Abstract
Formaldehyde (FA) is a common environmental toxin but is also endogenously produced through a diverse array of essential biological processes, including mitochondrial one-carbon metabolism, metabolite oxidation, and nuclear epigenetic modifications. Its high electrophilicity enables reactivity with a wide variety of biological nucleophiles, which can be beneficial or detrimental to cellular function depending on the context. New methods that enable detection of FA in living systems can help disentangle the signal/stress dichotomy of this simplest reactive carbonyl species (RCS), and fluorescent probes for FA with high selectivity and sensitivity have emerged as promising chemical tools in this regard.
Introduction
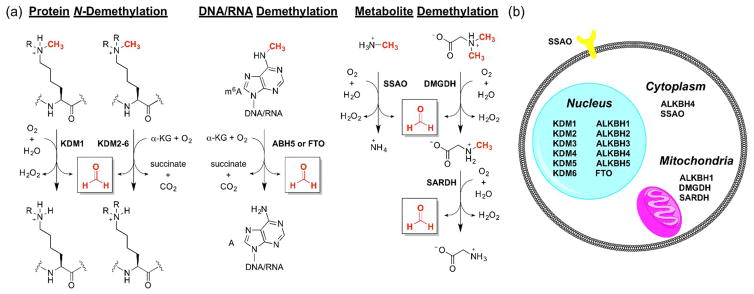
Formaldehyde (FA) is an endogenously-produced reactive carbonyl species (RCS) released in biological processes ranging from epigenetics to one carbon metabolism (Figure 1) [1,2]. Although more widely known as an environmental toxin and carcinogen [3], FA has been reported to exist at relatively high concentrations intracellularly under normal physiological conditions, reaching up to 500 μM in certain organelles [4,5••]. FA is a one carbon fuel involved in maintenance of cell homeostasis, and its abundance and high reactivity suggest a potential role as a physiological signaling molecule [6,7•,8]. FA is the product of demethylation events of N-methylated amino acid residues (e.g. lysine, arginine, histidine) mediated by demethylase enzymes [9], such as lysine specific demethylase 1 (KDM1) [10,11] and Jumonji domain-containing proteins (Figure 1) [12–14]. FA is also produced through N-demethylation of DNA and RNA bases, such as m6A, by AlkB homologues (ALKBH) [15]. Methylation and demethylation events signal transcription factors to promote or repress transcription, and disruption of normal methylation markers could have wide ranging effects in disease states, particularly cancer progression [16,17]. Similarly, metabolism of methylated amines, including the abundant endogenous metabolite methylamine, by semicarbazide-sensitive amine oxidases (SSAO) releases FA [18•,19]. In addition, several demethylase enzymes utilize tetrahydrofolate (THF) as a cofactor to bind FA, yielding 5,10-methylene-THF, known as “active formaldehyde” [20,21]. Folate derivatives are essential for mitochondrial one-carbon metabolism, in which demethylation events release FA which is further incorporated into important cellular building blocks such as amino acids, purines, and phospholipids [22]. A canonical example is the sarcosine pathway, where dimethylglycine dehydrogenase (DMGDH) and sarcosine dehydrogenase (SARDH) demethylate dimethylglycine sequentially, yielding two equivalents of FA and glycine [23•]. Owing to the toxic nature of FA, it is efficiently metabolized by the enzyme alcohol dehydrogenase 5 (ADH5, also known as formaldehyde dehydrogenase, S-nitrosogluthathione reductase, and alcohol dehydrogenase 3) as its glutathione adduct, S-(hydroxymethyl)glutathione [24–27].
Figure 1.
(a) Selected enzymatic pathways that generate FA in biological systems. (b) Subcellular localization of FA-generating enzymes. Abbreviations: α-KG, α-ketoglutarate; KDM, lysine demethylase; ALKBH, AlkB homologues; SSAO, semicarbazide-sensitive amine oxidases; DMGDH, dimethylglycine dehydrogenase; SARDH, sarcosine dehydrogenase; FTO, fat mass and obesity-associated protein.
