Abstract
The objectives of this article were: (1) to review common and rare manifestations of systemic and pulmonary Langerhans cell histiocytosis, Rosai–Dorfman disease, Erdheim–Chester disease and juvenile xanthogranuloma; (2) to provide the reader with important pathologic, epidemiologic and clinical features of these diseases. The histiocytoses are a diverse group of diseases which typically manifest with multiorgan involvement. Understanding the pathologic, epidemiologic and clinical features of these entities can help the radiologist suggest an accurate diagnosis of histiocytosis when typical imaging features are encountered.
INTRODUCTION
The histiocytoses form a disparate group of rare diseases that present most commonly in children, but may also be seen in adults. Histiocytes are a group of immune cells composed of macrophages and dendritic cells.1 The contemporary classification of histiocytic disorders, an effort by the World Health Organization's Committee on Histiocytic/Reticulum Cell Proliferations and the Reclassification Working Group of the Histiocyte Society, divides this group of diseases into two large categories: those with varied biological behaviour and those with malignant behaviour.
Histiocytic disorders of varied biological behaviour are further classified into dendritic cell- and macrophage-related disorders. The more common primary histiocytic disorders of varied biologic behaviour include Langerhans cell histiocytosis (LCH), pulmonary Langerhans cell histiocytosis (pLCH), Rosai–Dorfman disease (RDD), Erdheim–Chester disease (ECD) and juvenile xanthogranuloma (JXG).1,2 Histiocytic disorders of malignant behaviour are extremely rare and beyond the scope of this article. Cardiothoracic involvement may be present in the majority of these diseases. The purpose of this article was to review the pathophysiology, clinical manifestations and the spectrum of common and less frequently encountered manifestations within the chest for each listed entity.
SYSTEMIC LANGERHANS CELL HISTIOCYTOSIS
LCH is a systemic disease. It is the most frequently encountered dendritic cell disorder. The term Langerhans cell histiocytosis was adopted, given the similarities between the LCH cell and the normal, antigen-presenting Langerhans cell found in the skin, lymph nodes and bronchial mucosa.3 A Langerhans cell is a highly differentiated histiocyte derived from the stellate dendritic antigen-presenting cell and expresses CD1a, S100 and CD207 antigens. CD1a is a specific immunocytochemical marker for Langerhans cells.1,3 The LCH cell, however, differs from the normal Langerhans cell in many ways. Some of the differences include surface markers found in the LCH cell that are not present in the normal Langerhans cell, such as interferon γ receptor. In patients with LCH, the cells are found in organs that do not normally have a Langerhans cell population. Furthermore, unlike the Langerhans cell, the LCH cells are non-functional in terms of antigen presentation.4 Both normal Langerhans and LCH cells exhibit intracytoplasmic organelles known as Birbeck granules, a characteristic that helps in the differentiation of this disease from other histiocytic disorders.5
In the past, various nomenclatures were used to describe the various patterns of disease encountered including histiocytosis X, eosinophilic granuloma, Letterer–Siwe disease and Hand–Schüller–Christian disease; however, these terms are now considered obsolete.6 Systemic LCH is divided into three different disease forms based on the number of lesions and systems affected.6,7 Unifocal LCH is the most common manifestation (70% of cases), almost exclusively involves the skeletal structures and can be either monostotic or polyostotic. The lungs may also occasionally be affected. The second most common form of systemic LCH, accounting for approximately 20% of cases, is termed multifocal unisystem LCH. These patients may demonstrate reticuloendothelial system involvement (previously referred to as Letterer–Siwe disease) or combined osseous and pituitary gland involvement, leading to diabetes insipidus (previously referred to as Hand–Schüller–Christian disease). Multifocal unisystem LCH presents at an earlier age (under 5 years of age) than unifocal LCH. The least common form is termed multifocal multisystem LCH and accounts for approximately 10% of the cases. It is seen in patients less than 2 years of age and is often fatal. In this form of disease, the reticuloendothelial system is predominantly involved. The diagnosis of systemic LCH is based on clinical, histopathologic, radiologic and immunohistochemical data.6
In the lungs, multiple small nodules with early signs of cavitation and thin-walled air cysts (<10 mm) are the primary findings (Figure 1). Spontaneous pneumothorax may occur in cases of ruptured peripheral cysts. Pleural effusions and pneumomediastinum are rare complications. In the chronic stage, findings of irreversible fibrosis and emphysema may evolve. Advanced parenchymal destruction may subsequently lead to pulmonary hypertension (Table 1).8
Figure 1.
Systemic Langerhans cell histiocytosis (LCH) in an infant: chest radiograph (a) in an infant with systemic LCH is showing diffuse reticular opacities. Accompanying CT (b) is demonstrating extensive cysts and areas of architectural distortion.
Table 1.
