Abstract
Aminodecarboxylation of unactivated alkyl carboxylic acids has been accomplished utilizing an organic photocatalyst. This operationally simple reaction utilizes readily available carboxylic acids to chemoselectively generate reactive alkyl intermediates that are not accessible via conventional two-electron pathways. The organic radical intermediates are efficiently trapped with electrophilic diazo compounds to provide aminated alkanes.
Keywords: homogenous catalysis, photocatalysis, amination, carboxylic acids
Graphical Abstract
Aminodecarboxylation of unactivated alkyl carboxylic acids has been accomplished utilizing an organic photocatalyst. This operationally simple reaction utilizes readily available carboxylic acids to chemoselectively generate reactive alkyl intermediates that are not accessible via conventional two-electron pathways.
Aliphatic amines are ubiquitous across many chemical disciplines, especially in synthetic organic chemistry where they serve as versatile building blocks.1 For example, N-alkylation with organic halides is one of the top ten reactions performed by medicinal chemists.2 While substitution provides rapid access to a wide variety of amines, these reactions could undoubtedly be improved if more stable, less expensive and less toxic alkylating agents could be utilized in lieu of organic halides. The formation of C–N bonds using abundant carboxylic acids as alkylating agents would be a first step toward achieving this goal. Increased functional group diversity, higher atom economy, and trivial removal of the only stoichiometric byproduct (CO2) are potential additional advantages of replacing alkyl halides with carboxylic acids.
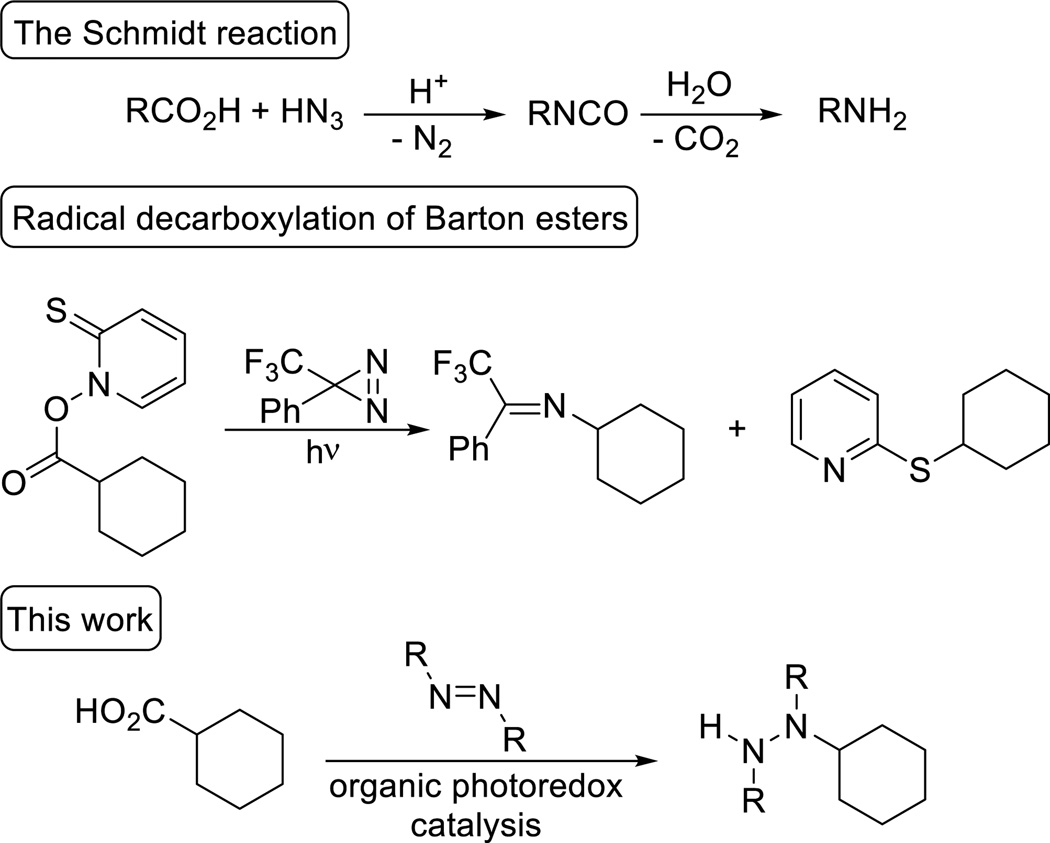
Classically, aliphatic amines can be prepared directly from carboxylic acids via the Schmidt reaction or variants of the Curtius rearrangment.3 We postulated that the radical decarboxylation of aliphatic carboxylic acids would be a complementary umpolung approach to regiospecific C–N bond formation that would allow the formation of amines as well as more oxidized hydrazine derivatives.4,5 The trapping of alkyl radicals generated by decarboxylation of pre-formed activated Barton esters with electrophilic diazirines and nitroso compounds provided important precedent.6 Unfortunately, such reactions suffer from a limited substrate scope, competitive byproduct formation, and require the preactivation of the carboxylic acid (Scheme 1). Our group and others have recently utilized photoredox catalysis7 to site-specifically generate alkyl radicals directly from carboxylic acids.8 Thus, we anticipated that photoredox catalysis would obviate the need for stoichiometric pre-activation and enable the use of carboxylic acids as traceless activating groups for selective C–N bond formation.9
Scheme 1.
