Abstract
Objective:
To determine the clinical and imaging implications of prominent cortical and medullary veins on susceptibility-weighted images (SWI) of patients with acute stroke.
Methods:
Consecutive patients with acute ischaemic stroke who had SWI scan within 24 h of symptom onset or time last-seen-well were included. The SWI series were reviewed for the presence of prominent cortical and medullary veins and were graded independently by two neuroradiologists. The correlations between prominent vein grades with different imaging and clinical variables were determined.
Results:
Among 213 patients, prominent SWI cortical and medullary veins were identified in 35 (16.4%) patients and 20 (9.4%) patients, respectively. There was fair interobserver agreement (k = 0.314–0.338, p ≤ 0.001) for grading, and moderate agreement (k = 0.406–0.413, p ≤ 0.001) for the presence of prominent veins. Both prominent cortical and medullary veins were associated with the presence of arterial occlusion (rho = 0.232, p = 0.001; rho = 0.180, p = 0.008; respectively) and larger infarct volume (rho = 0.445, p < 0.001; rho = 0.167, p = 0.015; respectively). However, neither cortical nor medullary cortical veins were associated with the severity of symptoms at admission or clinical outcome. Prominent cortical veins were independent predictors of arterial occlusion (p = 0.018), whereas prominent medullary veins were more strongly associated with larger infarct volumes (p < 0.001).
Conclusion:
There were small but significant correlations between cortical and medullary veins on SWI with arterial occlusion and large infarct volume in acute ischaemic stroke.
Advances in knowledge:
In consecutive patients with acute ischaemic stroke within anterior and posterior circulation territories, prominent cortical and medullary veins on SWI series are associated with imaging biomarkers of poor clinical outcome (i.e. large infarct volume and major arterial occlusion).
INTRODUCTION
With its high sensitivity for paramagnetic substances and high resolution, susceptibility-weighted imaging (SWI) has emerged as a crucial MRI technique that is becoming more routinely in use. The SWI sequence has developed into a useful clinical tool in the evaluation of cerebrovascular diseases, including detection of intraparenchymal haemorrhage, venous thrombosis and haemorrhagic transformation of stroke, and even assessment of brain tissue at risk of infarction.1–4 The presence of microhaemorrhage, prominent veins and susceptible vein sign on SWI series has been applied for evaluating stroke severity, treatment response and prognosis.5–8
Asymmetrically dilated cortical and medullary veins on SWI have been reported in patients with acute ischaemic stroke.9,10 The presence of prominent veins has been hypothesized to represent an uncoupling between oxygen supply and demand within the hypoperfused tissue, with a relative increase in the deoxyhaemoglobin to oxyhaemoglobin ratio.11 Prominent veins on SWI are thought to represent either ischaemic penumbra or poor collateralization of arterial supply.1,9,12,13 However, the appearance of prominent cortical and medullary veins seems to be pathophysiologically complex; thus, their clinical significance remains elusive.
The present study aimed to evaluate the frequency and severity of prominent cortical and medullary veins on SWI in patients with acute ischaemic stroke scanned within 24 h of symptom onset. We also evaluated the relationship between the presence of prominent cortical and medullary veins with arterial occlusion, severity of symptoms, infarct volume and clinical outcome.
METHODS AND MATERIALS
Patients
The prospectively collected clinical and imaging database of consecutive patients with acute ischaemic stroke, in two university-affiliated hospitals, was retrospectively reviewed, between January 2011 and December 2014, constituting a 4-year period. The inclusion criteria were (1) acute ischaemic stroke with no evidence of intracranial haemorrhage; (2) brain MRI performed within 24 h from witnessed symptom onset (or time last-seen-well); (3) interpretable-quality SWI sequence obtained as part of the MRI; (4) and had undergone either simultaneous Magnetic Resonance Angiography (MRA) or Computed tomography angiography (CTA) within 3 h of the MRI scan. Institutional review board approval at both centres was obtained.