The aforementioned examples provide motivation for developing new FA detection technologies for biological study. Owing to the prevalence of FA in industrial settings as well as in household items, several sensitive FA detection methods have been devised [28], including methods utilizing high performance liquid chromatography (HPLC) [29,30], gas chromatography (GC) [31], mass spectrometry (MS) [32], as well as preconcentration/chemical ionization MS [33]. However, the study of FA and its roles in physiology and pathology is still limited by a lack of detection methods that can give spatiotemporal resolution in living cells and more complex biological specimens. One emerging approach utilizes fluorescence-based probes, which offer high selectivity and sensitivity and can be used in situ. Reactivity-based fluorescent probes have been successfully employed to image a variety of biological analytes [34–36], including the carbonyl species carbon monoxide [37–41] and methylglyoxal [42], and recent efforts have focused on the development of reactivity-based probes for FA. This review aims to summarize the current progress toward creating responsive and selective fluorescent reporters for FA, along with an outlook toward improvements in the field.
Design considerations for fluorescent formaldehyde probes
Effective fluorescent probes for imaging FA in living cells and higher specimens must meet several criteria. Key considerations are fast reactivity with FA and selectivity of the reaction against other biological analytes, especially other biologically-relevant RCS which possess similar electrophilic carbonyl groups. These competing RCS, including acrolein, 4-hydroxynonenal, and methylglyoxal, as well as other aldehyde-containing metabolites such as acetaldehyde, are generated primarily during metabolism and oxidative stress conditions, such as ethanol metabolism and lipid peroxidation [43,44]. Compared to the high micromolar endogenous concentrations of FA, steady-state levels of other RCS range from high nanomolar to low micromolar [45] and are estimated to be higher in various disease states [46]. Thus, the large variety of continually-generated RCS and their similar electrophilic reactivity makes selectivity perhaps the most important hurdle to overcome for developing reliable FA detection methods.
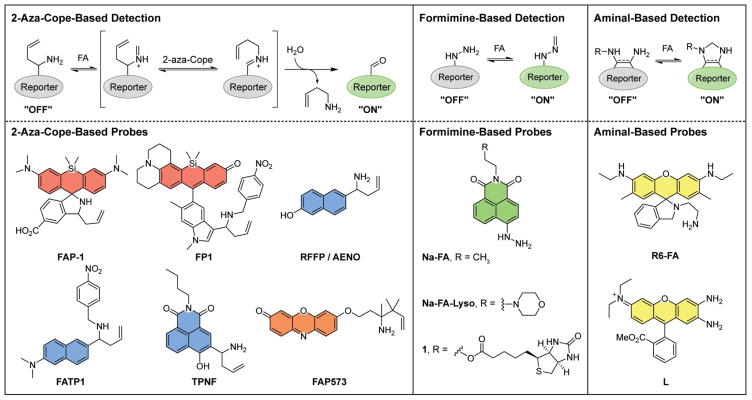
Several different but complementary mechanisms of FA detection have been reported, including 2-aza-Cope-, formimine-, and aminal-based detection methods (Figure 2), all offering unique advantages and disadvantages that are summarized below and in Table 1. In addition, such probes rely on a number of fluorescent scaffolds suitable for biological imaging with excitation wavelengths that span the visible to near-infrared spectrum and/or possess reasonable two-photon cross-sections [47]; ultraviolet excitation is less desirable owing to interference from native autofluorescence from chromophores in the cell such as NADH and flavins as well as the potential for photodamage and oxidative stress induced by irradiation with high-energy light. FA detection can be realized by attaching various reactivity switches to fluorophore platforms, utilizing well-developed fluorescent response mechanisms ranging from spirocyclization, photoinduced electron transfer (PET), internal charge transfer (ICT), fluorophore uncaging, and ratiometric detection [48].
Figure 2.
Molecular probes for the detection of FA. Three general detection strategies have been taken: 2-aza-Cope-based, formimine-based, and aminal-based. Coloring reflects excitation wavelengths.
Table 1.
