Abstract
Image-guided radiotherapy (IGRT) is an essential tool in the accurate delivery of modern radiotherapy techniques. Prostate radiotherapy positioned using skin marks or bony anatomy may be adequate for delivering a relatively homogeneous whole-pelvic radiotherapy dose, but these surrogates are not reliable when using reduced margins, dose escalation or hypofractionated stereotactic radiotherapy. Fiducial markers (FMs) for prostate IGRT have been in use since the 1990s. They require surgical implantation and provide a surrogate for the position of the prostate gland. A variety of FMs are available and they can be used in a number of ways. This review aimed to establish the evidence for using prostate FMs in terms of feasibility, implantation procedures, types of FMs used, FM migration, imaging modalities used and the clinical impact of FMs. A search strategy was defined and a literature search was carried out in Medline. Inclusion and exclusion criteria were applied, which resulted in 50 articles being included in this review. The evidence demonstrates that FMs provide a more accurate surrogate for the position of the prostate than either external skin marks or bony anatomy. A combination of FM alignment and soft-tissue analysis is currently the most effective and widely available approach to ensuring accuracy in prostate IGRT. FM implantation is safe and well tolerated. FM migration is possible but minimal. Standardization of all techniques and procedures in relation to the use of prostate FMs is required. Finally, a clinical trial investigating a non-surgical alternative to prostate FMs is introduced.
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed cancer in males and is often treated with radical external beam radiotherapy. Advances in linear accelerator technology and treatment planning software have made it possible to deliver highly conformal radiation dose distributions using techniques such as intensity-modulated radiotherapy (IMRT). Dose escalation studies have demonstrated improved outcomes for patients with PCa increasingly using hypofractionated stereotactic radiotherapy to exploit the sensitivity of PCa to large doses per fraction.1–4 Image-guided radiotherapy (IGRT) is considered an essential tool in ensuring the safe clinical application of these techniques.
Prostate radiotherapy positioned with reference skin marks and/or aligned to bony anatomy is dependent on stability and reproducibility. This may be adequate for delivering a relatively homogeneous dose to large pelvic fields. However, it is not a reliable approach for IMRT, volumetric arc therapy, dose-escalated or hypofractionated stereotactic treatment. Collectively, these advanced techniques require a greater degree of precision, given the decreased margin for error and the potential for radiation injury from overdosing critical structures or treatment failure due to underdosing the target. With the exception of MRI-guided treatment, technological advances which enable complex planning and delivery have surpassed that which aims to verify target position or dose delivery in patients. Two-dimensional (2D) megavoltage (MV) or kilovoltage (kV) prostate IGRT is reliant on pelvic bone alignment and while cone-beam CT (CBCT) enables three-dimensional (3D) imaging, prostate gland visualization remains challenging owing to inadequate soft-tissue contrast. Internal organ motion adds to the challenge of accurately targeting the prostate and is a limiting factor in margin reduction and dose escalation.
Fiducial markers (FMs) surgically implanted within the prostate gland prior to radiotherapy planning have been developed to improve contrast and therefore treatment setup and prostate targeting. Where employed in clinical practice, there are a variety of approaches in relation to how prostate FMs are utilized. This article aimed to review the evidence for the use of FMs in prostate IGRT in relation to feasibility and efficacy, implantation, number and type of FMs, migration, imaging modalities used and clinical impact. Furthermore, it outlines current evidence on a natural alternative to FMs and introduces a recently opened clinical trial investigating this.
METHODS AND MATERIALS
A literature search was carried out in Medline using the search strategy outlined in Figure 1.
Figure 1.
Medline search strategy.
Results of the initial literature search were subject to the following selection criteria:
Inclusion criteria: studies on humans, specifically investigating the technical aspects or clinical efficacy of FMs for prostate IGRT; studies of prostate motion based on FMs
Exclusion criteria: review articles, studies of <20 patients (where treatment setup data are reported), phantom studies, post-prostatectomy treatments.
Limiting articles to those reporting setup data on at least 20 patients resulted in a significant number of articles being automatically excluded from this review. This is justified on the basis that published guidance states that at least 20 patients and preferably 25 patients should be included in such studies to be representative of a given patient population and technique.5 A summary of the studies included in the review is presented in Table 1.
Table 1.
Summary of studies included in review
| Focus | Title | Authors | Year of publication | Study type | Number of patients/subjects | Main conclusions |
|---|---|---|---|---|---|---|
| Feasibility | Prostate motion during standard radiotherapy as assessed by FMs | Crook et al6 | 1995 | Retrospective cohort | 55 | Significant prostate motion during radiotherapy. Recommend using markers and EPIDs to verify position of target |
| Technical aspects of daily online positioning of the prostate for 3D conformal radiotherapy using an EPI device | Herman et al7 | 2003 | Prospective feasibility | 20 | EPID and intraprostatic markers can be used to precisely localize and correct variations in target position following setup to external reference marks | |
| Feasibility of insertion/implantation of 2.0-mm-diameter gold internal FMs for precise setup and real-time tumour tracking in radiotherapy | Shirato et al8 | 2003 | Prospective feasibility | 93 (31 prostate) | Internal FMs can be safely inserted into various organs. Three-marker method has been shown to be useful for spinal/paraspinal and prostate setup | |
| Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and EPI | Schallenkamp et al9 | 2005 | Prospective feasibility | 20 | Independent prostate motion is significant. FMs within the prostate are stable and facilitate margin reduction | |
| A comparison of the use of bony anatomy and internal markers for offline verification and an evaluation of the potential benefit of online and offline verification protocols for prostate radiotherapy | McNair et al10 | 2008 | Retrospective analysis | 30 | FMs and an offline imaging protocol are effective in reducing systematic errors | |
| Utilization of CBCT for reconstruction of dose distribution delivered in IGRT of prostate carcinoma—bony landmark setup compared with FM setup | Paluska et al11 | 2013 | Retrospective analysis | 59 | PTV margin reduction is feasible using FMs for image guidance | |
| Analysis of FM-based position verification in EBRT of patients with PCa | Van der Heide et al12 | 2007 | Retrospective analysis | 453 | FMs are stable. Identified time trends in prostate motion | |
| Prostate motion | Intrafraction motion of the prostate during EBRT: analysis of 427 patients with implanted FMs | Kotte et al13 | 2007 | Retrospective analysis | 427 | Frequent prostate motion observed during EBRT, which can be encompassed with a 2-mm margin |
| IMRT using implanted FMs with daily portal imaging: assessment of prostate organ motion | Chen et al14 | 2007 | Retrospective analysis | 33 | Prostate motion is significant. Daily imaging with FMs is necessary for the reduction of margins | |
| Intrafraction prostate displacement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted FMs | Kron et al15 | 2010 | Retrospective analysis | 184 | Prostate motion is a limiting factor when considering margins for radiotherapy | |
| Intrafraction motion during extreme hypofractionated radiotherapy of the prostate using pre- and post-treatment imaging | Quon et al16 | 2012 | Phase I/II trial | 53 | Prostate displacements during hypofractionated radiotherapy are comparable with intrafraction conventionally fractionated treatments | |
| Intrafractional motion of the prostate during hypofractionated radiotherapy | Xie et al17 | 2008 | Retrospective analysis | 21 | With monitoring and intervention prostate motion within the range of the Cyberknife tracking range; however, there is significant variation between patients | |
| Deformation of prostate and SVs relative to intraprostatic FMs | Van der Weilen et al18 | 2008 | Prospective clinical study | 21 | With respect to FMs, prostate deformation is small, SV deformation considerable | |
| An MRI study of prostate deformation relative to implanted gold FMs | Nichol et al21 | 2007 | Prospective clinical study | 25 | During radiotherapy, FMs in-migrated and prostate volume decreased. Patients undergoing TURP demonstrated greater deformation than those not undergoing TURP | |
| Hybrid registration of prostate and SVs for IGRT | De Boer et al19 | 2013 | Retrospective analysis | 20 | Substantial differences observed between SV and prostate orientations | |
| Margin evaluation in the presence of deformation, rotation and translation in prostate and entire SV irradiation with daily marker-based setup corrections | Mutanga et al20 | 2011 | Retrospective study | 21 | PTV margins based on FM, prostate 5 mm and >8 mm for SVs. Correction of rotational errors of little benefit | |
| Implantation | Technique for implantation of FMs in the prostate | Shinohara and Roach22 | 2008 | Retrospective study | 705 | TRUS-guided FM implantation is well tolerated. Experience provides a guide for clinicians |
| Technique of outpatient placement of intraprostatic FMs before EBRT | Linden et al23 | 2009 | Retrospective study | 98 | TRUS-guided FM implantation is safe and efficacious | |