Clinical data
The clinical data were obtained from electronic medical records and stroke registry. The initial stroke severity was assessed using the National Institutes of Health Stroke Scale score. Patients received i.v. tissue plasminogen activator and/or intra-arterial (IA) thrombolysis/thrombectomy based on national treatment guidelines in use at the time of presentation.14 Clinical outcome was assessed based on 3-month modified Rankin Scale (MRS). Poor outcome was defined by a MRS score of >2.
Image acquisition
Most patients were examined using a 1.5- or 3.0-T MR scanner. The SWI sequence was acquired with the following parameters at 1.5 T (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany): repetition time 34.6 ms; echo time 49.6 ms; flip angle 15°; slice thickness 2 mm; field of view 23 cm. At 3.0 T (Magnetom Tim Trio; Siemens Medical Solutions, Erlangen, Germany), the parameters were: repetition time 27 ms; echo time 20 ms; flip angle 15°; slice thickness 2 mm; field of view 23 cm.
Image analysis
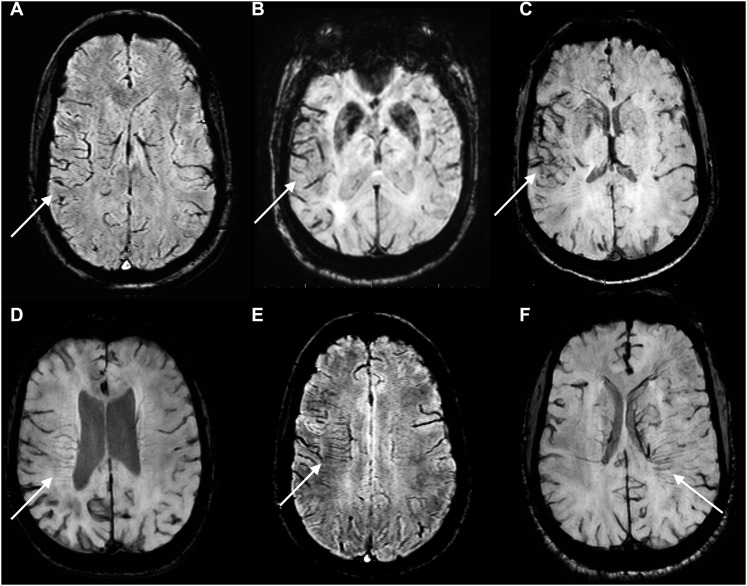
The SWI sequences of all patients were assessed for the presence of prominent cortical and/or medullary veins by two independent reviewers masked from clinical outcome. A 0–3 scoring system was used for the evaluation of prominence grades akin to prior studies: 0 = none, 1 = mild, 2 = moderate and 3 = distinct prominence (Figure 1).9,10,12 Arterial occlusion was determined on time-of-flight MRA obtained at the same time as SWI, or—if unavailable—on CTA performed within 3 h of the MRI examination. The diffusion-weighted imaging infarct lesion volumes were manually segmented and measured using the MRIcron software (McCausland Center for Brain Imaging, University of South Carolina, Columbia, SC).
Figure 1.
A 0–3 scoring system was applied for grading of prominent cortical and medullary veins on susceptibility-weighted imaging series compared with contralateral hemisphere. Upper row images from a to c are showing Grades I–III prominent cortical veins (arrows) and lower row images from d to f are showing Grades I–III prominent medullary veins (arrows).
Statistical analysis
The continuous, categorical and ordinal variables are expressed as mean ± standard deviation, number (percentage) and median (interquartile), respectively, and compared using Student's t, Mann–Whitney U and χ2 tests, respectively. Interobserver agreement was determined using Cohen's Kappa. Spearman's correlation test was used to analyze the correlation between ordinal variables (e.g. prominent vein grades). Stepwise variable selection was used with binary logistic and linear regression analysis in the determination of independent predictor variables, as appropriate. All analyses were performed using the SPSS® v. 21 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). The p-value threshold for significance was set to <0.05.
