201 (35.9%) |
| Received government assistance | n (%) | 1967 (35.2%) | 905 (30.9%) | 414 (36.9%) | 376 (28.2%) | 285 (39.6%) | 3947 (33.8%) |
NOTE: All tests for differences across trajectory groups are significant (p values <0.0001)
Table 3.
Participant Characteristics by Smoking Trajectory Group
| Smoking Trajectory Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Statistic | None n=6109 |
Moderate quit early n=871 |
High quit later n=264 |
Low continuous n=1105 |
Moderate continuous n=2135 |
High continuous n=991 |
Very high- continuous n=217 |
Overall n=11692 |
|
|
| |||||||||
| Maternal age (years) | n | 6109 | 871 | 264 | 1105 | 2135 | 991 | 217 | 11692 |
| mean (S.D.) | 26.4 (5.7) | 23.7 (5.3) | 24.5 (5.4) | 23.3 (5.3) | 24.6 (5.7) | 26.5 (5.9) | 28.5 (6.2) | 25.6 (5.8) | |
| median | 26.0 | 23.0 | 24.0 | 22.0 | 24.0 | 26.0 | 28.0 | 25.0 | |
| min : max | 16.0 : 44.0 | 15.0 : 44.0 | 16.0 : 42.0 | 16.0 : 43.0 | 15.0 : 43.0 | 16.0 : 45.0 | 16.0 : 42.0 | 15.0 : 45.0 | |
| Maternal race | |||||||||
| American Indian or Alaska Native | n (%) | 1198 (19.6%) | 344 (39.5%) | 76 (28.8%) | 185 (16.7%) | 140 (6.6%) | 68 (6.9%) | 10 (4.6%) | 2021 (17.3%) |
| Mixed Ancestry | n (%) | 2546 (41.7%) | 372 (42.7%) | 131 (49.6%) | 882 (79.8%) | 1941 (90.9%) | 840 (84.8%) | 176 (81.1%) | 6888 (58.9%) |
| White | n (%) | 2278 (37.3%) | 150 (17.2%) | 51 (19.3%) | 33 (3.0%) | 51 (2.4%) | 78 (7.9%) | 31 (14.3%) | 2672 (22.9%) |
| Other/Unknown | n (%) | 87 (1.4%) | 5 (0.6%) | 6 (2.3%) | 5 (0.5%) | 3 (0.1%) | 5 (0.5%) | 0 (0.0%) | 111 (0.9%) |
| Trimester participant was recruited | |||||||||
| First trimester | n (%) | 1567 (25.7%) | 294 (33.8%) | 72 (27.3%) | 274 (24.8%) | 476 (22.3%) | 216 (21.8%) | 40 (18.4%) | 2939 (25.1%) |
| Second trimester | n (%) | 4119 (67.4%) | 523 (60.0%) | 170 (64.4%) | 673 (60.9%) | 1324 (62.0%) | 643 (64.9%) | 134 (61.8%) | 7586 (64.9%) |
| Third trimester | n (%) | 423 (6.9%) | 54 (6.2%) | 22 (8.3%) | 158 (14.3%) | 335 (15.7%) | 132 (13.3%) | 43 (19.8%) | 1167 (10.0%) |
| Gestational age at recruitment (weeks) | n | 6109 | 871 | 264 | 1105 | 2135 | 991 | 217 | 11692 |
