Abstract
Ischemic stroke is one of the leading causes of morbidity and mortality worldwide. Females are protected against stroke before the onset of menopause. Menopause results in increased incidence of stroke when compared to men. The mechanisms of these differences remain to be elucidated. Considering that there is a postmenopausal phenomenon and females in general, are living longer sex hormone-dependent mechanisms have been postulated to be the primary factors responsible for the premenopausal protection from stroke and later to be responsible for the higher incidence and increased the severity of stroke after menopause. Animal studies suggest that administration of estrogen and progesterone is neuroprotective and decreases the incidence of stroke. However, the real-world outcomes of hormone replacement therapy have failed to decrease the stroke risk. Despite the multifactorial nature of sex differences in stroke, here, we briefly discuss the pathophysiology of sex steroid hormones, the molecular mechanisms of estrogen receptor-dependent signaling pathways in stroke, and the potential factors that determine the discrepant effects of hormone replacement therapy in stroke.
Keywords: Gender, Stroke, Epidemiology, Hormones, Receptors
Introduction
Ischemic stroke morbidity is a major health cost burden globally. The incidence of stroke has slightly improved in some parts of the world; however, there remains minimal therapeutic targets preventing this debilitating disease. Risk factors for ischemic stroke include aging, hypertension, diabetes, obesity especially with increased waist-to-hip ratio, dyslipidemia, smoking, chronic kidney disease, and other cardiovascular diseases [1]. Epidemiological studies demonstrate that younger females are better protected from stroke than men [2]. This difference reverses as age advances in females and menopause ensues, resulting in higher incidence of stroke in females [2]. Menopause increases the stroke risk in females [3] as evident from Framingham epidemiological study [4]. Reports from animal studies also corroborate these findings.
Young and middle aged female rats have neuroprotective effects after a transient ischemic event and are reported to have less stroke volume [5,6]. There are fundamental differences in the estrogen receptor-dependent and independent signaling on the cerebral vasculature in females compared to males. Estrogen plays a role in vascular smooth muscle cells (VSMC), endothelial cells, neurons, and glial cells. It also affects lipids levels, inflammation, and the thrombotic function [7]. Other factors, including anatomic factors, genetic factors, cell death pathways, microRNAs, and stroke-related specific genes on the X and/orY chromosomes, have been reported contribute to the differences in these outcomes [8–11].
Discussion
Menopause and ischemic stroke
Menopause is defined as the absence of menstruation over a period of 12 months. The average age of menopause is 51 years (30–60 years) [12]. The incidence of hypertension and ischemic stroke is lower in 20–54 year old women when compared to similarly aged men and post-menopausal women [13]. There is almost twice the risk of ischemic stroke in women with natural menopause before 42 years of age compared to those who developed menopause after 42 years of age [4] or between 50–54 years [14]. Menstruation has positive and protective effects on cardiac and cerebral vascular system as well. Female rats and those receiving an oophorectomy plus hormone replacement therapy (HRT) rats have less myogenic tone and less pronounced myogenic response than males or females with oophorectomy but without HRT due to estrogen-induced elevated nitric oxide (NO) release and activity [15]. The concept of HRT was based on the beneficial effects seen in various experiments on animals [15–17].
Sex hormones and ischemic stroke
Animal models have been designed to study sex differences in vivo and in vitro. Neurons derived from females have a higher tolerance to the toxic effects of dopamine compared to males [18] and in vivo studies using mice suggest that 24 after middle cerebral artery (MCA) stroke, young adult female mice have significantly smaller infarcts. Furthermore, surgical oophorectomy ameliorates this protective effect in stroke prone rats [19] and, estradiol replacement seems to have neuroprotective effects [16,17]. This evidence suggests that estradiol, a natural gonadotrophic hormone, provides a neuroprotective effect at normal physiological level in premenopausal females. Similarly, administration of progesterone resulted in reduced cortical infarct size in reproductive senescent female rats [20] as well in males rats [21]. The role of testosterone has been studied in both animals and humans. Castrated male rats showed no decrease in the size of stroke in a MCA occlusion model [22] whereas testosterone administration in non-castrated males enhanced infarct size [23]. The results in rats are variable based on the dosage of testosterone administration [24,25] as are the differences in the outcome in human males. The risk and severity of stroke increase with lower testosterone levels [26] whereas increased testosterone level tends to increase the risk of stroke in young boys [27].