RESULTS
Patient demographics
A total of 213 patients were included. The average patient age was 64.3 ± 17.4 years; 128 (60.0%) patients were male. The median admission National Institutes of Health Stroke Scale score was 4 (interquartile: 2–7). Of these, 173 (81.2%) patients had witnessed stroke; and the rest had wake-up stroke. The MRI scans were obtained 13.9 ± 7.2 h after the symptom onset or the time last-seen-well. Regarding reperfusion therapy, 50 (23.5%) patients received i.v. tissue plasminogen activator; 4 (1.9%) patients had combined IA thrombolysis and thrombectomy. Arterial occlusions were identified in 47 (22.1%) patients; these included 6 occlusions of the internal carotid artery, 29 occlusions of the middle cerebral artery (MCA), 1 occlusion of the anterior cerebral artery, 1 occlusion of the basilar artery and 10 occlusions of the posterior cerebral artery.
Prominent cortical veins
Regarding the grading, and the presence vs absence of prominent cortical veins, the interobserver kappa scores were 0.314 (p < 0.001) and 0.413 (p < 0.001), respectively. In 46/213 (22%) patients, the two reviewers had disagreement with regard to the presence of prominent cortical veins. According to the final consensus review, 17 patients had Grade I, 14 patients had Grade II and 4 patients had Grade III prominent cortical veins (Table 1). Table 2 summarizes the correlations between prominent cortical veins and different clinical and imaging findings. There was significant correlation between the prominent cortical vein grades and the presence of arterial occlusion (p = 0.001), as well as infarct volume (p < 0.001). An arterial occlusion was found in 15/35 (43%) patients having prominent cortical veins vs only 32/178 (18%) of patients lacking prominent cortical veins (p = 0.003). The average infarct volume in patients with prominent cortical veins was significantly higher (81.9 ± 144.4 ml) than that in those lacking prominent cortical veins (33.4 ± 71.3 ml, p = 0.003). The 1.5-T scanner was used in 5/35 (14%) patients with prominent cortical veins, and 54/178 (30%) patients without prominent cortical veins (p = 0.063).
Table 1.
Distribution of prominent cortical and medullary veins
| Prominent medullary vein (grade) |
Total | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Prominent cortical vein (grade) | 0 | 168 | 9 | 1 | 0 | 178 |
| 1 | 14 | 1 | 2 | 0 | 17 | |
| 2 | 10 | 1 | 2 | 1 | 14 | |
| 3 | 1 | 0 | 1 | 2 | 4 | |
| Total | 193 | 11 | 6 | 3 | 213 | |
Table 2.
Correlation between prominent cortical and medullary veins with different clinical and imaging findings
| Prominent cortical veins |
Prominent medullary veins |
|||
|---|---|---|---|---|
| Spearman rho | p-value | Spearman rho | p-value | |
| Age | −0.097 | 0.158 | −0.115 | 0.068 |
| Gender | 0.123 | 0.074 | 0.061 | 0.372 |
| i.v. tPA treatment | 0.016 | 0.814 | 0.004 | 0.959 |
| IA thrombolytic and thrombectomy | 0.045 | 0.510 | 0.192 | 0.005a |
| Onset to scan time gapb | −0.015 | 0.829 | −0.029 | 0.670 |
| Arterial occlusion | 0.232 | 0.001a | 0.180 | 0.008a |
| Infarct volume | 0.445 | <0.001c | 0.167 | 0.015d |
| Admission NIHSS score | 0.110 | 0.110 | 0.106 | 0.122 |
| 3-month mRS score | 0.115 | 0.095 | 0.057 | 0.411 |
IA, intra-arterial; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
p-value < 0.01.
The time gap between symptom onset or last-seen-well and MRI scan.
p-value < 0.001.
p-value < 0.05.