Non-malignant histiocytic disorders (adapted and simplified from Contemporary classification of histiocytic disorders. Med Pediatr Oncol 1997; 29: 157–66)
| Dendritic cell-related histiocytoses | Macrophage-related histiocytoses |
|---|---|
| LCH | Haemophagocytic syndromes (primary and secondary) |
| Secondary dendritic cell processes | RDD |
| JXG and related disorders | Solitary histiocytoma (macrophage) |
| Solitary histiocytomas of various dendritic cell phenotypes | |
| ECD |
ECD, Erdheim–Chester disease; JXG, juvenile xanthogranuloma; LCH, Langerhans cell histiocytosis; RDD, Rosai–Dorfman disease.
Mediastinal involvement is also rarely possible. The thymus is rarely involved in cases of LCH; however, its true incidence is likely underestimated. The majority of patients with thymic involvement have the multifocal multisystemic form of disease. As the histiocytic infiltration of the thymus progresses, there is progressive enlargement of the thymus that can be appreciated even on chest radiography. CT generally demonstrates diffuse enlargement and heterogeneous attenuation of the thymus. Calcifications may also be present. If i.v. contrast is administered, enhancing septations can be seen within the enlarged thymus. MRI shows areas of high T1 weighted signal intensity, which may represent fatty replacement due to fibroxanthomatous reaction.8 Other mediastinal manifestations of systemic LCH include diffuse lymphadenopathy and posterior mediastinal infiltration (Figure 2). Effects of mediastinal involvement may include tracheal compression, pericardial effusion, superior vena cava thrombosis and erosion of the sternum and vertebral bodies.9 Cases of thyroid goitre due to infiltration of Langerhans cells into the thyroid gland have also been reported.10
Figure 2.
Mediastinal and vertebral involvement in systemic Langerhans cell histiocytosis (LCH): contrast-enhanced chest CT (a) in an infant with systemic LCH is showing extensive coalescent lymphadenopathy throughout the mediastinum resulting in compression and displacement of vascular and other mediastinal structures. Sagittal T2 weighted MR image of the cervicothoracic spine (b) in a 6-year-old female is showing diffuse, severe loss of height of the T1 vertebral body (arrow) with mild retropulsion into the spinal canal. These findings are compatible with the vertebra plana and have been pathologically proven to represent LCH involvement.
Overall, osseous involvement is present in at least 80% of cases. Lesions are generally lytic; however, when chronic, these lytic lesions demonstrate a sclerotic margin indicative of healing. There is predilection for flat bones of the skull. In the chest, ribs are frequently involved, particularly in adults.6,11 The vertebral bodies are also commonly affected.11 Uniform collapse of the vertebral body with preservation of the intervertebral disc space has been termed vertebra plana or Calve disease (Figure 2).11
Positron emission tomography (PET)-CT is valuable in delineating sites of metabolically active disease (Figure 3). It has proven useful in the follow-up, as it can detect residual disease and new lesions that may be missed or overlooked by other diagnostic methods.12
Figure 3.
Adolescent male with systemic LCH. Coronal fused FDG PET-CT (a) and coronal CT (b) images demonstrating scattered upper lobe predominant FDG-avid irregular, slightly spiculated nodules with cavitation and cyst formation.
PULMONARY LANGERHANS CELL HISTIOCYTOSIS
The pLCH is a rare lung disorder seen primarily in young adult smokers.13 First described by Lichtenstein in 1951, pLCH is characterized by isolated pulmonary involvement and is recognized by the Histiocyte Society as a variant of systemic LCH.1,13 There are, however, significant epidemiologic, clinical and pathologic differences between systemic LCH and pLCH, suggesting that the pathogenetic pathways are likely different.14 Studies have shown that pLCH likely represents a reactive, non-clonal hyperplasia of Langerhans cells, with antigens in cigarette smoke being the likely stimulus, whereas systemic LCH appears to be a clonal, potentially neoplastic process.15,16
Histopathologically, Langerhans cells infiltrate the bronchiolar walls and epithelium, with subsequent development into discrete bronchiolocentric, stellate nodules, which also contain varying numbers of lymphocytes, fibroblasts, eosinophils, neutrophils, plasma cells and pigmented macrophages.17 Cavitation can develop within the nodules, which, as shown by Kambouchner et al,18 is secondary to bronchiolar luminal enlargement from inflammation, fibrosis and destruction of the bronchiolar wall, coalescence of adjacent affected airways and traction emphysema or paracicatricial airspace enlargement in peribronchiolar alveolar air spaces. A strong association has been established between cigarette smoking and pLCH, with 95% of patients being active or former smokers or having substantial second-hand smoke exposure.19 Cough and dyspnoea are the most commonly reported symptoms, although clinical presentation is variable, with one-third of patients being asymptomatic.20 A few patients present with spontaneous pneumothorax (4–17%), which can be recurrent.21
Chest radiography is often abnormal, showing middle and upper lung predominant nodular and reticular opacities, with reticular abnormality dominating later in the disease course.22–24 High-resolution CT is superior to radiography in depicting lung abnormalities, allowing a confident diagnosis.25 Early pLCH manifests as ill-defined bronchiolocentric nodules, potentially mimicking respiratory bronchiolitis.26 The nodular component of pLCH is characterized by nodules with irregular margins measuring between 1 and 10 mm in size. Occasionally, nodules larger than 10 mm in size may be seen.17 Cysts form with disease progression and can coalesce to form bizarre, irregular shapes, a characteristic feature of pLCH.26,27 Brauner et al28 have postulated the sequential progression of CT abnormality in pLCH as follows: nodules, cavitary nodules, thick-walled cysts, thin-walled cysts and finally, confluent cysts (Figure 4). Adult pLCH classically shows middle and upper lung predominance with sparing of the subpleural parenchyma at the costophrenic recesses, an important distinction from paediatric pLCH, which is typically diffuse.29 Patients with pLCH may develop pulmonary hypertension even after the lung disease has stabilized, which is thought to be secondary to arterial involvement. The degree of pulmonary hypertension in pLCH is severe and unrelated to pulmonary function. Pulmonary hypertension in patients with pLCH renders a poor prognosis.30
Figure 4.
