Abstract
Objective:
To develop a novel method for rapid myocardial T1 mapping at high spatial resolution.
Methods:
The proposed strategy represents a single-shot inversion recovery experiment triggered to early diastole during a brief breath-hold. The measurement combines an adiabatic inversion pulse with a real-time readout by highly undersampled radial FLASH, iterative image reconstruction and T1 fitting with automatic deletion of systolic frames. The method was implemented on a 3-T MRI system using a graphics processing unit-equipped bypass computer for online application. Validations employed a T1 reference phantom including analyses at simulated heart rates from 40 to 100 beats per minute. In vivo applications involved myocardial T1 mapping in short-axis views of healthy young volunteers.
Results:
At 1-mm in-plane resolution and 6-mm section thickness, the inversion recovery measurement could be shortened to 3 s without compromising T1 quantitation. Phantom studies demonstrated T1 accuracy and high precision for values ranging from 300 to 1500 ms and up to a heart rate of 100 beats per minute. Similar results were obtained in vivo yielding septal T1 values of 1246 ± 24 ms (base), 1256 ± 33 ms (mid-ventricular) and 1288 ± 30 ms (apex), respectively (mean ± standard deviation, n = 6).
Conclusion:
Diastolic myocardial T1 mapping with use of single-shot inversion recovery FLASH offers high spatial resolution, T1 accuracy and precision, and practical robustness and speed.
Advances in knowledge:
The proposed method will be beneficial for clinical applications relying on native and post-contrast T1 quantitation.
INTRODUCTION
Tissue characterization by native myocardial T1 mapping as well as quantitation of perfusion and extracellular volume after contrast administration are essential ingredients of Cardiovascular magnetic resonance imaging (CMR) investigations and commonly performed by inversion recovery (IR) methods using Fast low-angle shot (FLASH),1,2 Echo planar imaging (EPI)3 or Steady-state free precession (SSFP) readouts4 according to the Look-Locker technique.5 To date, respective applications commonly rely on a modified Look-Locker inversion (MOLLI) experiment6 or manifold derivatives therefrom (for a recent review of possibilities and limitations, see Kellman and Hansen7). In fact, despite widespread usage, most approaches still suffer from practical restrictions such as limited spatial resolution and/or compromised T1 accuracy, so that further technical improvements are warranted.
Following the recommendations of the T1 mapping Consensus Statement of the Society for Cardiovascular Magnetic Resonance and CMR Working Group of the European Society of Cardiology,8 the basic requirements and clinical needs for cardiac T1 mapping comprise (i) speed, i.e. single-shot applications with measuring times of a few seconds only, (ii) T1 accuracy, i.e. validated T1 values with small standard deviations and without dependency on heart rate, (iii) sufficiently high spatial resolution, i.e. about 1-mm in-plane resolution and (iv) practical robustness, i.e. no motion sensitivity and no image artefacts due to susceptibility problems, SSFP bandings or radial streakings.
This work describes a novel method which effectively meets all aforementioned challenges. It is based on a single-slice acquisition during a brief breath-hold (typically 3 s only) which combines a single-shot IR-FLASH technique with pronounced radial undersampling of individual frames, iterative reconstruction by non-linear inversion (NLINV)9 and conjugate gradient methods as previously described10,11 and fitting of a diastolic T1 map after deletion of systolic (i.e., motion-affected) frames. The entire procedure is fully automatic and only requires triggering of the initial inversion pulse to early diastole. The results comprise both an image series representing the entire IR experiment and a colour-coded T1 map where pixel intensities directly refer to T1 values in milliseconds. The proposed T1 mapping method complements previous real-time MRI acquisitions of cardiac function and flow12–15 which together bear the potential to develop a comprehensive real-time CMR examination.
METHODS AND MATERIALS
All measurements were performed on a human MRI system operating at 3 T (Magnetom® Prisma fit; Siemens Healthcare, Erlangen, Germany). Phantom measurements employed the 64-channel head coil, whereas human heart studies were performed using the 18-element thorax coil in combination with 12 elements of the 32-element spine coil. Six young subjects (two female, four male, age range 24–27 years) with no known illness [heart rate about 50–55 beats per minute (bpm)] were recruited among the students of the local university. Written informed consent, according to the recommendations of the local ethics committee, was obtained from all subjects prior to MRI.
According to the T1 mapping Consensus Statement,8 experimental validations of the proposed technique were performed at different simulated heart rates with the use of a commercial reference phantom (Diagnostic Sonar Ltd, Scotland, UK) consisting of six compartments with defined T1 values surrounded by water. As suggested, a long-repetition time (TR) IR fast spin echo (FSE) sequence with 13 logarithmically spaced inversion times between 50 and 2300 ms served for T1 determination [TR = 7.2 s, echo time (TE) = 12 ms, 6 echoes and measuring time = 50 min].