| Implantation of FMs for image guidance in prostate radiotherapy: patient-reported toxicity | Igdem et al24 | 2009 | Prospective clinical study | 177 | TRUS-guided FM implantation is safe and well tolerated | |
| Patient-reported complications from FM implantation for prostate IGRT | Gill et al26 | 2012 | Retrospective study (questionnaires) | 234 | TRUS-guided FM insertion for IGRT is well tolerated in the majority of patients with PCa | |
| Is periprostatic nerve block a gold standard in case of TRUS-guided prostate biopsy? | Kumar et al27 | 2013 | Prospective randomized double-blinded placebo-controlled study | 150 | Periprostatic nerve block provides better pain control in TRUS-guided prostate biopsy, but still there is need of additional analgesic in the form of tramadol or INB. Tramadol has advantage of oral intake and analgesic effect at time of probe insertion and at nerve block. Both tramadol and INB may be used in combination along with PNB | |
| Ultrasound-guided TR implantation of gold markers for prostate localization during EBRT: complication rate and risk factors | Langenhuijsen et al28 | 2007 | Retrospective analysis | 209 | TR gold marker implantation well tolerated. Moderate complication rate influenced by disease stage, ADT and age | |
| Single-centre experience in prostate fiducial placement: technique and mid-term follow-up | Kably et al30 | 2014 | Retrospective analysis | 75 | TR ultrasound guidance of FMs is feasible, well tolerated and safe | |
| Long-term experience with TR and TP implantations of gold FMs in the prostate for position verification in EBRT; feasibility, toxicity and quality of life | Moman et al33 | 2010 | Retrospective analysis | 914 | Clinical use of TP-implanted gold FMs for position verification in EBRT for PCa is a feasible and safe procedure without influencing patient quality of life | |
| Infections after FM implantation for prostate radiotherapy: are we underestimating the risks? | Loh et al31 | 2015 | Retrospective analysis | 285 | Overall rate of symptomatic infection with FM implantation is higher than other FM series at 7.7%. This is in keeping with prostate biopsy reports of infection | |
| Number and type of FM | Improving positioning in high-dose radiotherapy for PCa: safety and visibility of frequently used gold FMs | Fonteyne et al35 | 2012 | Prospective RCT | 25 | Stability and visibility of five different types of marker was proven. Larger markers facilitate automatic image fusion; however; they generate more scatter than smaller markers |
| Feasibility, detectability and experience with platinum seed internal FMs for CT–MRI fusion and real-time tumour tracking during SABR | Janardanan et al38 | 2012 | Retrospective study | 29 | Platinum seeds provide superior contrast to gold seeds on MR images and a better choice for CT–MRI fusion | |
| Clinical results from first use of prostate stent as FM for radiotherapy of PCa | Carl et al44 | 2011 | Prospective clinical study | 62 | Ni–Ti stents have potential as new prostate FM | |
| Influence of the number of elongated FMs on the localization accuracy of the prostate | De Boer et al42 | 2012 | Retrospective study | 24 | Two elongated markers placed at either side of the prostate can be used to accurately localize the prostate for IGRT | |
| Multi-institutional clinical experience with the Calypso system in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy | Kupelian et al46 | 2007 | Prospective clinical study | 41 | Using three implanted electromagnetic transponders provides clinically efficient, accurate and objective localization of the prostate | |
| Patient positioning based on a radioactive tracer implanted in patients with localized PCa: a performance and safety evaluation | De Kruijf et al48 | 2013 | Prospective single-arm multi-institutional study | 20 | Implantation of the tracer is safe and feasible and patients can be positioned and monitored accurately using RealEye | |
| Migration | (Non)-migration of radiopaque markers used for online localization of the prostate with an EPI device | Pouliot et al29 | 2003 | Retrospective analysis | 10 | None of the markers studied migrated significantly. The use of three markers provides a tool to monitor prostate position and volume changes that can occur over time owing to hormone or radiation therapy |
| Marker seed migration in prostate localization | Poggi et al49 | 2003 | Prospective clinical study | 9 | Negligible seed migration over the course of radiotherapy | |
| Intraprostatic fiducials for localization of the prostate gland: monitoring IMDs during radiation therapy to test for marker stability | Kupelian et al50 | 2005 | Retrospective analysis | 56 | Seed migration within the prostate during a course of radiotherapy is negligible. Prostate deformation rather than true migration results in observed marker position variations | |
| Migration of intraprostatic FMs and its influence on the matching quality in EBRT for PCa | Delouya et al51 | 2010 | Retrospective analysis | 31 | Average daily seed migration is often negligible. Migration >2 mm from planning to first treatment may require adjustment of PTV margin to account for this | |
| Impact of concurrent androgen deprivation on FM migration in EBRT for PCa | Tiberi et al52 | 2012 | Retrospective analysis | 37 | A delay between the start of ADT and the start of EBRT; prostate involution has little or no effect on FM positioning within the gland. <1% of treatments studied demonstrated significant marker migration | |
| Imaging modality used | IGRT for PCa comparing kV imaging of FMs with CBCT | Barney et al56 | 2011 | Retrospective study | 36 | Target verification for CBCT and kV imaging using FMs are similar; however, over 25% of shifts differed enough to affect target coverage |
| EPI vs kV imaging in FM IGRT for PCa: an analysis of setup uncertainties | Gill et al41 | 2012 | Prospective | 333 | Suggests a larger CTV–PTV margin is used in EPI-based IGRT for PCa | |
| Method comparison of ultrasound and kV X-ray FM imaging for prostate radiotherapy targeting | Fuller et al61 | 2006 | Prospective non-randomized study | 40 | Significant differences in ultrasound and FM IG setup data. Data between ultrasound and FM imaging not interchangeable | |
| Study of ExacTrac X-ray 6D IGRT setup uncertainty for marker-based prostate IMRT treatment | Shi et al60 | 2012 | Retrospective study | 43 | Overall interfraction mean error of 2 mm or less for 3D translations and 0.25° rotation, facilitating margin reduction | |
| Comparison of daily MV EPI or kV imaging with marker seeds with ultrasound imaging or skin marks for prostate localization and treatment positioning in patients with PCa | Serago et al43 | 2006 | Retrospective study | 35 | MV and kV EPI similar in terms of accuracy and superior to ultrasound | |
| Comparison of ultrasound and implanted seed marker prostate localization methods: implications for IGRT | Scarbrough et al62 | 2006 | Retrospective analysis | 40 | Ultrasound and FM setup data differ significantly, resulting in different PTV margins. More variation is seen in ultrasound data | |
| Comparison of TAUS and electromagnetic transponders for prostate localization | Foster et al63 | 2010 | Retrospective analysis | 41 | Ultrasound and electromagnetic transponder setup data differ significantly. Ultrasound-derived PTV margins 3–4 times smaller than Calypso data | |
| Clinical impact | Individualized PTVs for intrafraction motion during hypofractionated IMRT boost for PCa | Cheung et al64 | 2005 | Prospective clinical study | 33 | Acute toxicity using IMRT for hypofractionated boost was acceptable. Grade 3 urinary toxicity may be increased compared with standard fractionation |
| PTV margins for prostate radiotherapy using daily EPI and implanted FMs | Skarsgard et al65 | 2010 | Prospective Phase I/II study | 46 | Daily image guidance with FMs allows significant reduction of PTV margin, facilitating dose escalation which may improve outcomes for patients with PCa | |
| Does IGRT improve toxicity profile in whole pelvic-treated high-risk PCa? | Chung et al68 | 2009 | Prospective study | 25 | Rectal and bladder toxicity consistently lower for FM-guided radiotherapy group than that for non-FM-guided radiotherapy. This is likely attributable to reduced PTV margins in the FM-guided group | |
| Treatment-related morbidity in PCa: a comparison of 3D conformal radiation therapy with and without image guidance using implanted FMs | Singh et al69 | 2013 | Retrospective study | 282 | A significant reduction in bowel dysfunctional symptoms reported by the IGRT group vs the non-IGRT group | |
| Treatment outcome of high-dose IG-IMRT using intraprostate FMs for localized PCa at a single institute in Japan | Takeda et al67 | 2012 | Retrospective study | 141 | High-dose FM-guided IMRT well tolerated | |
| Late toxicity and biochemical control in 554 patients with PCa treated with and without dose-escalated IGRT | Kok et al71 | 2013 | Retrospective study | 186 | FM-guided IGRT associated with a reduction in late urinary toxicity and improved biochemical tumour control. Further studies required | |
| Improvement in toxicity in patients at high risk of PCa treated with IG-IMRT compared with 3D conformal radiotherapy without daily image guidance | Sveistrup et al72 | 2014 | Retrospective study | 311 | FM-IGRT can be an effective method of reducing GI and GU toxicity when treating PCa |
3D, three-dimensional; ADT, androgen deprivation therapy; CBCT, cone-beam CT; CTV, clinical target volume; EBRT, external beam radiotherapy; EPI, electronic portal imaging; EPID, electronic portal imaging device; FM, fiducial marker; GI, gastrointestinal; GU, genitourinary; IG, image-guided; IG-IMRT, image-guided intensity-modulated radiotherapy; IGRT, image-guided radiotherapy; IMD, intermarker distance; IMRT, intensity-modulated radiotherapy; INB, intraprostatic nerve block; kV, kilovoltage; MV, megavoltage; PCa, prostate cancer; PNB, periprostatic nerve block; PTV, planning target volume; RCT, randomized controlled trial; SABR, stereotactic ablative radiotherapy; SV, seminal vesicle; TAUS, transabdominal ultrasound; TP, transperineal; TR, transrectal; TRUS, transrectal ultrasound; TURP, transurethral resection of the prostate.
