Abstract
Objective:
To compare the diagnostic performance of MR and diffusion-weighted imaging (DWI), multidetector CT, endoscopic ultrasonography (EUS) and 18F-FDG (fluorine-18 fludeoxyglucose) positron emission tomography CT (PET-CT) in the pre-operative locoregional staging of oesophageal cancer.
Methods:
18 patients with oesophageal or Siewert I tumour (9 directly treated with surgery and 9 addressed to chemo-/radiotherapy before) underwent 1.5-T MR and DWI, 64-channel multidetector CT, EUS and PET-CT before (n = 18) and also after neoadjuvant treatment (n = 9). All images were analysed and staged blindly by dedicated operators (seventh TNM edition). Two radiologists calculated independently the apparent diffusion coefficient from the first scan. Results were compared with histopathological findings. After the population had been divided according to local invasion (T1–T2 vs T3–T4) and nodal involvement (N0 vs N+), sensitivity, specificity, accuracy, positive- and negative-predictive values were calculated and compared. Quantitative measurements from DWI and PET-CT were also analysed.
Results:
For T staging, EUS showed the best sensitivity (100%), whereas MR showed the highest specificity (92%) and accuracy (83%). For N staging, MR and EUS showed the highest sensitivity (100%), but none of the techniques showed adequate results for specificity. Overall, MR showed the highest accuracy (66%) for N stage, although this was not significantly different to the other modalities. The apparent diffusion coefficient was different between surgery-only and chemo-/radiotherapy groups (1.90 vs 1.30 × 10−3 mm2 s−1, respectively; p = 0.005)—optimal cut off for local invasion: 1.33 × 10−3 mm2 s−1 (p = 0.05). Difference in standardized uptake value was also very close to conventional levels of statistical significance (8.81 vs 13.97 g cm−3, respectively; p = 0.05)—optimal cut off: 7.97 g cm−3 (p = 0.44).
Conclusion:
In this pilot study, we have shown that MR with DWI could enrich the current pre-operative work-up for oesophageal cancer and could be used for T and N staging. However, larger studies will need to be carried out before introducing this technique in the standard diagnostic pathway, in order to understand if MR with DWI could change its management and replace more costly or invasive tests such as PET-CT or EUS.
Advances in knowledge:
This pilot study represents the first effort where the four techniques have been prospectively compared together for oesophageal cancer staging. The combination of MR and DWI could provide important, additional information for staging and initial treatment decision-making.
INTRODUCTION
Oesophageal cancer represents the third most frequently occurring gastrointestinal malignancy, with a 5-year survival rate between 4% and 10%.1
Tumour infiltration and nodal involvement represent important prognostic indicators and different treatment strategies can be taken following pre-operative staging.
Endoscopic mucosal resection is performed for early stages, whereas locally advanced cases with nodal involvement are usually treated with neoadjuvant chemo-/radiotherapy before surgery.2,3
However, as early tumour invasion of the adjacent structures is frequent, many patients with oesophageal cancer tend to have locally advanced disease at the time of presentation, especially in Western countries where mass screening is uncommon.4
Moreover, patients with poor prognostic features following initial chemo-/radiotherapy may warrant additional cycles, benefiting very little from surgery.
Thus, accurate pre-operative staging is imperative to determine the most appropriate treatments.
The most common diagnostic work-up for oesophageal cancer is based on endoscopic biopsy followed by endoscopic ultrasonography (EUS) for local staging, and multidetector CT (MDCT)5 and positron emission tomography CT (PET-CT) for distant metastases,6 each with both advantages and disadvantages.
Although EUS is an invasive technique, it detects all wall layers and is currently considered the most accurate imaging modality available for primary tumour staging. Overall accuracy increases with advanced T stages7 and values around 80% for T and up to 96% N stage have been reported.8,9 Additionally, EUS allows fine needle aspiration of the tumour when conventional endoscopic biopsies have been non-diagnostic, improving its value in staging. However, this technique has a restricted field of view for nodal involvement, is highly operator dependent and carries a high risk of perforation due to its invasiveness. EUS is also unable to detect distant metastases and evaluate stenotic lesions that prevent the passage of the endoscope.
By contrast, MDCT is a non-invasive technique that can assess the presence of distant metastases. The reported sensitivity and specificity for T stage in patients with oesophageal cancer range from 88% to 100% and 85–100%, respectively.6 For nodal involvement, MDCT has shown a sensitivity ranging from 30% to 60% and a specificity of up to 80%.6 However, this technique implies the use of ionizing radiation and does not differentiate oesophageal wall layers with accuracy. Initial studies on luminal distension of the oesophagus using oral effervescent powder on MDCT have shown remarkable improvements with regard to staging.10,11
PET-CT is useful for the detection of distant metastases but has a limited utility for T staging due to its poor spatial resolution.12 An additional limitation concerns the detection of pathological locoregional lymph nodes at initial staging, as fludeoxyglucose uptake within perioesophageal fat can be difficult to differentiate from uptake within the oesophagus itself.13–15
Recent evidence suggests that the use of MR is becoming increasingly frequent for oesophageal cancer, largely due to technical improvements (e.g. breath-hold sequences) and to the addition of new quantitative parameters [e.g. diffusion-weighted imaging (DWI)] to purely anatomical (T1 and T2 weighted) sequences.16
The apparent diffusion coefficient (ADC) allows investigation of microstructural changes in pathological tissue. Specifically, DWI provides a strong and easily visible contrast of tumour (higher signal intensity) with lower ADC values (from the reconstructed ADC map) than of normal tissue. DWI is also sensitive to microstructural cellular changes, occurring earlier than anatomical changes during neoadjuvant treatment. However, DWI requires long acquisition times and is subject to motion artefacts (e.g. cardiovascular pulsation), with the possibility of an impaired image quality. DWI lacks spatial resolution and for an accurate anatomical distinction of wall layers, it needs to be combined with T2 weighted imaging, having an intrinsic high soft-tissue contrast.