Pulmonary Langerhans cell histiocytosis (pLCH) in multiple patients: CT of the chest (a) in a 48-year-old female smoker is demonstrating upper lobe predominant bizarre cysts and scattered nodules (arrows). Coronal CT of the chest (b) in a different patient is showing more severe disease with replacement of parenchyma by multiple upper lobe predominant coalescent cysts, mimicking emphysema. It can be noted that the disease has spared the costophrenic angles. CT of the chest in a 44-year-old female (c) is showing a loculated pneumothorax complicating pLCH (star).
Smoking cessation is of paramount importance in the management of pLCH, often leading to clinical and radiographic stabilization and, in some cases, regression. The use of adjuvant corticosteroid or immunosuppressive therapy has been described, but its effectiveness is not well defined.19
The nodular aspect of pLCH may appear similar to other entities that present with nodules such as sarcoidosis, silicosis, metastatic disease and miliary tuberculosis. Cystic changes in patients with pLCH may resemble other cystic diseases such as pulmonary fibrosis (typically subpleural cystic change, basilar in distribution) or lymphangioleimyomatosis (classically diffuse lung involvement and almost exclusively seen in females).17 Clinical and radiologic findings are often very suggestive of pLCH, often obviating the need for invasive, diagnostic procedures. Definitive diagnosis can be achieved via bronchoscopy or surgical lung biopsy. In the past, the diagnostic yield of bronchoscopy has been reported as low; however, a more recent study has reported a diagnostic yield of 60% when combining the results of bronchoalveolar lavage and transbronchial biopsy.31
ROSAI–DORFMAN DISEASE
RDD is a rare, macrophage-related histiocytosis. RDD is also known as “sinus histiocytosis with massive lymphadenopathy” and was first recognized as a distinct entity in 1969.14 Although many theories have been discussed, the pathogenesis remains poorly understood. Epstein–Barr virus and human herpesvirus-6 have been implicated as possible causative agents.32 In recent studies, a link has been described between IgG4-related disease and RDD in up to 10–30% of RDD cases.33,34
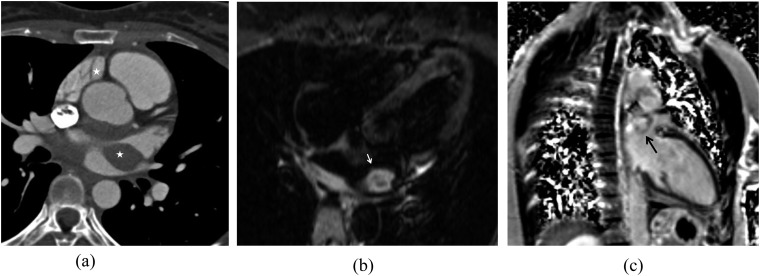
RDD is most commonly seen in young males and characterized primarily by painless cervical lymphadenopathy.14,32 However, up to 40% of patients present with extranodal involvement and in approximately 23% of patients, only extranodal disease is present.35,36 Extranodal involvement most commonly includes the skin, bone and soft tissue.33 Intrathoracic involvement is rare. When limited to intrathoracic lymph nodes, RDD is reminiscent of lymphoproliferative disorders (Figure 6). On fluorine-18-fludeoxyglucose (FDG) PET-CT, lymphadenopathy is hypermetabolic, although in contrast to lymphoma, lymph nodes in patients with RDD are not uniformly avid, but demonstrate intense central uptake with decreased activity peripherally.37,38 Approximately 3% of patients demonstrate lung involvement, most commonly affecting the major airways with associated nodal disease (Figure 5). Nodular lesions in the pulmonary parenchyma (Figure 5), as well as diffuse interstitial involvement, have also been described. Involvement of the pleura, with or without neighbouring lung disease, has also been noted.14 RDD may affect the heart and great vessels. Cardiac involvement in RDD is most often in the form of a myocardial mass.32,35,39 On CT, these have often been described as poorly defined, but may have well-defined borders (Figure 7). On MR, these lesions appear hyperintense on T2 weighted sequences and demonstrate delayed contrast enhancement (Figure 7).39 RDD may also affect the cardiac valves or pericardium.32 Involvement of the heart and great vessels may mimic a neoplastic process such as a sarcoma on CT or MR.40
Figure 6.
