Abstract
Introduction
We hypothesized that, like apolipoprotein E (APOE), other late-onset Alzheimer's disease (LOAD) genetic susceptibility loci predict mortality.
Methods
We used a weighted genetic risk score (GRS) from 21 non-APOE LOAD risk variants to predict survival in the Adult Changes in Thought and the Health and Retirement Studies. We meta-analyzed hazard ratios and examined models adjusted for cognitive performance or limited to participants with dementia. For replication, we assessed the GRS-longevity association in the Cohorts for Heart and Aging Research in Genomic Epidemiology, comparing cases surviving to age ≥90 years with controls who died between ages 55 and 80 years.
Results
Higher GRS predicted mortality (hazard ratio = 1.05; 95% confidence interval: 1.00–1.10, P = .04). After adjusting for cognitive performance or restricting to participants with dementia, the relationship was attenuated and no longer significant. In case-control analysis, the GRS was associated with reduced longevity (odds ratio = 0.64; 95% confidence interval: 0.41–1.00, P = .05).
Discussion
Non-APOE LOAD susceptibility loci confer risk for mortality, likely through effects on dementia incidence.
Keywords: Alzheimer's disease, Longevity, Mortality, Survival analysis, Genetic risk score, Selection bias, Collider stratification bias, Survivor bias, Genome-wide association study (GWAS), APOE, Adult Changes in Thought (ACT), Health and Retirement Study (HRS), Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE)
Highlights
-
•
A genetic risk score from 21 non-APOE late-onset Alzheimer's disease risk variants predicts mortality.
-
•
The genetic risk score likely confers risk for mortality through its effect on dementia incidence.
-
•
Late-onset Alzheimer's disease risk loci effect estimates from genome-wide association unlikely suffer from selection bias.
1. Introduction
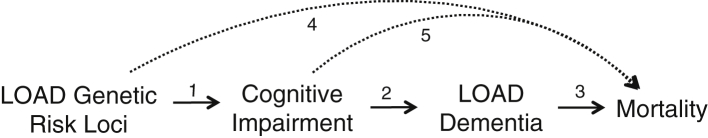
Risk variants from 22 genes have been identified for late-onset Alzheimer's disease (LOAD) incidence. Of these, apolipoprotein E ε4 (APOE ε4) has the largest effect [1]. Genome-wide association studies (GWASs) of longevity in older adults have identified APOE ε4 as a risk factor for mortality [2], [3], [4]. This risk may be partially mediated through dementia incidence [5], [6], as dementia is a leading cause of mortality in older adults [7]. Given the association of both APOE ε4 and dementia with mortality, we hypothesized that other genetic variants associated with LOAD incidence would also be associated with mortality in older adults. We anticipated that cognitive function and dementia incidence would mediate the relationship with mortality. In other words, we expected that LOAD genetic risk variants would increase risk for cognitive decline and LOAD dementia, which would in turn increase risk for death (Fig. 1, solid arrows).
Fig. 1.
Conceptual model. Solid lines indicate hypothesized path. Dashed lines indicate uncertain alternative paths. Selection bias (collider stratification bias) in LOAD genetic studies may be present if both the direct path to mortality and the indirect path through LOAD dementia exist. Abbreviation: LOAD, late-onset Alzheimer's disease.
Quantifying and explaining the link between LOAD genetic risk and mortality is critical for genetic research on determinants of LOAD incidence: severe survivor bias could make it difficult to identify genetic variants that increase disease incidence or could falsely implicate genetic variants that are not actually associated with disease incidence [8]. For example, LOAD cases who are APOE ε4 carriers may be omitted from research because they declined faster and died before study enrollment or disease ascertainment [9]. APOE has also been implicated in risk for other disease processes including cardiovascular disease [10], so it may influence mortality via mechanism(s) other than dementia, thereby introducing potential survivor bias in its relationship with LOAD. By assessing whether non-APOE genes associated with LOAD are also associated with increased mortality, we can provide researchers with tools to systematically evaluate the potential magnitude of survivor bias [11].