The procedures for cardiac T1 mapping described below (i.e. data acquisition, image reconstruction and T1 fitting) were implemented as an easy-to-use protocol on the MRI system by taking advantage of a bypass computer (sysGen/TYAN Octuple-GPU; Sysgen, Bremen, Germany) previously developed for real-time MRI9,15 and equipped with eight graphics processing units (NVIDIA® GeForce® GTX TITAN Black; NVIDIA, Santa Clara, CA). This bypass computer could be fully integrated into the reconstruction pipeline of the commercial MRI system (Magnetom Prisma fit) by a single network connection. If the system software is compatible, the implementation takes less than an hour including installation of ready-to-use measuring protocols for cardiac T1 mapping and other real-time CMR applications.
MRI acquisition and reconstruction
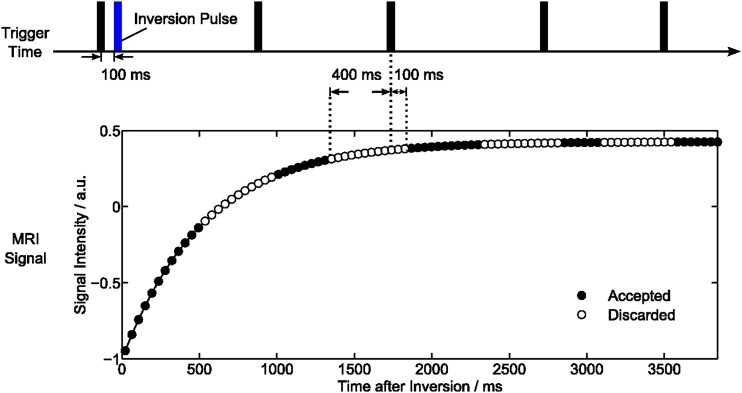
The chosen acquisition scheme for cardiac T1 mapping is illustrated in Figure 1. In order to achieve maximum robustness and T1 accuracy, a previously developed IR FLASH sequence10 was applied as a single-slice technique using a non-selective adiabatic 180° inversion pulse triggered to early diastole. The present study employed a simple and robust finger pulse trigger and a 100-ms delay to inversion. Although the method yields similar accuracy for a slice-selective inversion pulse when applied to stationary tissue (data not shown), cardiac T1 mapping exclusively used a non-selective inversion pulse to minimize the effects of through-plane motion and myocardial perfusion.
Figure 1.
Myocardial T1 mapping using single-shot inversion recovery FLASH with radial undersampling. Finger pulse trigger = black bar.
Continuous image readout after inversion was based on a radial FLASH sequence with pronounced undersampling. Time-efficient spoiling of residual transverse magnetizations was accomplished by random radiofrequency (RF) phases.16 Cardiac T1 maps were then acquired at a nominal in-plane resolution of 1.0 × 1.0 mm2 and 6-mm section thickness using a field of view = 256 × 256 mm2 in combination with a resolution of 512 complex data points per radial spoke (using two-fold oversampling). All spokes were homogeneously distributed over 360°, whereas five successive frames used complementary sets of spokes in sequential order. Other parameters were TR = 2.26 ms, TE = 1.47 ms and flip angle of 4°. The number of spokes per frame varied from 27 to 23 and finally 19 spokes yielding a temporal resolution of 61, 52 and 43 ms, respectively. The total acquisition time was initially chosen to be 8 s but later reduced to 4 and 3 s.
Image reconstruction has previously been described for the case of non-cardiac T1 mapping10,11 and employs the same iteratively regularized NLINV algorithm as developed for real-time MRI; for details, see Uecker et al.9 Apart from an advanced gradient-delay correction14 and data compression to 10 virtual channels based on a principal component analysis, the method takes advantage of some degree of spatial smoothness of coil sensitivities as well as of temporal regularization to the preceding frame. However, this latter term of the underlying cost function is downsized relative to the data consistency term by a factor of two during each iteration. The reconstruction ensures high temporal fidelity as demonstrated for a motion phantom rotating at defined speed17 and therefore does not compromise the resolution of contrast changes during inversion recovery.
The actual reconstruction process starts immediately after the end of data acquisition, first by a reverse NLINV reconstruction of the last 10 frames to obtain high-quality coil sensitivity maps using 6 iterations. Subsequently, the entire image series was reconstructed in the reverse order by fixing the coil sensitivities to those obtained by NLINV. The resulting linear inverse problem was solved by the iteratively regularized conjugate gradient method, again using six iterations.
Prior to T1 fitting, the images were spatially filtered by a recently developed modified non-local means algorithm.18 The filter preserves small isolated details and efficiently removes background noise [corresponding to a 60% signal-to-noise (SNR) improvement] without introducing blur, smearing or patch artefacts. This is accomplished by extending the conventional non-local means algorithm to adapt the influence of the original pixel value according to a simple measure for patch regularity. Detail preservation is improved by a compactly supported weighting kernel which closely approximates the commonly used exponential weight.
