Abstract
Stereotactic body radiotherapy (SBRT) is being increasingly utilized in the treatment of prostate cancer. With the advent of high-precision radiosurgery systems, it is possible to obtain dose distributions akin to high-dose rate brachytherapy with SBRT. However, urethral toxicity has a significant impact on the quality of life in patients with prostate cancer. Contouring the male urethra on a CT scan is difficult in the absence of an indwelling catheter. In this pictorial essay, we have used the MRI obtained for radiotherapy planning to aid in the delineation of the male urethra and have attempted to define guidelines for the same.
INTRODUCTION
The male urethra is approximately 17.5–20-cm long and extends from the bladder to the external urethral meatus. It is divided into posterior and anterior portions. The posterior urethra extends from the distal portion of bladder neck to the inferior urogenital diaphragm, and the anterior urethra extends from there to the external meatus distally. The posterior urethra consists of a prostatic segment and a membranous segment.1
Although there are isolated reports emphasizing the value of delineating the urethra as a volume at risk with the possibility of improving genitourinary (GU) toxicity, a large variation exists regarding the use of urethral sparing in practice, as no concrete contouring guideline is available.
The purpose of this pictorial essay was to use T2 weighted MRI, which is most commonly used for radiotherapy planning, to aid the radiation oncologist in delineating the male urethra.
METHODS AND MATERIALS
A representative MR image set for a normal scan of the pelvis was selected for the purpose of delineation. Scan parameters included an Intravenous gadolinium-enhanced MRI Scan on a 3.0T Siemens MRI scanner, axial plane images with continuous slices of thickness 1.5 mm and a T2 echo sequence (repetition time 14 s, echo time 7 s), with the upper limit at S1 vertebra and lower limit at mid-thigh.
The contours were defined with the help of a radiologist specializing in the urogenital system. The urethra appears more hyperintense on MRI T2 sequences than on T1 sequences or on the radiotherapy planning CT scan. This is one of the easiest and most reproducible ways to delineate the membranous urethra. Delineation is performed on the axial sections with the aid of the sagittal and coronal sections.
We also tried contouring the urethra on a representative pelvic CT scan with a Foley's catheter in situ. The parameters for this CT scan were: Siemens Biograph Simulator with 3-mm sections with Omnipaque i.v. contrast and Foley's catheter F-16, with scan limits at S1 vertebra superiorly and mid-thigh inferiorly. Delineation was performed on the axial sections with the aid of the sagittal and coronal sections.
We used the contouring tools of the CMS Monaco® treatment planning system v. 3 (Elekta, Stockholm, Sweden).
RESULTS
A step-by-step guide to delineate the male urethra
Step 1: identify the bladder neck and the prostate
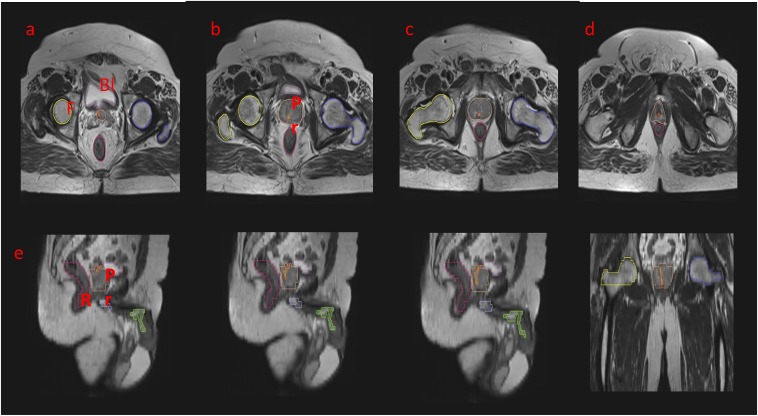
To begin, identify the MRI slice where the bladder neck starts. On T2 axial MRI slices, the prostatic urethra is seen as a moderately hyperintense region in the central to the posterior portion of the prostate, surrounded by the gland on all sides, and containing the intensely hyperintense urine within (Figure 1).
Figure 1.