Summary of Fluorescent FA probes
| Probe | λex (nm) | λem (nm) | Response to FA in vitro | Biological Application |
|---|---|---|---|---|
| aza-Cope-based probes | ||||
| FAP-1 [49] | 645 | 662 | 8-fold turn-on to 0.1 mM FA (1 h, 20 mM PBS) | Cells (HEK293T, MCF7) |
| FP1 [50] | 620 | 649 | 7-fold turn-on to 0.5 mM FA (3 h, PBS) | Cells (HEK293T.NS1) |
| RFFP [51] | 318 | 359 (451 post-FA) | 53-fold ratio change to 3 mM FA (4 h, 25 mM PBS/1% acetone) | Cells (HeLa) |
| AENO [52] | 319 | 513 | ca. 110-fold turn-on to 5 mM FA (3 h, 10 mM PBS/20% DMF) | Cells (HeLa) |
| FATP1 [53] | 390 | 526 | 25-fold turn-on to 0.2 mM FA (3 h, 20 mM PBS/5% DMSO) | Cells (HEK293T, MCF7), Tissue (liver) |
| TPNF [54] | 350 | 510 | 20-fold turn-on to 5 mM FA (3 h, 10 mM PBS/0.5% DMSO) | Cells (HeLa), Zebrafish |
| FAP573 [55] | 573 | 585 | 4.2-fold turn-on to 0.1 mM FA (2 h, 20 mM PBS/0.1% DMSO) | Cells (HEK293T, HAP1, MEF) |
| Formimine based probes | ||||
| Na-FA [58] | 440 | 543 | 900-fold turn-on to 0.1 mM FA (30 min, 10 mMPBS/l% DMSO) | Cells (HeLa), Tissue (liver) |
| Na-FA-Lyso [60] | 440 | 541 | 350-fold turn-on to 0.2 mM FA (30 min, 10 mM PBS/1% DMSO) | Cells (HeLa) |
| 1 [59] | 428 | 541 | 140-fold turn-on to 0.5 mM FA (8 min, 10 mM PBS/1% DMSO) | Cells (4T-1, 3T3), Tissue (tumor) |
| Aminal based probes | ||||
| R6-FA [61] | 530 | 560 | 7.4-fold turn-on to 0.01 mM FA (10 sec, 25 mM PBS/50% DMF) | Cells (HeLa) |
| L [62] | 520 | 620 | ca. 7-fold turn-on to 10 mMFA (10 mM Tris/30%EtOH) | Cells (L929) |
FA probes utilizing the 2-aza-Cope reaction
Our laboratory [49••] and Chan's laboratory [50••] independently and simultaneously developed the 2-aza-Cope-based strategy utilizing a homoallylic amine as a selective trigger for FA reactivity. In this scheme, the homoallylamine condenses with FA, undergoes the 2-aza-Cope rearrangement, and subsequent hydrolysis yields an aldehyde or ketone product (Figure 2). The 2-aza-Cope reaction exhibits high selectivity for FA over other RCS and carbonyl species, such as acetaldehyde, methylglyoxal, and 4-hydroxynonenal. The change in nucleophilicity and electronic properties on conversion of a homoallylamine to a carbonyl can be harnessed to provide a fluorescence turn-on or ratio change. Initial probes utilized near-infrared-emitting silicon rhodamine/rhodol scaffolds as the fluorophore with different sensing mechanisms to elicit a fluorogenic response. Our laboratory reported Formaldehyde Probe 1 (FAP-1), whose homoallylamine favors an initial spirocyclized, weakly emissive state [49••]. After FA reaction, the product aldehyde is incapable of spirocyclization, leading to an open, fluorescent product with an 8-fold turn-on response to 100 μM FA after 1 h. High sensitivity and selectivity allowed FAP-1 to respond to exogenously-added FA in living cells within 30 min, and was also able to detect a decrease in endogenous FA levels in cells upon LSD1 inhibition. Concurrent work by the Chan group reported FP-1, which utilizes a PET mechanism to elicit a fluorescent response [50••]. Specifically, a nitrobenzene moiety was appended to the homoallylamine, which quenches the rhodamine through donor-excited PET. After the 2-aza-Cope reaction, hydrolysis liberates the fluorophore from the nitrobenzene moiety, resulting in 7-fold fluorescence turn-on response to 250 μM FA after 3 h. In cells, FP-1 displayed a 1.2-fold turn-on to 1 mM FA after 3 h. These reports demonstrated the feasibility of utilizing fluorescence-based probes to detect FA in living cells, and specifically the utility of the 2-aza-Cope reaction as a detection platform.
The 2-aza-Cope strategy has subsequently been extended to other fluorophore scaffolds, establishing the generality of this reactive trigger for FA detection. Indeed, several groups have reported 2-aza-Cope probes for FA based on an ICT mechanism using an unfunctionalized homoallylamine switch. A homoallylamine appended to a naphthalene fluorophore furnished a UV-excitable ratiometric probe (reported by different groups as RFFP and AENO) [51,52]. These first-generation probes are highly selective for FA and exhibit good ratiometric responses, but are limited by relatively slow reaction kinetics, taking hours to display a statistically significant turn-on signal to millimolar levels of exogenously added FA and thus limiting their applicability to image endogenous FA pathways. The first examples of two-photon FA probes FATP-1 [53] and TPNF [54] were applied to image FA in tissue and zebrafish, respectively. Both FATP-1 and TPNF report a 3 h incubation in cells to show a statistically significant turn-on response but illustrate the compatibility of the 2-aza-Cope reaction for tissue and whole organism settings.