Hence, the combination of DWI and conventional MR sequences results in a promising tool for oncologic imaging.16
To date, there have been few studies on the application of MR and DWI in the staging of oesophageal cancer. Promising results have been reported,17,18 and a comparative study19 of PET/MR with MDCT, PET/CT, EUS and CT demonstrated an acceptable accuracy of PET/MR for T and N staging.
However, until now, studies have yet to compare MR and DWI, EUS, MDCT and PET-CT in the pre-operative locoregional staging of oesophageal cancer.
Therefore, the purpose of our pilot study was to prospectively compare the diagnostic performance of these techniques and also to investigate the potential added value of initial ADC derived from DWI in the staging of oesophageal cancer.
METHODS AND MATERIALS
Our prospective study fulfils the criteria set by the Standards for Reporting of Diagnostic Accuracy guidelines and follows the World Medical Association's Declaration of Helsinki and Good Clinical Practice Guidelines.
The medical ethics committee pertains to San Raffaele Hospital, Milan, Italy, and was approved by our medical ethics committee.
All patients provided written informed consent prior to participation.
Patient population
42 patients with evidence of oesophageal or oesophagogastric junction cancer (Siewert I) were referred to our institution San Raffaele Hospital, Milan, Italy between November 2009 and December 2013.
Inclusion criteria for this study were: (a) biopsy-proved oesophageal or Siewert I tumour (with the epicentre located 1–5 cm above the oesophagogastric junction), commonly staged as oesophageal cancer;20,21 (b) no contraindications to pre-operative imaging; (c) visible tumour on imaging; (d) fitness for surgery; and (e) no contraindications to neoadjuvant treatment.
The exclusion criteria were: (a) non-traversable lesions (i.e. impracticable EUS) (n = 2); (b) incomplete pre-operative imaging assessment (n = 8; specifically MR = 3; MDCT = 3; and EUS = 2); (c) MR, MDCT, EUS or PET-CT performed at another institution beforehand (n = 4); (d) no surgical intervention (comorbidity, contraindications or lack of consent) (n = 9); (e) no evidence of tumour at final histology after neoadjuvant treatment (i.e. pT0) (n = 1).
The final study population included 18 patients (12 males; 6 females; mean age: 60.24 ± 12.99 years; age range 33–76 years).
At the time of their first presentation at our institution, all patients underwent (i) EUS, (ii) 64-slice MDCT, (iii) 1.5-T MR with DWI and (iv) PET-CT in different days and were consequently treated with radical surgery (surgery-only group) or addressed to chemo-/radiotherapy (chemo-/radiotherapy group) according to EUS and MDCT staging, reflecting common clinical practice (i.e. chemo-/radiotherapy is commonly preferred over immediate surgery for patients with T3 or regional node-positive disease).5
Patients requiring chemo-/radiotherapy firstly underwent two cycles of cisplatin (60 mg m−2) and 5-fluorouracil (200 mg m−2 day−1) followed by 50.4 Gy/28 fractions and intravenous injection of cisplatin (75–100 mg m−2 of body surface area/day/28 days).
At the end of the neoadjuvant treatment, all patients (i.e. the chemo-/radiotherapy group, n = 9) were restaged with EUS, MDCT, MR with DWI and PET-CT, using the same protocol of the first scan, and then treated with radical surgery.
Surgical intervention was performed as soon as possible (preferably, within 4–6 weeks after baseline staging for the surgery group or after completion of post-treatment imaging for the chemo-/radiotherapy group).22
Oesophagectomy via laparotomy, right thoracotomy and intrathoracic anastomosis with nodal dissection (Ivor Lewis technique) was performed in all patients.
The final diagnosis of oesophageal cancer was assessed on the resected specimens by a dedicated histopathologist, based on the seventh TNM edition.23
MR protocol, analysis and interpretation
Patients included in the study underwent MR with DWI on a 1.5-T MR system (Achieva®; Philips Medical Systems, Best, Netherlands) using a five-channel phased-array cardiac coil. Gastric distension (for Siewert I tumours) was obtained by oral administration of 500 ml of water and ferumoxsil (Lumirem®; Guerbet, Villepinte, France) before imaging. According to literature,16 the protocol (Table 1) consisted in a T2 weighted study, with and without fat suppression, followed by a DWI (b = 0 and 600 s mm−2) and a T1 weighted study with fat suppression after intravenous injection of 0.1 ml kg−1 of body weight of gadobutrol (Gadovist®, 1 mmol ml−1; Bayer Schering Pharma, Berlin, Germany) using an automatic injector (Spectris MR; Medrad Europe, Maastricht, Netherlands) at a rate of 2 ml s−1.
Table 1.
MRI parameters
| Parameters | SS fat-suppressed T2 weighted | T2 weighted | SS EP diffusion-weighteda | Gadolinium contrast-enhanced |
|---|---|---|---|---|
| Plane TR (ms) |
Coronal, sagittal Shortest |
Axial 2400 |
Axial Single heartbeatb |
Axial Shortest |
| TE (ms) | 100 | 80 | 58 | Shortest |
| Slice thickness (mm) | 4 | 5 | 4 | 25 |
| Slice gap (mm) | 1 | 0.8 | 1 | Overcontiguous slice |
| Matrix size (reconstructed) | 336 | 288 | 336 | 288 |
| Field of view (mm) | 365 × 284 | 300 × 280 | 365 × 319 | 365 × 289 |
| Flip angle (degrees) | 90° | 90° | 90° | 10° |
| Acquisition time (s) | 14 | 150b | 104b | 94 |
| Number of slices | 35 | 18 | 30 | 65 |
| Number of signalsc | 2 | 4 | 6 | 2 |
EP, echo planar; SS, single-shot; TE, echo time; TR, repetition time.
b = 0 and 600 s mm−2.