| mean (S.D.) | 17.6 (6.1) | 16.8 (6.3) | 17.8 (6.4) | 19.8 (7.3) | 20.2 (7.2) | 20.0 (6.9) | 21.2 (7.6) | 18.5 (6.7) | |
| median | 16.6 | 16.0 | 16.9 | 19.4 | 20.0 | 19.9 | 20.0 | 17.4 | |
| min : max | 3.6 : 39.4 | 5.3 : 39.1 | 6.3 : 38.4 | 5.4 : 40.3 | 5.0 : 39.9 | 4.0 : 38.6 | 5.3 : 37.7 | 3.6 : 40.3 | |
| High school education | n (%) | 3946 (64.6%) | 446 (51.2%) | 121 (45.8%) | 274 (24.8%) | 419 (19.6%) | 246 (24.8%) | 47 (21.7%) | 5499 (47.0%) |
| Living with spouse or partner | n (%) | 5532 (90.6%) | 724 (83.1%) | 223 (84.5%) | 984 (89.0%) | 1930 (90.4%) | 889 (89.7%) | 185 (85.3%) | 10467 (89.5%) |
| Edinburgh Depression Scale >= 13 | n (%) | 1529 (25.0%) | 243 (27.9%) | 95 (36.0%) | 545 (49.3%) | 1141 (53.4%) | 531 (53.6%) | 117 (53.9%) | 4201 (35.9%) |
| Received government assistance | n (%) | 1811 (29.6%) | 402 (46.2%) | 119 (45.1%) | 375 (33.9%) | 756 (35.4%) | 390 (39.4%) | 94 (43.3%) | 3947 (33.8%) |
NOTE: All tests for differences across trajectory groups are significant (p values <0.0001)
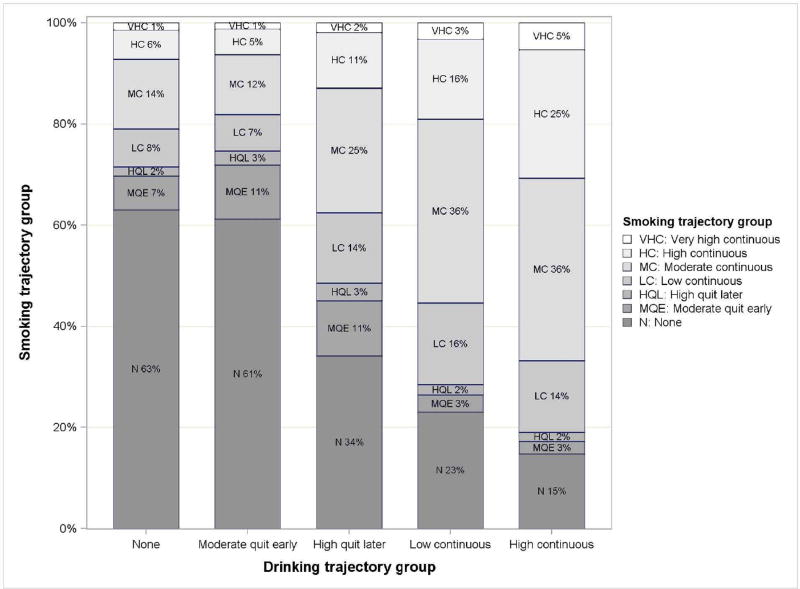
Dual Exposure
There is a statistically significant linear trend between drinking- and smoking-trajectory groups (p < 0.0001) (Fig. 4). Continuous drinkers were progressively more likely to smoke and to smoke higher quantities. Specifically, they were more likely to smoke at moderate-continuous, high-continuous, and very high-continuous levels as compared to quitters or those in the no-drinking trajectory group. For example, of pregnancies assigned to the high-continuous drinking group, 36%, 25%, and 5% smoked at moderate-, high-, and very high-continuous levels, respectively, compared to 14%, 6%, and 1% in the no-drinking group. Mixed ancestry women were more likely to report dual exposure (47.6%) as compared to American Indian (36.8%) and white women (13.0%).
Fig. 4.
Pregnancy smoking by drinking trajectory groups (n = 11,692 pregnancies). For the five drinking groups, values in the bars indicate the percentage of pregnancies assigned to each of the seven smoking groups (e.g., “N 63%”: of pregnancies assigned to the None drinking group, 63% were also assigned to the None smoking group).