Hormone replacement therapy and ischemic stroke
HRT is designed to reduce some of the post-menopausal symptoms such as heart disease after menopause. Depending on the formulation, it is comprised of either estrogen, progesterone, sometimes in combination with testosterone. It has been reported that there are additional benefits of HRT, such as boosting working memory [28,29]. However, HRT also comes with increased risks of breast cancer, endometrial cancer, and hypercoagulable state resulting in cerebral venous bleeding and infarction. Sex hormones are mainly metabolized in the liver. Thus any deviation of liver function or administration of drugs that are metabolized in the liver could result in metabolic derangement of HRT. Two large controlled trials from the Heart and Estrogen-Progestin Replacement Study (HERS) [30] and the Women’s Estrogen for Stroke Trial (WEST) [31] did not show any significant improvements in the risk of stroke. However, the data from the World Health Initiative Trial (WHI), and meta-analysis of 28 other randomized trials [32] suggests 29% increased risk of stroke in healthy postmenopausal women on standard hormone therapy, whereas the risk of stroke increases considerably in women >60 years of age [33].
In contrast, the results from animal studies universally suggest neuroprotective effects of HRT and decreased stroke risk [16,17]. This discrepancy is possibly multifactorial. Firstly, the majority of animals studied were young ovariectomized rodents rather than elderly postmenopausal animals. As aging is another one of the major risk factors for stroke, studies in young hormone deficient models might enhance the beneficial effects of HRT. Secondly, the enrollment criteria and the types of HRT were not uniform in the clinical trials. Thirdly, the population of patients enrolled in the clinical trials were different. Thus the genetic differences may contribute to the diversity of HRT outcomes and mask the significance. Finally, thrombotic mechanisms play a larger role in ischemic stroke, and estrogen has a prothrombogenic nature. The incidences of venous infarction [34] and ischemic stroke [34,35] are significantly higher in patients on HRT, especially when given to younger females. Experiments have shown that HRT decreases the fibrinolytic activity of tissue plasminogen activator (t-PA) in oophorectomized rats [36] whereas a randomized controlled study in healthy postmenopausal women shows a positive effect of HRT on the fibrinolytic system [37]. More validation of the usefulness of HRT is indeed required.
Molecular mechanisms of estrogen receptor-dependent signaling pathways in ischemic stroke
Despite our current understanding of the sex differences and HRT, there are several questions pertaining to the mechanism by which gonadotrophic hormones exert their effects remain unanswered. The role of estradiol on cerebral vascular disease is ambiguous based on the data from animal studies. Recent studies suggest that different subtypes of estrogen receptors (ER), including ERα and ERβ, play distinct roles in neuroprotection. In this regard, Dubal et al. [38] demonstrated that deletion of ERα resulted in abolishment of neuroprotective effect, whereas preservation of neuroprotective effect was observed in ERβ knock out (KO) mice, suggesting that ERα could be the mechanistic link to the protective effect of estradiol [38]. Administration of physiological level of 17β-estradiol results in flow-medicated relaxation of human coronary arteries [39]. This vasodilatory effect likely comes from activation of Na+/K+- ATPase (NKA). NKA is an enzyme found in the plasma membrane of all animal cells. It acts by utilizing adenosine triphosphate (ATP) to induce sodium efflux and potassium (K) influx, which hyperpolarizes the cell membrane and reduces vascular constriction. Functional activity of NKA in the aorta of female rats is more sensitive to the vasodilatory effect of acetylcholine (Ach) [40].