Here, we attempt to replicate the previously reported link between APOE ε4 and mortality and, in novel analyses, assess whether non-APOE loci, previously found to be associated with LOAD incidence, are also associated with mortality. Because the effect size for each non-APOE variant is small, we estimate the combined genetic risk that each subject had for developing LOAD by calculating a genetic risk score (GRS). In primary analyses in two longitudinal population-based prospective cohort studies, the Adult Changes in Thought (ACT) study and the Health and Retirement Study (HRS), we assessed the GRS relationship with time to death using survival analysis. In subsequent models, we tested whether cognition or dementia mediated or moderated the relationship between the GRS and mortality. We conducted a follow-up, confirmatory case-control analysis in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE), comparing the GRS among longevity cases age ≥90 years versus controls who died between ages 55 and 80 years. Our findings could suggest new genetic risk loci for longevity, add insight into how LOAD risk loci affect dementia incidence and disease course, and provide reassurance that the effect estimates of these loci on LOAD incidence do not suffer from selection bias.
2. Methods
2.1. Study populations
2.1.1. Adult Changes in Thought
The ACT cohort draws from a population base defined by the Group Health membership; the Group Health is an integrated healthcare delivery system in King County, WA. The ACT study enrolled 3392 cognitively normal community-dwelling adults 65 years or older in two enrollment phases (n = 2581 in 1994–1996 and n = 881 in 2001–2003) and initiated ongoing enrollments in 2004. After obtaining informed consent, in-person biennial interviews assessed participants' demographic, medical history, and functional status. ACT stopped assessing participants diagnosed with dementia but still obtained mortality information. For the current analyses, from 4131 individuals with ACT visits, we excluded participants who self-reported to be Hispanic or nonwhite (to avoid confounding from population stratification; n = 383) or who were not genotyped (n = 1418). This left an analytic sample of 2330 individuals.
2.1.2. Health and Retirement Study
The HRS is a nationally representative cohort study initiated in 1992 with enrollments in 1992, 1993, 1998, 2004, and 2010. The target population is all noninstitutionalized adults in the contiguous United States aged 50+ years. Biennial interviews (or proxy interviews for decedent or severely impaired participants) are available through 2012 [12]. Our analyses used a subsample with genetic data collected in 2006 or 2008. From 20,662 individuals alive in 2006 with HRS visits, we excluded participants who were not genotyped (n = 8580), who self-reported to be Hispanic or nonwhite (to avoid confounding from population stratification; n = 2894), or who were under age 65 years (to mimic ACT; n = 3224). This left an analytic sample of 5964 individuals.
2.1.3. Cohorts for Heart and Aging Research in Genomic Epidemiology
The CHARGE consortium includes participants from the following studies: Rotterdam Study, Study of Osteoporotic Fractures, Cardiovascular Health Study, Osteoporotic Fractures in Men Study, Framingham Heart Study, Health and Retirement Study, Age, Gene/Environment Susceptibility—Reykjavik Study, Religious Orders Study, Rush Memory and Aging Project, Invecchiare nel Chianti, Baltimore Longitudinal Study of Aging, New England Centenarians Study, Long Life Family Study, and European Union Longevity Consortium. The current analysis includes the same participants as a case-control GWAS of longevity reported previously [13]. Only the participants of European ancestry were included. There were 6036 cases, defined as participants who reached age ≥90 years, and 3757 controls, defined as participants who died between the ages of 55 and 80 years.
All individuals were recruited under the protocols approved by the appropriate Institutional Review Boards.
2.2. Measures
2.2.1. GRS for LOAD
Genotyping, imputation, and derivation of principal components to control for population substructure are described in the Supplementary Methods, and further details have been described previously for ACT [14], HRS [15], and CHARGE [13].
In ACT and HRS, we generated a weighted GRS for LOAD based on the effect size of single-nucleotide polymorphisms (SNPs) that achieved genome-wide significance. Twenty-one genetic loci, other than APOE, have been confirmed as genome-wide significant predictors of LOAD, with meta-analyzed odds ratios (ORs) reported most recently in the Lambert et al. meta-analysis [1]. Because some of the reported SNPs from Lambert et al. could not be confidently imputed, we used proxy SNPs (two in ACT; four in HRS) in high linkage disequilibrium (r2 > 0.8) identified using SNP Annotation and Proxy Search [16]. Supplementary Table 1 shows the SNPs used in each cohort and the respective β coefficients from the Lambert et al. meta-analysis. We calculated the GRS by multiplying each individual's predicted risk allele count for each locus by the β coefficient (expressed as the log OR) for that polymorphism as reported by Lambert et al. and summing the products for all 21 loci. This step weights each SNP in proportion to its anticipated effect on LOAD risk. Finally, we standardized the GRS using each sample's mean and standard deviation so that each GRS had a mean of 0 and a standard deviation of 1.
