Abstract
Autophagy is the process of cellular self-eating by a double-membrane organelle, the autophagosome. A range of signaling processes converge on two protein complexes to initiate autophagy: the ULK1 protein kinase complex and the PI3KC3-C1 lipid kinase complex. Some 90 % of the mass of these large protein complexes consists on non-catalytic domains and subunits, and the ULK1 complex has essential non-catalytic activities. Structural studies of these complexes have shed increasing light on the regulation of their catalytic and non-catalytic activities in autophagy initiation. The autophagosome is thought to nucleate from vesicles containing the integral membrane protein Atg9, COPII vesicles, and possibly other sources. In the wake of reconstitution and superresolution imaging studies, we are beginning to understand how the ULK1 and PI3KC3-C1 complexes might coordinate the nucleation and fusion of Atg9 and COPII vesicles at the start of autophagosome biogenesis.
Keywords: vesicle, membrane remodeling, tethering complex, membrane fusion, nanoscale biology, ULK1, Atg1, Vps34, phosphatidylinositol 3-kinase, COPII
Introduction
Macroautophagy (hereafter “autophagy”) is the main mechanism used by eukaryotic cells to degrade cargoes that are larger than individual proteins. It is also the main mechanism for eukaryotic cells to replenish pools of biosynthetic precursors and energy sources by recycling cytosolic contents during starvation. Both the process of autophagy and its machinery is conserved from yeasts (S. cerevisiae and S. pombe) (1) to mammals (2). Autophagy can be either selective or non-selective (“bulk”). Selective autophagy removes and recycles harmful or simply unneeded materials from the cell. These include protein aggregates, damaged mitochondria, unneeded peroxisomes, excess ribosomes, ER and endosomes, lipid droplets, and intracellular pathogens (3, 4). Failure to control the accumulation of any of these types of materials can lead to disease in humans. Excellent reviews cover the relationship of autophagy to neurodegeneration (5), cancer (6), aging (7), infection (8), and other diseases. Bulk autophagy is triggered by starvation, and is critical for maintaining a cellular supply of lipids, amino acids, carbohydrates, and nucleotides. Selective and bulk autophagy are triggered by different signals. Yet, these diverse signals are thought to funnel into a single pathway that initiates the mechanical events and membrane remodeling needed to create the autophagosome. This review will focus on the conserved and common events that initiate both selective and bulk autophagy. Far more is known about bulk autophagy initiation. The review will focus mostly on data from bulk autophagy research, with the understanding that most of the findings probably apply to selective autophagy.
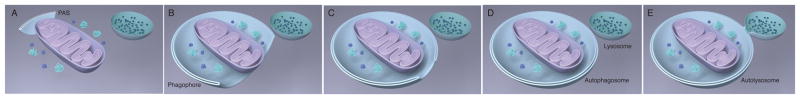
Autophagy is initiated in yeast at a punctate structure called the Pre-Autophagosomal Structure (9) (PAS, also sometimes called the Phagophore Assembly Site; Fig. 1). In mammals, initiation is associated with an endoplasmic reticulum (ER) subdomain enriched for the lipid phosphatidylinositol 3-phosphate (PI(3)P), known as the omegasome (10). From its inception at the PAS or omegasome, the phagophore elongates into a cup-shaped structure and begins to engulf cellular material. The membrane supply for phagophore growth can apparently be sourced to a variety of cellular reservoirs (11). In selective autophagy, the cargo itself templates the size and shape of the phagophore (4, 12, 13). In bulk autophagy, it is less clear how this occurs, but the actin cytoskeleton is involved (14). Finally, the cup closes upon itself. The narrow gap at the tip of the cup fuses, leading to the complete sequestration of the material inside. The outer membrane of the autophagosome then fuses with the lysosome (vacuole in yeast or plants) to form structure known as the autolysosome. At this stage, the inner membrane and all of its contents are degraded. This review will focus on the earliest steps in the process: the formation of the PAS in yeast and the formation of autophagy initiation sites in mammals, and the initial nucleation of the phagophore.
Figure 1. The autophagy program.
(A) Preautophagosome assembly site (PAS). (B) PAS elongates into phagophore to surround bulk cytosolic materials. (C) The geometry of phagophore closure. (D) The closed autophagosome. (E) The autophagosome merges with the lysosome; the new organelle is the autolysosome.
The conserved machinery for autophagosome formation (15–17) contains two major initiation complexes that are a central focus of this review: the ULK1 complex (known as the Atg1 complex in yeast) and the class III PI 3-kinase complex I (PI3KC3-C1) (Table 1). The sole conserved transmembrane protein in the core machinery, Atg9, is also closely connected to initiation. The PI3P binding WIPI 1–4 proteins and the Atg8-family and Atg12 conjugation systems (Atg3, Atg4, Atg5, Atg7, Atg10, Atg12, Atg16, LC3s, and GABARAPs; Table 1) function downstream and drive phagophore elongation. Conjugation of Atg8/LC3 family proteins to phosphatidylethanolamine, known as “LC3 lipidation” for short, is the hallmark downstream reaction driven by these proteins. These Atg proteins were discovered for their roles in autophagy and are primarily dedicated to this function, although “moonlighting” roles in non-autophagic functions have been reported (18). Multipurpose membrane trafficking factors also have essential roles in the pathway. In many cases, these roles are also conserved from yeast to humans. These factors include the coat complexes COPI and COPII, the vesicle- and organelle-identifying RAB GTPases, and SNARE proteins of vesicle fusion (19–21). In selective autophagy, a variety of adaptor proteins link cargoes to the autophagy machinery, including p62, Optineurin (OPTN), and NDP52 (3, 4, 22).
Table 1.
Autophagy genes implicated in autophagosome initiation, not a complete list of all autophagy genes.
| Autophagy complex | Mammals | Function | Yeast | Function | Protein interactors |
|---|---|---|---|---|---|
| Autophagic initating complex | ULK1, 2 | S/T kinase | Atg1 | S/T kinase | Atg13/ATG13 HORMA domain binds Atg9/ATG9 Atg1/ULK1 has Atg8/LC3 interacting motif (AIM/LIR) Atg1 is recruited by Ytp1 (TRAPPIII) |
| ATG13 | regulatory subunit | Atg13 | regulatory subunit | ||
| FIP200 | scaffold | Atg17, Atg11 | scaffold | ||
| ATG101 | regulatory | ||||
| Atg29 | budding yeast specific | ||||
| Atg31 | budding yeast specific | ||||
| PI(3)Kinase Complex | VPS34 | PI kinase | Vps34 | PI kinase | BECN1 interacts with BH3 domain containing proteins, AMBRA1 |
| VPS15 | scaffold | Vps15 | scaffold | ||
| BECN1 | regulatory subunit | Atg6/Vps30 | regulatory subunit | ||
| ATG14L | PAS targeting | Atg14 | PAS targeting | ||
| NRBF2 | activator | Atg38 | activator | ||
| ATG9 | ATG9 | transmembrane protein localizes to small vesicles | Atg9 | transmembrane protein localizes to small vesicles | Atg13 binds Atg9 |
| Rab1 | Rab1 | Rab GTPase | Ypt1 | Rab GTPase | Ypt1 Recruits Atg1 |
| TRAPPIII | TRAPPC8 | PAS targeting | Trs85 | PAS targeting | TRAPPI complexes binds COPII vesicles (Sec23) |
| UBL-like molecules | LC3 A-D, GABARAPs | Autophagosome marker | Atg8 | Autophagosome marker | Atg1/ULK1, selective autophagy adaptors |
The ULK1/Atg1 Complex
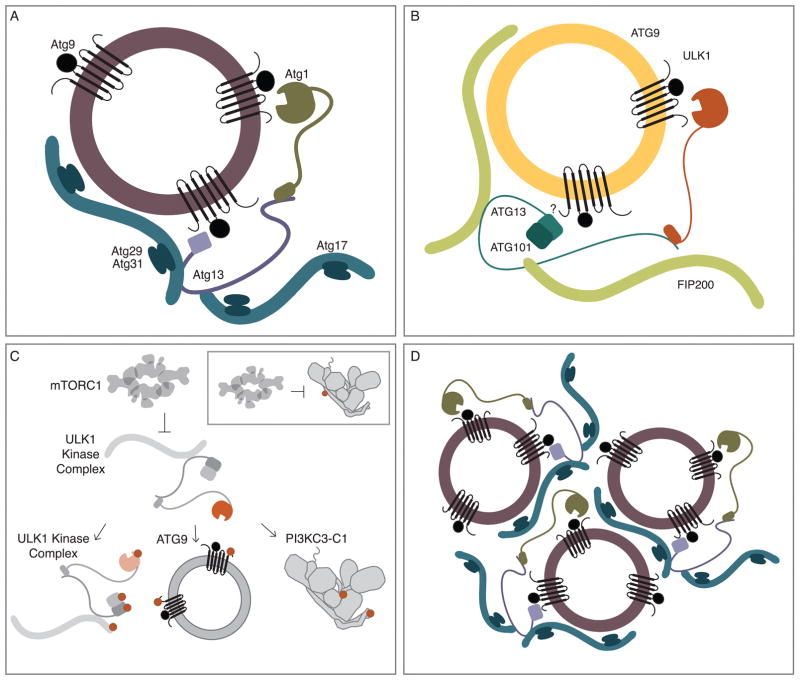
Autophagy initiation starts with the activation of the ULK1 complex (Atg1 complex in yeast) (23–25). ULK1 is part of a family of kinases ULK1–4 in humans. Isoform ULK1 is the most important of these in autophagy. In some cells lines it is necessary to block both ULK1 and ULK2 to completely shut down autophagy, however ULK2 is less characterized and will not be discussed further. The ULK1 complex consists of ULK1 itself, and the non-catalytic subunits FIP200, ATG13, and ATG101 (26–28) (Fig. 2B). FIP200 is a large predicted coiled coil protein involved in scaffolding (29). ATG13 and ATG101 contain HORMA (Hop/Rev7/Mad2) domains (30) which heterodimerize with each another (31–33). ATG13 contains a long IDR (intrinsically disordered region) following the HORMA domain, and the C-terminal part of its IDR contains motifs that bind to the C-terminal EAT/tMIT domain of ULK1 (34). The budding yeasts organize their Atg1 complexes in a uniquely complicated way. The Atg1 subunit itself is organized into the same domain structure as ULK1. Atg13 is conserved, but ATG101 is absent from budding yeasts. FIP200 is replaced by two scaffolding subunits: Atg11, which functions in selective autophagy (35), and Atg17, which functions in bulk autophagy (36, 37). Atg17 in turn co-assembles with two smaller subunits, Atg29 and Atg31 (38, 39) (Fig. 2A).