Prominent medullary veins
Regarding the grading of medullary vein prominence, and the determination of the presence vs absence of prominent medullary veins, the interobserver kappa values were 0.338 (p < 0.001) and 0.406 (p < 0.001), respectively. In 35/213 (15%) patients, the two reviewers had disagreement with regard to the presence of prominent medullary veins. A final consensus review found 11 patients with Grade I, 6 patients with Grade II and 3 patients with Grade III prominent medullary veins (Table 1). A significant correlation was noted between prominent medullary vein grades with the presence of arterial occlusion (p = 0.008), infarct volume (p = 0.015) and IA treatment (p = 0.005), as summarized in Table 2. An arterial occlusion was identified in 9/20 (45%) patients with prominent medullary veins vs 38/193 (20%) patients who lacked such veins (p = 0.019). The IA treatment was unevenly distributed, with 2/20 (10%) patients with and 2/193 (1%) patients without prominent medullary veins undergoing endovascular intervention. The average infarct volume in patients with prominent medullary cortical veins was significantly higher (120.2 ± 192.6 ml) than that in those without (33.2 ± 65.9 ml, p < 0.001). The 1.5-T scanner was used in 2/20 (10%) patients with prominent medullary veins and 57/193 (30%) patients without medullary cortical veins (p = 0.070).
Correlation between prominent cortical and medullary veins
There was a significant correlation between cortical and medullary prominent vein grades (rho = 0.325, p < 0.001). A total of 10 patients had both prominent cortical and medullary veins (Table 1); among those, 5/10 patients had an arterial occlusion (Figure 2). However, binary logistic regression analysis found that only prominent cortical vein presence had significant association with arterial occlusion (OR = 1.827, 95% confidence interval: 1.109–3.009; p = 0.018). Via stepwise linear regression analysis, there was a significant association between infarct volume with medullary vein grade (p < 0.001), but not with prominent cortical veins (p = 0.059).
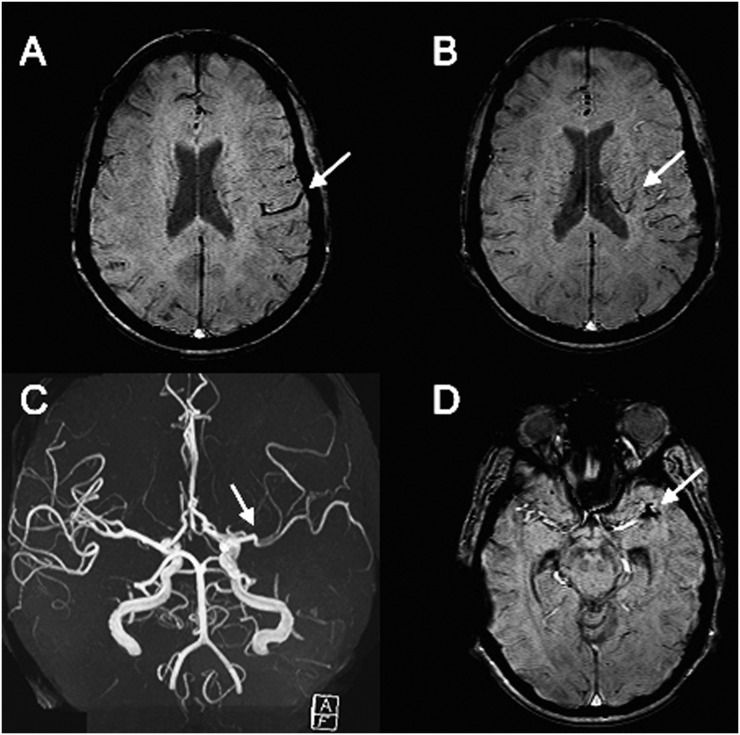
Figure 2.
A 34-year-old female presented with right hemiparesis and underwent MRI 11.8 h after symptom onset: the susceptibility-weighted imaging (SWI) series are demonstrating moderately prominent cortical (a) and medullary (b) veins (arrows). Notably, the arrow on MRA is pointing to MCA M2 segment occlusion (c), which has appeared as hypointense thrombus on SWI series (the arrow on d).