Mediastinal involvement in Rosai–Dorfman disease (RDD): axial CT images in two different patients with RDD at the level of the aortopulmonary window (a) and left main pulmonary artery (b) are showing coalescent lymphadenopathy and infiltrative soft tissue in the mediastinum (stars in a and b).
Figure 5.
Airways and lung involvement in Rosai-Dorfman disease. Coronal contrast enhanced CT image (a) through the laryngeal region demonstrating mass-like thickening of the right subglottic mucosa (arrow). Axial contrast enhanced CT image through the upper trachea (b) shows subtle mass-like thickening of the right lateral tracheal wall (arrow). Axial CT image through the upper lobes (c) shows a spiculated right upper lobe nodule (arrow).
Figure 7.
A cardiac mass in a patient with Rosai–Dorfman disease: contrast-enhanced electrocardiogram-gated CT of the heart (a) is demonstrating a soft-tissue density mass centred about the left atrium (stars). T2 weighted four-chamber MR image (b) of the heart is showing a T2 hyperintense left atrial mass (white arrow). After the injection of contrast on the phase-sensitive inversion-recovery sequence (c), this mass is demonstrating delayed enhancement (black arrow).
Nodal RDD is usually a self-limiting disease. Chemotherapy agents used in lymphoma as well as steroids are available treatment options. The prognosis of patients with extranodal involvement is dependent on the site and degree of involvement.41
ERDHEIM–CHESTER DISEASE
ECD is a rare, systemic, histiocytosis characterized by xanthomatous infiltration of tissues by lipid-laden histiocytes which, unlike Langerhans cells, lack Birbeck granules.1,42 Similar to other xanthogranulomatous disorders, sparse Touton giant cells (giant cells containing foamy cytoplasm) are also present and the lipid-laden histiocytes within the lesion are CD1- and S100-negative. In addition, these histiocytes are positive for CD68.2 All of these characteristics help differentiate ECD from LCH. A recent discovery of BRAFV600E mutations in ECD supports the clonal nature of this disease. There is also an inflammatory component of ECD characterized by hyperactivation of mitogen-activated protein kinase signalling. This plays a role in not only the pathogenesis of ECD, but also the clinical and radiological manifestations of the disease.43
ECD has been described in a wide age range (7–84 years) with a mean age at presentation of 53 years. The most common clinical manifestation is bone pain, as a consequence of osseous involvement. Typically, osseous involvement includes bilateral symmetrical sclerosis involving the diaphyses and metaphyses of long bones.42,44 Bone pain is more common in the lower extremities. Fever and weight loss are also common.
Approximately 50% of patients present with extraosseous involvement. Multiple organs and tissues may be affected including the central nervous, musculoskeletal, cardiovascular and pulmonary systems, as well as the mediastinum and retroperitoneum. Both neurologic and cardiac involvements are considered poor prognostic indicators.42,45 Overall, ECD is a progressive disease with a survival rate of 41–68% at 5 years.46,47
Cardiothoracic involvement is common. Pulmonary involvement has been described in up to 55% of patients.42,48 Dyspnoea is the most common respiratory complaint.48 On CT, the most common finding is smooth, symmetrical, interlobular septal thickening that can be diffuse or have upper or lower lung predominance (Figure 8). Subpleural thickening may also be present. All of these findings are the result of an inflammatory cellular infiltrate primarily composed of histiocytes.42,48 Other less common findings include ill-defined nodules, ground-glass opacities, subpleural consolidation and upper lobe predominant cysts.42 Histiocytic infiltration results in pleural and/or pericardial thickening.14,49 In a study by Brun et al,42 approximately 40% of patients with ECD had bilateral pleural involvement, most commonly pleural thickening. Pleural effusion was seen only in 3% of patients. In most patients, pleural involvement was present in the paravertebral region, and the majority of patients presented with associated lung involvement.
Figure 8.
Interstitial lung disease in two patients with Erdheim–Chester disease (ECD): CT images of the chest in a 74-year-old female (a) with ECD in the lung window is demonstrating smooth interlobular septal thickening and scattered areas of ground-glass opacity. Contrast CT of the chest in lung window in a 54-year-old male (b) is depicting more severe pulmonary involvement characterized by interlobular septal thickening, ill-defined ground-glass nodules, intralobular lines, ground-glass opacity and scattered bronchiectasis. Extensive pleural thickening can also be noted.