FIDUCIAL MARKERS FOR PROSTATE IMAGE-GUIDED RADIOTHERAPY
Feasibility and efficacy
The feasibility and efficacy of using FMs for prostate IGRT has been documented in the literature.6–11
Before in-room imaging was widely available, Crook et al6 demonstrated significant prostate gland motion based on FM positions relative to bony landmarks on repeat CT scans in a study of 55 patients. They found the average prostate displacement in the posterior and inferior directions to be 6 mm and that 30% had displacements >10 mm posteriorly, which standard treatment planning margins would not have encompassed. They strongly recommended the use of implanted FMs for treatment setup verification as opposed to alignment using bony landmarks.
Crook et al findings6 from 1995 were supported by Schallenkamp et al9 in 2005 using MV electronic portal imaging (MV EPI) and digitally reconstructed radiographs, and then reconfirmed in 2013 by Paluska et al11 using modern planning and imaging techniques. Paluska et al acquired weekly CBCT scans following setup to retrospectively reconstruct and verify dose delivered to patients receiving IMRT to the prostate with a simultaneous integrated boost to the proximal seminal vesicles (SVs). For bone setup using a 10-mm clinical target volume (CTV)–planning target volume (PTV) margin, inadequate prostate coverage was observed in 5 out of 29 patients, resulting in underdosing of the target. Despite a reduced CTV–PTV margin, the prostate coverage for FM-based setup was better. Schallenkamp et al and Paluska et al both proposed that prostate FMs, when used with a daily online imaging correction protocol, facilitate CTV–PTV margin reduction.
The largest study on the reliability of FMs was reported in a retrospective analysis of daily treatment setup data from 453 patients treated with IMRT using an offline correction protocol by van der Heide et al.12 They reported minimal marker migration, accurate marker detection and time trends in relation to prostate motion but recommended caution when considering reducing planning margins owing to other sources of uncertainty such as target delineation and SV motion.
Much of the evidence in relation to prostate motion is based on setup data acquired using FM position measured using a range of imaging modalities. Nine studies investigating prostate motion and otherwise satisfying the search criteria were included.
In a study of 427 patients undergoing prostate IMRT treated using FM IGRT, Kotte et al13 observed intrafraction motion >2 mm in a time frame of 5–7 min in 66% of patients. No correlation was found between fraction number and degree of motion. Motion was more frequent in the anteroposterior (AP) and superior–inferior directions. They recommended the use of FMs for prostate treatment setup and at least a 2-mm margin to account for intrafraction motion, not including other sources of uncertainty. These findings are supported by data from other studies of prostate motion also based on FMs.14,15
FMs have been employed in assessing the increased precision required for stereotactic ablative radiotherapy. Quon et al,16 using pre- and post-treatment 2D kV imaging of prostate FMs, concluded that intrafraction motion is small and that the degree of motion observed indicates that the time taken for delivery of step-and-shoot IMRT did not significantly increase prostate motion. However, it is possible that transient prostate displacements may have gone undetected. This idea is supported by Xie et al,17 who described another approach to monitoring FM position using stereoscopic (SC) X-ray imaging during treatment and pausing treatment if outside a predefined threshold. They found significant motion with an image sampling rate of every 40 s and suggest a higher sampling rate may be appropriate for some patients. These findings would indicate that prostate motion is more significant than observed by Quon et al16 and also that pre- and post-treatment images may not be adequate to assess intrafraction motion.
FMs combined with online correction can minimize the uncertainty associated with aligning the prostate for IGRT and act as an aid to assessing the impact of prostate intrafraction motion. However, the evidence suggests that the method used to assess motion using FMs can produce varying results and therefore, changes to dose, planning margins and correction protocols should allow for this.
Online correction based on prostate FM position will not necessarily ensure coverage of other critical structures, for example SVs, if included in the PTV. Van der Wielen et al18 used FMs to investigate prostate and SV deformation. In their study of 21 patients, analyzing prostate contours relative to FM position on repeat CT scans, they found prostate deformation to be small [standard deviation (SD) ≤ 1 mm] and SV deformation, relative to FMs, significant (SD ≤ 3 mm). De Boer et al19 also report significant differences in the position of the prostate and SVs and describe a method in which SVs are included to ensure registration of both based on CBCT imaging. FM corrections are generally translational corrections only. Despite the differences reported in prostate and SV deformation, the addition of a rotational correction has been shown to allow only a modest reduction in planning margins.18,20
Nichol et al21 used repeat MRI scans to assess prostate deformation relative to FMs in 25 patients and suggest that FMs are a good surrogate in the left–right direction but not as good in the AP or craniocaudal directions. They identified isolated cases of large changes in prostate volume and shape and suggest that increases in prostate volume may result in a geographic miss if using reduced margins.
The evidence spanning almost 20 years would indicate that alignment to FMs is feasible and more effective at localizing the prostate for radiotherapy than alignment to external skin marks or bony anatomy on planar imaging.
FIDUCIAL MARKER IMPLANTATION
Implantation of FMs into the prostate involves a surgical procedure which carries associated risks including pain, bleeding, inflammation and infection, but on balance, it is safe and well tolerated.
There are two main approaches: transrectal (TR) or transperineal (TP), both usually under TR ultrasound (TRUS) guidance. A TR technique is proposed by Shinohara and Roach22 as suitable for insertion of gold markers and electromagnetic transponders. The technique, developed over 10 years with 705 patients, is similar to a TRUS-guided biopsy procedure. They reported one case of urinary tract infection and no cases of bleeding or haematuria. Linden et al23 also report no complications using a similar technique in a retrospective review of 98 males.
Both studies describe preparation including prophylactic antibiotics, rectal enemas, cessation of anticoagulant/antiplatelet medication, local anaesthesia and preparation of markers and both employed a triangular arrangement of markers, i.e. right base, left mid-gland or base and right apex. Shinohara and Roach highlight the importance of avoiding of the urethra to ensure markers are not subsequently lost to voiding. They also refer to “tenting” of the prostate by the needle, which on TRUS may give the appearance of being in the prostate when in fact the needle has not penetrated the capsule. This, as well as placing within the urethra, may explain the “loss” of some markers reported by other investigators.6,24
Igdem et al24 prospectively quantified patient-reported morbidity with 135 out of 177 patients completing a questionnaire relating to side effects from TRUS-guided TR implantation of 3 gold FMs. No anaesthesia was used. 5 patients reported rectal bleeding and haematuria was experienced by 20 patients, although none required additional medication or intervention. Three patients experienced urinary infection requiring additional antibiotics. Using the Wong Baker pain scale,25 36% of patients indicated 2 (mild) on a 0–5 scale and the mean pain score was 1.7. Gill et al26 reported on 234 patients undergoing the same TRUS TR procedure with the addition of a periprostatic nerve block, which is considered the gold standard for TRUS-guided prostate biopsy.27 Of those patients who gave a pain score, only 21.9% of patients in total scored pain at 2 or higher and 75% patients scored pain as 1 or 0 (very mild or no pain), and the mean pain score was lower than that observed by Igdem et al at 1.1.
Langenhuijsen et al28 reported on 209 patients who underwent TRUS TR implantation of 4 gold markers without a preceding enema or local anaesthesia. Using a different pain scale, 50% of patients thought pain was less than that of biopsy, 40% patients thought it was comparable and 10% patients thought it was worse.
Many authors report low or moderate rates of complications following FM implantation and conclude that TRUS-guided implantation of FMs is safe and well tolerated in the majority of patients.23,24,28–30 One might expect a higher proportion of complication rates with such an invasive procedure. In general, these studies are based on retrospective voluntary patient reporting and therefore, less serious side effects may have been underreported. Another study by Loh et al31 suggests that the risk from FM implantation is underestimated. They report an infective complication rate of 5.6% minimum from 297 patients who underwent TR FM implantation, which is similar to that reported for prostate biopsy complication rates.32
Moman et al33 compared TR and TP routes of implantation in 402 and 512 patients, respectively. Toxicity and subsequent marker migration were similar. They have since continued to use the TP route and have omitted the use of antibiotic therapy since reporting their findings and report no infections several months following. It would be reasonable to suppose that TR implantation would result in a higher incidence of infection, particularly when no rectal evacuation is employed. However, the evidence does not seem to suggest this. This may be due to the use of prophylactic antibiotic therapy. A significant saving and benefit to the patient could be realized if TP implanting removed the need for antibiotics, as suggested by Moman et al.33 Indeed, the benefits of reducing the need for antibiotics may be more far reaching. Recent studies have demonstrated increasing rates of antibiotic-resistant infection following TR intraprostatic procedures, suggesting TP techniques may become more frequent in the future.32,34
Assuming the use of antibiotics is standard, there is no evidence to recommend one technique over the other. Based on the evidence presented, both approaches are safe and well tolerated. In clinical practice, the choice may be pragmatic and predetermined by the facilities and expertise available. Those with prostate brachytherapy expertise and capacity may choose the TP route, while those employing the skills of a biopsy clinic may rely on the TR route. Factors common to both should be standardized for the purpose of FM implantation, such as use of enemas, anaesthesia, patient follow-up, placement of markers and timing of implant prior to CT planning.