Temporal median filtering was only used for the purpose of displaying image series, whereas no temporal filter was used for T1 mapping. The median filter extended over five frames to match the number of frames with different sets of spokes, e.g. in the studies of Uecker et al9 and Frahm et al.17 As illustrated in Figure 1, the influence of systolic motion on the fitting of a diastolic T1 map was minimized by automatically deleting images over a period of 500 ms starting from 400 ms prior to each finger pulse trigger signal.
T1 quantitation
After reconstruction, spatial filtering and systolic deletion, the remaining complex images were fitted to the complex signal model2
| (1) |
where Mini is the initial complex signal after inversion; t is the central time point (i.e. radial spoke) of each frame during inversion recovery; γ is the ratio between the steady-state signal Mss and Mini; and T1∗ is the shortened apparent T1 due to multiple low flip-angle RF excitations. The same phase is assumed for Mss and Mini, which leads to four unknown real-valued parameters: Re{Mini}, Im{Mini}, γ and T1∗. A pixelwise estimation was performed using the Trust-Region algorithm (Chapters 4.1 and 4.3 in the study of Nocedal and Wright19) based on the Dlib C++ library.20 The algorithm performs an unconstrained minimization of the cost function defined by
| (2) |
where Y corresponds to the vector of pixel intensities during inversion recovery. The iterative optimization was stopped if the relative difference of the objective function values between successive iterations was <10−5. T1 was then calculated according to:21
| (3) |
with δt the delay between inversion and the start of data acquisition. In the present implementation, this period covered half of the inversion pulse (5 ms) and a following spoiler gradient (10 ms). For the assessment of myocardial T1 values, the regions of interest were carefully selected to exclude contributions from the blood pool. These analyses were accomplished using the arrayShow tool22 in MATLAB® (MathWorks®, Natick, MA).
RESULTS
Table 1 summarizes T1 relaxation times for a reference phantom. The data were acquired with the radial IR-FLASH method proposed for cardiac T1 mapping (43-ms resolution and 3-s duration) at different simulated heart rates ranging from 40 to 100 bpm. Zero heart rate refers to T1 fitting without deletion of any frames. A comparison with T1 relaxation times obtained by a long-TR IR-FSE technique reveals excellent agreement for most (long) values, whereas two tubes with shorter values are slightly underestimated (maximum deviation 5%).
Table 1.
T1 relaxation times for a reference phantom and simulated heart rates
| T1, msa | T2, msb | Heart ratec |
||||
|---|---|---|---|---|---|---|
| 0 | 40 | 60 | 80 | 100 | ||
| 331 ± 11 | 101 ± 2 | 315 ± 13 | 315 ± 13 | 314 ± 13 | 316 ± 13 | 317 ± 16 |
| 494 ± 22 | 46 ± 2 | 476 ± 18 | 475 ± 18 | 475 ± 18 | 476 ± 20 | 479 ± 23 |
| 676 ± 19 | 81 ± 3 | 660 ± 25 | 659 ± 26 | 661 ± 27 | 663 ± 29 | 665 ± 31 |
| 857 ± 25 | 132 ± 5 | 850 ± 28 | 850 ± 28 | 852 ± 30 | 853 ± 30 | 853 ± 35 |
| 1225 ± 20 | 138 ± 4 | 1227 ± 34 | 1226 ± 34 | 1230 ± 36 | 1230 ± 37 | 1236 ± 38 |
| 1501 ± 23 | 166 ± 5 | 1511 ± 42 | 1513 ± 43 | 1513 ± 44 | 1516 ± 46 | 1517 ± 50 |
Single-shot inversion recovery FLASH was performed at 43-ms resolution (19 spokes) for a duration of 3 s.
T1 values for a long-repetition time inversion recovery fast spin echo sequence.
T2 values according to Sumpf et al.29
Simulated heart rates (in beats per minute) correspond to the deletion of a 500-ms period (“systole”) in each cardiac cycle. No images are deleted for zero heart rate.