While highly selective, the use of a parent homoallylamine for the 2-aza-Cope reaction leaves room for improvement owing to its relatively slow reaction kinetics. Indeed, in organic synthesis, 2-aza-Cope reactions are often performed at higher temperatures to promote reactivity; however, biological constraints preclude altering temperature above physiological levels, and as such improving reactivity must be addressed through structural modifications of the homoallylamine trigger. Recent efforts from our lab led to the development of a 2-aza-Cope-based trigger for FA with improved kinetics through structural modification of the homoallylamine moiety [55•]. In particular, appending gem-dimethyl substituents accelerated the 2-aza-Cope reaction about 10-fold through the Thorpe-Ingold effect [56], allowing for a responsive and selective series of FA probes. One such probe, FAP-573, was capable of visualizing increased endogenous FA levels in ADH5 knockout cells compared to the wildtype counterpart. We have also utilized this motif to develop a first-generation ratiometric FA probe with visible excitation and emission profiles [57•]. Overall, this aforementioned collection of fluorescent probes demonstrates the broad applicability of the 2-aza-Cope reaction for detection of biological FA, and further extensions of this strategy will allow for tuning of excitation/emission wavelengths and subcellular localization, as well as expansion to other imaging modalities.
Formimine and aminal-based FA probes
Alternative reactivity-based approaches for FA detection exploit the formimine- or aminal-forming properties between FA and amines. Both approaches display fast reaction kinetics but are more challenging to tune for FA selectivity over other aldehydes, thus offering distinct advantages and disadvantages over the 2-aza-Cope strategy. Lin and colleagues have elegantly employed a hydrazine moiety to create a series of probes using the 1,8-naphthalimide fluorophore (Na-FA) [58••]. The free amine on the hydrazine initially quenches fluorescence through a PET mechanism, which is blocked after formimine formation. By modifying the fluorescent scaffold, biotin-guided (1) [59] and lysosome-targeting (Na-FA-Lyso) [60] FA probes were created. Na-FA displays an in vitro 900-fold increase in fluorescence to 100 μM FA in 30 min, but in cellulo reactivity appears to be more sluggish and comparable to 2-aza-Cope congeners. A key set of tissue imaging experiments include endogenous changes in FA from the addition of sodium bisulfite, a FA scavenger which lowers levels of free FA by sequestration as the bisulfite adduct. Bisulfite addition shows a decrease in fluorescence compared to basal conditions, suggesting the probes are capable of imaging changes in endogenous FA in cellulo. An important consideration in performing such FA sequestration experiments is to verify the in vitro response of FA probes toward bisulfite alone does not display fluorescence quenching. Formimines can also respond to simple aldehydes such as acetaldehyde, albeit with much less of a response compared to FA, and this promiscuity provides a promising starting point to create probes for acetaldehyde and other RCS in addition to FA.
Aminal formation-based probes exploit slightly different reactivity to detect FA. The Lin group reported R6-FA, which utilizes a spirocyclization method, similar to FAP-1, to induce a fluorescence turn-on [61••]. The spirocyclized amine moiety is linked to a free, FA-reactive amine. The resulting Schiff base is more electrophilic than the xanthone, resulting in an aminal heterocycle formation and concurrent fluorescent turn-on. While highly reactive (FA-induced turn-on occurs within 10 seconds), this method displays relatively poor selectivity for FA over other carbonyl-containing species. Along similar lines, the Zeng group utilized aminal heterocycle formation to create a dual methylglyoxal/FA probe (L) [62]. However, fluorescence turn-on by FA and turn-off by methylglyoxal makes in cellulo experiments difficult to interpret, as both analytes are present in the micromolar concentration range, limiting its application.