Total duration according to cardiac and respiratory frequency.
Averaged for each pulse sequence.
Cardiac and respiratory gating were performed.
Two experienced radiologists reviewed independently all the scans, blinded to all clinical information with the exception of tumour location, as small or superficial oesophageal tumours can be difficult to detect.
Both readers carried out the MR-TNM staging on the initial (surgery-only group) or post-treatment (chemo-/radiotherapy group) MR, based on the seventh TNM edition.23
In case of discordance, the opinion of a third radiologist was requested.
The ADC values of the lesion were deliberately determined on the initial DWI for both groups; in this way, we removed any potential, confounding biological change after neoadjuvant treatment in the chemo-/radiotherapy group, and we could investigate the role of ADC in the treatment decision-making.
ADC maps were examined using an appropriate workstation (Viewforum; Philips Medical Systems, Best, Netherlands).
With reference to T2 weighted images, multiple regions of interest were manually drawn on diffusion-weighted (DW) images along the tumour contour and automatically copied on the ADC map, section by section; if necrotic areas were present, regions of interest were drawn on DW images only referring to the enhancing areas identified on the T1 weighted images after contrast.
The mean tumour ADC values were calculated by averaging those obtained from each section.
The anatomical appearance of oesophageal wall layers and depth of infiltration (T) were evaluated according to earlier studies.17,24
On T2 weighted MR images, the mucosa shows high signal intensity; the muscularis propria is seen as a discrete low-signal intensity band; and the adventitia shows a high-signal intensity.
Based on T2 weighted images, tumours were staged as follows: thickening of the wall <5 mm as T1; >5 mm and <15 mm as T2; and >15 mm (with irregularity of the outer margin) as T3. If tumour invasion of adjacent structures (trachea, aorta or vertebral bodies) was recognizable, tumours were considered as T4.25
Round-shaped lymph nodes >10 mm on the short axis25 and showing hyperintensity on DWI were considered pathological.
Multidetector CT protocol, analysis and interpretation
MDCT scans were performed on a 64-slice scanner (Brilliance 64s; Philips Medical Systems, Best, Netherlands).
Patients with Sievert I tumours received 500 ml of water orally for adequate stomach distension. Intravenous injection of a non-ionic contrast agent (Iopromide, Ultravist 370®; Bayer Schering Pharma, Berlin, Germany; 120 ml) was administered using an automatic injector (Stellant; Medrad Europe, Maastricht, Netherlands) at a rate of 3 ml s−1.
MDCT scanning parameters were as follows: 64 detector rows; beam collimation: 64 × 0.62; pitch: 0.983; kVp/effective mA: 120/300; slice thickness: 2 mm; and slice interval: 1 mm. Multiplanar reconstruction images in the sagittal and coronal planes were obtained at 1-mm intervals (slice thickness of 3 mm).
Unenhanced scans were firstly acquired. Then, thoracic scans (from the sternal notch to below the hemidiaphragm) were obtained in the arterial phase (25 s after contrast administration) followed by upper abdominal scans performed in the portal venous phase (70 s).
MDCT examinations were reviewed independently by the same two radiologists who had analysed DW-MR images, blinded to all clinical information other than tumour location.
Both readers carried out the MDCT-TNM staging on the initial (surgery-only group) or post-treatment (chemo-/radiotherapy group) MDCT, based on the seventh TNM edition.23
In case of discordance, the opinion of a third radiologist was requested.
The depth of infiltration was evaluated applying the same criteria used for MR, as already reported.25 As with MR, round-shaped lymph nodes with a short axis diameter >10 mm and without a normal fatty hilum were considered pathological.25
Endoscopic ultrasonography protocol, analysis and interpretation
EUS was performed using a linear-array 5- to 10-MHz ultrasound video endoscope (Hitachi/Pentax EG3870UTK and Hitachi H900 HiVision; Hitachi, Tokyo, Japan). For Siewert I tumours, 500 ml of water was orally administered to improve the transmission of the ultrasound beam into the gastric cavity.
EUS was performed by one experienced gastrointestinal endoscopist, blinded to all clinical information apart from tumour location. The same operator also determined the EUS-TNM staging on the initial (surgery-only group) or post-treatment (chemo-/radiotherapy group) EUS, based on the seventh TNM edition.23
The depth of invasion of the primary tumour was assessed according to literature25 as follows: a tumour invading the lamina propria or submucosa as T1, invading the muscularis propria and leading to the loss of the layer structure (but with a smooth outer margin) as T2, invading the adventitia and surrounding fat tissues as T3 and invading adjacent structures as T4.
Similar to the MR and MDCT criteria, enlarged, round-shaped and hypoechoic lymph nodes with a short axis >10 mm, sharply demarcated from the surrounding fat and without a visible hilum were considered pathological.25
Positron emission tomography CT protocol, analysis and interpretation
Fluorine-18 fludeoxyglucose (18F-FDG) PET/CT studies were performed on four different scanners: Discovery LS, Discovery ST, Discovery STE, Discovery-690 (General Electric Medical Systems, Milwaukee, WI) and Gemini GXL PET-CT system (Philips Medical Systems, Best, Netherlands).
Patients fasted for 6 h before the intravenous injection of 1 mCi 10 kg−1 of 18F-FDG. The blood glucose level was initially measured, to ensure euglycaemic glucose metabolism (<140 ml mg−1). Information concerning amount and time of the administered and residual radioactivity, patient body weight and height were collected for glucose metabolism quantification.