Figure 2.
The autophagy initiation complex in yeast and mammals. (A) In budding yeast, vesicles containing Atg9 are phosphorylated by the S/T kinase Atg1, additionally, the N-terminus of Atg9 makes a direct contact with the HORMA domain Atg13. Atg13 in turn make contacts with the Atg17-29-31 scaffold. (B) Based on yeast observations, it is proposed that small vesicles containing ATG9 interact with the autophagy initiation complex- the S/T kinase ULK1, the HORMA domain containing ATG13 forms a heterodimer with the HORMA domain of ATG101, FIP200 is modeled on the structure of Atg17. However, the yeast interaction site in Atg9 is not conserved in humans. (C) ULK1 is inhibited by mTORC1. When mTORC1 is inactivated, ULK1 becomes activated, thereby leading to autophosphorylation within ULK1, ATG13, ATG101 and FIP200- multiple phosphorylation sites occur within ATG13, one is shown for clarity. ULK1 then phosphorylates ATG9, and PI3KC3-C1- at the N-terminus of BECN1 and within the C2 domain of VPS34. Inset: mTORC1 directly phosphorylates ATG14L of PI3KC3-C1. (D) From research in the budding yeast model system, the Intrinsically Disordered Regions (IDRs) within Atg1 and Atg13 as well as the position of Atg13-Atg17 contacts lead a proposed mechanism in which ATG proteins form a “meshwork.”
The large scaffolding subunits Atg11 and FIP200, whose structures are unknown, are similar to each other in size and predicted helical content. Their C-terminal domains are homologous, suggesting a common function. Atg17 is smaller than Atg11 and FIP200, and aside from a similar helical content, has little sequence similarity with Atg11 and FIP200. It is often stated in the literature that Atg17 and FIP200 are orthologs. It is important to bear in mind that this inference, while reasonable, is based on functional parallelism, not detailed sequence or structural similarity.
ULK1/Atg1 kinase activation and inactivation
ULK1/Atg1 is activated in at least three ways upon autophagy induction, and all three are essential. Protein kinase activity needs to be switched on, the active kinase needs to be recruited to the PAS, and essential – though still vaguely defined–non-catalytic scaffolding activities must be turned on. Autophosphorylation of the kinase domain’s activation loop at Thr180 of ULK1 (40, 41) (Thr226 in yeast Atg1 (42, 43)) is essential for activation. Autophosphorylation is promoted by conditions that induce autophagy and by co-assembly with other subunits of the complex (42, 44, 45). This co-assembly in turn increases the local concentration of Atg1 molecules and promotes their mutual autophosphorylation. This can occur both in selective autophagy under nutrient-rich conditions (44) and in starvation (45). Following activation, ULK1 (and PI3KC3-C1) can be ubiquitinated by the Cul3-KLHL20 ligase complex and degraded (46), thereby switching off the autophagy initiating signal.
How does starvation trigger Thr180 phosphorylation of ULK1? Autophosphorylation is usually promoted by the dimerization or higher-order oligomerization of kinases. The C-terminal EAT domain of Atg1 dimerizes in isolation (34, 39, 47), however, full-length Atg1 is reportedly a monomer in the absence of other subunits (48). It is currently not clear whether Atg1 undergoes regulated dimerization via its EAT domain under some conditions. ULK1 is bridged by ATG13 to the scaffolding subunit FIP200 (26–28). In yeast, Atg13 bridges to Atg11 (49) and Atg17 (36, 37). While the oligomeric state of FIP200 and Atg11 is unknown, Atg17 is a constitutive dimer (38, 39). The recruitment of ULK1 to FIP200 via the intermediation of ATG13 could in principle be a mechanism for ULK1 trans-autophosphorylation.
Whether and how starvation regulates formation of the Atg1 complex has been intensively investigated, yet consensus has been elusive. mTORC1 (TORC1 in yeast) is a master regulator of cell growth and metabolism, and its inactivation in response to amino acid depletion is a major trigger for autophagy (50). In the canonical model of the process in yeast, Atg13 is phosphorylated by TORC1 under non-starved conditions (51). The extensive phosphorylation inhibits the assembly of Atg13 with Atg1, and Atg13 with Atg17, by introducing steric and electrostatic repulsion into the binding sites on Atg1 and Atg17 (34). In a contrasting report, it was found that the Atg1 complex is assembled constitutively in yeast in both nutrient-rich and starved conditions (52). Of 51 reported phosphorylation sites within yeast Atg13, six fall within the crystallographically defined Atg1-binding site. Mutation of all of these sites from Ser to the phosphomimetic Asp only reduces Atg1 binding by a factor of three (34). The effect of Atg13 phosphorylation on Atg17 binding may be larger. Two of the 51 reported phosphorylation sites occur in the Atg17 binding site of Atg13. Phosphomimetic mutations in these two residues substantially disrupt binding (34). Phosphoregulation at the level of the Atg17-Atg13 interaction thus seems to lead to bigger affinity changes than for the Atg1-Atg13 interaction. FIP200 and Atg11 are less tractable biochemically than Atg17, and little is known at the quantitative and structural level about how ULK1 and Atg1 association with these two scaffolds is regulated. It is often stated that ULK1 is assembled constitutively in mammalian cells (26–29), yet on the other hand, the mammalian ATG13-ATG101 subcomplex appears to have autophagic functions that are independent of the ULK1 complex (53).