Conclusions and outlook
Development of a variety of fluorescent probes for FA has greatly expanded the capabilities to image FA in living biological systems. All three strategies show promise for further interrogation of the roles FA plays in biology. However, probe sensitivity remains hindered by either reaction kinetics for the 2-aza-Cope strategy or selectivity for the formimine- and aminal-based detection methods. Improving reaction kinetics is particularly important owing to the rapid elimination of FA, which has a measured half-life of approximately 90 seconds in organisms [63]. The presence of competing endogenously produced RCS also makes selectivity a key design consideration for confidence in interpreting the fluorescence response as a consequence of FA reactivity alone. Further investigation into the mechanisms of previously unstudied FA biological pathways will benefit from a method able to combine the advantages of the two approaches to create a responsive and selective modality. The tunability of the homoallylamine moiety for the 2-aza-Cope strategy, as demonstrated in FAP573, presages the potential for increased reactivity while conserving selectivity. Due to its generality, as showcased by the various probes discussed above, the 2-aza-Cope strategy shows promise for extension into alternate imaging modalities. Indeed, we recently reported a positron emission tomography-based probe, [18F]FAC-FDG, for in vivo FA imaging [64]. Likewise, the fast kinetics of formimine formation offer a large advantage which could be harnessed through further tuning to inhibit reactivity toward competing carbonyl species. In particular, increased steric encumbrance could afford enhanced selectivity over larger aldehydes. Future experiments with improved FA probes will help elucidate the roles FA may play in physiology and disease related to demethylation events, one-carbon metabolism, and downstream effects of FA metabolism.
Acknowledgments
K.J.B. was partially supported by an NSF graduate fellowship. T.F.B. was partially supported by a Chemical Biology Training Grant from the NIH (T32 GM066698). C.J.C. is an Investigator of the Howard Hughes Medical Institute and a CIFAR Senior Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
- 1.He RQ, Lu J, Miao JY. Formaldehyde stress. Sci China Life Sci. 2010;53:1399–1404. doi: 10.1007/s11427-010-4112-3. [DOI] [PubMed] [Google Scholar]
- 2.Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 3.Liteplo RG, Beauchamp R, Meek ME, Chénier R. Formaldehyde: Concise International Chemical Assessment Document 40. Geneva: WHO; 2002. [Google Scholar]
- 4.Tong Z, Han C, Luo W, Wang X, Li H, Luo H, Zhou J, Qi J, He R. Accumulated hippocampal formaldehyde induces age-dependent memory decline. Age. 2013;35:583–596. doi: 10.1007/s11357-012-9388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5(••).Heck HD, Casanova-Schmitz M, Dodd PB, Schachter EN, Witek TJ, Tosun T. Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rates exposed to CH2O under controlled conditions. AIHA J. 1985;46:1–3. doi: 10.1080/15298668591394275. This paper first reported FA concentrations in the blood of mice and humans. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CM, Ceder R, Grafström RC. Formaldehyde dehydrogenase: Beyond phase I metabolism. Toxicol Lett. 2010;193:1–3. doi: 10.1016/j.toxlet.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 7(•).Tulpule K, Dringen R. Formaldehyde in brain: An overlooked player in neurodegeneration? J Neurochem. 2013;127:7–21. doi: 10.1111/jnc.12356. This review highlights current knowledge on the production and metabolism of FA in the brain, along with a comprehensive review of FA producing and metabolizing enzymes. [DOI] [PubMed] [Google Scholar]
- 8.Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 9.Walport LJ, Hopkinson RJ, Schofield CJ. Mechanisms of human histone and nucleic acid demethylases. Curr Opin Chem Biol. 2012;16:525–534. doi: 10.1016/j.cbpa.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Luka Z, Pakhomova S, Loukachevitch LV, Calcutt MW, Newcomer ME, Wagner C. Crystal structure of the histone lysine specific demethylase LSD1 complexed with tetrahydrofolate. Protein Sci. 2014;23:993–998. doi: 10.1002/pro.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock RL, Dunne K, Walport LJ, Flashman E, Kawamura A. Epigenetic regulation by histone demethylases in hypoxia. Epigenomics. 2015;7:791–811. doi: 10.2217/epi.15.24. [DOI] [PubMed] [Google Scholar]