NUMBER AND TYPES OF FIDUCIAL MARKERS
FMs exist in a variety of shapes, sizes and materials and are commercially available from a variety of vendors. Gold markers are by far most frequently used for prostate IGRT. This is due to the fact that they are widely available and are visible using MV or kV image guidance. The basic specification for an FM includes radio-opacity for a given imaging modality (or preferably a number of imaging modalities), that they are readily available and that they are not prohibitively expensive.
This review elicited only one study specifically comparing types of markers. Fonteyne et al35 investigated five different gold FMs. They observed a better correlation between automatic CBCT image-matching results and manual match results for the largest FM, which was 20 mm × 0.75 mm, than for smaller FMs, suggesting that larger markers may result in more consistent setup data when comparing automatic and manual alignment. However, they also state the largest FM resulted in increased image artefact. Artefact created by FMs on CT and CBCT is one of the main disadvantages of using gold or entirely metallic markers and may add to the degree of uncertainty associated with contouring and matching the prostate36 and may also result in inaccurate treatment planning.37 One study suggests that platinum is superior to gold in terms of its visibility on MRI and therefore preferable as an aid for CT/MRI registration.38 This would have the disadvantage of being more expensive than gold.
Non-metallic FMs have been around for a number of years and claim to overcome the problem of metal-induced image artefacts. Despite this, in the evidence presented in this review, little attention has been given to what effect FMs may have on the dose distribution within the prostate. Dose and effects on dose distribution require modelling and measurement using geometrically appropriate phantoms and techniques and therefore, a full discussion is beyond the scope of this review. However, there is a growing body of evidence on the dosimetric consequences of various types of prostatic FMs. The effect may be minimal, but some reports suggest that using gold FM dose may be reduced by as much as 20% using photons39 and reduced by up to 85% in proton therapy in the area immediately around the FM.40
Many authors have reported studies based on the use of three or four implanted gold markers.6,7,9,12,14,41 Most studies employ three FMs placed in a 3D triangular arrangement to facilitate assessment in the three cardinal directions (Figure 2).
Figure 2.
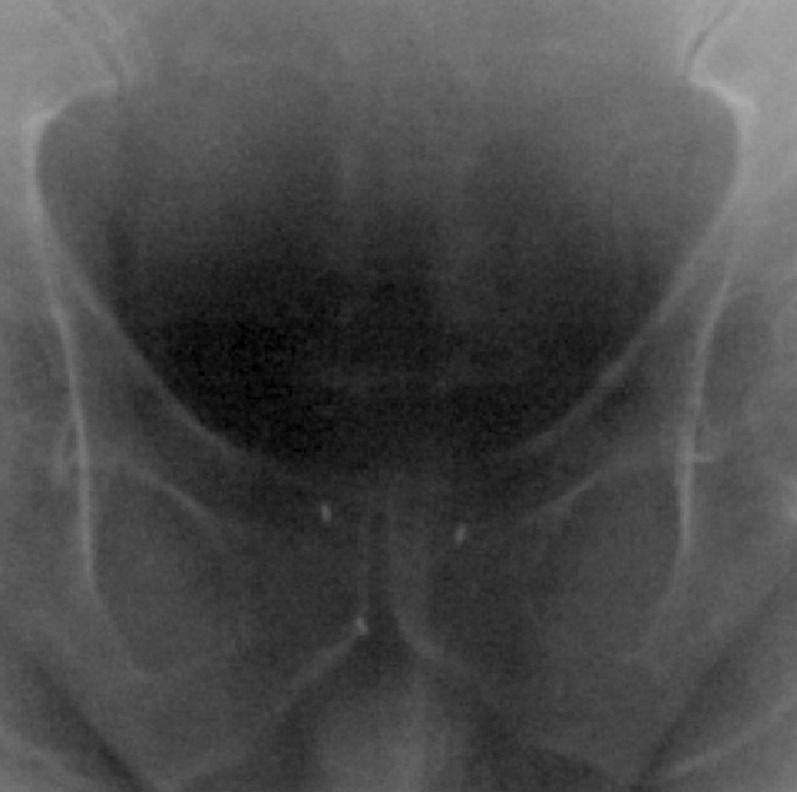
Three gold fiducial markers detected on anteroposterior megavoltage electronic portal imaging.
Using gold FM coils, de Boer et al42 evaluated the impact of the number of markers on matching and concluded that two accurately locate the prostate if adequately spaced. Other investigators favour three or more markers in the event that one or more migrate or are somehow lost.43
Nickel–titanium stents based on those used in the management of urinary obstruction have been adapted and tested for feasibility in prostate IGRT. They are visible on kV and MV images, create no artefact on CT and are suitable for MRI.44 Further evidence is needed to justify the complex surgical procedure and associated risks required to implant these.
OTHER TYPES OF MARKER
Real-time target tracking has been developed and used clinically using electromagnetic transponders.45,46 Radiofrequency-emitting transponders are implanted into the prostate similar to FMs. They are detected and monitored by an external magnetic array linked to the treatment room and located in relation to the isocentre and couch corrections applied. This system addresses intrafraction motion and theoretically eliminates operator subjectivity associated with image analysis. No ionizing radiation is received by the patient; however, this also means there is no radiographic record of treatment and no assessment of bladder, rectum or SV position is possible. Routine use of this technology is expensive.
An alternative type of surrogate first described by Shchory et al47 was clinically evaluated by de Kruijf et al,48 who used radioactive iridium-192 as an implanted tracer in 20 patients. They report this approach as feasible and safe. With the exception of reduced image artefact, no particular advantages are apparent over inert FMs. Given the additional radiation dose received by the patient, and the additional precautionary measures required during implantation, this method is not widely used.
Three gold markers as per Figure 2 are most commonly used. Whatever type and arrangement is used, each institution should be consistent, use standardized procedures and develop expertise in their chosen approach. Non-metallic markers offer an attractive alternative in terms of reduced cost, reduced image artefact and potentially reduced dosimetric consequences and should be further investigated.
PROSTATE FIDUCIAL MARKER MIGRATION
The efficacy of FMs for prostate IGRT is based on the assumption that each marker will remain fixed in position between planning and treatment and for the duration of radiotherapy. True migration of FMs is likely rare and when it does occur, the effect is minimal or negligible. Prostate gland distortion before or during radiotherapy can occur owing to: (1) post-implant oedema or bleeding, (2) changes in surrounding organs at risk (OARs) and (3) shrinkage due to androgen deprivation therapy.
A number of reports focused on this issue29,49–52 and collectively evaluated a combined total of 144 patients with between 3 and 5 gold FMs. Each group recorded intermarker distance (IMD) as a measure of FM migration, with the exception of Delouya et al,51 who compared the 3D position of the FMs from planar imaging with that at planning. Mean migration observed was between 0.8 mm (=/−0.3 mm SD)52 and 1.2 mm (±0.2 mm SD),49 (±0.6 mm SD).51 Pouliot et al29 report a mean SD of 1.3 mm (range 0.44–3.04 mm).
From their study of 453 patients, van der Heide et al12 report minimal changes in IMD, demonstrating that markers are stable in relation to each other and can be detected accurately.
In general, the evidence suggests that FM migration is not significant but raises a number of issues to consider when using FMs.