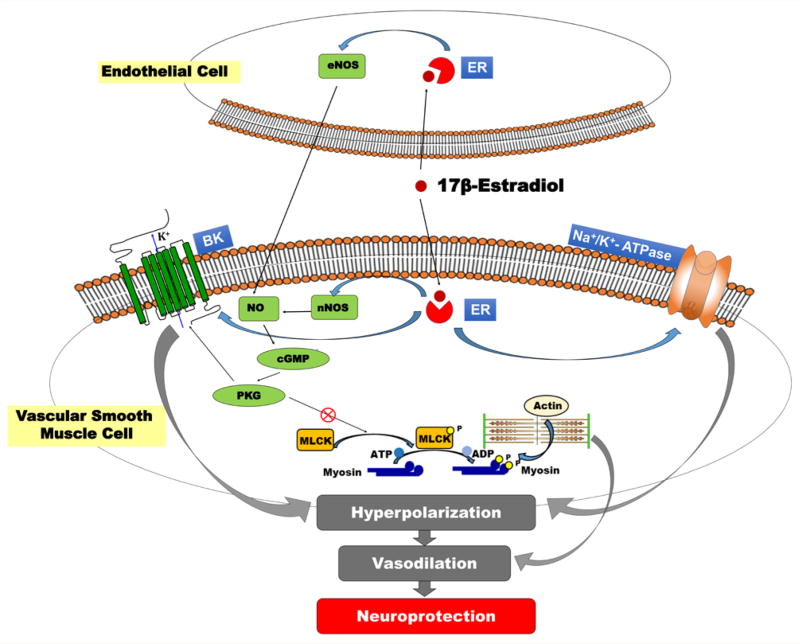
Blocking NKA with ouabain (OUA) increases vascular tone [41] moreover, reduces Ach-induced vascular relaxation in the aorta of both male and female rats, but less so in females suggesting lower NKA functional activity in females [42]. In another study, ovariectomized female rats do not exhibit differences in NKA function compared to the male counterparts, and chronic hormone replacement with 17β-estradiol restored the vasodilator effect of Ach on NKA [43]. Despite these findings, the effects of estradiol on NKA-dependent vascular relaxation in the central nervous system in postmenopausal females are not yet clear. (Figure 1) depicts the different molecular pathways through which estrogen exerts the neuroprotective role. VSMCs tend to contract with the rise in perfusion pressure. This effect is countered by transient outward K current initiated by the large conductance calcium-activated potassium (BK) channels in VSMCs resulting in hyperpolarization of these cells [44]. 17β-estradiol activates BK channels directly [45]. BKβ1 is one of the downstream targets of sex hormones including 17β-estradiol and progesterone [46] and specifically activated by 17β-estradiol in the VSMCs [46]; increases neuronal nitric oxide synthase (nNOS) as well endothelial nitric oxide synthase (eNOS) expression; and releases NO in the endothelial cells, all of which promotes vasodilation [47,48].
Figure 1.
Neuroprotective role of 17β-estradiol. 17β-estradiol binds to intracellular estrogen receptor (ER). It activates endothelial nitric oxide synthase (eNOS) in the endothelium and neuronal nitric oxide synthase (nNOS) in the vascular smooth muscle cells (VSMC). Thus, resulting in enhanced release of nitric oxide (NO) and inhibits actin-myosin based vasoconstriction through activation of cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) pathway that inhibits myosin light chain (MLC) phosphorylation and activates the large conductance calcium-activated potassium channel (BK). 17β-estradiol binds to the ER to increase the expression and activity of Na+/K+- ATPase and has a direct effect to activate BK channels, both of which cause membrane hyperpolarization and vascular relaxation. The vasodilation effect plays a critical role in neuroprotection.