- 12.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 13.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–95. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Walport LJ, Hopkinson RJ, Chowdhury R, Schiller R, Ge W, Kawamura A, Schofield CJ. Arginine demethylation is catalysed by a subset of JmjC histone lysine demethylases. Nat Commun. 2016;7:11974. doi: 10.1038/ncomms11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujikawa K, Koike K, Kitae K, Shinkawa A, Arima H, Suzuki T, Tsuchiya M, Makino Y, Furukawa T, Konishi N, Yamamoto H. Expression and sub-cellular localization of human ABH family molecules. J Cell Mol Med. 2007;11:1105–1116. doi: 10.1111/j.1582-4934.2007.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wissmann M, Yin N, Müller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Günther T, Buettner R, Metzger E, Schüle R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 17.Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu L, Swenberg JA, Crossan GP, Patel KJ. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol Cell. 2015;60:177–188. doi: 10.1016/j.molcel.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18(•).O’Sullivan J, Unzeta M, Healy J, O’Sullivan MI, Davey G, Tipton KF. Semicarbazide-sensitive amine oxidases: enzymes with quite a lot to do. NeuroToxicology. 2004;25:303–315. doi: 10.1016/S0161-813X(03)00117-7. This review of SSAO enzymes suggests a possible signaling role of FA and other metabolites produced from amine metabolism through discussion of enzyme activity and function. [DOI] [PubMed] [Google Scholar]
- 19.Andrés N, Lizcano JM, Rodríguez MJ, Romera M, Unzeta M, Mahy N. Tissue activity and cellular localization of human semicarbazide-sensitive amine oxidase. J Histochem Cytochem. 2001;49:209–217. doi: 10.1177/002215540104900208. [DOI] [PubMed] [Google Scholar]
- 20.Steenkamp DJ, Husain M. The effect of tetrahydrofolate on the reduction of electron transfer flavoprotein by sarcosine and dimethylglycine dehydrogenases. Biochem J. 1982;203:707–715. doi: 10.1042/bj2030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leys D, Basran J, Scrutton NS. Channelling and formation of “active” formaldehyde in dimethylglycine oxidase. EMBO J. 2003;22:4038–4048. doi: 10.1093/emboj/cdg395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scotti M, Stella L, Shearer EJ, Stover PJ. Modeling cellular compartmentation in one-carbon metabolism. Wiley Interdiscip Rev Syst Biol Med. 2013;5:343–365. doi: 10.1002/wsbm.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23(•).Tralau T, Lafite P, Levy C, Combe JP, Scrutton NS, Leys D. An internal reaction chamber in dimethylglycine oxidase provides efficient protection from exposure to toxic formaldehyde. J Biol Chem. 2009;284:17826–17834. doi: 10.1074/jbc.M109.006262. This paper provides an example for a FA channeling mechanism through active site coupling between dimethylglycine oxidation and 5,10-methylenetetrahydrofolate production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staab CA, Hellgren M, Höög JO. Dual functions of alcohol dehydrogenase 3: Implications with focus on formaldehyde dehydrogenase and S-nitrosoglutathione reductase activities. Cell Mol Life Sci. 2008;65:3950–3960. doi: 10.1007/s00018-008-8592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staab CA, Ålander J, Brandt M, Lengqvist J, Morgenstern R, Grafström RC, Höög JO. Reduction of S-nitrosoglutathione by alcohol dehydrogenase 3 is facilitated by substrate alcohols via direct cofactor recycling and leads to GSH-controlled formation of glutathione transferase inhibitors. Biochem J. 2008;413:493–504. doi: 10.1042/BJ20071666. [DOI] [PubMed] [Google Scholar]
- 26.Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, O’Brien PJ. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem Biol Interact. 2001;130:285–296. doi: 10.1016/s0009-2797(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 27.MacAllister SL, Choi J, Dedina L, O’Brien PJ. Metabolic mechanisms of methanol/formaldehyde in isolated rat hepatocytes: Carbonyl-metabolizing enzymes versus oxidative stress. Chem Biol Interact. 2011;191:308–314. doi: 10.1016/j.cbi.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Salthammer T, Mentese S, Marutzky R. Formaldehyde in the indoor environment. Chem Rev. 2010;110:2536–2572. doi: 10.1021/cr800399g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gubisne-Haberle D, Hill W, Kazachkov M, Richardson JS, Yu PH. Protein cross-linkage induced by formaldehyde derived from semicarbazide-sensitive amine oxidase-mediated deamination of methylamine. J Pharmacol Exp Ther. 2004;310:1125–1132. doi: 10.1124/jpet.104.068601. [DOI] [PubMed] [Google Scholar]