Exposure Metrics by Drinking and Smoking Trajectory Groups
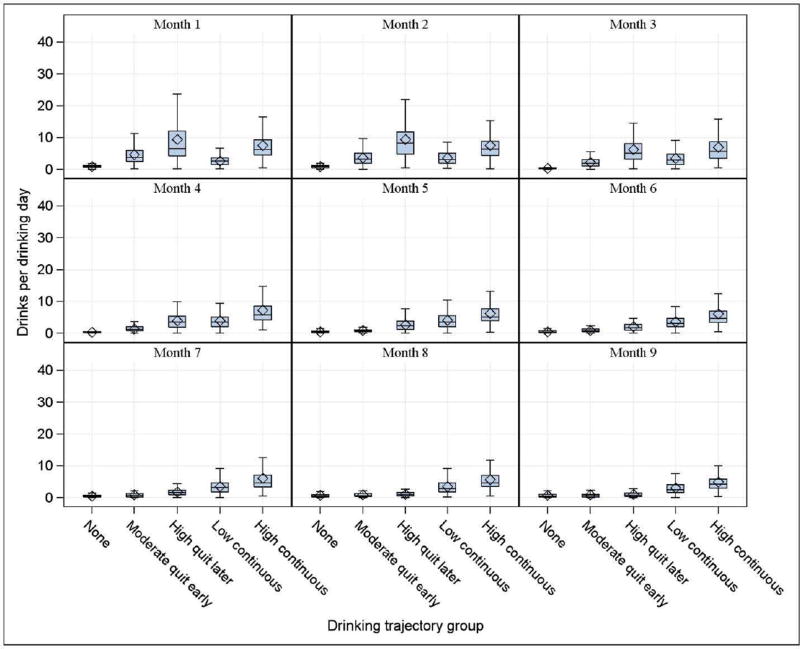
The quantity of drinking and smoking aligned with the exposure trajectories across the months of pregnancy (Figs. 5 & 6). Mean drinks per drinking day (DPDD) and cigarettes per day (CPD) were essentially zero in the no-smoking and no-drinking groups for all months of pregnancy (Fig. 5). After month 4, the moderate quit-early drinking group demonstrated a decline in DPDD to nearly correspond with the no-drinking group. The high quit-later drinking group showed a similar decline by month 7. Between months 5 and 9, women assigned to the high continuous-drinking trajectory group continued to report a higher number of drinks per drinking day compared to the other four trajectory groups, although there is some decline in the later months of pregnancy (high-continuous reported 4.3 ± 4.4 DPDD in month 5 and 2.8 ± 3.5 DPDD in month 9) (Appendix B, Table 1). Pregnancies with continuous exposure had fewer weeks with drinking status defined (low-continuous 26.4 ± 7.8, high-continuous: 22.0 ± 7.2) as compared to the no-drinking or quitting groups (no-drinking: 37.0 ± 6.2, moderate quit-early: 36.0 ± 6.3, high quit-later: 31.9 ± 7.1). The moderate quit-early smoking group looks similar to the no-smoking group by month 3, while the high quit-later group did not look similar to the no-smoking group until month 6 (Fig. 6). There does not appear to be much change in the distribution of CPD across pregnancy for the moderate-, high-, and very high-continuous smoking groups. Pregnancies assigned to the very high-continuous group involved smoking greater than 14 cigarettes per day, on average, during months 1 through 9 of pregnancy, as compared to 0 cigarettes per day in the none-trajectory group (Appendix B, Table 2). Pregnancies with continuous exposure had fewer weeks with smoking status defined as compared to the no-smoking or quitting groups.
Fig. 5.
Drinks per drinking day by drinking trajectory group in each month of pregnancy (n = 11,692 pregnancies).
Fig. 6.
Cigarettes per day by smoking trajectory group in each month of pregnancy (n = 11,692 pregnancies).