An axial T2 weighted sequence showing the stepwise delineation of the prostatic urethra: the prostatic urethra has been marked as a tubular structure in red–green. (a) Identifying the bladder neck: the hyperintense signal is representing the urine in the bladder. (b–d) It is traced further into the parenchyma of the prostate. This is representing the prostatic urethra. (e–g) The sagittal sectional correlation to aid in delineation: the urethra as a whole has been depicted. (g) The prostatic, bulbar and penile parts of the urethra are shown. (h) A coronal section is illustrating the complete prostatic urethra. Bl, bladder; F, femur; Pr, prostate; R, rectum.
However, the urethra is not always centrally located within the gland. The T2 MRI sequences can be a better tool to see the abnormal position of the urethra than a CT scan alone.
Step 2: identify the membranous urethra by further tracing the hyperintensity towards the urogenital diaphragm
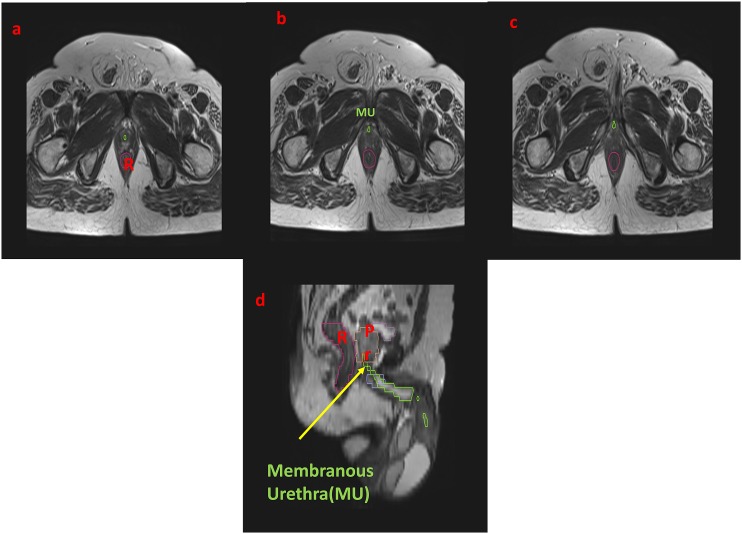
The membranous urethra, which extends for around 1 cm from the prostatic part, can be seen on axial T2 weighted images as a low-signal intensity ring surrounding the high-signal intensity epithelial surface. It ends at the inferior aspect of urogenital diaphragm (Figures 2 and 3).
Figure 2.
The axial T2 weighted sequence showing the stepwise delineation of the prostatic urethra: the membranous urethra (MU) has been marked as a tubular structure in green. (a–c) Urogenital diaphragm: the MU is traversing through it and ending at its inferior portion, beyond which it is continuing as the bulbar part. (d) The coronal section with an arrow is denoting the MU. Pr, prostate; R, rectum.
Figure 3.
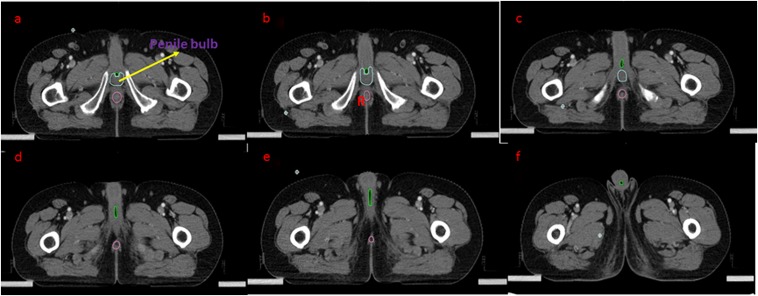
Axial T2 MRI: the penile bulb (corpus spongiosum) has been identified and the bulbar urethra is starting at the inferior border of the urogenital diaphragm. It is delineated in green. (a) The penile bulb has been marked in purple, with the bulbar urethra marked in green. (b, c) The bulbar urethra is continuing as the penile urethra.