- 30.Soman A, Qiu Y, Chan Li Q. HPLC-UV method development and validation for the determination of low level formaldehyde in a drug substance. J Chromatogr Sci. 2008;46:461–465. doi: 10.1093/chromsci/46.6.461. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi A, Takigawa T, Abe M, Kawai T, Endo Y, Yasugi T, Endo G, Ogino K. Determination of formaldehyde in urine by headspace gas chromatography. Bull Environ Contam Toxicol. 2007;79:1–4. doi: 10.1007/s00128-007-9172-0. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Sritharathikhun P, Motomizu S. Development of novel reagent for Hantzsch reaction for the determination of formaldehyde by spectrophotometry and fluorometry. Anal Sci. 2007;23:413–417. doi: 10.2116/analsci.23.413. [DOI] [PubMed] [Google Scholar]
- 33.Kato S, Burke PJ, Koch TH, Bierbaum VM. Formaldehyde in human cancer cells: detection by preconcentration-chemical ionization mass spectrometry. Anal Chem. 2001;73:2992–2997. doi: 10.1021/ac001498q. [DOI] [PubMed] [Google Scholar]
- 34.Chan J, Dodani SC, Chang CJ. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat Chem. 2012;4:973–984. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MH, Kim JS, Sessler JL. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev. 2015;44:4185–4191. doi: 10.1039/c4cs00280f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Zhao Q, Feng W, Li F. Luminescent chemodosimeters for bioimaging. Chem Soc Rev. 2013;113:192–270. doi: 10.1021/cr2004103. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Karpus J, Zhao BS, Luo Z, Chen PR, He C. A selective fluorescent probe for carbon monoxide imaging in living cells. Angew Chem Int Ed. 2012;51:9652–9656. doi: 10.1002/anie.201203684. [DOI] [PubMed] [Google Scholar]
- 38.Michel BW, Lippert AR, Chang CJ. A reaction-based fluorescent probe for selective imaging of carbon monoxide in living cells using a palladium-mediated carbonylation. J Am Chem Soc. 2012;134:15668–15671. doi: 10.1021/ja307017b. [DOI] [PubMed] [Google Scholar]
- 39.Zheng K, Lin W, Tan L, Chen H, Cui H. A unique carbazole–coumarin fused two-photon platform: development of a robust two-photon fluorescent probe for imaging carbon monoxide in living tissues. Chem Sci. 2014;5:3439–3448. [Google Scholar]
- 40.Chaves-Ferreira M, Albuquerque IS, Matak-Vinkovic D, Coelho AC, Carvalho SM, Saraiva LM, Romão CC, Bernardes GJL. Spontaneous CO release from RuII(CO)2-protein complexes in aqueous solution, cells, and mice. Angew Chem Int Ed. 2015;54:1172–1175. doi: 10.1002/anie.201409344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson JL, Kobeissi SF, Oudir S, Haas B, Michel B, Randé JLD, Ollivier A, Martens T, Rivard M, Motterlini R, Foresti R. Design and synthesis of new hybrid molecules that activate the transcription factor Nrf2 and simultaneously release carbon monoxide. Chem Eur J. 2014;20:14698–14704. doi: 10.1002/chem.201403901. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Douglass EF, Fitzgerald KJ, Spiegel DA. A “turn-on” fluorescent sensor for methylglyoxal. J Am Chem Soc. 2013;135:12429–12433. doi: 10.1021/ja406077j. [DOI] [PubMed] [Google Scholar]
- 43.Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2010;5:121–128. doi: 10.1007/s12263-009-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellis EM. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol Ther. 2007;115:13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Siegel D, Meinema AC, Permentier H, Hopfgartner G, Bischoff R. Integrated quantification and identification of aldehydes and ketones in biological samples. Anal Chem. 2014;86:5089–5100. doi: 10.1021/ac500810r. [DOI] [PubMed] [Google Scholar]
- 46.Aldini G, Dalle-Donne I, Colombo R, Facino RM, Milzani A, Carini M. Lipoxidation-derived reactive carbonyl species as potential drug targets in preventing protein carbonylation and related cellular dysfunction. ChemMedChem. 2006;1:1045–1058. doi: 10.1002/cmdc.200600075. [DOI] [PubMed] [Google Scholar]
- 47.Lavis LD, Raines RT. Bright ideas for chemical biology. ACS Chem Biol. 2008;3:142–155. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer; 2006. [Google Scholar]