The protocol began 60 min after injection and was performed according to the oncological clinical protocol, including a CT scan for the attenuation correction and PET scans (2.5 min/scan) for adjacent bed positions.
PET images were reconstructed by standard reconstruction algorithms and qualitatively examined by an experienced nuclear physician, blinded to all clinical information other than tumour location.
In case of focal uptake of radiotracer, quantification of 18F-FDG PET/CT studies was performed calculating body-weighted standardized uptake value (SUV) corrected for partial volume effect.26 Considering that the Discovery LS scanner is not equipped by the calibration needed for partial volume correction, SUV quantification was not available for the only patient acquired on this scanner.
Nodal involvement was reported when 18F-FDG uptake was present in at least one lymphnode.
Statistical analysis
Data were analysed using R statistical software (Foundation for Statistical Computing, Vienna, Austria). Continuous variables are presented as mean ± standard deviation. Categorical data are presented as frequencies and percentages.
The initial population was divided into two groups based on the local invasion (T1–T2 vs T3–T4) and nodal involvement (N0 vs N+). To evaluate the diagnostic performance for T and N staging, sensitivity, specificity, negative-predictive value (NPV), positive-predictive value (PPV) and accuracy for MR, MDCT and EUS were calculated using histopathological TNM as the gold standard.
Differences between these values were verified by means of McNemar test.
Interobservers' consensus and agreement in measuring ADC were evaluated by means of the Spearman's correlation coefficient and the intraclass correlation coefficient. Confidence intervals (CIs) at level 0.95 were evaluated by bootstrap with adjusted percentile.
Differences between mean of ADC and SUV in the two subgroups were verified by Mann–Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed to determine the overall performance of ADC and SUV to identify serosal invasion. ROC curves were based on densities fitted by means of Gaussian kernel estimators,27,28 with bandwidth selected by unbiased cross validation. The optimal cut-off was selected as the one minimizing the distance between the curve and the ideal performance (sensitivity = 1; specificity = 1). p-values were computed by means of permutation methods to avoid any distributional assumption and considered significant when <0.05.
RESULTS
All patients (n = 18) had visible lesions and were treated with radical surgery.
According to resected specimens, 1/18 (6%) patient had upper-third oesophageal cancer, 8/18 (44%) and 5/18 (28%) patients were classified as middle-third and distal oesophagus, respectively, and 4/18 (22%) as Siewert I.
Baseline demographic and clinical data are presented in Table 2.
Table 2.
Baseline characteristics of patients
| Characteristic (n) (%) | All population (n = 18) | Surgery-only group (n = 9) | Chemo-/radiotherapy group (n = 9) |
|---|---|---|---|
| Sex | |||
| Male | 12/18 (66) | 4/9 (44) | 8/9 (88) |
| Female | 6/18 (34) | 5/9 (56) | 1/9 (12) |
| Age,a (years) | 60.24 (±12.99) | 62.51 (±9.96) | 57.97 (±15.75) |
| Tumour site | |||
| Oesophagus | 14/18 (78) | 7/9 (78) | 7/9 (78) |
| Upper | 1/18 (6) | 1/9 (12) | – |
| Middle | 8/18 (44) | 3/9 (33) | 5/9 (56) |
| Lower | 5/18 (28) | 3/9 (33) | 2/9 (22) |
| Siewert I | 4/18 (22) | 2/9 (22) | 2/9 (22) |
| Pathological T stageb | |||
| T1 | 8/18 (44) | 7/9 (76) | 1/9 (12) |
| T2 | 4/18 (22) | 1/9 (12) | 3/9 (33) |
| T3 | 5/18 (28) | 1/9 (12) | 4/9 (43) |
| T4 | 1/18 (6) | – | 1/9 (12) |
| Pathological N stageb | |||
| N0 | 14/18 (78) | 8/9 (88) | 6/9 (67) |
| N+ | 4/18 (22) | 1/9 (12) | 3/9 (33) |
| Histology | |||
| Adenocarcinoma | 7/18 (39) | 3/9 (33) | 4/9 (44) |
| Squamous cell carcinoma | 11/18 (61) | 6/9 (67) | 5/9 (56) |
| Grade | |||
| 1 | 1/18 (6) | 1/9 (11) | – |
| 2 | 12/18 (66) | 5/9 (56) | 7/9 (78) |
| 3 | 5/18 (28) | 3/9 (33) | 2/9 (22) |
| ADC (×10−3 mm2 s−1)a | 1.60 (±0.50) | 1.90 (±0.46) | 1.30 (±0.34) |
| SUV (g cm−3)a,c | 11.71 (±5.58) | 8.81 (±5.58) | 13.97 (±4.69) |
ADC, apparent diffusion coefficient; SUV, standardized uptake value.
Data in parentheses are percentages, except for age, ADC and SUV showing standard deviation.
According to the seventh TNM edition.
Data available for 16 patients.
Mean interval times (in days) between pre-operative imaging and surgery were as follows: MR with DWI: 20.44 (±12.49), MDCT: 34 (±22.87), EUS: 26 (±13.04), PET-CT: 27.11 (±15.48) for the surgery-only group and MR with DWI: 8.56 (±5.96), MDCT: 13 (±9.03), EUS: 6.78 (±5.26), PET-CT: 13.56 (±14.81) for the chemo-/radiotherapy group.
Multimodality imaging performance
Table 3 shows the diagnostic performances for T and N stage.
Table 3.