Kupelian et al50 highlight one such issue when they observed two patients demonstrating IMD variation where the SD was >4 mm (maximum 4.2 mm). In each case, one particular IMD varied owing to variations in rectal filling. The proportion of IMD variation contributed by actual migration in such cases is difficult to establish. Migration may not reliably be measured unless all other surrounding physical conditions remain constant. Such cases, as observed by Kupelian et al,50 demonstrate that if the prostate has changed shape, the inconvenient reality is this cannot be corrected by standard IGRT conventions with or without the use of FMs. Deformable image registration and adaptive planning strategies are required to address this problem, a detailed discussion of which is beyond the scope of this review, but an overview of these topics is given by Maintz and Viergever,53 Kashani et al54 and Kupelian and Meyer.55
Pouliot et al29 measured IMDs of 3 gold implanted markers in 11 patients using orthogonal MV image pairs. They observed a time trend in three patients where the distance between all three markers decreased over the course of radiotherapy, suggesting overall shrinkage of the prostate. Volume reduction was subsequently confirmed on CT for one of the patients. They suggest that all three patients received neoadjuvant hormones, which may have caused prostate shrinkage. However, shrinkage was not observed in all patients who received hormone therapy, indicating there are other influencing factors. Details of the timing of fiducial implantation were not given. Anatomical or physiological changes have the potential to cause or mimic marker migration and therefore, approaches minimizing this should be considered. Oedema and inflammatory responses initiated during implantation may still be present during CT planning. These may then resolve before or during treatment, resulting in volume changes or deformation. This could potentially alter the position of the FMs and introduce systematic positioning error. This is supported by the findings of Delouya et al.51 Of 31 patients, 7% of patients demonstrated “significant shrinkage”. The maximum reduction was 95% of the volume at planning. They observed that of 31 patients included in their study, 23 patients had their CT planning scan the same day as FM implantation and had larger migrations than those whose CT planning was later than the day of implantation. The actual differences in migration are not reported, but this group subsequently changed their local practice to allow a minimum of at least 3 days between implantation and CT planning.
FM migration can occur, but prostate gland deformation is more commonly observed. However, in clinical practice, it is unlikely that migration or deformations are significant limitations to the utilization of FMs for prostate radiotherapy, but standardized approaches to minimize known causes should be incorporated.
IMAGING MODALITIES
Evidence supporting the use of prostate FM IGRT is largely based on the use of 2D imaging (MV or kV). There is significantly less evidence available on the use of FMs with 3D imaging such as CBCT or ultrasound.
In a study of 36 patients, Barney et al56 compared their standard technique of kV imaging of FMs with CBCT soft-tissue alignment to ascertain the degree of correlation. They reported mean and absolute differences in setup data between the two modalities and observed a mean difference of 3 mm in two of three dimensions, which affected target coverage in 25% of all shifts observed. In a larger study, Gill et al investigated interfraction prostate displacement in 333 patients and compared MV EPI and FMs with KV imaging of FMs. They found a statistically significant smaller setup error with MV imaging in all directions, but particularly so in the AP direction.41 It is possible that setup error was underestimated owing to the inferior image quality of MV vs kV images. This would be more apparent on lateral images (used to assess the AP position) owing to increased X-ray attenuation. Regardless of the reasons, the differences reported by these investigators may result in different planning margins being derived depending on which imaging modality is used to generate treatment setup data. According to Gill et al,41 this may be most likely in the AP direction which will impact clinically at the prostate–rectal interface. This is where the CTV–PTV margin is often reduced to avoid rectal toxicity, thus increasing this risk of missing the posterior aspect of the prostate or overdosing the rectal wall.
Soft-tissue contrast is not optimal on CT or CBCT; therefore, it can be reasoned that accurately identifying the position of the prostate based on CBCT is a subjective process and there is evidence to support this.57,58,36 A key issue when using CBCT without FM is interobserver variability due to poor soft-tissue contrast, which can introduce a source of systematic error in the application of treatment setup corrections, which by definition contribute to the error in margin calculations. FMs serve to eliminate a degree of the uncertainty associated with soft-tissue alignment, but cannot overcome other contributory factors including the variations associated with imaging modality.56,41
When used with 2D imaging alone, FMs do not enable visualization of the target or assessment of organ motion or deformation. It has been reported that a large rectal volume is predictive of poorer treatment outcome59 and therefore requires assessment in conjunction with accurate prostate alignment to ensure treatment efficacy. In this regard, CBCT may be preferable to 2D imaging, or it could be reasoned, should be used as an adjunct to planar imaging with FMs. While the accuracy of a CBCT prostate match may be inherently variable, it will nevertheless reveal gross anatomical differences in rectal and also bladder volumes that may significantly alter the planned dose distribution to the target and/or OARs. This information alerts therapists to take action to improve this, thereby optimizing treatment delivery. This review found no studies directly comparing ultrasound detection of FM with detection using another imaging modality. This may be because FMs are not easily visualized on transabdominal ultrasound (TAUS).35 Further research into the use of in-room ultrasound techniques for FM IGRT is warranted.
There are limited studies on the use of FMs with SC imaging and six degrees of freedom capability. Using the ExacTrac® 6D IGRT system (Brainlab AG, Feldkirchen, Germany), Shi et al60 report mean shifts of 0.2, −1.09 and −0.93 mm in the lateral, longitudinal and vertical directions, respectively, with corresponding rotational errors of 0.25, 0.1 and 0.02° for all fractions in 43 patients with PCa with FMs. Rotational corrections were not applied. Those exceeding 2° were investigated and repositioned.
In a series of 40 patients, Fuller et al61 compared treatment setup errors derived from in-room TAUS imaging of the prostate with those from kV SC images of prostate FMs for radiotherapy verification. As in Barney et al, the purpose of this comparison study was not to establish which modality was superior, although treatment was corrected and treated according to SC FM position. They concluded that concordance of resulting TAUS prostate and SC FM measurements was “poor” or “unacceptable” with 95% of measures within 15 mm.61 Numerous other groups have found significant differences in treatment setup data derived from TAUS soft-tissue assessment compared with FM data from other modalities, greater variation within ultrasound data and ultrasound-derived PTV margins may be up to 3–4 times smaller than those reported in the literature.43,62,63 Fuller et al61 also provide a useful narrative on the inherent difficulties of comparing IGRT modalities which briefly include the lack of an established ground truth and the statistical inaccuracies of comparing data generated by incomparable methods or devices.
The evidence presented here would suggest that treatment setup error data, with or without the use of FMs, are not interchangeable between imaging modalities and may generate different planning margins for the same treatment site and technique. Larger multicentre studies comparing standardized IGRT methods are needed to establish a gold standard IGRT approach that will enhance the benefits of FMs.
With currently available IGRT solutions, it is likely that a combined IGRT approach employing CBCT with FMs or CBCT in addition to FMs and planar imaging will provide the best possible assessment of prostate position and changes in OARs.
CLINICAL IMPACT OF PROSTATE FIDUCIAL MARKER IMAGE-GUIDED RADIOTHERAPY
Many studies demonstrate that the use of FMs improves prostate targeting during image-guided (IG) external beam radiotherapy. It is less clear what clinical impact this has in terms of patient-reported toxicity and overall survival and no prospective randomized controlled trials have been performed.
Paluska et al,11 Cheung et al64 and Skarsgard et al65 have all demonstrated the feasibility of reducing PTV margins with the use of FMs. Paluska et al proposed that a 10-mm CTV–PTV margin used with daily bone setup could be reduced to 7 mm using FMs and daily imaging. Furthermore, they observed better CTV coverage using FMs and reduced margins compared with bony setup plus a 10-mm margin. Toxicity was not reported in this study. However, by further limiting dose to normal surrounding structures, the PTV margin reduction theoretically reduces toxicity, since reducing the volume of rectum receiving 60 Gy or more has been shown to be associated with a reduction in rectal toxicity.66 In addition, reducing the PTV facilitates dose escalation and may improve progression-free survival.
Cheung et al64 prospectively assessed acute toxicity. They delivered a hypofractionated IMRT boost using patient-specific margins, the average of which was 3, 3 and 4 mm in the lateral, superior–inferior and AP directions, respectively. These margins were based on their assessment of intrafraction prostate motion using daily FM IGRT. They reported acceptable acute toxicity; however, Grade 3 genitourinary toxicity (National Cancer Institute Common Toxicity Criteria v. 2.0) was reported in 3 out of the 33 patients included in the study. In a more recent retrospective study, Takeda et al67 analyzed the outcome data for 141 patients treated using high-dose FM IG-IMRT and reported that treatment is well tolerated based on 5-year outcomes. Cheung et al and Takeda et al report findings which are reassuring in terms of demonstrating acceptable toxicity using reduced margins, but neither had a non-FM IGRT control group; therefore, it is unclear what contribution the use of FMs made to the outcome data.
The impact of IG-IMRT with FMs and reduced margins was compared with IMRT without FM IGRT by Chung et al68 in a prospective non-randomized study of 25 patients. Radiation Therapy Oncology Group and Common Terminology for Adverse Events scores for rectal and bladder toxicity were consistently lower in the FM IG-IMRT group. These findings are partially supported by Singh et al69 in relation to rectal toxicity in a large patient cohort, where 154 patients had FM IGRT and 128 patients had non-IGRT. There was no difference in reported urinary symptoms in each group.
Zelefsky et al,70 Kok et al71 and Sveistrup et al72 compared large cohorts of patients retrospectively, where the main difference was the use or not of FMs for image guidance. All report improvements in urinary and gastrointestinal toxicity for the FM groups. Kok et al observed a reduced incidence of gastrointestinal toxicity despite a higher dose of 78 Gy in the FM group compared with 74 Gy in the non-FM group, but no difference in biochemical failure-free survival was observed. Sveistrup et al observed a biochemical progression-free advantage for the FM group, but this was not statistically significant.