- 49(••).Brewer TF, Chang CJ. An aza-Cope reactivity-based fluorescent probe for imaging formaldehyde in living cells. J Am Chem Soc. 2015;137:10886–10889. doi: 10.1021/jacs.5b05340. The first example, along with Roth et. al., of an aza-Cope reactivity-based fluorescent probe for FA. [DOI] [PubMed] [Google Scholar]
- 50(••).Roth A, Li H, Anorma C, Chan J. A reaction-based fluorescent probe for imaging of formaldehyde in living cells. J Am Chem Soc. 2015;137:10890–10893. doi: 10.1021/jacs.5b05339. The first example, along with Brewer et. al., of an aza-Cope reactivity-based fluorescent probe for FA. [DOI] [PubMed] [Google Scholar]
- 51.He L, Xueling Y, Liu Y, Kong X, Lin W. A ratiometric fluorescent formaldehyde probe for bioimaging application. Chem Commun. 2016;52:4029–4032. doi: 10.1039/c5cc09796g. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Zhang Y, Zeng L, Liu J, Kinsella JM, Sheng R. A simple naphthalene-based fluorescent probe for high selective detection of formaldehyde in toffees and HeLa cells via aza-Cope reaction. Talanta. 2016;160:645–652. doi: 10.1016/j.talanta.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Li J-B, Wang Q-Q, Yuan L, Wu Y-X, Hu X-X, Zhang X-B, Tan W. A two-photon fluorescent probe for bio-imaging of formaldehyde in living cells and tissues. Analyst. 2016;141:3395–3402. doi: 10.1039/c6an00473c. [DOI] [PubMed] [Google Scholar]
- 54.Xie Z, Ge J, Zhang H, Bai T, He S, Ling J, Sun H, Zhu Q. A highly selective two-photon fluorogenic probe for formaldehyde and its bioimaging application in cells and zebrafish. Sensors Actuators B Chem. 2017;241:1050–1056. [Google Scholar]
- 55(•).Bruemmer KJ, Walvoord RR, Brewer TF, Burgos-Barragan G, Wit N, Pontel LB, Patel KJ, Chang CJ. Development of a general aza-Cope reaction trigger applied to fluorescence imaging of formaldehyde in living cells. J Am Chem Soc. 2017 doi: 10.1021/jacs.6b12460. This work provides a general FA trigger and can enable detection of endogenous FA pools in cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung ME, Piizzi G. gem-Disubstituent effect: theoretical basis and synthetic applications. Chem Rev. 2005;105:1735–1766. doi: 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
- 57(•).Brewer TF, Burgos-Barragan G, Wit N, Patel KJ, Chang CJ. A 2-aza-Cope reactivity-based platform for ratiometric fluorescence imaging of formaldehyde in living cells. Chem Sci. 2017 doi: 10.1039/C7SC00748E. The first example of a ratiometric FA probe with visible excitation/emission profiles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58(••).Tang Y, Kong X, Xu A, Dong B, Lin W. Development of a two-photon fluorescent probe for imaging of endogenous formaldehyde in living tissue. Angew Chem Int Ed. 2016;55:3356–3359. doi: 10.1002/anie.201510373. The first example of a formimine reactivity-based fluorescent probe for FA. [DOI] [PubMed] [Google Scholar]
- 59.Lee YH, Tang Y, Verwilst P, Lin W, Kim JS. A biotin-guided formaldehyde sensor selectively detecting endogenous concentrations in cancerous cells and tissues. Chem Commun. 2016;52:11247–11250. doi: 10.1039/c6cc06158c. [DOI] [PubMed] [Google Scholar]
- 60.Tang Y, Kong X, Liu Z, Xu A, Lin W. Lysosome-targeted turn-on fluorescent probe for endogenous formaldehyde in living cells. Anal Chem. 2016;88:9359–9363. doi: 10.1021/acs.analchem.6b02879. [DOI] [PubMed] [Google Scholar]
- 61(••).He L, Yang X, Ren M, Kong X, Liu Y, Lin W. An ultra-fast illuminating fluorescent probe for monitoring formaldehyde in living cells, shiitake mushrooms, and indoors. Chem Commun. 2016;52:9582–9585. doi: 10.1039/c6cc04254f. The first example of a aminal reactivity-based fluorescent probe for FA. [DOI] [PubMed] [Google Scholar]
- 62.Liu C, Jiao X, He S, Zhao L, Zeng X. A reaction-based fluorescent probe for the selective detection of formaldehyde and methylglyoxal via distinct emission patterns. Dyes Pigm. 2017;138:23–29. [Google Scholar]
- 63.McMartin KE, Martin-Amat G, Noker PE, Tephly TR. Lack of a role for formaldehyde in methanol poisoning in the monkey. Biochem Pharmacol. 1979;28:645–649. doi: 10.1016/0006-2952(79)90149-7. [DOI] [PubMed] [Google Scholar]
- 64.Liu W, Truillet C, Flavell RR, Brewer TF, Evans MJ, Wilson DM, Chang CJ. A reactivity-based [18F]FDG probe for in vivo formaldehyde imaging using positron emission tomography. Chem Sci. 2016;7:5503–5507. doi: 10.1039/c6sc01503d. [DOI] [PMC free article] [PubMed] [Google Scholar]