Performance characteristics of MR, multidetector CT (MDCT) and endoscopic ultrasonography (EUS) for local invasion (T stage) and metastatic lymph nodes (N stage)
| Preoperative T and N staging | MR | MDCT | EUS | MR vs MDCT |
MR vs EUS |
MDCT vs EUS |
|---|---|---|---|---|---|---|
| p-value | p-value | p-value | ||||
| T stage | ||||||
| Sensitivity | 67 | 83 | 100 | 0.56 | 0.16 | 0.32 |
| Specificity | 92 | 75 | 67 | 0.16 | 0.08 | 0.56 |
| Accuracy | 83 | 78 | 78 | 1 | 1 | 1 |
| PPV | 80 | 62 | 60 | 0.68 | 0.12 | 0.29 |
| NPV | 85 | 90 | 100 | 0.68 | 0.12 | 0.29 |
| N stage | ||||||
| Sensitivity | 100 | 75 | 100 | 0.31 | 1 | 0.31 |
| Specificity | 57 | 57 | 36 | 1 | 0.08 | 0.08 |
| Accuracy | 66 | 61 | 50 | 1 | 0.25 | 0.61 |
| PPV | 40 | 33 | 31 | 0.29 | 1 | 0.29 |
| NPV | 100 | 89 | 100 | 0.29 | 1 | 0.29 |
NPV, negative-predictive value; PPV, positive-predictive value.
Data are percentages and p-values are in bold.
For T staging, EUS showed the best sensitivity (100%) and NPV (100%), higher than MR and MDCT. Conversely, MR showed the highest specificity (92%) and PPV (80%) compared with EUS and MDCT. In terms of overall accuracy for T stage, MR showed the highest value (83%), although not significantly different to EUS and CT.
For N staging, MR and EUS showed the highest sensitivity and NPV (100%) but none of the three techniques showed satisfactory results for specificity and PPV.
Similar to T stage, MR showed the highest accuracy (66%) for nodal involvement, although not significantly different to the other two modalities.
It is important to stress that in this pilot study, we could not perform a PET/CT analysis for the N stage, as 17/18 patients did not show any pathological nodal uptake at baseline, preventing any further statistical analysis.
Diffusion-weighted imaging and positron emission tomography CT quantitative analysis
The interobserver reproducibility for ADC was very good (Spearman's rho = 0.939, CI = 0.841–0.977; intraclass correlation coefficient = 0.942, CI = 0.881–0.976) as shown in Figure 1.
Figure 1.
Bland–Altman plot of the interobserver reproducibility between the two readers for ADC values. The centre solid line represents the mean of differences. The top solid line shows the upper 95% limit of agreement and the bottom solid line shows the lower 95% limit of agreement, with the mean difference between the long-axis and short-axis measurements (±1.96 times the standard deviation). Obs, observer.
Quantitative analysis for SUV was available for 16 patients, as 1 patient did not show any uptake at baseline and the other was scanned on a system not equipped by the calibration needed for partial volume correction.
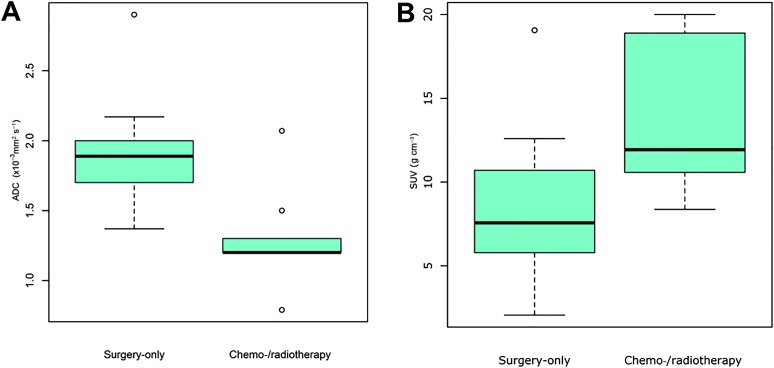
The mean ADC and SUV for the overall population and for the two subgroups (surgery vs chemo-/radiotherapy) are presented in Table 2; of note, the ADC showed a significant difference between the two subgroups (1.90 vs 1.30 × 10−3 mm2 s−1; p = 0.005) (Figure 2a). The difference for SUV was also very close to conventional levels of statistical significance (p = 0.05) (Figure 2b).
Figure 2.
Box and whisker plots showing apparent diffusion coefficient (ADC; ×10−3 mm2 s−1) (a) and standardized uptake value (SUV; g cm−3) (b) values in the surgery-only and chemo-/radiotherapy group. Centre line = median, top of box = 75th percentile, bottom of box = 25th percentile, whiskers = 10th and 90th percentiles. ° = outliers (between 1.5 and 3.0 interquartile ranges).
Moreover, comparing ADC and SUV with the absence (pT1–pT2) or presence (pT3–pT4) of local invasion at final histology, we observed an optimal cut-off of 1.33 × 10−3 mm2 s−1 for ADC (p = 0.05) and of 7.97 g cm−3 for SUV (p = 0.44).
The ROC curves shown in Figure 3 (area under the curve: 0.78 and 0.62 for ADC and SUV, respectively) suggest that patients with ADC values >1.33 (Figure 4) may be directly treated with surgery (differently from those with lower ADC values, Figure 5) with a sensitivity of 66%, a specificity of 75%, a PPV of 57%, a NPV of 81% and an accuracy of 72%.
Figure 3.
Receiver operating characteristic curves to identify the invasion of adventitia on the basis of apparent diffusion coefficient (ADC) and standardized uptake value (SUV). The optimal cut-off was 1.33 × 10−3 mm2 s−1 for ADC and 7.97 g cm−3 for SUV.
Figure 4.