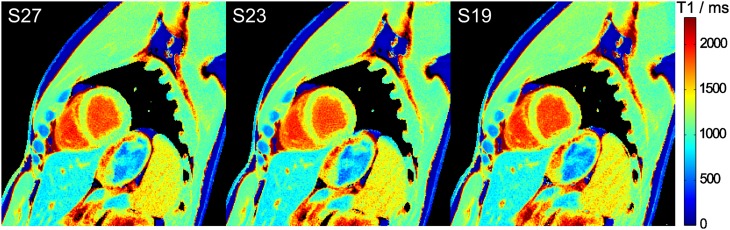
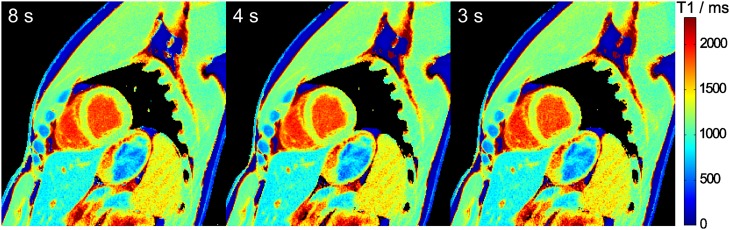
Figures 2 and 3 demonstrate cardiac T1 maps for different numbers of spokes per frame, i.e. different temporal resolution, and different durations of the IR-FLASH measurement, respectively. In all cases, visual inspection reveals no detectable difference. This qualitative finding is confirmed by the quantitative analysis in Table 2.
Figure 2.
Myocardial T1 maps for different temporal resolutions. Single-shot inversion recovery FLASH was performed with 27, 23 and 19 spokes per frame (i.e. acquisition times of 63, 52 and 43 ms) for a duration of 8 s.
Figure 3.
Myocardial T1 maps for different measurement durations. Single-shot inversion recovery FLASH was performed at 43 ms temporal resolution (19 spokes) for durations of 8, 4 and 3 s.
Table 2.
Myocardial T1 relaxation times for different temporal resolutions and measuring times
| Spokes per frame | 27 | 23 | 19 |
|---|---|---|---|
| Temporal resolutiona | 61 ms | 52 ms | 43 ms |
| IR-FLASH measurementb | T1, msc | ||
| 8 s | 1253 ± 29 | 1254 ± 29 | 1253 ± 31 |
| 4 s | 1252 ± 30 | 1254 ± 30 | 1253 ± 31 |
| 3 s | 1254 ± 32 | 1257 ± 33 | 1256 ± 33 |
IR, inversion recovery.
Number of spokes and acquisition time per frame.
Total measuring time.
Averaged across subjects (mean ± standard deviation, n = 6).
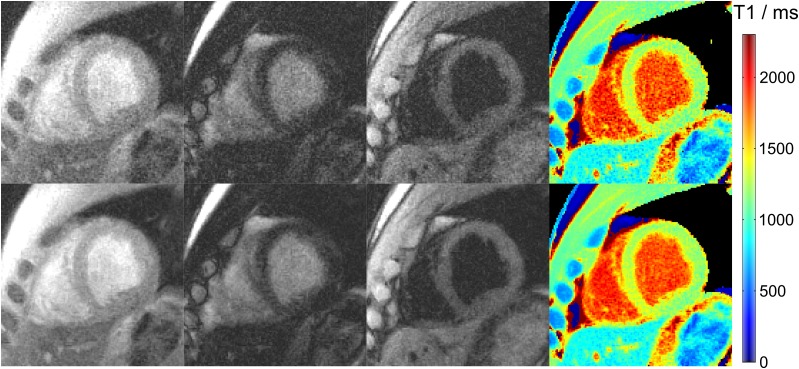
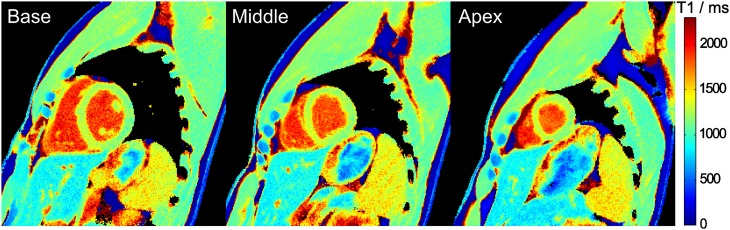
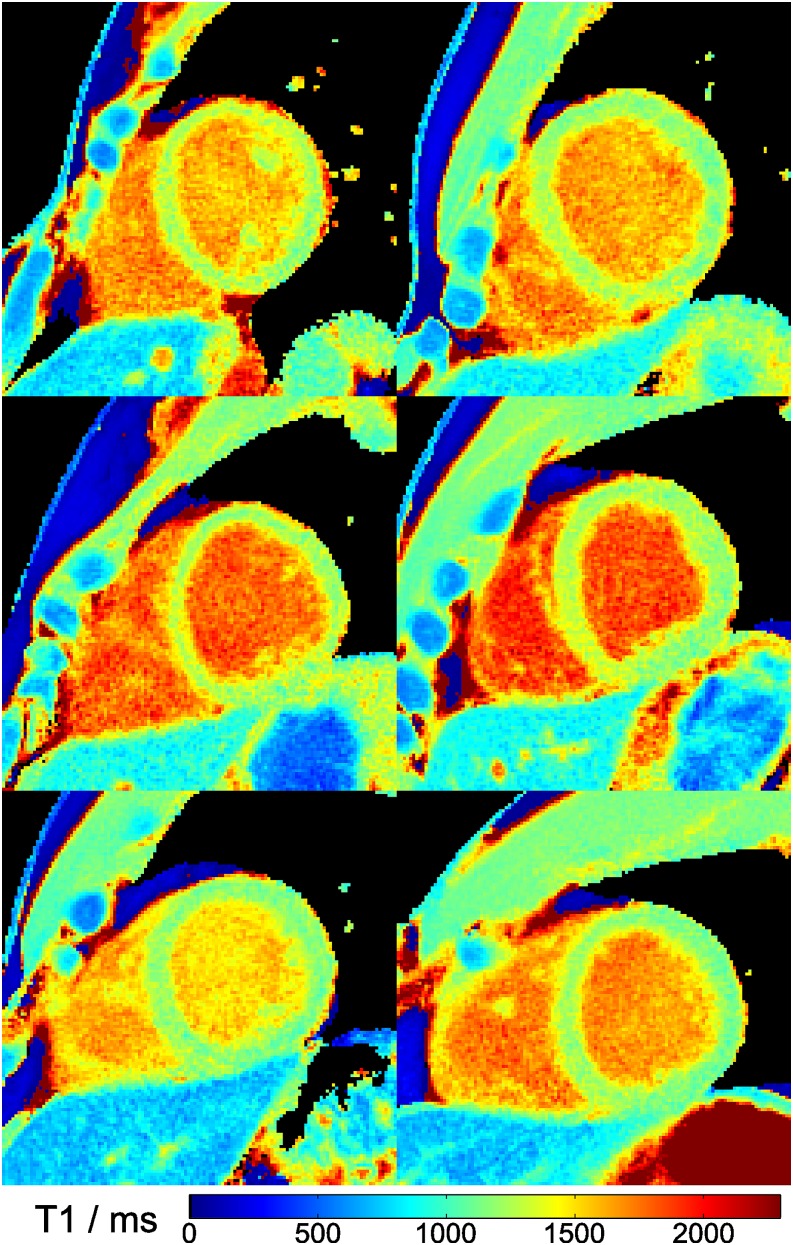
The effect of filtering prior to T1 fitting is demonstrated in Figure 4 comparing raw images and T1 maps with and without application of a spatial filter. Figure 5 shows three T1 maps for a single subject in a basal, mid-ventricular and apical short-axis section, whereas Figure 6 summarizes the mid-ventricular T1 maps of all six subjects. A full cardiac image series which corresponds to the T1 map in the mid-ventricular section of Figure 5 is available as Supplementary Video A. The quantitative results for all six subjects are summarized in Table 3 (septal T1 values in a basal, mid-ventricular and apical section) and Table 4 (segmental T1 values in a mid-ventricular section), respectively.