Other regulatory mechanisms function in parallel to, or even antagonize mTORC1/TORC1 regulation (Fig. 2C). AMPK (AMP-activated protein kinase) upregulates autophagy in response to energy depletion as detected by an increase in cytosolic AMP (54). AMPK directly phosphorylates ULK1 at multiple sites in its central IDR (40, 55–59), leading to its activation. The details of how these IDR phosphorylation sites communicate with the catalytic domain remain to be elucidated. In selective autophagy, ULK1 must be locally active even under fed conditions when mTORC1/TORC1 is also active. Relatively little is known about how the ULK1 complex is sheltered from inactivation under these conditions. Huntingtin, the protein product of the gene mutated in Huntington’s disease, interacts with ULK1 and has been proposed to have such an ULK1-shielding function (60)
ULK1 recruitment to initiation sites
Recruitment of the ULK1 complex to sites of autophagy initiation is the second regulated step in its activation. In bulk autophagy in yeast, PAS recruitment is regulated at the level of Atg13 phosphorylation. Atg17, along with its accessory proteins Atg29 and Atg31, is the first protein to arrive at the PAS is yeast (61), which set the stage for the recruitment of Atg13 and Atg1 as described above. The EAT domain of ULK1, the locus for ATG13 binding, is essential for its recruitment to the sites of phagophore initiation in human cells (62). This suggests that the principles for recruitment are similar in this respect in yeast and mammals.
A number of other protein-protein interactions influence ULK1 localization to the site of phagophore initiation. The LC3 family proteins bind to LIR/AIM motifs in both human and yeast ULK1/Atg1 (52, 63) and in human ATG13 (63). LC3 conjugation is thought to occur downstream of ULK1 activation, however, and the LIR/AIM motifs seem likely to be involved in later events in autophagosome biogenesis. The yeast Atg1 complex binds to Atg9 through a direct interaction between the HORMA domain of Atg13 and the N-terminal soluble domain of Atg9 (64), and perhaps also through Atg17 (48, 65). Yeast Rab1 (Ypt1) is a small G-protein better known as a regulator of ER-Golgi and intra-Golgi traffic, binds to Atg1 and helps recruit it to the PAS (66). Ypt1 is activated and recruited to the PAS by the TRAPPIII complex, which binds to Atg9 (67) and Atg17 (66). TRAPPIII is also implicated in mammalian autophagy (68, 69).
C9orf72 is a protein that is mutated in the most common hereditary forms of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). C9orf72 was very recently shown to be important for the RAB1A dependent recruitment of ULK1 to the sites of phagophore initiation in human cells (70). C9orf72 contains a DENN domain, and in many cases DENN domain proteins act as RAB guanine nucleotide exchange factors (GEFs). Apparently the C9orf72 DENN binds to RAB1A but lacks GEF activity, making C9orf72 a RAB1A effector rather than a RAB1A GEF. It has been proposed that impairment of ULK1 recruitment to autophagy initiation sites in C9orf72 mutants is responsible for their disease phenotype (70). Thus, a circuit involving ATG9, RAB1A, and TRAPPIII appears to be important for the recruitment of ULK1 to autophagy initiation sites.
ULK1 substrates
The ULK1 kinase transduces pro-autophagic signals by phosphorylating many substrate proteins (25). The ULK1 consensus site is characterized by a preference for hydrophobic residues surrounding the serine phosphoacceptor (71, 72). This is not a rare sequence, and the numerous substrates of ULK1 include itself and other subunits of the ULK1 complex; other elements of the core autophagy machinery, including PI3KC3-C1 subunits and Atg9; and other proteins whose connections to autophagy are understood to varying extents (25, 71). Within the ULK1 complex, there are phosphorylation sites in ATG101 and multiple sites in FIP200 and ATG13 (72). The ATG101 phosphorylation sites, Ser11 and Ser203, are at the start of the ATG101 HORMA domain, and in a flexible region just past the end of the HORMA domain, respectively.
The PI3KC3-C1 complex, another pivotal autophagy initiating complex, is one of the most important and best understood targets of ULK1 phosphorylation. ULK1 phosphorylates Ser15 and other sites in BECN1, activating the PI3KC3-C1 complex and promoting autophagy (72, 73). The PI3KC3-C1 catalytic subunit VPS34 contains a major ULK1 phosphorylation site at Ser249 (72). The consequences of these phosphorylations are discussed further in the PI3KC3-C1 section. The massive PI3KC3-C1-associated IDR protein AMBRA1 is another ULK1 substrate (74). In yeast, Atg9 is an important substrate for Atg1 (71). ULK1 is critical for selective as well as bulk autophagy. It phosphorylates the cargo adaptor protein p62, increasing the binding affinity of p62 for ubiquitin (75). ULK1 also phosphorylates FUNDC1 to promote mitophagy (76). Many of these phosphorylations, including those of ULK1 and PI3KC3-C1 subunits, seem to be very important in autophagy initiation, while in other cases such as Atg9, the effect is further downstream.
Non-catalytic functions of the ULK1/Atg1 complex
ULK1/Atg1 probably regulates autophagy induction at a third level, through its non-catalytic activities. These are essential for autophagy initiation, at least in yeast (77–79). Some 90 % of the mass of the ULK1/Atg1 complex consists of non-catalytic domains (24). We will enumerate some of the non-catalytic functions imputed to these domains. The ULK1/Atg1 EAT domain dimerizes and is capable of tethering high curvature lipid vesicles in vitro (39, 47), which could be relevant to vesicle clustering at the PAS. The dimeric Atg17 subunit of the yeast Atg1 complex has a double crescent shape (39) that suggests it could not just tether vesicles, but scaffold them rigidly into a specific geometry preceding cup formation. The combination of Atg1 and Atg17 dimers provides a mechanism for higher-order assembly of daisy-chained or branched assemblies of Atg1 complexes at the PAS (80). Moreover, the C-terminal IDR of Atg13 has at least two Atg17 binding sites, located such that they must bridge different Atg17 dimers (45). Taken together, the dimerization data and the crosslinking ability of Atg13 suggests a meshwork model for organization of the PAS (Fig 2D).
Mammalian ATG13 and ATG101 form a heterodimeric subcomplex consisting of the HORMA domains of the two proteins. ATG101 has a hydrophobic binding site revealed by the crystal structures that has been dubbed the “WF finger” (31–33). The binding partner of the WF finger is not known, however it is clear that the ATG13, ATG101 and FIP200 have functions in autophagy that are fundamentally important and independent of the ULK1 (and ULK2) kinases (53, 81).
The PI3KC3-C1 Complex
The class III Phosphatidylinositol 3-kinase (PI3KC3) phosphorylates the lipid head group of phosphatiylinositol to generate phosphatidylinositol 3-phosphate (PI(3)P) (82). Formation of PI(3)P is an essential early event in autophagy initiation, occurring just downstream of ULK1 (83, 84). PI3KC3 forms at least two distinct complexes, known as complexes–I and II (PI3KC3-C1 and –C2) (83, 84). Both complexes contain the catalytic subunit VPS34/Vps34, the putative protein kinase VPS15/Vps15, and BECN1/Atg6. PI3KC3-C1 contains ATG14L/Atg14 (84–89), which directs the complex to phagophore initiation sites. PI3KC3-C1 facilitates elongation while complex II containing UVRAG directs endosome and autophagosome maturation, reviewed elsewhere (82).
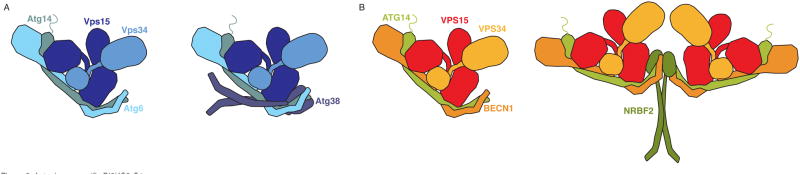
For many years, progress in understanding the structure of PI3KC3-C1 was gradual and fragmentary. Structures of the VPS34 catalytic and associated helical domain (90), the central coiled coil (91) and C-terminal BARA (92, 93) domains of Vps30/BECN1, and the WD40 domain of Vps15 (94) were all solved separately. More recently, the structure of the complete human complex I was solved by electron microscopy (95), revealing a V-shaped architecture. The x-ray crystal structure of yeast complex II showed a conserved architecture and domain placement (96) and added new information, including the presence of a BARA-like domain in Vps38 (yeast UVRAG). The left hand side of the complex (as seen in the canonical view, Fig. 3A) includes the central coiled coils and C-terminal domains of BECN1 and ATG14L. The N-termini of BECN1 and ATG14L are at the base of the V. A number of regulatory signals, described below, converge on these sites. On the right hand side is the catalytic subunit VPS34 and the protein kinase domain of VPS15. VPS15 bridges the left and right arms of the complex. Its WD40 domain is part of the left arm, and serves as a docking site for BECN1 and ATG14L, while its HEAT domain spans the two arms. Similar to the situation with the ULK1 complex, the catalytic domain of VPS34 comprises just ~10 % of the mass of the PI3KC3-C1 complex. Its placement within the context of the rest of the much larger V-shaped complex highlights the likely importance of the regulatory and scaffolding roles of the non-catalytic ~90%.