Lesion involving the lower third of the oesophagus in a 46-year-old male. The arrows indicate on MR a slight thickening of the oesophageal wall on T2 weighted images (a), diffusion-weighted imaging (b) and apparent diffusion coefficient (ADC) map (c). The ADC of the lesion was 1.7 × 10−3 mm2 s−1. The lesion was also recognisable on multidetector CT (d), endoscopic ultrasonography (e) and positron emission tomography CT (f, g) images (arrows), showing invasion of submucosal and muscular layers and a standardized uptake value of 2.06 g cm−3. The tumour was staged as “non-invasive” (pT1) at final histology.
Figure 5.
Lesion involving the middle third of the oesophagus in a 67-year-old male. The arrows indicate on MR the massive presence of an obstructing tumour on T2 weighted images (a), diffusion-weighted imaging (DWI) (b) and apparent diffusion coefficient (ADC) map (c). The ADC of the lesion was 1.2 × 10−3 mm2 s−1. The lesion was also recognisable on multidetector CT (MDCT) (d), endoscopic ultrasonography (EUS) (e) and positron emission tomography CT (f, g) images, showing invasion of perioesophageal tissue (arrows) and a standardized uptake value of 20 g cm−3, and therefore addressed to chemo-/radiotherapy. After completion of neoadjuvant treatment, the lesion was considerably reduced in size, as shown by T2 (h), DWI (i), ADC map (j), MDCT (k) and EUS (l) images, and staged as “organ-confined” (pT2) at final histology.
For SUV analysis, the sensitivity was 100%, specificity was 40%, PPV was 50%, NPV was 100% and accuracy was 62%.
DISCUSSION
The pre-operative management of oesophageal cancer strongly depends on the invasion of the adventitia and nodal involvement. With the growing importance of neoadjuvant treatments, the aim of reliably improving T and N assessment by imaging is becoming more and more challenging.
This pilot study had been designed to investigate the diagnostic performance of MR with DWI, MDCT, EUS and PET-CT on the same cohort of patients, so that each diagnostic modality faced the same case load (e.g. peritumoural inflammation, adventitia and nodal involvement).
Our main findings were: (i) MR showed the highest specificity for T stage and the same sensitivity of EUS for N stage and (ii) a significant different initial ADC from DWI was found in the two subgroups, with higher values for patients directly treated with surgery.
Together, these results indicate that MR with DWI could potentially enrich the pre-operative work-up in oesophageal cancer.
At present, few studies have evaluated the use of MR with DWI for staging oesophageal cancer,18,29 and current literature on its application for T staging is considerably limited.30
The role of MR in oesophageal cancer has been scarce due to numerous technical challenges but a recent systematic review by van Rossum et al31 emphasized the idea that MR could improve the pre-operative staging and the assessment of treatment response.
In our study, EUS showed the highest sensitivity for T staging, highlighting its primary role for detecting locally advanced tumours. Hence, we confirmed that EUS is fundamental in the diagnostic pathway of oesophageal cancer.
Interestingly, MR showed the highest specificity compared with MDCT and EUS, presenting as a potential, reliable technique to identify organ-confined tumours, where results from the other techniques were not satisfactory.
This is of paramount importance as patients with non-invasive tumours are commonly treated with immediate surgical resection. MRI could therefore prevent them from the risks correlated to neoadjuvant therapy (e.g. bone marrow toxicity).
Additionally, our results in terms of accuracy for MR (83%) are in line with those reported in literature31 and very similar to the pooled accuracy for EUS (81–92%) reported in a systematic review by Puli et al.9
MR and EUS showed a higher sensitivity than MDCT for N staging but none of the three techniques had satisfactory results in terms of specificity and accuracy. This may be partly attributed to the fact that pathological nodes are not necessarily enlarged and swollen lymph nodes might be also just inflammatory (especially after chemo-/radiotherapy).
Moreover, owing to the notable interobserver variation and lack of standardised guidelines, lymph nodes should be assessed using a reproducible method (i.e. the short axis diameter should be measured perpendicular to the longest one). This is why, in order to ensure the reproducibility of our results, in this pilot study, we deliberately chose a common cut-off of >10 mm on the short axis in all the three techniques to define pathological lymph nodes.
We acknowledge that the choice of this cut-off is unusual for EUS, as most operators would consider nodes of 5–10 mm as pathological in the presence of other distinctive features (round-shaped, hypoechoic, without a visible hilum).
Moreover, in this pilot study, we could not perform a PET/CT analysis for N stage, preventing any further comparison between the techniques.
We are therefore confirming the need for improvement of imaging techniques for nodal involvement, integrating, as an example, MRI with other modalities such as PET/CT, as pointed out by van Rossum et al.29
We believe that our pilot study also adds to current literature by proving that the use of DWI, namely the ADC, assists in addressing patients to the correct treatment. Specifically, it has been demonstrated16 that pre-treatment ADC of responders to neoadjuvant therapy in gastro-oesophageal cancer is significantly lower than that of non-responders.
This may be due to the presence of necrotic areas, which are less responsive to cytotoxic treatments, accounting for the higher ADC values of non-responders. In our study, patients undergoing surgical resection had a significant higher ADC than those addressed to neoadjuvant therapy.
This implies that only patients with low ADC could benefit from chemo-/radiotherapy, whereas those with higher values, if treated with immediate surgical resection, might avoid the side effects of neoadjuvant therapy (oesophagitis, pericarditis and pneumonia).
Our results also suggest that an ADC cut-off of 1.33 × 10−3 mm2 s−1 may assist the pre-operative work-up of oesophageal cancer to recommend adequate treatment (surgery vs chemo-/radiotherapy), according to what has been previously described.2,3
Interestingly, a similar value has been reported in literature (1.50 × 10−3 mm2 s−1)16 to discriminate between patients responding or not to neoadjuvant treatment, although that study also included gastric cancer.