Figure 4.
Cardiac images and T1 maps (top) without and (bottom) with spatial filtering. Single-shot inversion recovery FLASH was performed at 43 ms temporal resolution (19 spokes) for a duration of 3 s. The images (magnified views) refer to an early time point after inversion, nulling of myocardial tissue and nulling of blood signal, respectively.
Figure 5.
Myocardial T1 maps of a basal, mid-ventricular and apical section. Single-shot inversion recovery FLASH was performed at 43 ms resolution (19 spokes) for a duration of 3 s.
Figure 6.
Myocardial T1 maps of all six subjects (mid-ventricular section, magnified view). Single-shot inversion recovery FLASH was performed at 43 ms resolution (19 spokes) for a duration of 3 s.
Table 3.
T1 relaxation times of the septal wall
| Subject | Basal T1, #framesa |
Mid-ventricular T1, #frames |
Apical T1, #frames |
|---|---|---|---|
| #1 | 1250 ± 69/48 | 1256 ± 64/48 | 1298 ± 72/38 |
| #2 | 1237 ± 60/48 | 1263 ± 54/47 | 1291 ± 58/47 |
| #3 | 1270 ± 74/35 | 1287 ± 69/35 | 1332 ± 60/38 |
| #4 | 1277 ± 71/47 | 1295 ± 61/49 | 1298 ± 50/47 |
| #5 | 1215 ± 68/49 | 1209 ± 51/47 | 1256 ± 48/47 |
| #6 | 1227 ± 61/49 | 1227 ± 51/47 | 1253 ± 46/48 |
| Meanb | 1246 ± 24 | 1256 ± 33 | 1288 ± 30 |
| Kawel et al23 | 1286 | ||
| von Knobelsdorff-Brenkenhoff et al24 | 1157 | 1159 | 1181 |
| Lee et al25 | 1315 |
T1 (in milliseconds, mean ± standard deviation in a region of interest covering most of the septal wall) for single-shot inversion recovery-FLASH at 43 ms resolution (19 spokes) and 3 s duration. #Frames refers to the number of images retained after deletion of systolic frames.