Figure 3. Autophagy specific PI3KC3-C1.
(A) Yeast PI3KC3-C1 is composed of the lipid kinase Vps34, the scaffold and potential protein kinase Vps15, the regulatory subunit Atg6, the phagophore targeting subunit Atg14. A dimer of Atg38 binds to one heterotetramer. (B) Mammalian PI3KC3-C1 is composed of the lipid kinase VPS34, the scaffold and potential protein kinase VPS15, the regulatory subunit BECN1, the phagophore targeting subunit ATG14L. The binding of dimer of NRBF2 to the heterotetramer creates leads to the dimerization and the formation of a homopentamer.
PI3KC3-C1 recruitment to the PAS
The functioning of PI3KC3-C1 in autophagy requires its translocation to the PAS, which is driven by its unique ATG14L subunit (84–89). A cysteine-rich domain near the N-terminus of ATG14L is essential for its starvation-induced translocation to the phagophore initiation sites (88). The structure of this domain is unknown, as is its putative interaction partner at phagophore initiation sites. A C-terminal amphiphatic helix deemed the BATS domain (Barkor/ATG14L Autophagosome Targeting Sequence) (97), which is an example of the larger class of amphipathic lipid packing sensor (ALPS) motifs (98), is also important for targeting. This is thought to be due to its ability to bind high-curvature lipids, although it is important to note that ALPS motifs can also bind loosely packed low curvature membranes such as are found at the ER (99).
In addition to the complex I- and autophagy-specific PAS- and ER-targeting motifs in ATG14L, other regions of the PI3KC3-C1 and –C2 have been implicated in binding less specifically to membranes. The C-terminal BARA domain of BECN1, at the tip of the left arm, is proposed to insert into membranes via an aromatic finger (93). On the opposite side of the V-shape is VPS34, whose final kα12 helix must bind membranes in order for lipid phosphorylation to take place (90). On the other hand, although it had been expected that the C2 domain of VPS34 would binds membranes, the structure of complex II suggests that the C2 domain is only involved in protein-protein interactions. Finally, the N-myristoylation of VPS15 provides one more membrane contact (100). The actual geometry of PI3KC3-C1 and –C2 docking on membranes through the various known and putative anchoring motifs is still unknown, and will be important to clarify.
PI3KC3-C1 regulatory proteins
The kinase activity of PI3KC3-C1 is regulated through post-translational modifications and a variety of protein-protein interactions. The cast of players that interact with PI3KC3-C1 is extensive and leads to the suggestion that the “two-complex” model is an oversimplification. PI3KC3-C1 associates tightly with a fifth subunit, known as NRBF2 (Nuclear Receptor Binding Factor 2) (101–103) in mammals and Atg38 (104) in yeast. NRBF2/Atg38 contain an N-terminal three-helix bundle MIT (Microtubule-Interacting and Transport) domain and a coiled-coil-containing domain at the C-terminus (105) that induces dimerization. NRBF2 binds to the base of complex I through interactions with the N-terminus of ATG14L and BECN1, enhances kinase activity in vitro and leads to the dimerization of human complex I (106) (Fig 3B). Although yeast Atg38 is dimeric as well, it does not facilitate yeast PI3KC3-C1 dimerization (105). Mammalian PI3KC3-C1 dimerization and kinase activation by NRBF2 seem to be completely decoupled from one another (106). Since kinase dimerization is not required for its enzymatic activation, both the mechanism of allosteric activation and the biological function of dimerization remain to be clarified.
The anti-apoptotic factor Bcl-2 (B-Cell lymphoma 2) binds to the BH3 (Bcl-2 Homology domain) of BECN1 (107), which places its location in the complex close to, and perhaps even overlapping, with the NRBF2 binding site (106). The affinity of Bcl-2 is fifty time lower than that of NRBF2 (106). Unlike NRBF2, Bcl-2 rapidly exchanges on and off PI3KC3-C1 (106). Bcl-2 binding to BECN1 inhibits VPS34 kinase activity and antagonizes autophagy (108). Because the binding site is remote from the lipid kinase domain, the mechanism for this inhibition is unknown, but presumably must involve long-range allosteric communication. Additionally, BECN1 is capable of binding with other anti-apoptotic Bcl-2 family members (Bcl-XL, Bcl-w, Mcl-l) through its BH3 domain (107). Only ER-localized Bcl-2 is capable of inhibiting autophagy, and mitochondrial Bcl-2 was not (108).
AMBRA1 (Autophagy and Beclin 1 Regulator 1) binds to PI3KC3-C1 in cells and promotes autophagy (109). A full biochemical characterization with recombinant proteins is still lacking, as the size of this IDR protein makes it challenging to study. AMBRA1 is phosphorylated by ULK1 as described above, which is proposed to activate PI3KC3-C1 by releasing it from microtubules (74). The most recent addition to the family of PI3KC3-C1 interactors is PAQR3 (progestin and adipoQ receptor 3), a Golgi localized multipass transmembrane protein that has been proposed to promote autophagy by helping to assemble PI3KC3-C1 (110). The degree to which PI3KC3-C1 assembly (as opposed to the better-studied topics of the acute regulation of its enzyme activity and localization) as a regulated step has been relatively less explored.
PI3KC3-C1 phosphoregulation
BECN1 is phosphoregulated by at least six kinases: ULK1, MAPKAP2 (mitogen-activated protein kinase-activated protein kinase 2), AMPK, and DAPK (death-associated protein kinase) that promote autophagy activation while Akt and EGFR phosphorylations suppress autophagy. ULK1 activates PI3KC3-C1 by phosphorylating BECN1 at Ser15 (73). Upon stress, the kinases MAPKAPK2/3 phosphorylate BECN1 at Ser90, leading to autophagy activation (111). Upon glucose starvation, AMPK enhances autophagy by phosphorylating BECN1 at Ser90 and Ser94 (112). Ser90 phosphorylation is reversed by protein phosphatase 2A (PP2A) (113), which is upregulated by starvation (114). Ser15, Ser90, and Ser94 are all located in the flexible N-terminal portion of BENC1, and it is still unknown how these signals are communicated to the catalytic domain.
DAPK phosphorylates BECN1 at Thr119, which is located within the BH3 domain, and thereby inhibits binding of Bcl-2 and Bcl-X-L (115). AMPK, which is generally pro-autophagic, seems to selectively suppress the activity of the endosomal and late autophagy PI3KC3-C2 by phosphorylating Thr163 and Ser165 in VPS34 (112). EGFR suppresses autophagy through phosphorylation of BECN1 at Tyr229, Tyr233, Tyr352 (116), which might, in principle, regulate assembly of the coiled-coil complex. Additionally, autophagy suppression can occur through phosphorylation at the C-terminus of ATG14L by mTORC1 (mechanistic Target Of Rapamycin Complex 1) at positions Ser3, Ser223, Ser233, Ser383, and Ser440 (117). Akt catalyzes yet another negative regulatory event, phosphorylation of Ser295 on an exposed loop of the BECN1 BARA domain. Ser295 phosphorylation seems to inhibit autophagy by promoting PI3KC3 association with 14-3-3 proteins and vimentin, rather than by directly modulating lipid kinase activity (118). Phosphatases that remove these signals have not been reported, however it has been reported that the Cul3-KLHL20 ligase complex promotes the ubiquitination of BECN1 and VPS34, thereby targeting PI3KC3-C1 for degradation (46). It is still difficult to rationalize many of these effects at the structural level. This will require obtaining structures at higher resolution and comparison of phosphorylated and dephosphorylated states at the structural level.
Role of vesicles in autophagy initiation
In yeast, a growing body of evidence suggests that the autophagosome is nucleated by the coalescence of Atg9 and COPII vesicles (119–122). In yeast, Atg9 vesicles are derived from the trans-Golgi network (TGN) (119), while COPII vesicles emerge from ER exit sites (ERES) (123). Both Atg9 and COPII vesicles seem to have central roles in autophagy initiation in mammals, as well. In mammals, the phagophore begins at a tubular outgrowth (124–126) of the PI(3)P-positive domain of the ER known as the omegasome (10). The relationship between the tubular outgrowth and the pool of COPII vesicles is still unclear. Here we focus on recent advances in understanding the roles of the vesicles themselves in initiating autophagy.