Periaortic infiltration is common in ECD and its CT appearance has been termed the “coated aorta”.50 Soft-tissue infiltration about the aorta is greatest at the level of the aortic arch and presents in up to 85% of patients with ECD. Extension along the arch vessels or coronary arteries may also be present.42 Myocardial infarction as a result of coronary artery involvement has been described.43 FDG PET-CT is helpful in delineating these regions of infiltration, which are metabolically active.51
Cardiac involvement may be seen as pericardial, epicardial or myocardial infiltration (Figure 9).42 Involvement of the epicardial fat presents as a soft-tissue attenuation infiltration that extends along the epicardial fat, often in the region of the right atrioventricular groove. The right coronary artery may be encased by this infiltrative soft tissue. Epicardial infiltration may result in mass effect upon the underlying chamber, most commonly the right atrium or right ventricular free wall. In some cases, epicardial infiltration can lead to tamponade physiology.52 Infiltration of the myocardium is possible and has been described in up to 31% of cases. Furthermore, in a small percentage of patients, cardiac involvement may appear as a right atrial mass.52 As with periaortic involvement, this infiltrative tissue is metabolically active on FDG PET-CT. Although epicardial and myocardial involvement in patients with ECD mimics other diseases, such as angiosarcoma and lymphoma, the constellation of intrathoracic findings (periaortic, lung and pleural involvement) is distinctive. Infiltration of the posterior mediastinum is present in approximately 50% of patients and commonly seen as an extension of retroperitoneal, pleural or periaortic disease.42 Evaluation of the retroperitoneum, in particular for the presence of bilateral perinephric fat infiltration, is key in distinguishing ECD from other entities such as lymphoma.
Figure 9.
Cardiomediastinal involvement in three patients with Erdheim–Chester disease: non-contrast CT of the chest through the heart (a) is showing epicardial and posterior mediastinal infiltrative soft tissue (arrows). Fused positron emission tomography-CT image in a different patient (b) is demonstrating a highly hypermetabolic infiltrative soft tissue in an epicardial distribution about the right atrium (arrows). The patient additionally has a small right pleural effusion as well as a fludeoxyglucose-avid right lower lobe histopathologically confirmed primary lung neoplasm (arrowhead). CT of the chest at the level of the heart in another patient (c) is demonstrating extensive soft tissue infiltrating the epicardium. This soft tissue is encasing the right coronary artery (arrow) in the right atrioventricular groove. There is extensive posterior mediastinal soft tissue that has displaced the descending aorta anteriorly. In addition, significant left pleural thickening is present.
JUVENILE XANTHOGRANULOMA
JXG is a non-LCH in which the principal cell involved is considered to be dermal and interstitial dendrocytes.53 JXG occurs primarily in childhood, often presenting in the first year of life, with occasional reports of the disease presenting in the adult population.54 In the vast majority of cases, JXG is a cutaneous disorder, with the characteristic lesion being a red-brown papular cutaneous nodule. Diagnosis is based on biopsy of these lesions which demonstrate an infiltrate of foamy histiocytes and Touton giant cells.55
Although JXG rarely presents as a non-cutaneous disorder, JXG tumoral involvement of other organs has been described in up to 4% of patients. The more commonly involved visceral organs include the liver and lungs (Figure 10). Pulmonary involvement is seen in about 38% of patients with visceral disease, the typical lesion being nodules of varying sizes.56 Cardiac involvement is extremely rare and has been described only once in the literature.54 Although visceral lesions regress without therapy, death in patients with visceral disease has been reported occasionally as a result of hepatic failure.56
Figure 10.
Juvenile xanthogranuloma in a 3-month-old infant: coronal (a) and axial (b) T2 weighted MR images of the chest are demonstrating multiple T2 hyperintese nodules throughout the lung parenchyma as well as in the liver.
CONCLUSION
Cardiothoracic involvement in patients with primary histiocytoses is not uncommon. Imaging findings are widely varied and, with the exception of pulmonary LCH, often non-specific. When encountering patients with known systemic LCH, or in those patients with osseous lesions that suggest this diagnosis, the radiologist must be aware of other possible sites of intrathoracic disease. Similarly, the radiologist must be aware of the constellation of findings in patients with RDD or ECD, as characteristic clues to the diagnosis of these patients are often present in sites of cardiothoracic involvement.
Contributor Information
Daniel Vargas, Email: daniel.vargas@ucdenver.edu.
J Caleb Richards, Email: johncalebrichards@gmail.com.
Daniel Ocazionez, Email: danielocazionez@gmail.com.
Arlene Sirajuddin, Email: asirajuddin@radiology.arizona.edu.
Lorna Browne, Email: lorna.browne@childrenscolorado.org.
Carlos S Restrepo, Email: crestr@gmail.com.