Re-enrollments (n = 1,581 women)
Of the women participating more than one time in the Safe Passage Study, 50% were assigned to the same drinking trajectory group and 67% were assigned to the same smoking trajectory group in all pregnancies. Restricting the sample to women who enrolled exactly twice with singleton pregnancies for both (n = 1,345), 26.3% increased drinking, 22.8% decreased drinking, and 50.9% did not change drinking patterns or trajectory group assignment in the second pregnancy as compared to the first. Of these 1,345 women, 17.0% increased, 14.0% decreased, and 69.0% remained unchanged with respect to smoking trajectories in the second pregnancy as compared to the first.
Sensitivity Analysis
The two sensitivity analyses performed to examine the effect of missing data showed nearly identical results for both drinking and smoking trajectory classifications (Appendix B, Figs. 3–6). The first subset included pregnancies that resulted in a miscarriage, stillbirth, or delivery prior to 28 weeks or were enrolled in the third trimester (incomplete study, n = 1,425). The second subset included those with drinking and smoking status defined for all three trimesters of pregnancy (all trimesters defined, n = 10,720). In all cases, the plots look nearly identical to those from the full cohort. There is some shift in percentages, as well as more variability in the data for the ‘incomplete study’ subset, due to the smaller sample size, but percentages and variability are nearly identical in the ‘all trimesters defined’ subset.
Discussion
When investigating the role of prenatal drinking and smoking in poor fetal and postnatal outcome, it is critical to examine the effects of quantity, frequency, and timing of exposure during pregnancy. We used group-based trajectory modeling to develop distinct trajectories of maternal drinking and smoking exposure during pregnancy, derived from the detailed exposure information captured in the Safe Passage Study. Specifically, we identified 5 distinct patterns of drinking exposures and 7 distinct patterns of smoking exposures in a diverse group of pregnant women. Several published studies classify women as exposed or not exposed, and provide estimates of the average quantity of exposure during a specified time window (e.g., first trimester) of pregnancy. These studies have observed that higher quantities of exposure, such as binge drinking episodes, are associated with fetal alcohol syndrome, as well as poor neurological outcomes in the offspring (May et al., 2013), and an increased risk for stillbirth and SIDS (Bailey & Sokol, 2011). A major objective of the Safe Passage Study is to improve on current knowledge by examining the role of different patterns of exposure over time in poor outcome.
There are a number of methods available to take full advantage of the detailed quantity, frequency, and timing exposure data captured in the Safe Passage study, including growth curve modeling (GCM), Markov modeling (Titman & Sharples, 2008), and group-based trajectory modeling (Nagin, 1999; Nagin & Odgers, 2010). GCM approaches capture the mean developmental trend and individual departures from mean developmental trend. Additionally, GCM approaches assume random variation and assume that development over time can be described using one set of parameters for the population. Markov modeling captures proportions of the population transitioning from one state to another state (Titman & Sharples, 2008). GBTM, an application of finite mixture modeling, employs a multinomial modeling strategy to approximate a discrete number of homogenous subpopulations; population variability is captured by differences across groups, not distinguishable on measured patient characteristics or inter-individual variability provided by random effects. In GBTM, the interest is in determining the number of and shape of patterns of change over time.
GBTM approaches have been in existence since the 1990s, but this approach has been under-utilized to classify prenatal exposures. In 2013, Eiden utilized a GBTM approach to characterize maternal smoking patterns in pregnancy of women who were current smokers at enrollment (n = 215), yielding four distinct trajectories: non-persistent light smokers, non-persistent moderate smokers, persistent-moderate smokers, and persistent-heavy smokers (Eiden et al., 2013). Our findings are comparable, but because we included non-smokers and possibly more variation among a much larger sample, we were able to identify 7 smoking trajectory groups in the Safe Passage Study. Studies among HIV-positive men and women utilized GBTM to determine smoking- and drinking-trajectory groups, respectively (Akhtar-Khaleel et al., 2016; Cook et al., 2013), but these trajectory groups are not applicable to our study because cessation is more prevalent during pregnancy and learning of an HIV diagnosis may ultimately increase exposure in a subset of participants (Akhtar-Khaleel et al., 2016; Cook et al., 2013).