There is convincing evidence that FM IGRT improves the accuracy of prostate targeting and some evidence that using FM IGRT reduces treatment-related toxicity without compromising treatment outcomes; therefore, it is unlikely a prospective randomized control trial comparing FM IGRT with non-FM IGRT will ever be performed.
PROSTATE CALCIFICATIONS AS FIDUCIAL MARKERS
The risks of prostate FM implantation described earlier are justified, provided a similar non-invasive approach does not exist. Alternatives that remove the need for artificial FMs would also eliminate the use of anaesthesia and antibiotics and save significant health service time and resources. For example, MRI-based radiotherapy using MRI linear accelerators may in the future offer a non-invasive and also non-ionizing approach to prostate IGRT which may not require FMs.
Prostate calcifications (PCs) are small ovoid or round bodies impregnated with calcium phosphate and calcium carbonate and are reported to be present in almost 90% of prostatectomy specimens.73 It has been reported that up to 35% of patients undergoing prostate radiotherapy have calcifications visible on CBCT.74 PCs may be detected coincidentally from histological or radiological findings, and patients are often asymptomatic. The significance of PCs is not well understood, but there is some evidence to suggest an association of PCs and prostatic inflammation, lower urinary tract symptoms and PCa.75–77 Their relevance here is that PCs visible on radiographic images may present a natural alternative to surgically implanted markers.
Zeng et al74 investigated the stability of PCs with a view to using them as a surrogate for prostate position during IGRT and found that they were stable. A subsequent study recommended the use of central intraprostatic PCs for IGRT.78 More encouraging is the work by Kim et al,79 who used PC mismatch as the end point in assessing similarity metrics on CBCT. They found the presence of PCs increased the success of CT/CBCT image registrations. These findings are based on 4, 10 and 14 patients, respectively, and therefore, investigation using larger patient numbers is warranted.
Given the potential for reducing risk and inconvenience to patients of surgically implanted FMs and the potential cost savings, PCs as a natural FM justifies more in-depth research. A prospective feasibility study investigating this is under way at our centre. The study Calcifications as an Alternative to Surgically Implanted Fiducial Markers for Prostate IGRT will directly compare FMs and PCs for the purpose of online image matching and correction. This study will also prospectively collect patient feedback regarding their experience of FM implant.
CONCLUSION
FMs present a good surrogate for the position of the prostate and may reflect prostate gland motion or deformation. Changes in rectal and bladder volume, prostate deformation and SV motion are not detected by FM imaging alone.
A combination of FM alignment and soft-tissue analysis is currently the most effective and widely available approach to ensuring accuracy in prostate IGRT. Standardized clinical protocols are needed to enable comparison of treatment accuracy data, toxicity and treatment outcomes. Further research into the use of TAUS with FMs and the dosimetric implications of FMs is warranted.
There is a lack of randomized studies on the benefits of FM IGRT that control for other parameters such as the effect of hormone therapy and pre-existing urinary symptoms.
Marker migration is minimal. Anatomical or physiological changes such as rectal and bladder filling may cause deformation or distortion of the prostate gland and result in the apparent migration of FMs. Other factors such as the timing of CT planning following implantation and the use of androgen deprivation therapy may influence the apparent stability of markers.
Implantation of FMs is well tolerated, but surgical techniques and toxicity data require standardization. Development of FM implantation techniques that facilitate a reduction in the use of prophylactic antibiotic therapy should be considered in light of the global problem of multiresistant bacteria.
Organ deformation and conflicting alignment of multiple targets requires 3D verification, deformable image registration and adaptive planning techniques and cannot be addressed by conventional IGRT with or without the use of FMs.
Despite the advent of MR-guided radiotherapy, such technology is unlikely to become the standard of care in most centres for some time. Also, while superior in terms soft-tissue contrast, MRI is contraindicated for some patients and since dose escalation, hypofractionation and stereotaxy are already here, the authors suggest that the future of FMs in prostate IGRT is secure for the foreseeable.
It is clear from the evidence that FMs are a useful aid to enhancing the accuracy of prostate IGRT. However, given the issues in relation to FM implantation including cost, risk of infection and antibiotic resistance, there is merit, perhaps also a duty, to investigate a natural alternative to FMs that has the potential to benefit a significant proportion of the PCa population.
FUNDING
The authors of this article are supported by grants from external funding bodies including the R&D Division of the Northern Ireland Public Health Agency and Northern Ireland charity, Friends of the Cancer Centre.
Contributor Information
Angela G M O'Neill, Email: angela.o'neill@belfasttrust.hscni.net.
Suneil Jain, Email: s.jain@qub.ac.uk.
Alan R Hounsell, Email: a.hounsell@qub.ac.uk.
Joe M O'Sullivan, Email: joe.osullivan@qub.ac.uk.
REFERENCES
- 1.Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007; 8: 475–87. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 2008; 71: 1028–33. doi: https://doi.org/10.1016/j.ijrobp.2007.11.066 [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low α/β ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002; 52: 6–13. [DOI] [PubMed] [Google Scholar]
- 4.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2012; 82: e17–24. doi: https://doi.org/10.1016/j.ijrobp.2010.10.075 [DOI] [PubMed] [Google Scholar]
- 5.BIR. Geometric uncertainties in radiotherapy: defining the planning target volume. London, UK: British Institute of Radiology; 2003. [Google Scholar]
- 6.Crook JM, Raymond Y, Salhani D, Yang H, Esche B. Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother Oncol 1995; 37: 35–42. doi: https://doi.org/10.1016/0167-8140(95)01613-L [DOI] [PubMed] [Google Scholar]
- 7.Herman MG, Pisansky TM, Kruse JJ, Prisciandaro JI, Davis BJ, King BF. Technical aspects of daily online positioning of the prostate for three-dimensional conformal radiotherapy using an electronic portal imaging device. Int J Radiat Oncol Biol Phys 2003; 57: 1131–40. doi: https://doi.org/10.1016/S0360-3016(03)00766-1 [DOI] [PubMed] [Google Scholar]
- 8.Shirato H, Harada T, Harabayashi T, Hida K, Endo H, Kitamura K, et al. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 240–7. doi: https://doi.org/10.1016/S0360-3016(03)00076-2 [DOI] [PubMed] [Google Scholar]
- 9.Schallenkamp JM, Herman MG, Kruse JJ, Pisansky TM. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys 2005; 63: 800–11. [DOI] [PubMed] [Google Scholar]
- 10.McNair HA, Hansen VN, Parker CC, Evans PM, Norman A, Miles E, et al. A comparison of the use of bony anatomy and internal markers for offline verification and an evaluation of the potential benefit of online and offline verification protocols for prostate radiotherapy. Int J Radiat Oncol Biol Phys 2008; 71: 41–50. [DOI] [PubMed] [Google Scholar]
- 11.Paluska P, Hanus J, Sefrova J, Rouskova L, Grepl J, Jansa J, et al. Utilization of cone beam CT for reconstruction of dose distribution delivered in image-guided radiotherapy of prostate carcinoma—bony landmark setup compared to fiducial markers setup. J Appl Clin Med Phys 2013; 14: 4203. doi: https://doi.org/10.1120/jacmp.v14i3.4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heide UA, Kotte AN, Dehnad H, Hofman P, Lagenijk JJ, van Vulpen M. Analysis of fiducial marker-based position verification in the external beam radiotherapy of patients with prostate cancer. Radiother Oncol 2007; 82: 38–45. doi: https://doi.org/10.1016/j.radonc.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Kotte AN, Hofman P, Lagendijk JJ, van Vulpen M, van der Heide UA. Intrafraction motion of the prostate during external-beam radiation therapy: analysis of 427 patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys 2007; 69: 419–25. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Lee RJ, Handrahan D, Sause WT. Intensity-modulated radiotherapy using implanted fiducial markers with daily portal imaging: assessment of prostate organ motion. Int J Radiat Oncol Biol Phys 2007; 68: 912–19. [DOI] [PubMed] [Google Scholar]