DISCUSSION
This study set out to determine the frequency and severity of prominent cortical and medullary veins on SWI in patients with acute ischaemic stroke and to determine whether a relationship exists between the presence or grade of prominent cortical and medullary veins with several other clinical and imaging findings. In this regard, prominent cortical veins on SWI were found in 16.4% of patients with acute ischaemic stroke, with prominent medullary veins in 9.4% patients. There was fair to moderate interobserver agreement in the determination of such prominent vein presence and grade. Interestingly, both prominent cortical and medullary veins have small but significant correlations with larger infarct volumes and the presence of an arterial occlusion. However, we found no statistically significant association with severity of symptoms at presentation or 3-month clinical outcome.
This may be the first study to demonstrate a correlation between the presence of prominent cortical and medullary veins on SWI with infarct volume in patients with acute phase stroke. While the presence of prominent cortical and medullary veins was not directly associated with clinical outcome in this study cohort, such prominent veins correlate with imaging biomarkers of severe hypoperfusion—i.e. arterial occlusion and infarct volume. Large infarct lesions on diffusion-weighted imaging have a high risk of developing worse clinical outcomes and symptomatic intracranial haemorrhage.15,16 Also, proximal intracranial arterial occlusions are independent predictors of poor outcome in acute ischaemic stroke.17 Hence, a prospective clinical study with uniform field strength and inclusion criteria may identify an association between prominent vessels on admission MRI/SWI scan and clinical outcome in patients with acute strokes.
The presence of asymmetrically prominent cortical or medullary veins on SWI of patients with acute ischaemic stroke has been described previously.4,9,10,18,19 Although the correlation between prominent veins with infarct volume or arterial occlusion has barely been tested before, some authors have evaluated the association between the prominent cortical or medullary veins and clinical outcome.4,9,10 For example, in a cohort of 44 patients with acute ischaemic stroke scanned within 48 h of onset with 75% rate of MCA occlusion, Huang et al4 found 15 (34.1%) patients with prominent veins on SWI. In their series,4 the presence of prominent veins was not associated with haemorrhagic transformation and oedema, stroke worsening or improving, or clinical outcome. In contrast, Sun et al9 reported a 39/572 (6.8%) rate of asymmetric cortical veins on SWI of patients with stroke, which was associated with early neurological deterioration, the presence of arterial occlusion and poor clinical outcome. Also, Mucke et al10 reported a 55/86 (64%) rate of prominent medullary veins in patients with MCA territory infarct, which was associated with more severe symptoms at presentation and worse outcome at discharge. Thus, the presence of cortical or medullary veins may still have promise in portending a poor long-term prognosis, given their correlation with imaging biomarkers of severe stroke.
Regarding the pathophysiologic explanation for the presence of prominent veins on SWI, early theories suggest that the phenomenon may be caused by an imbalance between oxygen supply and demand in the hypoperfused tissue and increased oxygen extraction fraction.11 The locally increased ratio of deoxyhaemoglobin to oxyhaemoglobin in cortical and medullary veins might be an expression of ischaemia and represent the degree of hypoperfusion and oxygen extraction.20,21 Several studies have indeed shown an association between T2* signal drop and increased oxygen extraction fraction.20,21 Since the oxygen extraction fraction is increased in the ischaemic penumbra, some authors suggested that prominent hypointense veins on SWI could possibly represent the penumbra.4,7 Such a penumbra may diminish, as reperfusion is achieved via intervention, or a new perfusion balance is achieved through collateral veins. Prior studies, using gradient-echo T2* weighted imaging, have shown that abnormal prominent deep cerebral and superficial cortical veins correlate with perfusion defects and increased cerebral blood volume, suggestive of misery perfusion.22,23 Our findings are consistent with the general consensus that prominent cortical and medullary veins on SWI reflect a state of hypoperfusion in acute ischaemic stroke that usually leads to a relatively large core of infarct volume—commonly in the context of major arterial occlusion. Thus, given the general association between larger infarct volume size and arterial occlusion with poor outcome, the presence of prominent cortical or medullary veins on SWI may help with prognostication of acute ischaemic stroke, although this needs to be validated prospectively.