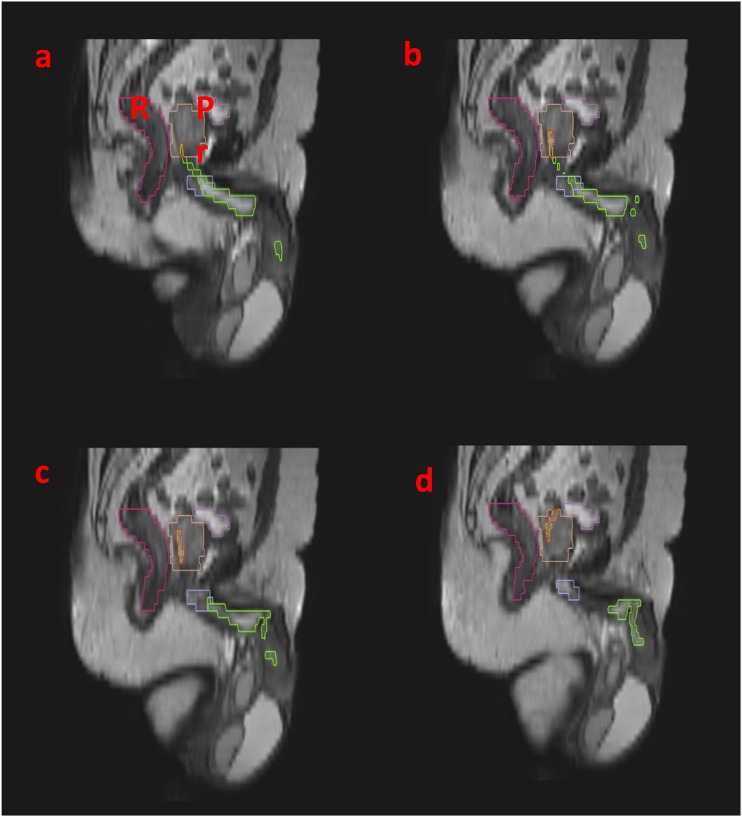
Step 3: identify the bulbar urethra and continue on to penile urethra
The bulbar urethra is located between the inferior margin of the urogenital diaphragm and the penoscrotal junction. It courses within the root of the penis and is seen as a hypointense tubular structure in the midline within the bulb of the corpus spongiosum. The penile urethra extends from the penoscrotal junction to the external meatus. The distal penile urethra is often not visible on MRI without a Foley's catheter in situ Figure 4.
Figure 4.
Sagittal T2 MRI: for correlating with the axial sections, (a–d) the penile bulb has been marked in purple. The bulbar urethra and penile urethra have been marked in green. Bl, bladder; Pr, prostate.
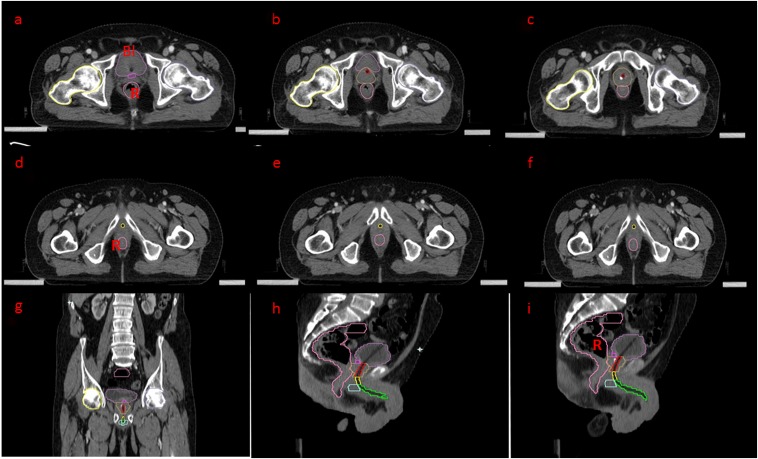
We have also tried contouring the urethra on a CT scan in patients who were catheterized, particularly in situations where MRI is not available, and the radiation oncologist often has only the CT scans for delineation, and this technique has its own limitations. The idea here is to trace the catheter from the bladder neck. Although it is a simple procedure, it has its own demerits, such as the need for catheterization in all cases and increased urethra volume with the catheter in situ. In rare cases where patients present with an indwelling catheter placed earlier for either bladder outlet obstruction or urinary incontinence, this practice can be undertaken (Figures 5 and 6).