Moreover, we found a higher SUV for patients addressed to chemo-/radiotherapy, suggesting that more aggressive tumours are related to a higher metabolic activity.
As a result, our key findings suggest the potential role of combining MR and DWI in the pre-operative staging of oesophageal cancer and could have a potential bearing on its clinical management.
The important limitations of this pilot study must be reported.
Firstly, in order to ensure a methodologically proofed population for our analysis, a significant number of patients (n = 24) were excluded due to our restrictive criteria; we believe that those results not reaching conventional levels of statistical significance could be mainly related to this limitation.
From the above, it is reasonable to conclude that larger population-based studies will be informative and should be further evaluated.
Another limitation is the lack of standardized imaging guidelines to stage oesophageal cancer, both in the use of b-values (we used only two different b-values) and for pathological nodal size. However, to overcome this drawback, we deliberately chose a common dimensional cut-off of 10 mm on the short axis for all techniques, ensuring the reproducibility of our results.
Another significant caveat is that EUS and PET-CT findings were analysed only by one operator, whereas MR and MDCT findings were analysed by two radiologists.
Despite the aforementioned limitations, we believe that our initial results are promising and could lead the way to further investigations on this topic.
This pilot study represents the first effort where the four techniques have been prospectively compared together in oesophageal cancer staging. All data had been collected and analysed by a multidisciplinary team that was highly experienced in the field.
We analysed two subgroups initially managed with different approaches (surgery vs chemo-/radiotherapy) according to common clinical practice,2,3 investigating the differences in terms of quantitative measurements from DWI and PET-CT, with significant results for ADC values.
Histopathological correlation (considered the gold standard) had been available for all patients, whereas a similar previous report was primarily based on clinical staging.30
Moreover, two experienced radiologists performed DWI analysis using a reliable and reproducible method, with important results in terms of agreement.
In summary, this pilot study has provided initial evidence that the use of MR with DWI could enrich the current pre-operative work-up for oesophageal cancer and could be used for T and N staging, showing important, additional information for both staging and initial treatment decision-making. However, larger studies have to be carried out before introducing this technique in the standard diagnostic pathway of oesophageal cancer, in order to understand if MR with DWI could change its management and replace more costly or invasive tests such as PET-CT or EUS.
Acknowledgments
ACKNOWLEDGMENTS
The authors are indebted to all the patients, families and healthcare assistants (nurses and radiographers) who greatly contributed to the realization of this study.
Contributor Information
Francesco Giganti, Email: giganti.francesco@hsr.it, giganti.fra@gmail.com.
Alessandro Ambrosi, Email: ambrosi.alessandro@hsr.it.
Maria C Petrone, Email: petrone.mariachiara@hsr.it.
Carla Canevari, Email: canevari.carla@hsr.it.
Damiano Chiari, Email: chiari.damiano@hsr.it.
Annalaura Salerno, Email: annalaurasalerno@hotmail.it.
Paolo G Arcidiacono, Email: arcidiacono.paologiorgio@hsr.it.
Roberto Nicoletti, Email: nicoletti.roberto@hsr.it.
Luca Albarello, Email: albarello.luca@hsr.it.
Elena Mazza, Email: mazza.elena@hsr.it.
Francesca Gallivanone, Email: gallivanone.francesca@hsr.it.
Luigi Gianolli, Email: gianolli.luigi@hsr.it.
Elena Orsenigo, Email: orsenigo.elena@hsr.it.
Antonio Esposito, Email: esposito.antonio@hsr.it.
Carlo Staudacher, Email: staudacher.carlo@hsr.it.
Alessandro Del Maschio, Email: delmaschio.alessandro@hsr.it.
Francesco De Cobelli, Email: decobelli.francesco@hsr.it.
REFERENCES
- 1.Keighley MR. Gastrointestinal cancers in Europe. Aliment Pharmacol Ther 2003; 18(Suppl. 3): 7–30. doi: https://doi.org/10.1046/j.0953-0673.2003.01722.x [DOI] [PubMed] [Google Scholar]
- 2.Courrech Staal EF, Aleman BM, Boot H, van Velthuysen ML, van Tinteren H, van Sandick JW. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg 2010; 97: 1482–96. doi: https://doi.org/10.1002/bjs.7175 [DOI] [PubMed] [Google Scholar]
- 3.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta analysis. Lancet Oncol 2011; 12: 681–92. doi: https://doi.org/10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 4.Urschel J, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2002; 185: 538–43. doi: https://doi.org/10.1016/S0002-9610(03)00066-7 [DOI] [PubMed] [Google Scholar]
- 5.Jamil LH, Gill KR, Wallace MB. Staging and restaging of advanced esophageal cancer. Curr Opin Gastroenterol 2008; 24: 530–34. doi: https://doi.org/10.1097/MOG.0b013e3283025c91 [DOI] [PubMed] [Google Scholar]
- 6.Kim TJ, Kim HY, Lee KW, Kim MS. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. RadioGraphics 2009; 29: 403–21. doi: https://doi.org/10.1148/rg.292085106 [DOI] [PubMed] [Google Scholar]
- 7.Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol 2005; 23: 4483–9. doi: https://doi.org/10.1200/JCO.2005.20.644 [DOI] [PubMed] [Google Scholar]