We found that women who were classified as continuous drinkers and smokers were less educated, more likely to be depressed, and enrolled later in pregnancy, as compared to women classified as no/minimal or quit-exposure groups. Further, women who were high- or low-continuous drinkers were more likely to smoke at moderate, high-continuous, and very high-continuous levels, as compared to the women who were categorized as non-drinkers and quitters. The same pattern held for women reporting high- and very high-continuous smoking levels; they were more likely to be high-continuous and low-continuous drinkers, supporting findings in literature (Flynn, Marcus, Barry, & Blow, 2003). Women engaging in drinking or smoking were more likely to participate in dual exposures, and the quantity of each exposure increased when used in a dual fashion. We also found that women of mixed ancestry were more likely to be low- or high-continuous drinkers as compared to American Indian or white women, and moderate, high- or very high-continuous smokers, as compared to American Indians and white women. Mixed ancestry women were also more likely to report dual exposure as compared to American Indian and white women.
Limitations
First, women may be likely to under-report drinking and smoking exposure during pregnancy due to social stigma and recall error. The prospective design of the Safe Passage study reduced recall error, and a significant effort was made to reduce social stigma by building rapport and a sense of trust between the participants, community, and study staff and utilizing a certificate of confidentiality (Dukes et al., this issue). We found that 61% of women reported a positive history of drinking, 56% reported a positive history of smoking, and 38% reported a positive history of dual exposure during pregnancy, compared to lower prevalences of 9.4% and 15.4% drinking and smoking during pregnancy, respectively, reported in the literature (Slater et al., 2015). Our study populations consisted of women from communities historically known to have high exposures in pregnancy compared to the general population; however, we also observed higher alcohol exposures in white women (23.4%) than previously reported. This population allowed for a larger variety of patterns than if we had been restricted to a population more likely to quit after pregnancy confirmation. Discrepancies in rates between our findings and those from published reports may be due to the timing of assessments. Changes in exposure would not have been captured without serial assessments and subsequent analysis of patterns over the course of pregnancy, as well as enrollment early in pregnancy for the majority of participants.
Second, there were challenges in collecting exposure data at pre-determined time points in pregnancy. Specifically, there may be gaps in exposure information due to late enrollments or missed assessments. Missing exposure information is problematic because it may bias research findings, particularly if the data are not missing completely at random, and GBTM approaches are not invariant to issues of missing data. However, sensitivity analyses yielded nearly identical results in two subsets of women who: 1) had a miscarriage, stillbirth, or delivery prior to 28 weeks or who enrolled in the third trimester of pregnancy, and 2) had complete exposure status defined for all three trimesters of pregnancy.
Third, there was some concern that the study design could allow for therapeutic drift (i.e., changes in exposure behavior as a result of study participation). However, the results seen in the subset of women who enrolled more than once show that only 22.8% and 14% decreased their drinking and smoking patterns, respectively, in the second pregnancy compared to the first.
Fourth, the determination of trajectory groups has subjective aspects, specifically in the selection of the number of trajectory groups and the determination of the order of each trajectory group. With our large sample size, some of the statistical tests were not valid, forcing us to rely more upon substantive criteria. However, sensitivity analyses yielded consistent results with respect to hypothesized exposure patterns. Further, exposure metrics at the week level of pregnancy also yielded equivalent results. However, for model stability and to facilitate interpretation, we determined trajectories using data aggregated at the month level of pregnancy. In addition, we successfully validated self-reported alcohol exposure in a study of 108 Safe Passage study women for which a meconium sample was collected (Himes et al., 2015).