Figure 5.
Axial and sagittal CT scans with catheter in situ: (a–c) An axial CT scan—the bulb of Foley's catheter is seen in the bladder. The bladder neck has been delineated in pink, the prostate in yellow. The prostatic urethra is traced along the catheter (red). (d–f) The prostatic urethra is continuing as the membranous part, delineated in yellow. (g) The coronal section is correlating the delineation. (h, i) Sagittal sections for correlating the course of the urethra along Foley's catheter. Bl, bladder; R, rectum.
Figure 6.
Axial CT scan with catheter in situ: (a–c) the bulbar urethra has been contoured in blue. (d–f) The penile urethra is traced till the meatus (green). R, rectum.
DISCUSSION
Imaging studies like voiding cystourethrography, retrograde urethrography or transrectal ultrasonography can help in assessing the urethra. However, these investigations are invasive and have a limited field of view, making it difficult to appreciate the details of the periurethral tissues.2 It is difficult to delineate the male urethra on CT images without aids like an indwelling Foley's catheter. On the other hand, MRI studies have multiplanar capability with excellent tissue contrast, providing anatomical details of the urethra as well as the periurethral tissues.
GU toxicity is a common issue for patients undergoing prostate cancer external beam radiation therapy. With dose escalation aiming at higher locoregional control, at least half of all patients require symptomatic management of acute dysuria, frequency or urgency.3 Late urinary toxicity is also a relatively common problem, ranging from hesitancy to urinary retention and gross haematuria. Although numerous studies have been published investigating dosimetric parameters as predictors of late urinary toxicity, almost all of these studies have focused on the urinary bladder. Quantitative Analyses of Normal Tissue Effects in the Clinic meta-analysis points out that late urinary toxicity is heterogeneous, and the different types of complications may have unique predisposing factors.4 In particular, urethral stricture formation may not be adequately predicted by bladder dosimetry.
GU symptoms can originate in any part along the GU tract, including the prostate, bladder and intraprostatic urethra, and it is difficult to identify which organs at risk are ultimately responsible for a given toxicity in isolation.
Further, prostatic urethra and the bladder neck lie within the target volume for prostate cancer. It is difficult to segregate the bladder- and urethra-related toxicities. The low rate of GU toxicity observed in post-prostatectomy series suggests that the prostate and prostatic urethra may be responsible for most of the GU symptoms.5,6 Stereotactic body radiotherapy (SBRT) plans are more heterogeneous; thus, a temporal variation in bladder and urethral dose volume is an important determinant for acute and late Radiation Therapy Oncology Group Grade 2 or above GU toxicity.7,8
In addition, there are limitations in the extent of dose optimization possible for the bladder based on the interface with the prostate, which would increase with a larger prostate gland size, an inherent confounding factor that limits bladder dose–volume histogram analysis.9 These results imply that correlating GU toxicity considering only the bladder as the organ at risk may not be justifiable and that a large component of GU toxicity may be attributable to the urethra.
The quantum of radiobiological advantage resulting from escalating the intraprostatic dose remains unclear. However, extrapolating from the dose distributions and consequent favourable outcomes with high-dose rate brachytherapy in the intraprostatic region, Fuller et al10 employed SBRT with CyberKnife (CK; Accuray Inc., Sunnyvale, CA) for treatment of carcinoma prostate using a urethra-sparing technique. They were able to achieve where maximal and fractional doses in urethra that (Dmax, D10 and D50) that were better than the brachytherapy plans by creating “horseshoe-shaped” dose escalation volumes within the lateral and posterior aspects of the prostate gland with CyberKnife planning.