Several limitations were present in this study besides the inherent limitation of its retrospective nature. First, both 1.5- and 3.0-T magnet scanners were utilized for MRI acquisition, which could result in difference in sensitivities of SWI in detecting such veins; hence, the true frequency of such prominent veins in acute stroke would optimally be determined prospectively via a single scanner, preferably at 3.0-T field strength or above with a homogeneous protocol, given the higher degree of magnetic susceptibility with increasing field strength. However, we found no significant difference in the rate of prominent veins among patients scanned with 1.5-T vs 3.0-T scanner. Second, there could be improved detection of such prominent veins with other sequence parameters besides field strength; however, the authors acknowledge that SWI sequence parameters in this study generally followed the routine settings by the vendor, with very little changes. Third, the presence of prominent veins on SWI series may depend on the delay between symptom onset and imaging, although no significant correlation between these imaging findings and the time gap to scan was identified. Fourth, the wide range of reported rate of prominent cortical and medullary veins in the literature and fair to moderate interreviewer agreement in our cohort may reflect the subjectivity of the evaluation and limit its clinical application before standardization of image evaluation. Finally, given that many patients were scanned out of the reperfusion therapy window, extrapolation of data for any treatment decision would be limited, and as mentioned earlier, this necessitates a dedicated, prospective study.
CONCLUSION
In this retrospective study of consecutive patients with acute stroke, a correlation was found between the presence and grade of prominent cortical and medullary veins with larger infarct volumes and the presence of arterial occlusion, but not with outcome. Thus, the findings support previously described theories regarding the pathophysiology of prominent cortical and medullary veins as signs of ischaemia and severe hypoperfusion. Given that large infarct volume and major arterial occlusion are accepted secondary signs of poor clinical outcome, a prospective study with more selective inclusion criteria at higher field strengths could better determine whether there is a true association between the presence of such prominent veins with clinical outcome.
Contributor Information
Seyedmehdi Payabvash, Email: spayab@gmail.com.
John C Benson, Email: benso905@umn.edu.
Shayandokht Taleb, Email: staleb@umn.edu.
Jeffrey B Rykken, Email: jrykken@umn.edu.
Benjamin Hoffman, Email: hoffm969@umn.edu.
Mark C Oswood, Email: mark.oswood@hcmed.org.
Alexander M McKinney, Email: mckinrad@umn.edu.
REFERENCES
- 1.Fujioka M, Okuchi K, Iwamura A, Taoka T, Siesjo BK. A mismatch between the abnormalities in diffusion- and susceptibility-weighted magnetic resonance imaging may represent an acute ischemic penumbra with misery perfusion. J Stroke Cerebrovasc Dis 2013; 22: 1428–31. doi: https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 2.Lou M, Chen Z, Wan J, Hu H, Cai X, Shi Z, et al. Susceptibility-diffusion mismatch predicts thrombolytic outcomes: a retrospective cohort study. AJNR Am J Neuroradiol 2014; 35: 2061–7. doi: https://doi.org/10.3174/ajnr.A4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 2009; 30: 19–30. doi: https://doi.org/10.3174/ajnr.A1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol 2012; 259: 1426–32. doi: https://doi.org/10.1007/s00415-011-6369-2 [DOI] [PubMed] [Google Scholar]
- 5.Kesavadas C, Thomas B, Pendharakar H, Sylaja PN. Susceptibility weighted imaging: does it give information similar to perfusion weighted imaging in acute stroke? J Neurol 2011; 258: 932–4. doi: https://doi.org/10.1007/s00415-010-5843-6 [DOI] [PubMed] [Google Scholar]