- 15.Kron T, Thomas J, Fox C, Thompson A, Owen R, Herschtal A, et al. Intra-fraction prostate displacement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted fiducial markers. Radiother Oncol 2010; 95: 191–7. doi: https://doi.org/10.1016/j.radonc.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 16.Quon H, Loblaw DA, Cheung PC, Holden L, Tang C, Pang G, et al. Intra-fraction motion during extreme hypofractionated radiotherapy of the prostate using pre- and post-treatment imaging. Clin Oncol (R Coll Radiol) 2012; 24: 640–5. doi: https://doi.org/10.1016/j.clon.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 17.Xie Y, Djajaputra D, King CR, Hossain S, Ma L, Xing L. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Wielen GJ, Mutanga TF, Incrocci L, Kirkels WJ, Vasquez Osorio EM, Hoogeman MS, et al. Deformation of prostate and seminal vesicles relative to intraprostatic fiducial markers. Int J Radiat Oncol Biol Phys 2008; 72: 1604–11. doi: https://doi.org/10.1016/j.ijrobp.2008.07.023 [DOI] [PubMed] [Google Scholar]
- 19.de Boer J, van Herk M, Pos FJ, Sonke JJ. Hybrid registration of prostate and seminal vesicles for image guided radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86: 177–82. doi: https://doi.org/10.1016/j.ijrobp.2012.11.034 [DOI] [PubMed] [Google Scholar]
- 20.Mutanga TF, de Boer HC, van der Wielen GJ, Hoogeman MS, Incrocci L, Heijmen BJ. Margin evaluation in the presence of deformation, rotation, and translation in prostate and entire seminal vesicle irradiation with daily marker-based setup corrections. Int J Radiat Oncol Biol Phys 2011; 81: 1160–7. doi: https://doi.org/10.1016/j.ijrobp.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 21.Nichol AM, Brock KK, Lockwood GA, Moseley DJ, Rosewall T, Warde PR, et al. A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys 2007; 67: 48–56. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara K, Roach M, 3rd. Technique for implantation of fiducial markers in the prostate. Urology 2008; 71: 196–200. doi: https://doi.org/10.1016/j.urology.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 23.Linden RA, Weiner PR, Gomella LG, Dicker AP, Suh DB, Trabulsi EJ, et al. Technique of outpatient placement of intraprostatic fiducial markers before external beam radiotherapy. Urology 2009; 73: 881–6. doi: https://doi.org/10.1016/j.urology.2008.10.071 [DOI] [PubMed] [Google Scholar]
- 24.Igdem S, Akpinar H, Alco G, Agacayak G, Turkan S, Okkan S. Implantation of fiducial markers for image guidance in prostate radiotherapy: patient-reported toxicity. Br J Radiol 2009; 82: 941–5. doi: https://doi.org/10.1259/bjr/14201041 [DOI] [PubMed] [Google Scholar]
- 25.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs 1988; 14: 9–17. [PubMed] [Google Scholar]
- 26.Gill S, Li J, Thomas J, Bressel M, Thursky K, Styles C, et al. Patient-reported complications from fiducial marker implantation for prostate image-guided radiotherapy. Br J Radiol 2012; 85: 1011–17. doi: https://doi.org/10.1259/bjr/68127917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Griwan MS, Singh SK, Sen J, Pawar DS. Is periprostatic nerve block a gold standard in case of transrectal ultrasound-guided prostate biopsy? Urol Ann 2013; 5: 152–6. doi: https://doi.org/10.4103/0974-7796.115732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langenhuijsen JF, van Lin EN, Kiemeney LA, van der Vight LP, McColl GM, Visser AG, et al. Ultrasound-guided transrectal implantation of gold markers for prostate localization during external beam radiotherapy: complication rate and risk factors. Int J Radiat Oncol Biol Phys 2007; 69: 671–6. [DOI] [PubMed] [Google Scholar]
- 29.Pouliot J, Aubin M, Langen KM, Liu YM, Pickett B, Shinohara K, et al. (Non)-migration of radiopaque markers used for on-line localization of the prostate with an electronic portal imaging device. Int J Radiat Oncol Biol Phys 2003; 56: 862–6. doi: https://doi.org/10.1016/S0360-3016(03)00267-0 [DOI] [PubMed] [Google Scholar]
- 30.Kably I, Bordegaray M, Shah K, Salsamendi J, Narayanan G. Single-center experience in prostate fiducial marker placement: technique and midterm follow-up. J Vasc Interv Radiol 2014; 25: 1125–32. doi: https://doi.org/10.1016/j.jvir.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 31.Loh J, Baker K, Sridharan S, Greer P, Wratten C, Capp A, et al. Infections after fiducial marker implantation for prostate radiotherapy: are we underestimating the risks? Radiat Oncol 2015; 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Çek M, Grabe M, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol 2013; 63: 521–7. doi: https://doi.org/10.1016/j.eururo.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 33.Moman MR, van der Heide UA, Kotte AN, van Moorselaar RJ, Bol GH, Franken SP, et al. Long-term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother Oncol 2010; 96: 38–42. doi: https://doi.org/10.1016/j.radonc.2010.02.027 [DOI] [PubMed] [Google Scholar]
- 34.Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and “superbugs”: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int 2014; 114: 384–8. doi: https://doi.org/10.1111/bju.12536 [DOI] [PubMed] [Google Scholar]
- 35.Fonteyne V, Ost P, Villeirs G, Oosterlinck W, Impens A, De Gersem W, et al. Improving positioning in high-dose radiotherapy for prostate cancer: safety and visibility of frequently used gold fiducial markers. Int J Radiat Oncol Biol Phys 2012; 83: 46–52. doi: https://doi.org/10.1016/j.ijrobp.2011.05.058 [DOI] [PubMed] [Google Scholar]
- 36.Rasch C, Barillot I, Remeijer P, Touw A, van Herk M, Lebesque JV. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys 1999; 43: 57–66. doi: https://doi.org/10.1016/S0360-3016(98)00351-4 [DOI] [PubMed] [Google Scholar]
- 37.Sibata CH, Mota HC, Higgins PD, Gaisser D, Saxton JP, Shin KH. Influence of hip prostheses on high energy photon dose distributions. Int J Radiat Oncol Biol Phys 1990; 18: 455–61. [DOI] [PubMed] [Google Scholar]
- 38.Janardanan Nair V, Szanto J, Vandervoort E, Henderson E, Avruch L, Malone S, et al. Feasibility, detectability, and experience with platinum seed internal fiducial markers for CT-MRI fusion and real-time tumor tracking during stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys 2012; 84: S821. doi: https://doi.org/10.1016/j.ijrobp.2012.07.2200 [Google Scholar]
- 39.Chow JC, Grigorov GN. Dose measurements near a non-radioactive gold seed using radiographic film. Phys Med Biol 2005; 50: N227–34. doi: https://doi.org/10.1088/0031-9155/50/18/N02 [DOI] [PubMed] [Google Scholar]
- 40.Newhauser W, Fontenot J, Koch N, Dong L, Lee A, Zheng Y, et al. Monte Carlo simulations of the dosimetric impact of radiopaque fiducial markers for proton radiotherapy of the prostate. Phys Med Biol 2007; 52: 2937–52. doi: https://doi.org/10.1088/0031-9155/52/11/001 [DOI] [PubMed] [Google Scholar]
- 41.Gill S, Thomas J, Fox C, Kron T, Thompson A, Chander S, et al. Electronic portal imaging vs kilovoltage imaging in fiducial marker image-guided radiotherapy for prostate cancer: an analysis of set-up uncertainties. Br J Radiol 2012; 85: 176–82. doi: https://doi.org/10.1259/bjr/13553326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Boer J, de Bois J, van Herk M, Sonke JJ. Influence of the number of elongated fiducial markers on the localization accuracy of the prostate. Phys Med Biol 2012; 57: 6211–26. doi: https://doi.org/10.1088/0031-9155/57/19/6211 [DOI] [PubMed] [Google Scholar]
- 43.Serago CF, Buskirk SJ, Igel TC, Gale AA, Serago NE, Earle JD. Comparison of daily megavoltage electronic portal imaging or kilovoltage imaging with marker seeds to ultrasound imaging or skin marks for prostate localization and treatment positioning in patients with prostate cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1585–92. [DOI] [PubMed] [Google Scholar]
- 44.Carl J, Nielsen J, Holmberg M, Larsen EH, Fabrin K, Fisker RV. Clinical results from first use of prostate stent as fiducial for radiotherapy of prostate cancer. Acta Oncol 2011; 50: 547–54. doi: https://doi.org/10.3109/0284186X.2010.541935 [DOI] [PubMed] [Google Scholar]
- 45.Balter JM, Wright JN, Newell LJ, Friemel B, Dimmer S, Cheng Y, et al. Accuracy of a wireless localization system for radiotherapy. Int J Radiat Oncol Biol Phys 2005; 61: 933–7. [DOI] [PubMed] [Google Scholar]
- 46.Kupelian P, Willoughby T, Mahadevan A, Djemil T, Weinstein G, Jani S, et al. Multi-institutional clinical experience with the Calypso system in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys 2007; 67: 1088–98. [DOI] [PubMed] [Google Scholar]