Future Considerations
In GBTM, the goal is to determine the number and shape of exposure patterns over time. Groups derived from GBTM are not distinguishable on measured participant characteristics or inter-individual variability provided by random effects. Thus, in utilizing trajectory groups in analyses investigating the effect of exposure and outcome in the Safe Passage prospective study, appropriate adjustment for non-random allocation of exposure at baseline will be performed using techniques such as the development of propensity scores or adjustment for individual confounders. Since there is substantial imbalance in the population of women who were highly exposed, particularly those with high dual exposure (higher proportion of mixed ancestry), adjustment to parse out the independent effect of exposure(s) with outcome is a necessity. In addition, in analyses involving rare outcomes, the exposure trajectories may require aggregation (e.g., dichotomize by combining the no-exposure and quit-trajectory groups vs. the continuous-exposure groups) in order to make meaningful comparisons.
Conclusion
This is the first study to examine prenatal drinking and smoking patterns from longitudinal data in a large, diverse cohort of pregnant women. We successfully developed and validated the distinct drinking and smoking trajectory groups that will be utilized in the Safe Passage Study, using study populations with higher and more variable levels of prenatal exposures compared to the general US population, to assess the effects of quantity, frequency, and timing of drinking and smoking during pregnancy on adverse outcomes. Exposure validation metrics confirmed the appropriateness of the trajectories defined. These trajectories will be used in future analyses to more accurately determine the exposure patterns that subsequently manifest as poor pre-, peri-, and postnatal outcomes. The GBTM method used here can contribute to a more accurate way to classify exposure patterns and can be applied to populations other than pregnant women. It will be, however, important when using the exposure trajectories in analyses, to adjust for non-random allocation of exposure at baseline.
Supplementary Material
Highlights.
Group-based trajectory modeling identified prenatal drinking and smoking patterns.
Five unique drinking trajectories (1 none/min, 2 quit, 2 continuous) are identified.
Seven unique smoking trajectories (1 none/min, 2 quit, 4 continuous) are identified.
Continuous patterns linked with profiles (e.g., less educated, more depressed) are identified.
Future analyses will link trajectories to peri- and postnatal outcomes.
Acknowledgments
The research reported in this publication was supported by National Institutes of Health grants U01HD055154, U01HD045935, U01HD055155, U01HD045991, and U01AA016501 funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders.
The PASS Network is solely responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The following researchers compose the PASS Network:
PASS Steering Committee Chair (University of Texas Medical Branch): Gary DV Hankins, MD
Data Coordinating & Analysis Center (DM-STAT, Inc.): PI: Kimberly A Dukes, PhD; Co-PI: Lisa M Sullivan, PhD; Biostatistics: Tara Tripp, MA; Fay Robinson, MPH; Cheri Raffo, MPH; Project Management/Regulatory Affairs: Julie M Petersen, MPH; Rebecca A Young, MPH; Statistical Programming/Data Management: Cindy Mai, BA; Elena Grillo, MBA, BS, BBA; Data Management/Information Technology: Travis Baker, BS; Patti Folan; Gregory Toland, MS; Michael Carmen, MS
Developmental Biology & Pathology Center (Children’s Hospital Boston): PI: Hannah C Kinney, MD; Assistant Director: Robin L Haynes, PhD; Co-investigators: Rebecca D Folkerth, MD; Ingrid A Holm, MD; Theonia Boyd, MD; David S Paterson, PhD; Hanno Steen, PhD; Kyriacos Markianos, PhD; Drucilla Roberts, MD; Kevin G Broadbelt, PhD; Richard G Goldstein, MD; Laura L. Nelsen, MD; Jacob Cotton, BS; Perri Jacobs, BS