Conclusion
Epidemiologic studies reveal that age and sex differences play a role in the onset and outcomes of ischemic stroke. The levels of sex hormones, including estrogen and progesterone, are reduced in the elderly females. These changes alter estrogen receptor-dependent signaling pathways and increase the incidence and severity of stroke in the postmenopausal females. Animal studies demonstrate that postmenopausal HRT has beneficial effects on stroke, but the outcomes are not consistent in human studies due to multifactorial mechanisms. Further investigations are in need to elucidate the mechanisms and to develop novel therapeutic targets to reduce the risk of stroke in the postmenopausal females.
Acknowledgments
This study was supported by grants AG050049 (Fan), P20GM104357 (Fan and Roman), HL36279 (Roman) and DK104184 (Roman) from the National Institutes of Health and 16GRNT31200036 (Fan) from the American Heart Association.
Abbreviations
- VSMC
Vascular Smooth Muscle Cells
- HRT
Hormone Replacement Therapy
- NO
Nitric Oxide
- MCA
Middle Cerebral Artery
- HERS
Heart and Estrogen-Progestin Replacement Study
- WEST
Women’s Estrogen for Stroke Trial
- WHI
World Health Initiative Trial
- T-PA
Tissue Plasminogen Activator
- ER
Estrogen Receptor
- KO
Knock Out
- Na+/K+- ATPase
NKA
- ATP
Adenosine Triphosphate
- K
Potassium
- OUA
Ouabain
- Ach
Acetylcholine
- BK
large conductance calcium-activated potassium
- nNOS
Neuronal Nitric Oxide Synthase
- eNOS
Endothelial Nitric Oxide Synthase
References
- 1.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 2.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28(3):491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 3.Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol. 2012;11(1):82–91. doi: 10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, et al. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YQ, Shi J, Rajakumar G, Day AL, Simpkins JW. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784(1–2):321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- 6.Li K, Futrell N, Tovar S, Wang LC, Wang DZ, et al. Gender influences the magnitude of the inflammatory response within embolic cerebral infarcts in young rats. Stroke. 1996;27(3):498–503. doi: 10.1161/01.str.27.3.498. [DOI] [PubMed] [Google Scholar]
- 7.Henderson VW, Lobo RA. Hormone therapy and the risk of stroke: perspectives 10 years after the Women’s Health Initiative trials. Climacteric. 2012;15(3):229–234. doi: 10.3109/13697137.2012.656254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J Cereb Blood Flow Metab. 2012;32(12):2100–2107. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TH, Vemuganti R. Effect of sex and age interactions on functional outcome after stroke. CNS neuroscience & therapeutics. 2015;21(4):327–336. doi: 10.1111/cns.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, et al. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43(2):326–334. doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian Y, Stamova B, Jickling GC, Xu H, Liu D, et al. Y chromosome gene expression in the blood of male patients with ischemic stroke compared with male controls. Gend Med. 2012;9(2):68–75e63. doi: 10.1016/j.genm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 14.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang A, Sun D, Koller A, Kaley G. Gender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. Am J Physiol. 1997;272(4 Pt 2):H1804–H1809. doi: 10.1152/ajpheart.1997.272.4.H1804. [DOI] [PubMed] [Google Scholar]
- 16.Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M, et al. Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Brain Res Rev. 2001;37(1–3):313–319. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 17.Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22(1):33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 18.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279(37):38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 19.Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278(1):H290–H294. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 20.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 21.Brands MW, Bell TD, Gibson B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension. 2004;43(1):57–63. doi: 10.1161/01.HYP.0000104524.25807.EE. [DOI] [PubMed] [Google Scholar]
- 22.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796(1–2):296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 23.Yang SH, Perez E, Cutright J, Liu R, He Z, et al. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol (1985) 2002;92(1):195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 24.Frye CA, McCormick CM. Androgens are neuroprotective in the dentate gyrus of adrenalectomized female rats. Stress. 2000;3(3):185–194. doi: 10.3109/10253890009001122. [DOI] [PubMed] [Google Scholar]