Strategies for aminodecarboxylation.
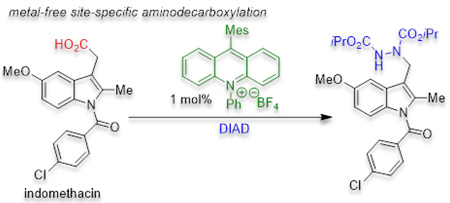
Optimization studies began with aliphatic carboxylic acids which do not undergo anionic decarboxylation. Given that alkyl carboxylates typically have oxidation potentials of +1.1 – 1.3 V vs. SCE,10 the Fukuzumi acridinium family11,8p of photocatalysts with excited state reduction potentials > 2.0 V vs. SCE seemed promising. Electrophilic dialkyl azodicarboxylates12 are effective radical traps that can be subsequently transformed to the corresponding hydrazine, amine or protected amide.4e,4f,14 Thus, diisopropyl azodicarboxylate (DIAD) was chosen to forge the desired C–N bond upon reaction with the saturated aliphatic acid 1. Irradiation of an Ar-sparged solution of 1, the non-nucleophilic base 1,8-diazabicylo[5.4.0]undec-7-ene (DBU), DIAD, and 1 mol % of the photocatalyst (Mes-Acr-Ph) with 450 nm LEDs provided the desired product 2a (eq 1). While initial studies were conducted with one equivalent of DBU, brief optimization revealed that the reaction still reached complete conversion when a sub-stoichiometric amount of DBU was added. When DIAD was replaced with di-tert-butyl azidocarboxylate or di(2,2,2-trichloroethyl) aziodocarboxylate the reaction became sluggish and did not reach completion, even after extended reactions times. Lastly, no conversion to 2a was detected by
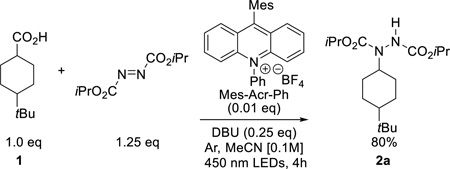 |
(1) |
GC/MS analysis of the crude reaction mixture when light or photocatalyst were omitted from the standard reaction conditions.