Atg9 vesicles
Atg9 is the only integral membrane protein that is essential for autophagy (127, 128). This large protein (997 residues in yeast) contains six predicted transmembrane helices spread through its central region, and has large cytosolic IDRs in its N- and C-terminal regions. Atg9 self-associates within membranes into a higher-order assembly (129). Atg9 is incorporated in small vesicles of diameter 30–40 nm (119) or 30–60 nm (120). While other Atg proteins are often diffusely distributed in the cytosol under nutrient rich conditions, as a membrane protein, Atg9 vesicles reside in a vesicular reservoir (130). Atg9 vesicles are generated and transported from the Golgi (131, 132). In yeast, the process depends on Atg23 and Atg27 (131) and the Rab/Rab GEF pair Sec4 and Sec2 (133). In nutrient rich conditions, Atg9 localizes to the trans-Golgi network and early and late post-Golgi endosomes (134).
In starvation, Atg9 vesicles are mobilized to the PAS by TRAPPIII (67, 69, 134) and, in yeast (64) but apparently not mammals (135), by the Atg1 complex. Specifically, the yeast Atg13 HORMA domain binds to the N-terminus of Atg9, which is much shorter in mammals. In one estimate, three Atg9 vesicles containing ~27 molecules of Atg9 each assemble at the PAS (120). In yeast, Atg9 is important for recruiting Atg2 (136, 137) and PI3KC3-C1 (64) to the PAS. The amount of Atg9 expressed in cells appears to control the frequency of autophagosome formation (138). Phosphorylation of the N-terminal domain of Atg9 by an unknown kinase upregulates the recruitment of Atg9 to the PAS and its ability to initiate autophagy (139). Clearly, the bulk lipid contributed by such a small number of vesicles is a miniscule fraction of what is needed to generate an autophagosome. Consistent with this idea, mammalian ATG9A is not a bulk component of autophagosomes (140), but rather seems to recycle out of the phagophore prior to closure. Thus, the importance of Atg9 seems to more as an organizing center for the initial nucleation of the phagophore.
COPII vesicles in autophagy
The COPII coat consists of the Sar1 GTPase, the inner membrane- and cargo-binding Sec23 and Sec24 subunits, and the outer cage-forming Sec13 and Sec31 subunits (141). COPII vesicles have a long-established role, unrelated to autophagy, as the initial carriers of secretory cargo out of the ER (123). COPII was first implicated in autophagosome biogenesis when mutants in Sec23 and Sec24 and in the COPII associated ERES proteins Sec12 and Sec16 were shown to be defective in autophagy (142). COPII was subsequently found to be physically connected to other core elements of autophagy initiation in yeast, including the Atg1 and PI3KC3-C1 complexes (121). In yeast, the Rab1 ortholog Ypt1 activates the casein kinase 1 (CK1) ortholog Hrr25 to phosphorylate Sec23, which is required for COPII vesicle sorting to the PAS (143). COPII vesicles are targeted for membrane fusion in autophagy by the ER SNARE protein Ufe1, which is a Q(a) SNARE and a member of the syntaxin family (144). The COPII vesicle tethering TRAPPIII complex is implicated in autophagy initiation in both yeast (134, 145, 146) and mammals (68, 69).
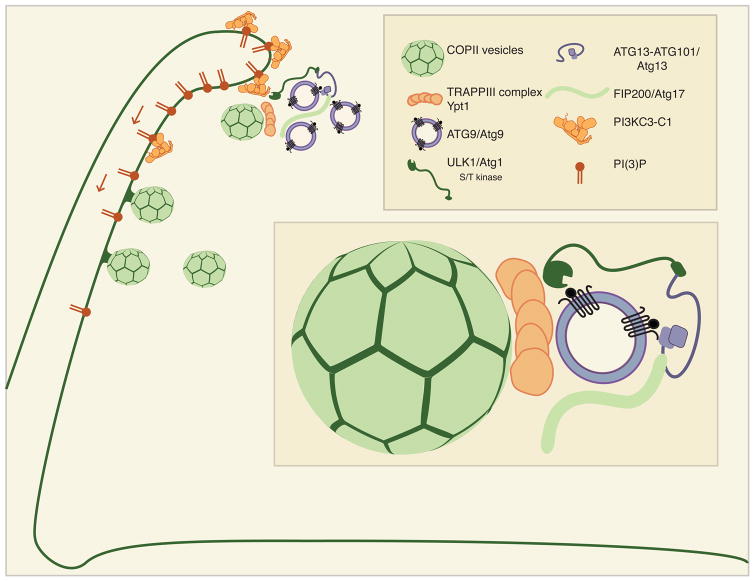
In mammals, most of the output of ERES is directed to the cis-Golgi via the ER-Golgi intermediate compartment (ERGIC) (123). The ERGIC is the depot for sorting by both the COPI and COPII coats in mammals (123). ERGIC membranes support LC3 lipidation in a cell-free system (147, 148). In human cells, the ERES protein tectonin β-propeller containing protein 2 (TECPR2), which is mutated in a hereditary spastic paraplegia, connects the COPII subunit SEC24D to LC3C (149). Super-resolution imaging of mammalian autophagy initiation sites shows that ATG9 vesicles and the ULK1 complex subunit ATG13 coalesce with ERGIC components COPI, COPII, and ERGIC53 (150) (Fig. 4). Collectively, these data provide compelling evidence that COPII vesicles have a conserved role in autophagy initiation. The ER is closely associated with autophagosome biogenesis, and COPII vesicle production provides an appealing mechanism for the exchange of membrane from the ER to the phagophore. In contrast to the situation with ATG9 vesicles, COPII vesicles can be formed in large amounts and so could presumably account for the bulk membrane needed for autophagosome biogenesis, but this has yet to be confirmed quantitatively.
Figure 4. Model for Autophagy initiation at ER Exit Sites, the Omegasome.
The omegasome provides for the initiation of autophagy. The omegasome is enriched with the lipid PI3P that is generated by PI3KC3-C1. The ULK1 complex activates PI3KC3-C1 through phosphorylation. Arrows denote that PI3P can diffuse through the membrane. PI3P is also enriched at ER Exit Sites (ERES), where COPII vesicles emerge. Inset: COPII vesicles interact with the TRAPPIII complex, which binds to ATG9/Atg9 and ULK1/Atg1.
Conclusion
The “parts list” of autophagy initiation is becoming increasingly well defined. Building on the identification of the key initiating proteins in yeast (1, 15), proteomics and mammalian cell biology have fleshed out many additional components (15, 68). With the parts list approaching, perhaps, completion, the big question is how all these parts work together to create the phagophore de novo. As with many other complex processes of cells, the mechanics of how Atg proteins come together with vesicles to create phagophores occurs on the nanoscale, i.e., the length scale of 10s on nm. In the recent past, the experimental autophagy research has relied extensively on diffraction-limited fluorescence microscopy, co-immunoprecipitation, and protein phosphorylation studies. These now-traditional approaches have yielded many important discoveries, yet they will not be able to break through to resolving the nanoscale mechanisms involved in phagophore initiation. Superresolution and cryo-electron microscopy methodologies are advancing quickly, and the advances are quickly being adopted in autophagy research (151). The imaging approaches will generate more insights that can be contemplated on the structural scale, and the structural biology studies are expanding in scope to approach the size scales reachable by superresolution imaging. Thus far the insights generated by cell imaging and structural biology have yet to truly converge. Yet it seems that such a convergence will be inevitable. At that point, the rate-limiting step may change from technical challenges to the conceptual challenge of embracing the complexity that these methods are bound to reveal.
Acknowledgments
We thank members of the Hurley lab for discussions and Liang Ge for critically reading the manuscript. Autophagy research in the Hurley lab is supported by NIH grants GM111730 and GM051487.