- 25.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, et al. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29(8):1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeppesen LL, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS, et al. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16(6):749–754. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- 27.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond) 2011;7(3):319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens C, Bristow V, Pachana NA. HRT and everyday memory at menopause: a comparison of two samples of mid-aged women. Women Health. 2006;43(1):37–57. doi: 10.1300/J013v43n01_03. [DOI] [PubMed] [Google Scholar]
- 29.Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann NY Acad Sci. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 30.Simon JA, Hsia J, Cauley JA, Richards C, Harris F, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen-progestin Replacement Study (HERS) Circulation. 2001;103(5):638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 31.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, et al. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 32.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ. 2005;330(7487):342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 Suppl 1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29(16):2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 36.Topcuoglu A, Albayrak M, Erman H, Balci H, Karakus M, et al. Effects of hormone replacement therapy on plasma and tissue fibrinolytic activity in a rat model of surgically induced menopause. Clin Invest Med. 2014;37(2):E85–92. doi: 10.25011/cim.v37i2.21090. [DOI] [PubMed] [Google Scholar]
- 37.Madsen JS, Kristensen SR, Gram J, Bladbjerg EM, Henriksen FL, et al. Positive impact of hormone replacement therapy on the fibrinolytic system: a long-term randomized controlled study in healthy postmenopausal women. J Thromb Haemost. 2003;1(9):1984–1991. doi: 10.1046/j.1538-7836.2003.00362.x. [DOI] [PubMed] [Google Scholar]
- 38.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98(4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, et al. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;289(4):H1468–1475. doi: 10.1152/ajpheart.01173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuma I, Liu MY, Sato A, Hayashi T, Iguchi A, et al. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: influence of oestrogen. Br J Pharmacol. 2002;135(1):48–54. doi: 10.1038/sj.bjp.0704444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossoni LV, Salaices M, Marín J, Vassallo DV, Alonso MJ. Alterations in phenylephrine-induced contractions and the vascular expression of Na+,K+-ATPase in Ouabain-induced hypertension. Br J Pharmacol. 2002;135(3):771–781. doi: 10.1038/sj.bjp.0704501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias FM, Ribeiro RF, Fernandes AA1, Fiorim J, Travaglia TC, et al. Na+K+-ATPase activity and K+ channels differently contribute to vascular relaxation in male and female rats. PloS one. 2014;9(9):e106345. doi: 10.1371/journal.pone.0106345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palacios J, Marusic ET, Lopez NC, Gonzalez M, Michea L. Estradiol-induced expression of N(+)-K(+)-ATPase catalytic isoforms in rat arteries: gender differences in activity mediated by nitric oxide donors. Am J Physiol Heart Circ Physiol. 2004;286(5):H1793–1800. doi: 10.1152/ajpheart.00990.2003. [DOI] [PubMed] [Google Scholar]
- 44.Fan F, Pabbidi MR, Ge Y, Li L, Wang S, et al. Knock down of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol. 2017;312(6):F971–F981. doi: 10.1152/ajprenal.00529.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hristov KL, Parajuli SP, Provence A, Rovner ES, Petkov GV. Nongenomic modulation of the large conductance voltage- and Ca2+-activated K+ channels by estrogen: A novel regulatory mechanism in human detrusor smooth muscle. Physiol Rep. 2017;5(14):e13351. doi: 10.14814/phy2.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granados ST, González-Nilo D, Gonzalez J, Latorre R, Torres Y, et al. 17β-Estradiol Binds and Modulates BK Channel through its β1 Auxiliary Subunit. Biophysical Journal. 2016;110(3):280a–281a. [Google Scholar]
- 47.Nevzati E, Shafighi M, Bakhtian KD, Treiber H, Fandino J, et al. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir Suppl. 2015;120:141–145. doi: 10.1007/978-3-319-04981-6_24. [DOI] [PubMed] [Google Scholar]
- 48.Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE. Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol. 2012;302(1):115–123. doi: 10.1152/ajpheart.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]