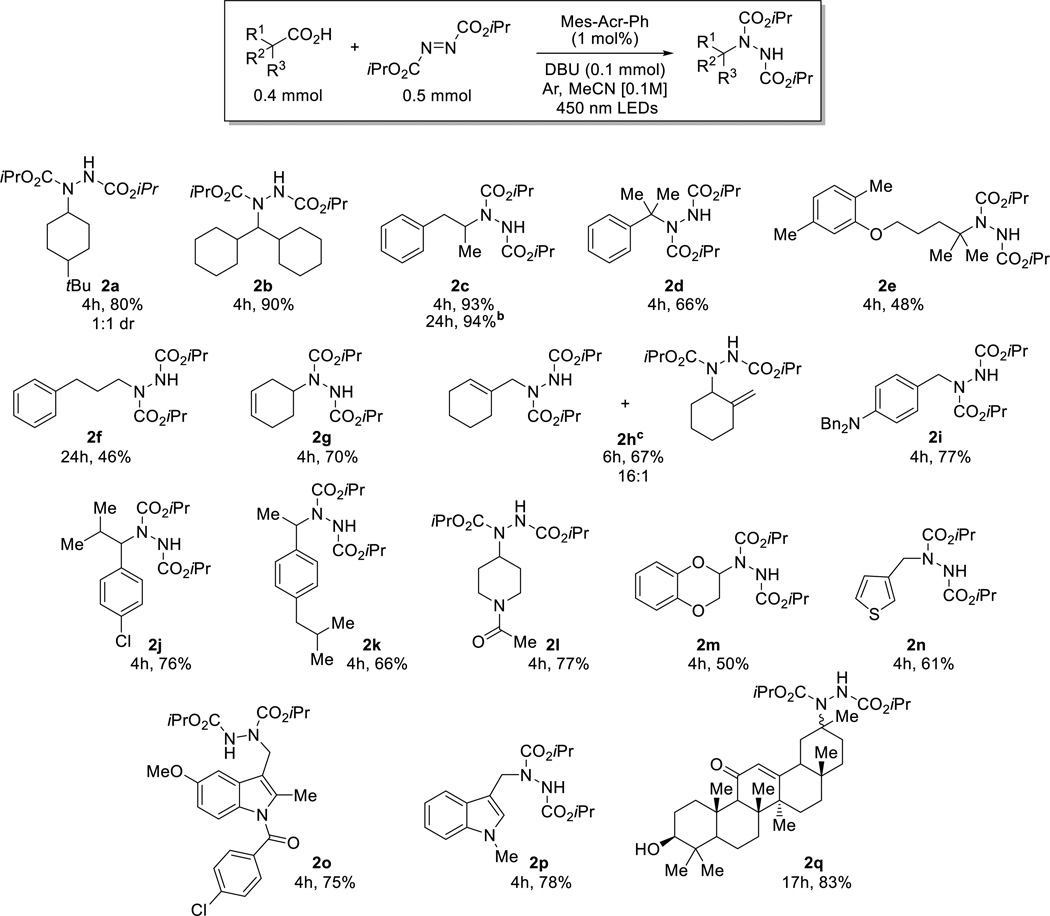
The scope of the metal-free aminodecarboxylation was then explored (Scheme 2). Both cyclic (2a) and acyclic (2b, 2c) secondary alkyl carboxylic acids were readily aminated in high yields under the standard reaction conditions. The reaction could also be performed on a 5 mmol scale but required 24h to reachcompletion when conducted under more convenient concentrated conditions (2c). Aminodecarboxylation also occurred at a sterically congested tertiary benzylic position (2d). Additionally, the remote tertiary center of the phenolic ether-containing hypolipidemic agent, Gemfibrozil, was successfully aminated (2e). A primary carboxylic acid (2f) required extended reaction times to reach complete conversion, presumably due to the slow kinetics of formation of the corresponding primary radical. An unconjugated cyclic alkene (2g) was also compatible with the reaction conditions. When 1-cyclohexene acetic acid was employed, selective amination of the primary over the secondary allylic position was observed (2h), suggesting that the reaction is influenced by steric hindrance. Para amino- (2i), chloro- (2j), and alkyl (ibuprofen, 2k) substituted phenylacetic acids afforded the desired aminated products. Additional functional groups including an N-acyl protected amine (2l) and a catechol derivative (2m) participated in aminodecarboxylation. Heteroaromatics such as 3-thiophenyl- (2n) and 3-N-methylindolyl acetic acid (2p) were also tolerant of the reaction conditions. Indomethacin, an anti-inflammatory 3-indolacetic acid bearing substituents at the 1,2 and 5 positions was aminated in good yield (2o). Finally, enoxolone, which contains a secondary alcohol and an α,β-unsaturated ketone was well tolerated (2q). It should be noted that all but one of the carboxylic acids employed are commercially available. Conversely, only four of the corresponding alkyl bromides or iodides are available, highlighting an advantage of utilizing carboxylic acids for alkylation chemistry.
Scheme 2.