- 6.Rovira A, Orellana P, Alvarez-Sabin J, Arenillas JF, Aymerich X, Grive E, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology 2004; 232: 466–73. doi: https://doi.org/10.1148/radiol.2322030273 [DOI] [PubMed] [Google Scholar]
- 7.Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol 2009; 64: 74–83. doi: https://doi.org/10.1016/j.crad.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 8.Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol 2015; 22: 967–72. doi: https://doi.org/10.1111/ene.12693 [DOI] [PubMed] [Google Scholar]
- 9.Sun W, Liu W, Zhang Z, Xiao L, Duan Z, Liu D, et al. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: a novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur J Neurol 2014; 21: 1411–18. doi: https://doi.org/10.1111/ene.12510 [DOI] [PubMed] [Google Scholar]
- 10.Mucke J, Mohlenbruch M, Kickingereder P, Kieslich PJ, Baumer P, Gumbinger C, et al. Asymmetry of deep medullary veins on susceptibility weighted MRI in patients with acute MCA stroke is associated with poor outcome. PLoS One 2015; 10: e0120801. doi: https://doi.org/10.1371/journal.pone.0120801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 2009; 30: 232–52. doi: https://doi.org/10.3174/ajnr.A1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma RK, Hsieh K, Gratz PP, Schankath AC, Mordasini P, Zubler C, et al. Leptomeningeal collateralization in acute ischemic stroke: impact on prominent cortical veins in susceptibility-weighted imaging. Eur J Radiol 2014; 83: 1448–54. doi: https://doi.org/10.1016/j.ejrad.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Luo S, Yang L, Wang L. Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol 2015; 42: 255–60. doi: https://doi.org/10.1016/j.neurad.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 14.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. doi: https://doi.org/10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–17. doi: https://doi.org/10.1002/ana.20976 [DOI] [PubMed] [Google Scholar]
- 16.Singer OC, Kurre W, Humpich MC, Lorenz MW, Kastrup A, Liebeskind DS, et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke 2009; 40: 2743–8. doi: https://doi.org/10.1161/STROKEAHA.109.550111 [DOI] [PubMed] [Google Scholar]
- 17.Smith WS, Tsao JW, Billings ME, Johnston SC, Hemphill JC, 3rd, Bonovich DC, et al. Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care 2006; 4: 14–17. doi: https://doi.org/10.1385/NCC:4:1:014 [DOI] [PubMed] [Google Scholar]
- 18.Baik SK, Choi W, Oh SJ, Park KP, Park MG, Yang TI, et al. Change in cortical vessel signs on susceptibility-weighted images after full recanalization in hyperacute ischemic stroke. Cerebrovasc Dis 2012; 34: 206–12. doi: https://doi.org/10.1159/000342148 [DOI] [PubMed] [Google Scholar]
- 19.Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol 2008; 29: 9–17. doi: https://doi.org/10.3174/ajnr.A0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An H, Lin W. Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cereb Blood Flow Metab 2000; 20: 1225–36. doi: https://doi.org/10.1097/00004647-200008000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Wang Y, Waight DJ. Blood oxygen saturation assessment in vivo using T2* estimation. Magn Reson Med 1998; 39: 685–90. doi: https://doi.org/10.1002/mrm.1910390503 [DOI] [PubMed] [Google Scholar]
- 22.Hermier M, Nighoghossian N, Derex L, Adeleine P, Wiart M, Berthezene Y, et al. Hypointense transcerebral veins at T2*-weighted MRI: a marker of hemorrhagic transformation risk in patients treated with intravenous tissue plasminogen activator. J Cereb Blood Flow Metab 2003; 23: 1362–70. doi: https://doi.org/10.1097/01.WCB.0000091764.61714.79 [DOI] [PubMed] [Google Scholar]
- 23.Hermier M, Nighoghossian N, Derex L, Wiart M, Nemoz C, Berthezene Y, et al. Hypointense leptomeningeal vessels at T2*-weighted MRI in acute ischemic stroke. Neurology 2005; 65: 652–3. doi: https://doi.org/10.1212/01.wnl.0000173036.95976.46 [DOI] [PubMed] [Google Scholar]