Mean ± standard deviation across subjects.
Table 4.
Regional myocardial T1 relaxation times
| Subject | Anterior T1, ms |
Septal T1, ms |
Inferior T1, ms |
Lateral T1, ms |
|---|---|---|---|---|
| #1 | 1295 ± 70 | 1261 ± 70 | 1223 ± 95 | 1218 ± 70 |
| #2 | 1191 ± 60 | 1259 ± 56 | 1245 ± 91 | 1157 ± 64 |
| #3 | 1212 ± 62 | 1291 ± 68 | 1270 ± 89 | 1238 ± 69 |
| #4 | 1224 ± 64 | 1304 ± 64 | 1230 ± 92 | 1217 ± 68 |
| #5 | 1169 ± 56 | 1206 ± 59 | 1155 ± 79 | 1166 ± 57 |
| #6 | 1173 ± 56 | 1234 ± 58 | 1171 ± 66 | 1156 ± 69 |
| Meana | 1211 ± 47 | 1259 ± 36 | 1216 ± 44 | 1192 ± 36 |
T1 (in milliseconds, mean ± standard deviation per standardized region of interest in a mid-ventricular section) for single-shot inversion recovery-FLASH at 43-ms resolution (19 spokes) and 3-s duration.
Mean ± standard deviation across subjects.
DISCUSSION
This work describes a novel method for myocardial T1 mapping which offers accuracy, high spatial resolution, practical robustness and speed. The results indicate that myocardial T1 mapping by IR-FLASH may be performed at a nominal resolution of 1.0 mm, a temporal resolution of 43 ms per frame and within a measuring time of only 3 s.
T1 accuracy was confirmed in a phantom study providing reference T1 values for a long-TR IR-FSE sequence. A slight underestimation of T1 relaxation times for two tubes with values of about 500 and 675 ms coincides with the occurrence of the shortest T2 relaxation times of 46 and 81 ms, respectively (compare Table 1). The slight deviation of T1 values may therefore be due to a partial failure of the “reference” IR-FSE acquisition which extends to a maximum TE of 72 ms and thus may affect the image of compounds and tissues with short T2 relaxation times. Similar effects are to be expected for IR methods with a SSFP readout module, because such sequences require relatively long T2 relaxation times to build up sufficiently strong transverse coherences.
Apart from T1 accuracy, the results in Table 1 confirm the independence of T1 quantitation on the heart rate up to 100 bpm which effectively refers to the independence of T1 on the number of fitted images after elimination of “systolic” frames. This advantageous behaviour reflects the fact that the highly undersampled radial FLASH readout ensures a sufficiently large number of frames for a proper sampling of the IR signal time course. T1 precision was also demonstrated to be high both in vitro and in vivo. It is characterized by small standard deviation values of 3–5% of the mean for phantom measurements and 4–8% for septal T1 values (compare Tables 1–4). Moreover, the achieved T1 mapping quality not necessarily depends on the use of filtering as shown by the comparison in Figure 4. Nevertheless, although high-quality T1 maps may be obtained by fitting unfiltered images, the use of a new modified non-local means filter18 further improves the SNR of T1 maps without the expense of blurring.
Although myocardial T1 relaxation times found here were in general agreement with literature values, comparisons to previous results are compromised by numerous technical differences or even inadequacies. As an example, the present values are in the range of those reported in the study by Kawel et al23 but slightly higher than in that by von Knobelsdorff-Brenkenhoff et al24 and lower than in the study by Lee et al,25 who all used similar MOLLI sequences. Of course, all techniques including the one proposed here suffer from some general limitations of the Lock-Locker approach which often are due to the presence of residual motion both in plane and through plane. For example, diastolic circulation of blood within the ventricles leads to image intensities which violate the expected IR signal model and preclude a reliable fitting of blood T1 relaxation times. Even myocardial movements may play a role during early diastolic phases. However, this mainly becomes a problem for motion-sensitive readouts such as SSFP sequences, whereas short-TE FLASH sequences as used here and recently proposed by others26,27 are much less affected. This is because SSFP sequences inherently rely on the establishment of phase coherence over multiple repetition intervals which is commonly precluded (i.e. spoiled) in the presence of motion, whereas FLASH sequences interrogate a pool of longitudinal magnetization with independent low-flip angle excitations that give rise to spin-density weighted images for the low flip angles used for T1 mapping. In fact, when exploiting the additional motion robustness of radial encodings in the present implementation, preliminary trials of myocardial T1 mapping during free breathing showed little if any qualitative and quantitative difference to breath-hold scans (data not shown). Thus, the proposed method seems to be robust enough to even work in patients who are unable to perform any breathing protocol.