Comprehensive Clinical Site Northern Plains (Sanford Research): PI: Amy J Elliott, PhD; Co-PI: Larry Burd, Ph.D.; Co-investigators: Jyoti Angal, MPH; Elizabeth Berg, RN; Jessica Gromer, RN; H Eugene Hoyme, MD; Margaret Jackson, BA; Luke Mack, MA; Bethany Norton, MA; Bradley B Randall, MD; Mary Ann Sens, MD; Liz Swenson, RN; Deborah Tobacco, MA; Peter Van Eerden, MD
Comprehensive Clinical Site South Africa (Stellenbosch University): PI: Hendrik Odendaal, MBChB, FRCOG, MD; Co-PI: Colleen Wright, MD, FRCPath, PhD; Co-Investigators: Lut Geerts, MD, MRCOG; Greetje de Jong, MBChB, MMed, MD; Pawel Schubert, FCPath (SA) MMed; Shabbir Wadee, MMed; Johan Dempers, FCFor Path (SA); Elsie Burger, FCFor Path (SA), MMed Forens Path; Janetta Harbron, PhD; Co-investigator & Project Manager: Coen Groenewald, MBChB, MMed, FCOG, M Comm; Project Manager: Erna Carstens, RN
Physiology Assessment Center (Columbia University): Co-PIs: William Fifer, PhD; Michael Myers, PhD; Co-investigators: Joseph Isler, PhD; Yvonne Sininger, PhD; Project Management: J David Nugent, MA; Carmen Condon, BA; Data Analysis: Margaret C Shair, BA; Tracy Thai, MA
NIH Project Scientists: Marian Willinger, PhD (NICHD); Dale Hereld, MD, PhD (NIAAA); Howard J Hoffman, MA (NIDCD); Chuan-Ming Li, MD, PhD (NIDCD)
The authors gratefully acknowledge the cooperation of the study participants, PASS investigators, and members of the NICHD Advisory and Safety Monitoring Board: Elizabeth Thom, PhD (Chair); The Reverend Phillip Cato, PhD; James W Collins, Jr, MD, MPH; Terry Dwyer, MD, MPH; George Macones, MD; Philip A May, PhD; Richard M Pauli, MD, PhD; Raymond W Redline, MD; and Michael Varner, MD.
Additional Acknowledgments
The following individuals made significant contributions to the research and warrant recognition:
DCAC: Idania Ramirez, MPH; Laura Spurchise, MPH; Jamie Collins, PhD; Derek Petersen, BS
DBPC: Hoa Tran, FNP PhD; Richard A Belliveau, BA; Kristin Rivera, BA; Megan Minter, MS; Kathryn Schissler, BS
PAC: Johnston T Grier, BA; Emilia F Vignola, BA; Joseph J Violaris, BA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Indian Health Service (IHS).
References
- Akhtar-Khaleel WZ, Cook RL, Shoptaw S, Surkan PJ, Teplin LA, Stall R, et al. Long-Term Cigarette Smoking Trajectories Among HIV-Seropositive and Seronegative MSM in the Multicenter AIDS Cohort Study. AIDS and Behavior. 2016;20:1713–1721. doi: 10.1007/s10461-016-1343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angal J, Petersen JM, Tobacco D, Elliott AJ. Ethics Review for a Multi-Site Project Involving Tribal Nations in the Northern Plains. Journal of Empirical Research on Human Research Ethics. 2016;11:91–96. doi: 10.1177/1556264616631657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrandale V, Koehoorn M, MacNab Y, Kennedy SM. How to use SAS® Proc Traj and SAS® Proc Glimmix in Respiratory Epidemiology: CHER, SOEH School of Occupational & Environmental Health. University of British Columbia; 2006. [Google Scholar]
- Bailey BA, Sokol RJ. Prenatal alcohol exposure and miscarriage, stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Research & Health. 2011;34:86–91. [PMC free article] [PubMed] [Google Scholar]
- Brick J. Standardization of alcohol calculations in research. Alcoholism: Clinical and Experimental Research. 2006;30:1276–1287. doi: 10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- Cook RL, Zhu F, Belnap BH, Weber KM, Cole SR, Vlahov D, et al. Alcohol consumption trajectory patterns in adult women with HIV infection. AIDS and Behavior. 2013;17:1705–1712. doi: 10.1007/s10461-012-0270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Methodological issues in measuring alcohol use. Alcohol Research & Health. 2003;27:18–29. [PMC free article] [PubMed] [Google Scholar]
- Day NL, Wagener DK, Taylor PM. Measurement of substance use during pregnancy: methodologic issues. NIDA Research Monograph. 1985;59:36–47. [PubMed] [Google Scholar]
- Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GD, et al. The safe passage study: design, methods, recruitment, and follow-up approach. Paediatric and Perinatal Epidemiology. 2014;28:455–465. doi: 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes KA, Tripp T, Petersen J, Robinson F, Odendaal H, Elliott A, et al. A Modified Timeline Followback Assessment to Capture Alcohol Exposure in Pregnant Women: Application in the Safe Passage Study. Alcohol. doi: 10.1016/j.alcohol.2017.02.174. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukic VM, Niessner M, Benowitz N, Hans S, Wakschlag L. Modeling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: a deterministic approach. Nicotine & Tobacco Research. 2007;9:453–465. doi: 10.1080/14622200701239530. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Homish GG, Colder CR, Schuetze P, Gray TR, Huestis MA. Changes in smoking patterns during pregnancy. Substance Use & Misuse. 2013;48:513–522. doi: 10.3109/10826084.2013.787091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn HA, Marcus SM, Barry KL, Blow FC. Rates and correlates of alcohol use among pregnant women in obstetrics clinics. Alcoholism: Clinical and Experimental Research. 2003;27:81–87. doi: 10.1097/01.ALC.0000046595.47491.37. [DOI] [PubMed] [Google Scholar]
- Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, et al. Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy. Clinical Chemistry. 2015;61:523–532. doi: 10.1373/clinchem.2014.233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICAP. ICAP Reports. Vol. 5. Washington, DC: International Center for Alcohol Policies; 1998. What is a "standard drink"? [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Joya X, Friguls B, Ortigosa S, Papaseit E, Martinez SE, Manich A, et al. Determination of maternal-fetal biomarkers of prenatal exposure to ethanol: a review. Journal of Pharmaceutical and Biomedical Analysis. 2012;69:209–222. doi: 10.1016/j.jpba.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Cook MB, Pandeya N, Vaughan TL, Abnet CC, Giffen C, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett's Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiology. 2012;36:306–316. doi: 10.1016/j.canep.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Gaudet MM, Olshan AF, Kelsey K, Boffetta P, Brennan P, et al. Body mass index, cigarette smoking, and alcohol consumption and cancers of the oral cavity, pharynx, and larynx: modeling odds ratios in pooled case-control data. American Journal of Epidemiology. 2010;171:1250–1261. doi: 10.1093/aje/kwq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Muscat J, Gaudet MM, Olshan AF, Curado MP, Dal Maso L, et al. An examination of male and female odds ratios by BMI, cigarette smoking, and alcohol consumption for cancers of the oral cavity, pharynx, and larynx in pooled data from 15 case-control studies. Cancer Causes & Control. 2011;22:1217–1231. doi: 10.1007/s10552-011-9792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, et al. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug and Alcohol Dependence. 2013;133:502–512. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin DS. Analyzing Developmental Trajectories: A Semiparametric, Group-Based Approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- NIAAA. Council Approves Definition of Binge Drinking. NIAAA Newsletter. 2004;3 [Google Scholar]
- Odendaal HJ, Steyn DW, Elliott A, Burd L. Combined effects of cigarette smoking and alcohol consumption on perinatal outcome. [Review] Gynecologic and Obstetric Investigation. 2009;67:1–8. doi: 10.1159/000150597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatric and Perinatal Epidemiology. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Slater ME, Haughwout SP, Castle I-JP. Surveillance Report #103: Trends in Substance Use among Reproductive-Age Females in the United States, 2002–2013: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, Division of Epidemiology and Prevention Research. Alcohol Epidemiologic Data System 2015 [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Instructional Training Video. Toronto, Canada: Addiction Research Foundation; 1979. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption; Psychosocial and Biochemical Methods. Vol. 17. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- Titman AC, Sharples LD. A general goodness-of-fit test for Markov and hidden Markov models. Statistics in Medicine. 2008;27:2177–2195. doi: 10.1002/sim.3033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.