References
- 1.Wen X, Klionsky DJ. An overview of macroautophagy in yeast. J Mol Biol. 2016;428:1681–99. doi: 10.1016/j.jmb.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, et al. In: Annual Review of Biochemistry. Kornberg RD, editor. Vol. 85. Palo Alto: Annual Reviews; 2016. pp. 685–713. [DOI] [PubMed] [Google Scholar]
- 3.Shaid S, Brandts CH, Serve H, Dikic I. Ubiquitination and selective autophagy. Cell Death Differ. 2013;20:21–30. doi: 10.1038/cdd.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaffagnini G, Martens S. Mechanisms of selective autophagy. J Mol Biol. 2016;428:1714–24. doi: 10.1016/j.jmb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nature Reviews Neuroscience. 2015;16:345–57. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–80. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N, Komatsu M. Autophagy: Renovation of Cells and Tissues. Cell. 2011;147:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Gomes LC, Dikic I. Autophagy in Antimicrobial Immunity. Mol Cell. 2014;54:224–33. doi: 10.1016/j.molcel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–81. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, et al. Autophagosome formation from compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nature Reviews Molecular Cell Biology. 2013;14:759–74. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 12.Sawa-Makarska J, Abert C, Romanov J, Zens B, Ibiricu I, Martens S. Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane-cargo apposition during selective autophagy. Nat Cell Biol. 2014;16:425–33. doi: 10.1038/ncb2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurzer B, Zaffagnini G, Fracchiolla D, Turco E, Abert C, et al. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife. 2015;4:e08941. doi: 10.7554/eLife.08941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi N, Chen Y, Wang S, Chen MR, Zhao MK, et al. CapZ regulates autophagosomal membrane shaping by promoting actin assembly inside the isolation membrane. Nat Cell Biol. 2015;17:1112–23. doi: 10.1038/ncb3215. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima N, Yoshimori T, Ohsumi Y. Annual Review of Cell and Developmental Biology. 2011;27:107–32. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 16.Hurley JH, Schulman BA. Atomistic Autophagy: The Structures of Cellular Self-Digestion. Cell. 2014;157:300–11. doi: 10.1016/j.cell.2014.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Osawa T, Fujioka Y, Noda NN. Structural biology of the core autophagy machinery. Curr Opin Struct Biol. 2016;43:10–7. doi: 10.1016/j.sbi.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Joo JH, Wang B, Frankel E, Ge L, Xu L, et al. The Noncanonical Role of ULK/ATG1 in ER-to-Golgi Trafficking Is Essential for Cellular Homeostasis. Mol Cell. 2016;62:491–506. doi: 10.1016/j.molcel.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair U, Jotwani A, Geng JF, Gammoh N, Richerson D, et al. SNARE Proteins Are Required for Macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome Precursor Maturation Requires Homotypic Fusion. Cell. 2011;146:303–17. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau K, Renna M, Rubinsztein DC. Connections between SNAREs and autophagy. Trends Biochem Sci. 2013;38:57–63. doi: 10.1016/j.tibs.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Mancias JC, Kimmelman AC. Mechanisms of selective autophagy in normal physiology and cancer. J Mol Biol. 2016;428:1659–80. doi: 10.1016/j.jmb.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–9. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Lin MG, Hurley JH. Structure and function of the ULK1 complex in autophagy. Curr Opin Cell Biol. 2016;39:61–8. doi: 10.1016/j.ceb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papinski D, Kraft C. Regulation of autophagy by signalling through the Atg1/ULK1 complex. J Mol Biol. 2016;428:1725–41. doi: 10.1016/j.jmb.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1-ATG13-FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, et al. Nutrient-dependent mTORC1 Association with the ULK1-Atg13-FIP200 Complex Required for Autophagy. Molecular Biology of the Cell. 2009;20:1981–91. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, et al. ULK-Atg13-FIP200 Complexes Mediate mTOR Signaling to the Autophagy Machinery. Molecular Biology of the Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara T, Takamura A, Kishi C, Iemura SI, Natsume T, et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jao CC, Ragusa MJ, Stanley RE, Hurley JH. A HORMA domain in Atg13 mediates PI 3-kinase recruitment in autophagy. Proc Natl Acad Sci U S A. 2013;110:5486–91. doi: 10.1073/pnas.1220306110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki H, Kaizuka T, Mizushima N, Noda NN. Structure of the Atg101-Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat Struct Mol Biol. 2015;22:572–80. doi: 10.1038/nsmb.3036. [DOI] [PubMed] [Google Scholar]
- 32.Michel M, Schwarten M, Decker C, Nagel-Steger L, Willbold D, Weiergraber OH. The mammalian autophagy initiator complex contains 2 HORMA domain proteins. Autophagy. 2015;11:2300–8. doi: 10.1080/15548627.2015.1076605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi SQ, Kim DJ, Stjepanovic G, Hurley JH. Structure of the Human Atg13-Atg101 HORMA Heterodimer: an Interaction Hub within the ULK1 Complex. Structure. 2015;23:1848–57. doi: 10.1016/j.str.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujioka Y, Suzuki SW, Yamamoto H, Kondo-Kakuta C, Kimura Y, et al. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat Struct Mol Biol. 2014;21:513–21. doi: 10.1038/nsmb.2822. [DOI] [PubMed] [Google Scholar]
- 35.Yorimitsu T, Klionsky DJ. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Molecular Biology of the Cell. 2005;16:1593–605. doi: 10.1091/mbc.E04-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheong H, Yorimitsu T, Reggiori F, Legakis JE, Wang CW, Klionsky DJ. Atg17 regulates the magnitude of the autophagic response. Molecular Biology of the Cell. 2005;16:3438–53. doi: 10.1091/mbc.E04-10-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabeya Y, Kamada Y, Baba M, Takikawa H, Sasaki M, Ohsumi Y. Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Molecular Biology of the Cell. 2005;16:2544–53. doi: 10.1091/mbc.E04-08-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabeya Y, Noda NN, Fujioka Y, Suzuki K, Inagaki F, Ohsumi Y. Characterization of the Atg17-Atg29-Atg31 complex specifically required for starvation-induced autophagy in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2009;389:612–5. doi: 10.1016/j.bbrc.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 39.Ragusa MJ, Stanley RE, Hurley JH. Architecture of the Atg17 Complex as a Scaffold for Autophagosome Biogenesis. Cell. 2012;151:1501–12. doi: 10.1016/j.cell.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach M, Larance M, James DE, Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem J. 2011;440:283–91. doi: 10.1042/BJ20101894. [DOI] [PubMed] [Google Scholar]
- 41.Lazarus MB, Novotny CJ, Shokat KM. Structure of the Human Autophagy Initiating Kinase ULK1 in Complex with Potent Inhibitors. ACS chemical biology. 2015;10:257–61. doi: 10.1021/cb500835z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh YY, Wrasman K, Herman PK. Autophosphorylation Within the Atg1 Activation Loop Is Required for Both Kinase Activity and the Induction of Autophagy in Saccharomyces cerevisiae. Genetics. 2010;185:871–82. doi: 10.1534/genetics.110.116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kijanska M, Dohnal I, Reiter W, Kaspar S, Stoffel I, et al. Activation of Atg1 kinase in autophagy by regulated phosphorylation. Autophagy. 2010;6:1168–78. doi: 10.4161/auto.6.8.13849. [DOI] [PubMed] [Google Scholar]
- 44.Kamber RA, Shoemaker CJ, Denic V. Receptor-Bound Targets of Selective Autophagy Use a Scaffold Protein to Activate the Atg1 Kinase. Mol Cell. 2015;59:372–81. doi: 10.1016/j.molcel.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto H, Fujioka Y, Suzuki SW, Noshiro D, Suzuki H, et al. The Intrinsically Disordered Protein Atg13 Mediates Supramolecular Assembly of Autophagy Initiation Complexes. Dev Cell. 2016;38:86–99. doi: 10.1016/j.devcel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Liu CC, Lin YC, Chen YH, Chen CM, Pang LY, et al. Cul3-KLHL20 Ubiquitin Ligase Governs the Turnover of ULK1 and VPS34 Complexes to Control Autophagy Termination. Mol Cell. 2016;61:84–97. doi: 10.1016/j.molcel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Stjepanovic G, Davies CW, Stanley RE, Ragusa MJ, Kim DJ, Hurley JH. Assembly and dynamics of the autophagy initiating Atg1 complex. Proc Natl Acad Sci U S A. 2014;111:12793–8. doi: 10.1073/pnas.1407214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao Y, Perna MG, Hofmann B, Beier V, Wollert T. The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy. Nature Communications. 2016;7:10338. doi: 10.1038/ncomms10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–13. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24:400–6. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamada Y, Yoshino K, Kondo C, Kawamata T, Oshiro N, et al. Tor Directly Controls the Atg1 Kinase Complex To Regulate Autophagy. Mol Cell Biol. 2010;30:1049–58. doi: 10.1128/MCB.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraft C, Kijanska M, Kalie E, Siergiejuk E, Lee SS, et al. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 2012;31:3691–703. doi: 10.1038/emboj.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hieke N, Loffler AS, Kaizuka T, Berleth N, Bohler P, et al. Expression of a ULK1/2 binding-deficient ATG13 variant can partially restore autophagic activity in ATG13-deficient cells. Autophagy. 2015;11:1471–83. doi: 10.1080/15548627.2015.1068488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egan DF, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7:645–6. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science. 2011;331:456–61. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang LB, Chen S, Du FH, Li S, Zhao LP, Wang XD. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci U S A. 2011;108:4788–93. doi: 10.1073/pnas.1100844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mack HID, Zheng B, Asara JM, Thomas SM. AMPK-dependent phosphorylation of ULK1 regulates ATG9 localization. Autophagy. 2012;8:1197–214. doi: 10.4161/auto.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rui Y-N, Xu Z, Patel B, Chen Z, Chen D, et al. Huntingtin functions as a scaffold for selective macroautophagy. Nat Cell Biol. 2015;17:262–75. doi: 10.1038/ncb3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–18. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 62.Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29:157–71. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alemu EA, Lamark T, Torgersen KM, Birgisdottir AB, Larsen KB, et al. ATG8 Family Proteins Act as Scaffolds for Assembly of the ULK Complex SEQUENCE REQUIREMENTS FOR LC3-INTERACTING REGION (LIR) MOTIFS. J Biol Chem. 2012;287:39275–90. doi: 10.1074/jbc.M112.378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki SW, Yamamoto H, Oikawa Y, Kondo-Kakuta C, Kimura Y, et al. Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc Natl Acad Sci U S A. 2015;112:3350–5. doi: 10.1073/pnas.1421092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sekito T, Kawamata T, Ichikawa R, Suzuki K, Ohsumi Y. Atg17 recruits Atg9 to organize the pre-autophogsomal structure. Genes Cells. 2009;14:525–38. doi: 10.1111/j.1365-2443.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Menon S, Yamasaki A, Chou H-T, Walz T, et al. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc Natl Acad Sci U S A. 2013;110:9800–5. doi: 10.1073/pnas.1302337110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakuta S, Yamamoto H, Negishi L, Kondo-Kakuta C, Hayashi N, Ohsumi Y. Atg9 Vesicles Recruit Vesicle-tethering Proteins Trs85 and Ypt1 to the Autophagosome Formation Site. J Biol Chem. 2012;287:44261–9. doi: 10.1074/jbc.M112.411454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamb CA, Nuhlen S, Judith D, Frith D, Snijders AP, et al. TBC1D14 regulates autophagy via the TRAPP complex and ATG9 traffic. EMBO J. 2016;35:281–301. doi: 10.15252/embj.201592695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Webster CP, Smith EF, Bauer CS, Moller A, Hautbergue GM, et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J. 2016;35:1656–76. doi: 10.15252/embj.201694401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papinski D, Schuschnig M, Reiter W, Wilhelm L, Barnes CA, et al. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol Cell. 2014;53:471–83. doi: 10.1016/j.molcel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Egan DF, Chun MG, Vamos M, Zou H, Rong J, et al. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell. 2015;59:285–97. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Russell RC, Tian Y, Yuan HX, Park HW, Chang YY, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–50. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–68. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim J, Lachenmayer ML, Wu S, Liu WC, Kundu M, et al. Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates. PLoS Genet. 2015;11:e1004987. doi: 10.1371/journal.pgen.1004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu WX, Tian WL, Hu Z, Chen G, Huang L, et al. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. Embo Reports. 2014;15:566–75. doi: 10.1002/embr.201438501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abeliovich H, Zhang C, Dunn WA, Jr, Shokat KM, Klionsky DJ. Chemical genetic analysis of Apg1 reveals a non-kinase role in the induction of autophagy. Molecular Biology of the Cell. 2003;14:477–90. doi: 10.1091/mbc.E02-07-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheong H, Nair U, Geng JF, Klionsky DJ. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Molecular Biology of the Cell. 2008;19:668–81. doi: 10.1091/mbc.E07-08-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stanley RE, Ragusa MJ, Hurley JH. The beginning of the end: how scaffolds nucleate autophagosome biogenesis. Trends Cell Biol. 2014;24:73–81. doi: 10.1016/j.tcb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Köfinger J, Ragusa MJ, Lee IH, Hummer G, Hurley JH. Solution structure of the Atg1 complex: Implications for the architecture of the phagophore assembly site. Structure. 2015;23:809–18. doi: 10.1016/j.str.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alers S, Loffler AS, Paasch F, Dieterle AM, Keppeler H, et al. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1424–33. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Backer JM. The intricate regulation and complex functions of the Class III phosphoinositide 3-kinase Vps34. Biochem J. 2016;473:2251–71. doi: 10.1042/BCJ20160170. [DOI] [PubMed] [Google Scholar]
- 83.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–30. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Molecular Biology of the Cell. 2008;19:5360–72. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kametaka S, Okano T, Ohsumi M, Ohsumi Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J Biol Chem. 1998;273:22284–91. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- 86.Obara K, Sekito T, Ohsumi Y. Assortment of phosphatidylinositol 3-kinase complexes-Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Molecular Biology of the Cell. 2006;17:1527–39. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci US A. 2008;105:19211–6. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11:385–96. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 89.Zhong Y, Wang QJ, Li XT, Yan Y, Backer JM, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–76. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, et al. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science. 2010;327:1638–42. doi: 10.1126/science.1184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li XH, He LQ, Che KH, Funderburk SF, Pan LF, et al. Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG. Nature Communications. 2012;3:662. doi: 10.1038/ncomms1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noda NN, Kobayashi T, Adachi W, Fujioka Y, Ohsumi Y, Inagaki F. Structure of the Novel C-terminal Domain of Vacuolar Protein Sorting 30/Autophagy-related Protein 6 and Its Specific Role in Autophagy. J Biol Chem. 2012;287:16256–66. doi: 10.1074/jbc.M112.348250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang W, Choi W, Hu W, Mi N, Guo Q, et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012;22:473–89. doi: 10.1038/cr.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heenan EJ, Vanhooke JL, Temple BR, Betts L, Sondek JE, Dohlman HG. Structure and Function of Vps15 in the Endosomal G Protein Signaling Pathway. Biochemistry. 2009;48:6390–401. doi: 10.1021/bi900621w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baskaran S, Carlson LA, Stjepanovic G, Young LN, Kim DJ, et al. Architecture and Dynamics of the Autophagic Phosphatidylinostol 3-Kinase Complex. eLife. 2014 Dec;9:3. doi: 10.7554/eLife.05115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rostislavleva K, Soler N, Ohashi Y, Zhang LF, Pardon E, et al. Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350 doi: 10.1126/science.aac7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan W, Nassiri A, Zhong Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L) Proc Natl Acad Sci U S A. 2011;108:7769–74. doi: 10.1073/pnas.1016472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–46. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 99.Vanni S, Vamparys L, Gautier R, Drin G, Etchebest C, et al. Amphipathic Lipid Packing Sensor Motifs: Probing Bilayer Defects with Hydrophobic Residues. Biophys J. 2013;104:575–84. doi: 10.1016/j.bpj.2012.11.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stack JH, DeWald DB, Takegawa K, Emr SD. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J Cell Biol. 1995;129:321–34. doi: 10.1083/jcb.129.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao Y, Wang Y, Saab WFA, Yang F, Pessin JE, Backer JM. NRBF2 regulates macroautophagy as a component of Vps34 Complex I. Biochem J. 2014;461:315–22. doi: 10.1042/BJ20140515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu J, He L, Behrends C, Araki M, Araki K, et al. NRBF2 regulates autophagy and prevents liver injury by modulating Atg14L-linked phosphatidylinositol-3 kinase III activity. Nature Communications. 