Another factor contributing to myocardial T1 values is the different access to high spatial resolution and the concomitant consideration of partial volume effects. Such problems have been reported for thin myocardial walls7 including the assessment of fibrosis in the peri-infarct zone as well as for the right ventricle.28 These T1 mapping studies using MOLLI techniques were performed at 1.4 × 1.9 × 8.0 mm3 for low heart rates and 1.9 × 2.3 × 8.0 mm3 at high heart rates.7 A higher resolution of 1.2 × 1.2 × 4 mm2 was only achieved with the use of a segmented readout module after inversion which therefore required repetitive acquisitions and very long measuring times of 2.5 min per T1 map.28 Another recent work using IR-FLASH employed a sliding-window reconstruction27 at 1.17 × 1.17 × 8 mm3 resolution, which was achieved by twofold zero-padding, i.e. an interpolation of the acquired resolution. To the best of our knowledge, the method proposed here is the first technique for myocardial T1 mapping which offers 1.0 × 1.0 × 6.0 mm3 resolution within a measuring time of only 3 s.
The most important limitation of this study is the small sample size. This is because the work represents a new technical development which requires basic validation with use of a T1 reference phantom and a group of normal subjects. Obviously, this precedes any evaluation of the clinical utility of the proposed T1 mapping in a large cohort of patients. Moreover, at this stage, widespread clinical applications are hampered by the fact that the technical solution requires dedicated software and hardware which so far is only available for MRI systems of the same manufacturer as used here. A remaining temporary restriction is the computational time needed for image reconstruction and T1 fitting which currently takes about 13 s per T1 map. Nevertheless, this may not necessarily block the clinical workflow, because a delayed reconstruction does not interfere with continuous acquisitions.
CONCLUSION
Myocardial T1 mapping based on single-shot IR-FLASH with radial undersampling and iterative reconstruction as well as T1 fitting with automated deletion of systolic frames meets most current clinical challenges. The proposed method warrants extensive clinical trials as it promises significant advantages for CMR studies which rely on native or post-contrast T1 quantitation.
FUNDING
AAJ is supported by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research).
Contributor Information
Xiaoqing Wang, Email: xwang1@gwdg.de.
Arun A Joseph, Email: ajoseph@gwdg.de.
Oleksandr Kalentev, Email: okalent@gwdg.de.
Klaus-Dietmar Merboldt, Email: kmerbol@gwdg.de.
Dirk Voit, Email: dvoit@gwdg.de.
Volkert B Roeloffs, Email: vroelof@gwdg.de.
Maaike van Zalk, Email: mvanzal@gwdg.de.
Jens Frahm, Email: jfrahm@gwdg.de.
REFERENCES
- 1.Haase A. Snapshot FLASH MRI. Applications to T1, T2, and chemical-shift imaging. Magn Reson Med 1990; 13: 77–89. doi: https://doi.org/10.1002/mrm.1910130109 [DOI] [PubMed] [Google Scholar]
- 2.Deichmann R, Haase A. Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson 1992; 96: 608–12. [Google Scholar]
- 3.Ordidge RJ, Gibbs P, Chapman B, Stehling MK, Mansfield P. High-speed multislice T1 mapping using inversion-recovery echo-planar imaging. Magn Reson Med 1990; 16: 238–45. doi: https://doi.org/10.1002/mrm.1910160205 [DOI] [PubMed] [Google Scholar]
- 4.Scheffler K, Hennig J. T1 quantification with inversion recovery TrueFISP. Magn Reson Med 2001; 45: 720–3. doi: https://doi.org/10.1002/mrm.1097 [DOI] [PubMed] [Google Scholar]
- 5.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 1970; 41: 250–1. doi: https://doi.org/10.1063/1.1684482 [Google Scholar]
- 6.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified look-locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004; 52: 141–6. doi: https://doi.org/10.1002/mrm.20110 [DOI] [PubMed] [Google Scholar]
- 7.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014; 16: 2. doi: https://doi.org/10.1186/1532-429X-16-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013; 15: 92. doi: https://doi.org/10.1186/1532-429X-15-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uecker M, Zhang S, Voit D, Karaus A, Merboldt KD, Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed 2010; 23: 986–94. doi: https://doi.org/10.1002/nbm.1585 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Roeloffs V, Merboldt KD, Voit D, Schätz S, Frahm J. Single-shot multi-slice T1 mapping at high spatial resolution—inversion-recovery FLASH with radial undersampling and iterative reconstruction. Open Med Imaging J 2015; 9: 1–8. doi: https://doi.org/10.2174/1874347101509010001 [Google Scholar]