REFERENCES
- 1.Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, et al. Contemporary classification of histiocytic disorders. The WHO committee on histiocytic/reticulum cell proliferations. Reclassification working group of the histiocyte society. Med Pediatr Oncol 1997; 29: 157–66. doi: https://doi.org/10.1002/(SICI)1096-911X(199709)29:3<157::AID-MPO1>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- 2.Jaffe R. The histiocytoses. Clin Lab Med 1999; 19: 135–55. [PubMed] [Google Scholar]
- 3.Chu T, Jaffe R. The normal langerhans cell and the LCH cell. Br J Cancer Suppl 1994; 23: S4–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Katz SI, Tamaki K, Sachs DH. Epidermal langerhans cells are derived from cells originating in bone marrow. Nature 1979; 282: 324–6. doi: https://doi.org/10.1038/282324a0 [DOI] [PubMed] [Google Scholar]
- 5.Lieberman PH, Jones CR, Steinman RM, Erlandson RA, Smith J, Gee T, et al. Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol 1996; 20: 519–52. doi: https://doi.org/10.1097/00000478-199605000-00001 [DOI] [PubMed] [Google Scholar]
- 6.Zaveri J, La Q, Yarmish G, Neuman J. More than just langerhans cell histiocytosis: a radiologic review of histiocytic disorders. Radiographics 2014; 34: 2008–24. doi: https://doi.org/10.1148/rg.347130132 [DOI] [PubMed] [Google Scholar]
- 7.Badalian-Very G, Vergilio JA, Fleming M, Rollins BJ. Pathogenesis of langerhans cell histiocytosis. Annu Rev Pathol 2013; 8: 1–20. doi: https://doi.org/10.1146/annurev-pathol-020712-163959 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt S, Eich G, Geoffray A, Hanquinet S, Waibel P, Wolf R, et al. Extraosseous langerhans cell histiocytosis in children. Radiographics 2008; 28: 707–26. doi: https://doi.org/10.1148/rg.283075108 [DOI] [PubMed] [Google Scholar]
- 9.Ducassou S, Seyrig F, Thomas C, Lambilliotte A, Marec-Berard P, Berger C, et al. Thymus and mediastinal node involvement in childhood langerhans cell histiocytosis: long-term follow-up from the French national cohort. Pediatr Blood Cancer 2013; 60: 1759–65. doi: https://doi.org/10.1002/pbc.24603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia CX, Li R, Wang ZH, Lv FJ, Tang XQ, Li QF, et al. A rare cause of goiter: langerhans cell histiocytosis of the thyroid. Endocr J 2012; 59: 47–54. doi: https://doi.org/10.1507/endocrj.EJ11-0243 [DOI] [PubMed] [Google Scholar]
- 11.Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics 1992; 12: 801–23. doi: https://doi.org/10.1148/radiographics.12.4.1636041 [DOI] [PubMed] [Google Scholar]
- 12.Kaste SC, Rodriguez-Galindo C, McCarville ME, Shulkin BL. PET-CT in pediatric langerhans cell histiocytosis. Pediatr Radiol 2007; 37: 615–22. doi: https://doi.org/10.1007/s00247-007-0467-4 [DOI] [PubMed] [Google Scholar]
- 13.Friedman PJ, Liebow AA, Sokoloff J. Eosinophilic granuloma of lung. Clinical aspects of primary histiocytosis in the adult. Medicine 1981; 60: 385–96. doi: https://doi.org/10.1097/00005792-198111000-00001 [PubMed] [Google Scholar]
- 14.Nagarjun Rao R, Moran CA, Suster S. Histiocytic disorders of the lung. Adv Anat Pathol 2010; 17: 12–22. doi: https://doi.org/10.1097/PAP.0b013e3181c6a524 [DOI] [PubMed] [Google Scholar]
- 15.Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, et al. Langerhans'-cell histiocytosis (histiocytosis X)—a clonal proliferative disease. N Engl J Med 1994; 331: 154–60. doi: https://doi.org/10.1056/NEJM199407213310303 [DOI] [PubMed] [Google Scholar]
- 16.Yousem SA, Colby TV, Chen YY, Chen WG, Weiss LM. Pulmonary langerhans' cell histiocytosis: molecular analysis of clonality. Am J Surg Pathol 2001; 25: 630–6. doi: https://doi.org/10.1097/00000478-200105000-00010 [DOI] [PubMed] [Google Scholar]
- 17.Abbott GF, Rosado-de-Christenson ML, Franks TJ, Frazier AA, Galvin JR. From the archives of the AFIP: pulmonary langerhans cell histiocytosis. Radiographics 2004; 24: 821–41. doi: https://doi.org/10.1148/rg.243045005 [DOI] [PubMed] [Google Scholar]
- 18.Kambouchner M, Basset F, Marchal J, Uhl JF, Hance AJ, Soler P. Three-dimensional characterization of pathologic lesions in pulmonary langerhans cell histiocytosis. Am J Respir Crit Care Med 2002; 166: 1483–90. doi: https://doi.org/10.1164/rccm.2201050 [DOI] [PubMed] [Google Scholar]