Substrate scope [a] isolated yields [b] 5 mmol scale, 2 blue LEDs [0.38M] [c] from 1-cyclohexene-1-acetic acid
Next, experiments were conducted to support the formation of radical intermediates. First, when cyclopropane acetic acid was subjected to the standard reaction conditions, the ring-opened product 2r was isolated in a 35% yield after 24h (eq 2). Second, the addition of two equivalents of TEMPO as a radical trap afforded 2s in 86% yield (eq 3). Further analysis of the products in Chart 1 revealed that decarboxylation results in the site-specific contra-thermodynamic formation of radicals at the carbon bearing the carboxylate with isomerization to a more stable species prior to amination (e.g. 2a–c, 2f, 2g, 2i, 2l).
Although substituted hydrazines are found in a range of drugs and insecticides, they are also versatile intermediates used in the synthesis of azapeptides and a variety of heterocycles, including commercially relevant pyrazoles and triazoles.5,13 The N–N bond can also be readily cleaved to afford the corresponding protected carbamate utilizing an E1cB reaction developed by Magnus (eq 4).14
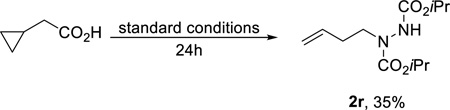 |
(2) |
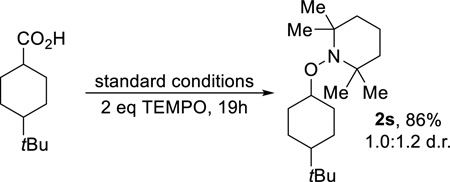 |
(3) |
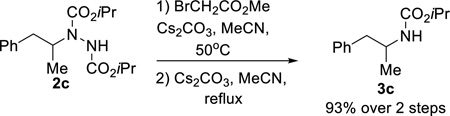 |
(4) |
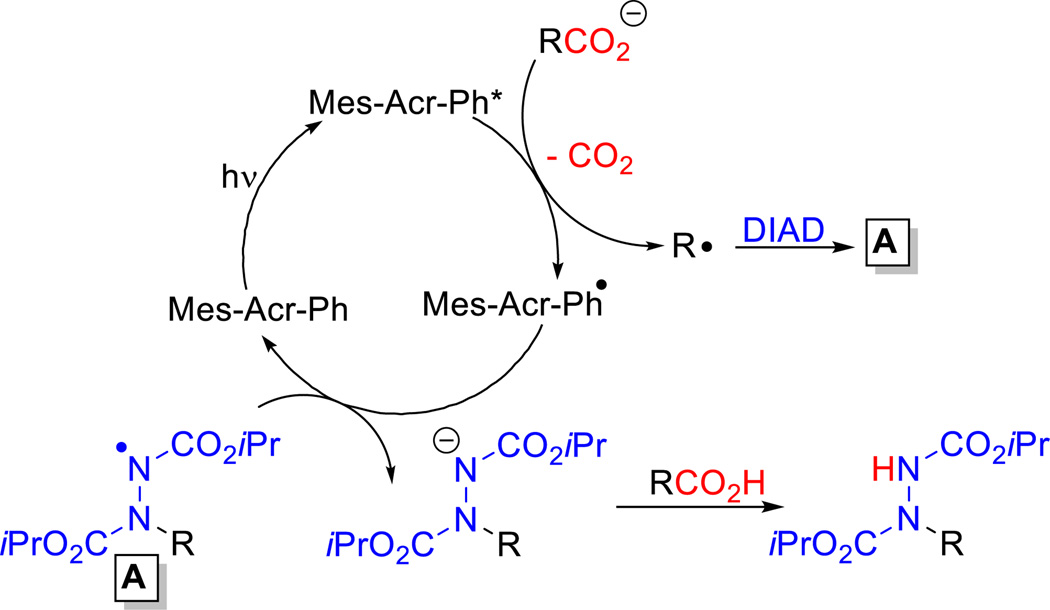
An idealized mechanism is presented in Scheme 3. Irradiation of the photocatalyst provides the oxidizing species Mes-Acr-Ph* which removes an electron from an alkyl carboxylate (generated with the strong base DBU). This triggers radical decarboxylation to provide an alkyl radical and the reduced photocatalyst. The resulting alkyl radical is trapped by DIAD to provide the N-centered radical species A which undergoes reduction by Mes-Acr-Ph• to regenerate the ground state photocatalyst and provide the corresponding amide anion. Another carboxylic acid can quench this charge providing an alkyl carboxylate to enter the catalytic cycle.