- 8.Richards DG, Brown TH, Manson JM. Endoscopic ultrasound in the staging of tumours of the oesophagus and gastro-oesophageal junction. Ann R Coll Surg Engl 2000; 82: 311–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008; 14: 1479–90. doi: https://doi.org/10.3748/wjg.14.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringe KI, Meyer S, Ringe BP, Winkler M, Wacker F, Raatschen HJ. Value of oral effervescent powder administration for multidetector CT evaluation of esophageal cancer. Eur J Radiol 2015; 84: 215–20. doi: https://doi.org/10.1016/j.ejrad.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 11.Jin GY, Park SH, Han YM. Usefulness of MDCT evaluation of the intraluminal surface of esophageal masses using only effervescent powder without injection of hypotonic agent. Abdom Imaging 2009; 34: 424–9. doi: https://doi.org/10.1007/s00261-008-9398-2 [DOI] [PubMed] [Google Scholar]
- 12.Hong SJ, Kim TJ, Nam KB, Lee S, Yang HC, Cho S, et al. New TNM staging system for esophageal cancer: what chest radiologists need to know. RadioGraphics 2014; 34: 1722–40. doi: https://doi.org/10.1148/rg.346130079 [DOI] [PubMed] [Google Scholar]
- 13.van Westreenen HL, Westerterp M, Bossuyt PM. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 2004; 22: 3805–12. doi: https://doi.org/10.1200/JCO.2004.01.083 [DOI] [PubMed] [Google Scholar]
- 14.Rice TW. Clinical staging of esophageal carcinoma: CT, EUS, and PET. Chest Surg Clin N Am 2000; 10: 471–85. [PubMed] [Google Scholar]
- 15.Heeren PA, Jager PL, Bongaerts F, van Dullemen H, Sluiter W, Plukker JT. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J Nucl Med 2004; 45: 980–7. [PubMed] [Google Scholar]
- 16.De Cobelli F, Giganti F, Orsenigo E, Cellina M, Esposito A, Agostini G, et al. Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology. Eur Radiol 2013; 23: 2165–74. doi: https://doi.org/10.1007/s00330-013-2807-0 [DOI] [PubMed] [Google Scholar]
- 17.Riddell AM, Hillier J, Brown G, King DM, Wotherspoon AC, Thompson JN, et al. Potential of surface-coil MRI for staging of esophageal cancer. AJR Am J Roentgenol 2006; 187: 1280–7. doi: https://doi.org/10.2214/AJR.05.0559 [DOI] [PubMed] [Google Scholar]
- 18.Sakurada A, Takahara T, Kwee TC, Yamashita T, Nasu S, Horie T, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in esophageal cancer. Eur Radiol 2009; 19: 1461–9. doi: https://doi.org/10.1007/s00330-008-1291-4 [DOI] [PubMed] [Google Scholar]
- 19.Lee G, Kim SJ, Jeong YJ, Kim IJ, Pak K, Park DY, et al. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med 2014; 55: 1242–7. doi: https://doi.org/10.2967/jnumed.114.138974 [DOI] [PubMed] [Google Scholar]
- 20.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85: 1457–9. doi: https://doi.org/10.1046/j.1365-2168.1998.00940.x [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa S, Yoshikawa T, Aoyama T, Hayashi T, Yamada T, Tsuchida K, et al. Esophagus or stomach? The seventh TNM classification for Siewert type II/III junctional adenocarcinoma. Ann Surg Oncol 2013; 20: 773–9. doi: https://doi.org/10.1245/s10434-012-2780-x [DOI] [PubMed] [Google Scholar]
- 22.van Hagen P, Hulshof MC, van Lanschot JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. doi: https://doi.org/10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 23.Peng CW, Wang LW, Zeng WJ, Yang XJ, Li Y. Evaluation of the staging systems for gastric cancer. J Surg Oncol 2013; 108: 93–105. doi: https://doi.org/10.1002/jso.23360 [DOI] [PubMed] [Google Scholar]
- 24.Dave UR, Williams AD, Wilson JA, Amin Z. Esophageal cancer staging with endoscopic MR imaging: pilot study. Radiology 2004; 230: 281–6. doi: https://doi.org/10.1148/radiol.2301021047 [DOI] [PubMed] [Google Scholar]
- 25.Wu LF, Wang BZ, Feng JL, Cheng WR, Liu GR, Xu XH, et al. Preoperative TN staging of esophageal cancer: comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol 2003; 9: 219–24. doi: https://doi.org/10.3748/wjg.v9.i2.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallivanone F, Stefano A, Grosso E. PVE correction in PET-CT whole-body oncological studies from PVE-affected images images. IEEE Trans Nucl Sci 2011; 58: 736–47. doi: https://doi.org/10.1109/TNS.2011.2108316 [Google Scholar]
- 27.Zou KH, Hall WJ, Shapiro DE. Smooth non-parametric receiver operating characteristic (ROC) curves for continuous diagnostic tests. Stat Med 1997; 16: 2143–56. doi: https://doi.org/10.1002/(SICI)1097-0258(19971015)16:19<2143::AID-SIM655>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 28.Sheater S. Density estimation. Stat Sci 2004; 19: 588–97. doi: https://doi.org/10.1214/088342304000000297 [Google Scholar]
- 29.van Rossum PS, van Lier AL, Lips IM, Meijer GJ. Imaging of oesophageal cancer with FDG-PET/CT and MRI. Clin Radiol 2015; 70: 81–95. doi: https://doi.org/10.1016/j.crad.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 30.Aoyagi T, Shuto K, Okazumi S, Shimada H, Nabeya Y, Kazama T, et al. Evaluation of the clinical staging of esophageal cancer by using diffusion-weighted imaging. Exp Ther Med 2010; 1: 847–51. doi: https://doi.org/10.3892/etm.2010.112 [Google Scholar]
- 31.van Rossum PS, van Hillegersberg R, Lever FM, Lips IM, van Lier AL, Meijer GJ, et al. Imaging strategies in the management of oesophageal cancer: what's the role of MRI? Eur Radiol 2013; 23: 1753–65. doi: https://doi.org/10.1007/s00330-013-2773-6 [DOI] [PubMed] [Google Scholar]