2014;5:3920. doi: 10.1038/ncomms4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhong Y, Morris DH, Jin L, Patel MS, Karunakaran SK, et al. Nrfb2 suppresses Autophagy by Modulating Atg14L-containing Beclin 1-Vps34 Protein Complex Architecture and Reducting Intracellular Phosphatidylinositol-3 Phosphate Levels. J Biol Chem. 2014;289:26021–37. doi: 10.1074/jbc.M114.561134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Araki Y, Ku WC, Akioka M, May AI, Hayashi Y, et al. Atg38 is required for autophagy-specific phosphatidylinositol 3-kinase complex integrity. J Cell Biol. 2013;203:299–313. doi: 10.1083/jcb.201304123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohashi Y, Soler N, Ortegon MG, Zhang L, Kirsten ML, et al. Characterization of Atg38 and NRBF2, a fifthe subunit of the autophagic Vps34/PIKC3C complex. Autophagy. 2016 doi: 10.1080/15548627.2016.1226736. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Young LN, Cho K, Lawrence R, Zoncu R, Hurley JH. Dynamics and architecture of the NRBF2-containing phosphatidylinositol 3-kinase complex I of autophagy. Proc Natl Acad Sci U S A. 2016;113:8224–9. doi: 10.1073/pnas.1603650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oberstein A, Jeffrey PD, Shi YG. Crystal structure of the Bcl-X-L-beclin 1 peptide complex - Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–32. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 108.Pattingre S, Tassa A, Qu XP, Garuti R, Liang XH, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–5. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 110.Xu DQ, Wang Z, Wang CY, Zhang DY, Wan HD, et al. PAQR3 controls autophagy by integrating AMPK signaling to enhance ATG14L-associated PI3K activity. EMBO J. 2016;35:496–514. doi: 10.15252/embj.201592864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei YJ, An ZY, Zou ZJ, Sumpter R, Su MF, et al. The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. Elife. 2015;4:05289. doi: 10.7554/eLife.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim J, Kim YC, Fang C, Russell RC, Kim JH, et al. Differential Regulation of Distinct Vps34 Complexes by AMPK in Nutrient Stress and Autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fujiwara N, Usui T, Ohama T, Sato K. Regulation of Beclin 1 Protein Phosphorylation and Autophagy by Protein Phosphatase 2A (PP2A) and Death-associated Protein Kinase 3 (DAPK3) J Biol Chem. 2016;291:10858–66. doi: 10.1074/jbc.M115.704908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong PM, Feng Y, Wang JR, Shi R, Jiang XJ. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nature Communications. 2015;6:8048. doi: 10.1038/ncomms9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-X-L and induction of autophagy. Embo Reports. 2009;10:285–92. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei YJ, Zou ZJ, Becker N, Anderson M, Sumpter R, et al. EGFR-Mediated Beclin 1 Phosphorylation in Autophagy Suppression, Tumor Progression, and Tumor Chemoresistance. Cell. 2013;154:1269–84. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan HX, Russell RC, Guan KL. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy. 2013;9:1983–95. doi: 10.4161/auto.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang RC, Wei YJ, An ZY, Zou ZJ, Xiao GH, et al. Akt-Mediated Regulation of Autophagy and Tumorigenesis Through Beclin 1 Phosphorylation. Science. 2012;338:956–9. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–22. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, et al. Atg9 Vesicles are an Important Membrane Source During Early Steps of Autophagosome Formation. J Cell Biol. 2012;198:219–33. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Graef M, Friedman JR, Graham C, Babu M, Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013;24:2918–31. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ge L, Baskaran S, Schekman R, Hurley JH. The Protein-Vesicle Network of Autophagy. Curr Opin Cell Biol. 2014;29:18–24. doi: 10.1016/j.ceb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 123.Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nature Reviews Molecular Cell Biology. 2013;14:382–92. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–7. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 125.Yla-Anttila P, Vihinen H, Jokita E, Eskelinen E-L. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5:1180–5. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
- 126.Biazik J, Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. Ultrastructural relationship of the phagophore with surrounding organelles. Autophagy. 2015;11:439–51. doi: 10.1080/15548627.2015.1017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lang T, Reiche S, Straub M, Bredschneider M, Thumm M. Autophagy and the cvt pathway both depend on AUT9. J Bacteriol. 2000;182:2125–33. doi: 10.1128/jb.182.8.2125-2133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noda T, Kim J, Huang WP, Baba M, Tokunaga C, et al. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J Cell Biol. 2000;148:465–79. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He CC, Baba M, Cao Y, Klionsky DJ. Self-Interaction Is Critical for Atg9 Transport and Function at the Phagophore Assembly Site during Autophagy. Molecular Biology of the Cell. 2008;19:5506–16. doi: 10.1091/mbc.E08-05-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse Autophagosome Membrane Sources Coalesce in Recycling Endosomes. Cell. 2013;154:1285–99. doi: 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 132.Young ARJ, Chan EYW, Hu XW, Koch R, Crawshaw SG, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 133.Geng JF, Nair U, Yasumura-Yorimitsu K, Klionsky DJ. Post-Golgi Sec Proteins Are Required for Autophagy in Saccharomyces cerevisiae. Molecular Biology of the Cell. 2010;21:2257–69. doi: 10.1091/mbc.E09-11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shirahama-Noda K, Kira S, Yoshimori T, Noda T. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J Cell Sci. 2013;126:4963–73. doi: 10.1242/jcs.131318. [DOI] [PubMed] [Google Scholar]
- 135.Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci. 2012;125:1488–99. doi: 10.1242/jcs.094110. [DOI] [PubMed] [Google Scholar]
- 136.Shintani T, Suzuki K, Kamada Y, Noda T, Ohsumi Y. Apg2p functions in autophagosome formation on the perivacuolar structure. J Biol Chem. 2001;276:30452–60. doi: 10.1074/jbc.M102346200. [DOI] [PubMed] [Google Scholar]
- 137.Wang CW, Kim J, Huang WP, Abeliovich H, Stromhaug PE, et al. Apg2 is a novel protein required for the cytoplasm to vacuole targeting, autophagy, and pexophagy pathways. J Biol Chem. 2001;276:30442–51. doi: 10.1074/jbc.M102342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin MY, He D, Backues SK, Freeberg MA, Liu X, et al. Transcriptional Regulation by Pho23 Modulates the Frequency of Autophagosome Formation. Curr Biol. 2014;24:1314–22. doi: 10.1016/j.cub.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feng YC, Backues SK, Baba M, Heo JM, Harper JW, Klionsky DJ. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Autophagy. 2016;12:648–58. doi: 10.1080/15548627.2016.1157237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, et al. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Molecular Biology of the Cell. 2012;23:1860–73. doi: 10.1091/mbc.E11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Faini M, Beck R, Wieland FT, Briggs JAG. Vesicle coats: structure, function, and general principles of assembly. Trends Cell Biol. 2013;23:279–88. doi: 10.1016/j.tcb.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 142.Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, et al. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Molecular Biology of the Cell. 2001;12:3690–702. doi: 10.1091/mbc.12.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J, Davis S, Menon S, Zhang JZ, Ding JZ, et al. Ypt1/Rab1 regulates Hrr25/CK1 delta kinase activity in ER-Golgi traffic and macroautophagy. J Cell Biol. 2015;210:273–85. doi: 10.1083/jcb.201408075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lemus L, Ribas JL, Sikorska N, Goder V. An ER-Localized SNARE Protein Is Exported in Specific COPII Vesicles for Autophagosome Biogenesis. Cell Reports. 2016;14:1710–22. doi: 10.1016/j.celrep.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 145.Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai HQ, et al. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A. 2010;107:7811–6. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan D, Cai Y, Wang J, Zhang J, Menon S, et al. The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc Natl Acad Sci U S A. 2013;110:19432–7. doi: 10.1073/pnas.1316356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ge L, Melville D, Hang ZM, Schekman R. The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. ELife. 2013;2 doi: 10.7554/eLife.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ge L, Zhang M, Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. Elife. 2014;3:04135. doi: 10.7554/eLife.04135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stadel D, Millarte V, Tillmann KD, Huber J, Tamin-Yecheskel BC, et al. TECPR2 Cooperates with LC3C to Regulate COPII-Dependent ER Export. Mol Cell. 2015;60:89–104. doi: 10.1016/j.molcel.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 150.Karanasios E, Walker SA, Okkenhaug H, Manifava M, Hummel E, et al. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nature Communications. 2016;7:12420. doi: 10.1038/ncomms12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hurley JH, Nogales E. Next-generation electron microscopy in autophagy research. Curr Opin Struct Biol. 2016;41:211–6. doi: 10.1016/j.sbi.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]