Scheme 3.
A plausible mechanism
In conclusion, a metal-free protocol for the site-specific amination of a wide variety of alkanes utilizing carboxylic acids as traceless directing groups was developed. This operationally simple method utilizes commercially available reagents and offers a potentially attractive alternative to alkyl halides for site-selective C–N bond formation.
Supplementary Material
Acknowledgments
Support for this research was provided by the National Science Foundation (CHE-1465172) and the Kansas Bioscience Authority. Support for the NMR instrumentation was provided by NIH Shared Instrumentation Grant # S10RR024664 and NSF Major Research Instrumentation Grant # 0320648.
References
- 1.Lawrence SA. Amines- Synthesis, Properties and Applications. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 2.Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 3.a) Wolff H. Org. React. 1946:307–336. [Google Scholar]; b) Shioiri T. Comp. Org. Synth. Vol. 6. Pergamom, Oxford: 1991. pp. 795–828. [Google Scholar]; c) Lebel H, Leogane O. Org. Lett. 2005;7:4107–4110. doi: 10.1021/ol051428b. [DOI] [PubMed] [Google Scholar]
- 4.For electrophilic amination strategies see Fisher DJ, Burnett GL, Velasco R, de Alaniz JR. J. Am. Chem. Soc. 2015;137:11614–11617. doi: 10.1021/jacs.5b07860. Starkov P, Jamison TF, Marek I. Chem. Eur. J. 2015;21:5278–5300. doi: 10.1002/chem.201405779. Yang X, Toste FD. J. Am. Chem. Soc. 2015;137:3205–3208. doi: 10.1021/jacs.5b00229. Ryu I, Tani A, Fukuyama T, Ravelli D, Montanaro S, Fagnoni M. Org. Lett. 2013;10:2554–2557. doi: 10.1021/ol401061v. Amaoka Y, Kamiio S, Hoshikawa T, Inoue M. J. Org. Chem. 2012;77:9959–9969. doi: 10.1021/jo301840e. Waser J, Gaspar B, Nambu H, Carreira EM. J. Am. Chem. Soc. 2006;128:11693–11712. doi: 10.1021/ja062355+. Miyake Y, Nakajima K, Nishibayashi Y. Chem. Eur. J. 2012;18:16473–16477. doi: 10.1002/chem.201203066.
- 5.Blair LM, Sperry J. J. Nat. Prod. 2013;76:794–812. doi: 10.1021/np400124n. [DOI] [PubMed] [Google Scholar]
- 6.a) Barton DHR, Jaszberenyi JC, Throforakis EA. J. Am. Chem. Soc. 1992;114:5904–5905. [Google Scholar]; b) Girard P, Guillot N, Motherwell WB, Potier P. J. Chem. Soc. Chem. Commun. 1995:2385–2386. [Google Scholar]
- 7.For reviews see: Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/b913880n. Xuan J, Xiao W-J. Angew. Chem. Int. Ed. 2012;51:6828–6838. doi: 10.1002/anie.201200223. Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. Chen J-R, Hu X-Q, Lu L-Q, Xiao W-J. Chem. Soc. Rev. 2016
- 8.a) Xuan J, Zhang Z-G, Xiao W-J. Angew. Chem. Int. Ed. 2015;54:15632–15641. doi: 10.1002/anie.201505731. [DOI] [PubMed] [Google Scholar]; b) Miyake Y, Nakajima K, Nishibayashi Y. Chem. Commun. 2013;49:7854–7856. doi: 10.1039/c3cc44438d. [DOI] [PubMed] [Google Scholar]; c) Zuo Z, MacMillan DWC. J. Am. Chem. Soc. 2014;136:5257–5260. doi: 10.1021/ja501621q. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lang SB, O’Nele KM, Tunge JA. J. Am. Chem. Soc. 2014;136:13606–13609. doi: 10.1021/ja508317j. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Cassani C, Bergonzini G, Wallentin C-J. Org. Lett. 2014;16:4228–4231. doi: 10.1021/ol5019294. [DOI] [PubMed] [Google Scholar]; g) Ventre S, Petronijevic FR, MacMillan DWC. J. Am. Chem. Soc. 2015;137:5654–5657. doi: 10.1021/jacs.5b02244. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Yoshimi Y, Masuda M, Mizunashi T, Nishikawa K, Maeda K, Koshida N, Itou T, Mortia T, Hatanaka M. Org. Lett. 2009;11:4652–4655. doi: 10.1021/ol9019277. [DOI] [PubMed] [Google Scholar]; i) Liu J, Liu Q, Yi H, Qin C, Bai R, Qi X, Lan Y, Lei A. Angew. Chem. Int. Ed. 2014;53:502–507. doi: 10.1002/anie.201308614. [DOI] [PubMed] [Google Scholar]; j) Rueda-Becerril M, Mahé O, Drouin M, Majewski MB, West JG, Wolf MO, Sammis GM, Paquin J-F. J. Am. Chem. Soc. 2014;136:2637–2641. doi: 10.1021/ja412083f. [DOI] [PubMed] [Google Scholar]; k) Huang H, Jia K, Chen Y. Angew. Chem. Int. Ed. 2015;54:1881–1884. doi: 10.1002/anie.201410176. [DOI] [PubMed] [Google Scholar]; l) Zhou Q-Q, Guo W, Ding W, Wu X, Chen X, Lu L-Q, Xia W-J. Angew. Chem. Int. Ed. 2015;54:11196-1628–11199-1628. doi: 10.1002/anie.201504559. [DOI] [PubMed] [Google Scholar]; m) Xuan J, Zeng T-T, Feng Z-J, Deng Q-H, Chen J-R, Lu L-Q, Xiao W-J, Alper H. Angew. Chem. Int. Ed. 2015;54:1625–1628. doi: 10.1002/anie.201409999. [DOI] [PubMed] [Google Scholar]; n) Lang SB, O’Nele KM, Douglas JT, Tunge JA. Chem. Eur. J. 2015;21:18589–18593. doi: 10.1002/chem.201503644. [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Candish L, Pitzer L, Gómez-Suárez A, Glorius F. Chem. Eur. J. 2016 doi: 10.1002/chem.201600421. [DOI] [PubMed] [Google Scholar]; p) Griffin JD, Zeller MA, Nicewicz DA. J. Am. Chem. Soc. 2015;137:11340–11348. doi: 10.1021/jacs.5b07770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For a singular example of a para-amino phenylacetic acid used as a traceless activator for C–N bond formation see the Supplementary Information of Ref 8b. To our knowledge, a general system for alkyl carboxylic acids has not been developed.
- 10. Galicia M, Gonzalez FJ. J. Electrochem. Soc. 2002;149:D46–D50. b) Ref 8p.
- 11.a) Fukuzumi S, Kotani H, Ohkubo K, Ogo S, Tkachenko NV, Lemmetyinen H. J. Am. Chem. Soc. 2004;126:1600–1601. doi: 10.1021/ja038656q. [DOI] [PubMed] [Google Scholar]; b) Kotani H, Ohkubo K, Fukuzumi S. J. Am. Chem. Soc. 2004;126:15999–16006. doi: 10.1021/ja048353b. [DOI] [PubMed] [Google Scholar]; c) Fukuzumi S, Ohkubo K. Org. Biomol. Chem. 2014;12:6059–6071. doi: 10.1039/c4ob00843j. [DOI] [PubMed] [Google Scholar]
- 12.Kanzian T, Mayr H. Chem. Eur. J. 2010;16:11670–11677. doi: 10.1002/chem.201001598. [DOI] [PubMed] [Google Scholar]
- 13.a) Ragnarsson U. Chem. Soc. Rev. 2001;30:205–213. [Google Scholar]; b) Narang R, Narasimhan B, Sharma S. Curr. Med. Chem. 2012;19:569–612. doi: 10.2174/092986712798918789. [DOI] [PubMed] [Google Scholar]
- 14.Magnus P, Garizi N, Seibert KA, Ornholt A. Org. Lett. 2009;11:5646–5648. doi: 10.1021/ol902313v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.