There are isolated reports regarding the value of the urethra as a volume at risk and that dose–volume optimization could improve GU toxicity especially in SBRT and brachytherapy. A study by Seymour et al7 reported late GU toxicity Grades 1, 2 and 3 in 19.6, 19.6 and 3.6% of cases treated with radical SBRT, respectively. Overall risk of any Grade 2 and above GU toxicity was associated with baseline International Prostate Symptom Scores >7, prostate volume ≥50 ml, urethral volume receiving 44 Gy or above and bladder volume receiving 19 Gy or above. Another similar study by Chen et al11 reported a 31% 2-year actuarial incidence of GU toxicity Grade 2 or higher.
One of the limitations of urethral delineation is that most contouring and planning systems allow only the axial sections to be imported and the sagittal and coronal sections are reconstructed from the axial sections. Development of better contouring software, improvement in imaging techniques coupled with sparing of the urethra as well as dose escalation may circumvent these limitations and are expected to yield promising results in future.
CONCLUSION
Guidelines for urethra delineation, especially in the era of hypofractionation, are an imperative but unmet need so far. This diagrammatic approach is a simple tool aiding the radiation oncologists in contouring the male urethra and needs to be validated through further studies.
Contributor Information
Tejinder Kataria, Email: tejinderkataria@medanta.org.
Deepak Gupta, Email: deepakonco@gmail.com.
Shikha Goyal, Email: shikha.goyal@medanta.org.
Shyam S Bisht, Email: shyam.bisht@medanta.org.
Ravi Chaudhary, Email: ravi.chaudhary@medanta.org.
Kushal Narang, Email: kushal.narang@medanta.org.
Susovan Banerjee, Email: susovan.banerjee@medanta.org.
Trinanjan Basu, Email: trinanjan.basu@medanta.org.
Ashu Abhishek, Email: ashu.abhishek@medanta.org.
Sasikumar Sambasivam, Email: sasikumarsms@gmail.com.
Nisha T Vishnu, Email: nisha.vishnu@medanta.org.
REFERENCES
- 1.Gray H, Clemente C. Anatomy of the human body. Philadelphia, PA: Lea & Febiger; 1985. [Google Scholar]
- 2.Ryu J, Kim B. MR imaging of the male and female urethra. Radiographics 2001; 21: 1169–85. doi: https://doi.org/10.1148/radiographics.21.5.g01se121169 [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005; 294: 1233–9. doi: https://doi.org/10.1001/jama.294.10.1233 [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys 2010; 76(Suppl. 3): S116–22. doi: https://doi.org/10.1016/j.ijrobp.2009.02.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Aschkenasy E, Kelsen S, Leibel SA. Tolerance and early outcome results of postprostatectomy three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 1997; 39: 327–33. doi: https://doi.org/10.1016/S0360-3016(97)00056-4 [DOI] [PubMed] [Google Scholar]
- 6.Feng M, Hanlon AL, Pisansky TM, Kuban D, Catton CN, Michalski JM, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 1417–23. doi: https://doi.org/10.1016/j.ijrobp.2007.01.049 [DOI] [PubMed] [Google Scholar]
- 7.Seymour Z, Chang A, Zhang L, Kirby N, Descovich M, Roach M, et al. Dose-volume analysis and the temporal nature of toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol 2015; 5: e465–72. doi: https://doi.org/10.1016/j.prro.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Descovich M, Carrara M, Morlino S, Pinnaduwage DS, Saltiel D, Pouliot J, et al. Improving plan quality and consistency by standardization of dose constraints in prostate cancer patients treated with CyberKnife. J Appl Clin Med Phys 2013; 14: 162–72. doi: https://doi.org/10.1120/jacmp.v14i5.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu IC, Hunt D, Straube W, Pouliot J, Cunha A, Krishnamurthy D, et al. Dosimetric analysis of radiation therapy oncology group 0321: the importance of urethral dose. Pract Radiat Oncol 2014; 4: 27–34. doi: https://doi.org/10.1016/j.prro.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller DB, Naitoh J, Lee C, Hardy S, Jin H. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys 2008; 70: 1588–97. doi: https://doi.org/10.1016/j.ijrobp.2007.11.067 [DOI] [PubMed] [Google Scholar]
- 11.Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol 2013; 8: 58. doi: https://doi.org/10.1186/1748-717X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]