- 19.Vassallo R, Ryu JH. Smoking-related interstitial lung diseases. Clin Chest Med 2012; 33: 165–78. doi: https://doi.org/10.1016/j.ccm.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 20.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary langerhans'-cell histiocytosis in adults. N Engl J Med 2002; 346: 484–90. doi: https://doi.org/10.1056/NEJMoa012087 [DOI] [PubMed] [Google Scholar]
- 21.Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary langerhans cell histiocytosis. Chest 2004; 125: 1028–32. doi: https://doi.org/10.1378/chest.125.3.1028 [DOI] [PubMed] [Google Scholar]
- 22.Fraser RS. Fraser and Paré's diagnosis of diseases of the chest. 4th edn. Philadelphia, PA: W.B. Saunders; 1999. [Google Scholar]
- 23.Moore AD, Godwin JD, Muller NL, Naidich DP, Hammar SP, Buschman DL, et al. Pulmonary histiocytosis X: comparison of radiographic and CT findings. Radiology 1989; 172: 249–54. doi: https://doi.org/10.1148/radiology.172.1.2787035 [DOI] [PubMed] [Google Scholar]
- 24.Greiwe AC, Miller K, Farver C, Lau CT. AIRP best cases in radiologic-pathologic correlation: pulmonary langerhans cell histiocytosis. Radiographics 2012; 32: 987–90. doi: https://doi.org/10.1148/rg.324115015 [DOI] [PubMed] [Google Scholar]
- 25.Grenier P, Valeyre D, Cluzel P, Brauner MW, Lenoir S, Chastang C. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology 1991; 179: 123–32. doi: https://doi.org/10.1148/radiology.179.1.2006262 [DOI] [PubMed] [Google Scholar]
- 26.Castoldi MC, Verrioli A, De Juli E, Vanzulli A. Pulmonary langerhans cell histiocytosis: the many faces of presentation at initial CT scan. Insights Imaging 2014; 5: 483–92. doi: https://doi.org/10.1007/s13244-014-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonelli FS, Hartman TE, Swensen SJ, Sherrick A. Accuracy of high-resolution CT in diagnosing lung diseases. AJR Am J Roentgenol 1998; 170: 1507–12. doi: https://doi.org/10.2214/ajr.170.6.9609163 [DOI] [PubMed] [Google Scholar]
- 28.Brauner MW, Grenier P, Mouelhi MM, Mompoint D, Lenoir S. Pulmonary histiocytosis X: evaluation with high-resolution CT. Radiology 1989; 172: 255–8. doi: https://doi.org/10.1148/radiology.172.1.2787036 [DOI] [PubMed] [Google Scholar]
- 29.Seely JM, Salahudeen S, Sr, Cadaval-Goncalves AT, Jamieson DH, Dennie CJ, Matzinger FR, et al. Pulmonary langerhans cell histiocytosis: a comparative study of computed tomography in children and adults. J Thorac Imaging 2012; 27: 65–70. doi: https://doi.org/10.1097/RTI.0b013e3181f49eb6 [DOI] [PubMed] [Google Scholar]
- 30.Fartoukh M, Humbert M, Capron F, Maitre S, Parent F, Le Gall C, et al. Severe pulmonary hypertension in histiocytosis X. Am J Respir Crit Care Med 2000; 161: 216–23. doi: https://doi.org/10.1164/ajrccm.161.1.9807024 [DOI] [PubMed] [Google Scholar]
- 31.Baqir M, Vassallo R, Maldonado F, Yi ES, Ryu JH. Utility of bronchoscopy in pulmonary langerhans cell histiocytosis. J Bronchology Interv Pulmonol 2013; 20: 309–12. doi: https://doi.org/10.1097/LBR.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 32.Maleszewski JJ, Hristov AC, Halushka MK, Miller DV. Extranodal Rosai-Dorfman disease involving the heart: report of two cases. Cardiovasc Pathol 2010; 19: 380–4. doi: https://doi.org/10.1016/j.carpath.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Perry AM, Cao W, Smith LM, Hsi ED, Liu X, et al. Relationship between Rosai-Dorfman disease and IgG4-related disease: study of 32 cases. Am J Clin Pathol 2013; 140: 395–402. doi: https://doi.org/10.1309/AJCPFH0SJ6YILXJU [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Hyjek E, Vardiman J. A subset of Rosai-Dorfman disease exhibits features of IgG4-related disease. Am J Clin Pathol 2013; 139: 622–32. doi: https://doi.org/10.1309/AJCPARC3YQ0KLIOA [DOI] [PubMed] [Google Scholar]
- 35.Ajise OE, Stahl-Herz J, Goozner B, Cassai N, McRae G, Wieczorek R. Extranodal Rosai-Dorfman disease arising in the right atrium: a case report with literature review. Int J Surg Pathol 2011; 19: 637–42. doi: https://doi.org/10.1177/1066896911409577 [DOI] [PubMed] [Google Scholar]
- 36.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol 1990; 7: 19–73. [PubMed] [Google Scholar]
- 37.Karunanithi S, Singh H, Sharma P, Naswa N, Kumar R. 18F-FDG PET/CT imaging features of Rosai Dorfman disease: a rare cause of massive generalized lymphadenopathy. Clin Nucl Med 2014; 39: 268–9. doi: https://doi.org/10.1097/RLU.0b013e31828731da [DOI] [PubMed] [Google Scholar]
- 38.Liu B, Lee NJ, Otero HJ, Servaes S, Zhuang H. Rosai-Dorfman disease mimics lymphoma on FDG PET/CT in a pediatric patient. Clin Nucl Med 2014; 39: 206–8. [DOI] [PubMed] [Google Scholar]