- 11.Hofer S, Wang X, Roeloffs V, Frahm J. Single-shot T1 mapping of the corpus callosum: a rapid characterization of fiber bundle anatomy. Front Neuroanat 2015; 9: 57. doi: https://doi.org/10.3389/fnana.2015.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Uecker M, Voit D, Merboldt KD, Frahm J. Real-time cardiovascular magnetic resonance at high temporal resolution: radial FLASH with nonlinear inverse reconstruction. J Cardiovasc Magn Reson 2010; 12: 39. doi: https://doi.org/10.1186/1532-429X-12-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voit D, Zhang S, Unterberg-Buchwald C, Sohns JM, Lotz J, Frahm J. Real-time cardiovascular magnetic resonance at 1.5 T using balanced SSFP and 40 ms resolution. J Cardiovasc Magn Reson 2013; 15: 79. doi: https://doi.org/10.1186/1532-429X-15-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Untenberger M, Tan Z, Voit D, Joseph AA, Roeloffs V, Merboldt KD, et al. Advances in real-time phase-contrast flow MRI using asymmetric radial gradient echoes. Magn Reson Med 2016; 75: 1901–8. doi: https://doi.org/10.1002/mrm.25696 [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Joseph AA, Voit D, Schaetz S, Merboldt KD, Unterberg-Buchwald C, et al. Real-time magnetic resonance imaging of cardiac function and flow-recent progress. Quant Imaging Med Surg 2014; 4: 313–29. doi: https://doi.org/10.3978/j.issn.2223-4292.2014.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeloffs V, Voit D, Frahm J. Spoiling without additional gradients: radial FLASH MRI with randomized radiofrequency phases. Magn Reson Med 2016; 75: 2094–9. doi: https://doi.org/10.1002/mrm.25809 [DOI] [PubMed] [Google Scholar]
- 17.Frahm J, Schätz S, Untenberger M, Zhang S, Voit D, Merboldt KD, et al. On the temporal fidelity of nonlinear inverse reconstructions for real-time MRI—the motion challenge. Open Med Imaging J 2014; 8: 1–7. doi: https://doi.org/10.2174/1874347101408010001 [Google Scholar]
- 18.Klosowski J, Frahm J. Image denoising for real-time MRI. Magn Reson Med 2016; doi: https://doi.org/10.1002/mrm.26205 [DOI] [PubMed] [Google Scholar]
- 19.Nocedal J, Wright SJ. Numerical Optimization. In: Mikosch TV, Sidney S, Resnick I, Robinson SM, eds. Springer Series in Operations Research. New York, NY: Springer Science+Business Media; 2006. pp. 66–98. [Google Scholar]
- 20.King D. Dlib-ml: A Machine Learning Toolkit. J Mach Learn Res 2009; 10: 1755–1758. [Google Scholar]
- 21.Deichmann R. Fast high-resolution T1 mapping of the human brain. Magn Reson Med 2005; 54: 20–7. doi: https://doi.org/10.1002/mrm.20552 [DOI] [PubMed] [Google Scholar]
- 22.Sumpf T, Unterberger M. arrayShow: a guide to an open source matlab tool for complex MRI data analysis. In: ISMRM; 2013. p. 2719. [Google Scholar]
- 23.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson 2012; 14: 27. doi: https://doi.org/10.1186/1532-429X-14-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, et al. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson 2013; 15: 53. doi: https://doi.org/10.1186/1532-429X-15-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, et al. Myocardial T1 and extracellular volume fraction mapping at 3 Tesla. J Cardiovasc Magn Reson 2011; 13: 75. doi: https://doi.org/10.1186/1532-429X-13-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao J, Rapacchi S, Nguyen KL, Hu P. Myocardial T1 mapping at 3.0 Tesla using an inversion recovery spoiled gradient echo readout and Bloch equation simulation with slice profile correction (BLESSPC) T1 estimation algorithm. J Magn Reson Imaging 2016; 43: 414–25. doi: https://doi.org/10.1002/jmri.24999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gensler D, Mörchel P, Fidler F, Ritter O, Quick HH, Ladd ME, et al. Myocardial T1: quantification by using an ECG-triggered radial single-shot inversion-recovery MR imaging sequence. Radiology 2015; 274: 879–87. doi: https://doi.org/10.1148/radiol.14131295 [DOI] [PubMed] [Google Scholar]
- 28.Mehta BB, Chen X, Bilchick KC, Salerno M, Epstein FH. Accelerated and navigator-gated look-locker imaging for cardiac T1 estimation (ANGIE): development and application to T1 mapping of the right ventricle. Magn Reson Med 2015; 73: 150–60. doi: https://doi.org/10.1002/mrm.25100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumpf TJ, Petrovic A, Uecker M, Knoll F, Frahm J. Fast T2 mapping with improved accuracy using undersampled spin-echo MRI and model-based reconstructions with a generating function. IEEE Trans Med Imaging 2014; 33: 2213–22. doi: https://doi.org/10.1109/TMI.2014.2333370 [DOI] [PMC free article] [PubMed] [Google Scholar]