- 47.Shchory T, Schifter D, Lichtman R, Neustadter D, Corn BW. Tracking accuracy of a real-time fiducial tracking system for patient positioning and monitoring in radiation therapy. Int J Radiat Oncol Biol Phys 2010; 78: 1227–34. doi: https://doi.org/10.1016/j.ijrobp.2010.01.067 [DOI] [PubMed] [Google Scholar]
- 48.de Kruijf WJ, Verstraete J, Neustadter D, Corn BW, Hol S, Venselaar JL, et al. Patient positioning based on a radioactive tracer implanted in patients with localized prostate cancer: a performance and safety evaluation. Int J Radiat Oncol Biol Phys 2013; 85: 555–60. [DOI] [PubMed] [Google Scholar]
- 49.Poggi MM, Gant DA, Sewchand W, Warlick WB. Marker seed migration in prostate localization. Int J Radiat Oncol Biol Phys 2003; 56: 1248–51. doi: https://doi.org/10.1016/S0360-3016(03)00328-6 [DOI] [PubMed] [Google Scholar]
- 50.Kupelian PA, Willoughby TR, Meeks SL, Forbes A, Wagner T, Maach M, et al. Intraprostatic fiducials for localization of the prostate gland: monitoring intermarker distances during radiation therapy to test for marker stability. Int J Radiat Oncol Biol Phys 2005; 62: 1291–6. [DOI] [PubMed] [Google Scholar]
- 51.Delouya G, Carrier JF, Béliveau-Nadeau D, Donath D, Taussky D. Migration of intraprostatic fiducial markers and its influence on the matching quality in external beam radiation therapy for prostate cancer. Radiother Oncol 2010; 96: 43–7. doi: https://doi.org/10.1016/j.radonc.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 52.Tiberi DA, Carrier JF, Beauchemin MC, Nguyen TV, Béliveau-Nadeau D, Taussky D. Impact of concurrent androgen deprivation on fiducial marker migration in external-beam radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012; 84: e7–12. doi: https://doi.org/10.1016/j.ijrobp.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 53.Maintz JB, Viergever MA. A survey of medical image registration. Med Image Anal 1998; 2: 1–36. doi: https://doi.org/10.1016/S1361-8415(01)80026-8 [DOI] [PubMed] [Google Scholar]
- 54.Kashani R, Hub M, Balter JM, Kessler ML, Dong L, Zhang L, et al. Objective assessment of deformable image registration in radiotherapy: a multi-institution study. Med Phys 2015; 35: 5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kupelian P, Meyer JL. Image-guided, adaptive radiotherapy of prostate cancer: toward new standards of radiotherapy practice. Front Radiat Ther Oncol 2011; 43: 344–68. doi: https://doi.org/10.1159/000322485 [DOI] [PubMed] [Google Scholar]
- 56.Barney BM, Lee RJ, Handrahan D, Welsh KT, Cook JT, Sause WT. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 2011; 80: 301–5. doi: https://doi.org/10.1016/j.ijrobp.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 57.Liszewski B, Choo E, D'Alimonte L. A retrospective analysis of prostate cone beam computed tomography (CBCT) image registration: a tale of two techniques. J Med Imaging Radiat Sci 2010; 41: 207–14. doi: https://doi.org/10.1016/j.jmir.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 58.Moseley DJ, White EA, Wiltshire KL, Rosewall T, Sharpe MB, Siewerdsen JH, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007; 67: 942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Crevoisier R, Tucker SL, Dong L, Mohan R, Cheung R, Cox JD, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 2005; 62: 965–73. [DOI] [PubMed] [Google Scholar]
- 60.Shi C, Tazi A, Fang DX, Iannuzzi C. Study of ExacTrac X-ray 6D IGRT setup uncertainty for marker-based prostate IMRT treatment. J Appl Clin Med Phys 2012; 13: 3757. doi: https://doi.org/10.1120/jacmp.v13i3.3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuller CD, Thomas CR, Schwartz S, Golden N, Ting J, Wong A, et al. Method comparison of ultrasound and kilovoltage X-ray fiducial marker imaging for prostate radiotherapy targeting. Phys Med Biol 2006; 51: 4981–93. doi: https://doi.org/10.1088/0031-9155/51/19/016 [DOI] [PubMed] [Google Scholar]
- 62.Scarbrough TJ, Golden NM, Ting JY, Fuller CD, Wong A, Kupelian PA, et al. Comparison of ultrasound and implanted seed marker prostate localization methods: Implications for image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2006; 65: 378–87. [DOI] [PubMed] [Google Scholar]
- 63.Foster RD, Solberg TD, Li HS, Kerkhoff A, Enke CA, Willoughby TR, et al. Comparison of transabdominal ultrasound and electromagnetic transponders for prostate localization. J Appl Clin Med Phys 2010; 11: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung P, Sixel K, Morton G, Loblaw DA, Tirona R, Pang G, et al. Individualized planning target volumes for intrafraction motion during hypofractionated intensity-modulated radiotherapy boost for prostate cancer. Int J Radiat Oncol Biol Phys 2005; 62: 418–25. [DOI] [PubMed] [Google Scholar]
- 65.Skarsgard D, Cadman P, El-Gayed A, Pearcey R, Tai P, Pervez N, et al. Planning target volume margins for prostate radiotherapy using daily electronic portal imaging and implanted fiducial markers. Radiat Oncol 2010; 5: 52. doi: https://doi.org/10.1186/1748-717X-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 2010; 76(Suppl. 3): S123–9. doi: https://doi.org/10.1016/j.ijrobp.2009.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda K, Takai Y, Narazaki K, Mitsuya M, Umezawa R, Kadoya N, et al. Treatment outcome of high-dose image-guided intensity-modulated radiotherapy using intra-prostate fiducial markers for localized prostate cancer at a single institute in Japan. Radiat Oncol 2012; 7: 105. doi: https://doi.org/10.1186/1748-717X-7-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung HT, Xia P, Chan LW, Park-Somers E, Roach M. Does image-guided radiotherapy improve toxicity profile in whole pelvic-treated high-risk prostate cancer? Comparison between IG-IMRT and IMRT. Int J Radiat Oncol Biol Phys 2009; 73: 53–60. doi: https://doi.org/10.1016/j.ijrobp.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 69.Singh J, Greer PB, White MA, Parker J, Patterson J, Tang CI, et al. Treatment-related morbidity in prostate cancer: a comparison of 3-dimensional conformal radiation therapy with and without image guidance using implanted fiducial markers. Int J Radiat Oncol Biol Phys 2013; 85: 1018–23. doi: https://doi.org/10.1016/j.ijrobp.2012.07.2376 [DOI] [PubMed] [Google Scholar]
- 70.Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2012; 84: 125–9. doi: https://doi.org/10.1016/j.ijrobp.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 71.Kok D, Gill S, Bressel M, Byrne K, Kron T, Fox C, et al. Late toxicity and biochemical control in 554 prostate cancer patients treated with and without dose escalated image guided radiotherapy. Radiother Oncol 2013; 107: 140–6. doi: https://doi.org/10.1016/j.radonc.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 72.Sveistrup J, af Rosenschöld PM, Deasy JO, Oh JH, Pommer T, Petersen PM, et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol 2014; 9: 44. doi: https://doi.org/10.1186/1748-717X-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suh JH, Gardner JM, Kee KH, Shen S, Ayala AG, Ro JY. Calcifications in prostate and ejaculatory system: a study on 298 consecutive whole mount sections of prostate from radical prostatectomy or cystoprostatectomy specimens. Ann Diagn Pathol 2008; 12: 165–70. doi: https://doi.org/10.1016/j.anndiagpath.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 74.Zeng GG, McGowan TS, Larsen TM, Bruce LM, Moran NK, Tsao JR, et al. Calcifications are potential surrogates for prostate localization in image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 963–6. doi: https://doi.org/10.1016/j.ijrobp.2008.07.021 [DOI] [PubMed] [Google Scholar]
- 75.Klimas R, Bennett B, Gardner WA, Jr. Prostatic calculi: a review. Prostate 1985; 7: 91–6. [DOI] [PubMed] [Google Scholar]
- 76.Venyo AK. Prostatic calculi: a review of the literature. Urology 2012; 3: 3463. doi: https://doi.org/10.9754/journal.wmc.2012.003463 [Google Scholar]
- 77.Smolski M, Turo R, Whiteside S, Bromage S, Collins GN. Prevalence of prostatic calcification subtypes and association with prostate cancer. Urology 2015; 85: 178–81. doi: https://doi.org/10.1016/j.urology.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 78.Hanna SA, Neves-Junior WF, Marta GN, Haddad CM, da Silva JL. Role of intra- or periprostatic calcifications in image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82: 1208–16. doi: https://doi.org/10.1016/j.ijrobp.2011.03.059 [DOI] [PubMed] [Google Scholar]
- 79.Kim J, Hammoud R, Pradhan D, Zhong H, Jin RY, Movsas B, et al. Prostate localization on daily cone-beam computed tomography images: accuracy assessment of similarity metrics. Int J Radiat Oncol Biol Phys 2010; 77: 1257–65. doi: https://doi.org/10.1016/j.ijrobp.2009.09.068 [DOI] [PubMed] [Google Scholar]