- 39.Sarraj A, Zarra KV, Jimenez Borreguero LJ, Caballero P, Nuche JM. Isolated cardiac involvement of Rosai-Dorfman disease. Ann Thorac Surg 2012; 94: 2118–20. doi: https://doi.org/10.1016/j.athoracsur.2012.04.134 [DOI] [PubMed] [Google Scholar]
- 40.Morsolini M, Nicola M, Paulli M, D'Armini AM. Primary pulmonary artery Rosai-Dorfman disease mimicking sarcoma. J Thorac Cardiovasc Surg 2013; 146: e57–9. doi: https://doi.org/10.1016/j.jtcvs.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 41.Bi Y, Huo Z, Meng Y, Wu H, Yan J, Zhou Y, et al. Extranodal Rosai-Dorfman disease involving the right atrium in a 60-year-old male. Diagn Pathol 2014; 9: 115. doi: https://doi.org/10.1186/1746-1596-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brun AL, Touitou-Gottenberg D, Haroche J, Toledano D, Cluzel P, Beigelman-Aubry C, et al. Erdheim-Chester disease: CT findings of thoracic involvement. Eur Radiol 2010; 20: 2579–87. doi: https://doi.org/10.1007/s00330-010-1830-7 [DOI] [PubMed] [Google Scholar]
- 43.Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 2014; 124: 483–92. doi: https://doi.org/10.1182/blood-2014-03-561381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittenberg KH, Swensen SJ, Myers JL. Pulmonary involvement with Erdheim-Chester disease: radiographic and CT findings. AJR Am J Roentgenol 2000; 174: 1327–31. doi: https://doi.org/10.2214/ajr.174.5.1741327 [DOI] [PubMed] [Google Scholar]
- 45.Gabbay LB, Leite Cda C, Andriola RS, Pinho Pda C, Lucato LT. Histiocytosis: a review focusing on neuroimaging findings. Arq Neuropsiquiatr 2014; 72: 548–58. doi: https://doi.org/10.1590/0004-282X20140063 [DOI] [PubMed] [Google Scholar]
- 46.Arnaud L, Hervier B, Neel A, Hamidou MA, Kahn JE, Wechsler B, et al. CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood 2011; 117: 2778–82. doi: https://doi.org/10.1182/blood-2010-06-294108 [DOI] [PubMed] [Google Scholar]
- 47.Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, Wechsler J, Brun B, Remy M, et al. Erdheim-Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine 1996; 75: 157–69. [DOI] [PubMed] [Google Scholar]
- 48.Egan AJ, Boardman LA, Tazelaar HD, Swensen SJ, Jett JR, Yousem SA, et al. Erdheim-Chester disease: clinical, radiologic, and histopathologic findings in five patients with interstitial lung disease. Am J Surg Pathol 1999; 23: 17–26. doi: https://doi.org/10.1097/00000478-199901000-00002 [DOI] [PubMed] [Google Scholar]
- 49.Walker CM, Takasugi JE, Chung JH, Reddy GP, Done SL, Pipavath SN, et al. Tumorlike conditions of the pleura. Radiographics 2012; 32: 971–85. doi: https://doi.org/10.1148/rg.324115184 [DOI] [PubMed] [Google Scholar]
- 50.Serratrice J, Granel B, De Roux C, Pellissier JF, Swiader L, Bartoli JM, et al. “Coated aorta”: a new sign of Erdheim-Chester disease. J Rheumatol 2000; 27: 1550–3. [PubMed] [Google Scholar]
- 51.Girszyn N, Arnaud L, Villain D, Kahn JE, Piette AM, Bletry O. Usefulness of combined positron emission tomography and computed tomography imaging in Erdheim-Chester disease. [In French.] Rev Med Interne 2007; 28: 770–4. doi: https://doi.org/10.1016/j.revmed.2007.05.036 [DOI] [PubMed] [Google Scholar]
- 52.Haroche J, Cluzel P, Toledano D, Montalescot G, Touitou D, Grenier PA, et al. Images in cardiovascular medicine. Cardiac involvement in Erdheim-Chester disease: magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients. Circulation 2009; 119: e597–8. doi: https://doi.org/10.1161/CIRCULATIONAHA.108.825075 [DOI] [PubMed] [Google Scholar]
- 53.Lin L, Salisbury EL, Gardiner I, Varikatt W. Solitary juvenile xanthogranuloma in the lung of a young adult. Pathology 2011; 43: 503–7. doi: https://doi.org/10.1097/PAT.0b013e328348900d [DOI] [PubMed] [Google Scholar]
- 54.Lehrke HD, Johnson CK, Zapolanski A, Kasatki A, Grau JB, Maleszewski JJ. Intracardiac juvenile xanthogranuloma with presentation in adulthood. Cardiovasc Pathol 2014; 23: 54–6. doi: https://doi.org/10.1016/j.carpath.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 55.Murphy JT, Soeken T, Megison S, Perez E. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol 2014; 36: 641–5. doi: https://doi.org/10.1097/MPH.0000000000000027 [DOI] [PubMed] [Google Scholar]
- 56.Dehner LP. Juvenile xanthogranulomas in the first two decades of life: a clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol 2003; 27: 579–93. doi: https://doi.org/10.1097/00000478-200305000-00003 [DOI] [PubMed] [Google Scholar]