2.2.2. Mortality
In ACT, we used mortality information obtained via death certificates, state death registry data, annual telephone contact with family members of study participants with dementia, quarterly mailings to participants, and thorough review of local obituaries. In HRS, we used mortality information obtained via National Death Index linkage from 2008 to 2012. Information from proxy interviews was used for individuals without National Death Index information.
2.2.3. Cognitive performance
In ACT, cognitive performance was assessed using the Cognitive Abilities Screening Instrument (CASI) [17]. Raw scores range from 0 to 100, with higher scores indicating better performance. Because of concerns about nonlinear scaling, we used CASI scores that were previously derived using the item response theory [18]. In HRS, we used a previously developed memory composite score combining direct and proxy memory assessments for longitudinal analyses. All HRS participants who were interviewed directly were asked to complete an immediate and delayed recall test based on a 10-word list. For individuals too impaired to directly participate in memory assessments, the informants, typically spouses, assessed the participants' memory on a 5-item Likert scale and completed a 16-item version of the Informant Questionnaire for Cognitive Decline (IQCODE). We used an algorithm to integrate direct and proxy assessments to retain severely impaired individuals in longitudinal studies of cognitive function. The composite score was derived in an 856-subject subsample who participated in a comprehensive neuropsychological battery as part of the Aging, Demographics, and Memory Study and then uses regression models to calibrate the direct and proxy reports from the prior HRS core interview to predict the Aging, Demographics, and Memory Study outcomes. We then apply these prediction rules to estimate memory in the entire HRS sample [19]. We standardized the memory score by dividing each score by the 1995 standard deviation so that every unit change in memory score corresponded to one standard deviation in the population before baseline.
2.2.4. Dementia diagnosis in ACT
At baseline and every 2 years, ACT participants were administered the CASI. Those with raw scores below 86 received a diagnostic evaluation that included physical and neurological examinations, neuropsychological testing, and laboratory studies, if not already available. A consensus panel, which included study physicians, a neuropsychologist and a research nurse, determined whether these participants met criteria for dementia based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria [20]. Participants remained in the cohort until they were diagnosed with dementia or, if not demented, were censored as a result of death or discontinuation of participation in the study.
2.2.5. Covariates
All models that included ACT and HRS participants were adjusted for time constant covariates: sex, intake age, intake age2, wave of genetic data collection, and the first three (in ACT) or six (in HRS) principal components used to adjust for population substructure in genetic analyses.
2.3. Analytic plan
In ACT, we used Cox proportional hazards models to estimate the association between the GRS and APOE ε4 with dementia onset date. We did not evaluate this relationship in HRS because we recently published these analyses for HRS separately, reporting that the GRS and APOE ε4 both predicted dementia probability and memory score [21]. Next, in ACT and HRS, we used Cox models to estimate the independent effects of the GRS and APOE ε4 on time to death. We used time from study baseline as the timescale in the Cox models. In subsequent models in both ACT and HRS, we adjusted for cognitive performance using either time-dependent CASI-item response theory in ACT or time-dependent memory score in HRS. To further explore the role of dementia in explaining the link between the GRS and mortality, we stratified follow-up time for ACT participants by dementia diagnosis. We defined the dementia-free group as anyone who had not been diagnosed with dementia by the current visit, even if they were diagnosed at a later date. Our dementia-diagnosed group was defined as anyone with a dementia diagnosis at the current or previous visit, and in these analyses, follow-up time for the Cox models began at diagnosis instead of study baseline. Therefore, some people contributed person-time to both categories, that is, before and after diagnosis. If dementia incidence was the primary mechanism linking the GRS to mortality, we would not expect the GRS to have a large effect on mortality in either the dementia-free or the dementia-diagnosed groups, assuming no misclassification.
We meta-analyzed the estimated effect of the GRS on time to death in ACT and HRS from both the unadjusted model and the model adjusting for time-dependent cognitive performance, using a fixed effects model with inverse variance weights, as implemented in the metan package in Stata 11.2 [22].
In CHARGE, because individual-level data were not available, we reanalyzed the published summary statistics including ORs and standard errors from a case-control GWAS meta-analysis of longevity in CHARGE participants of European ancestry [13]. We estimated the coefficient “a” for the GRS as a linear predictor of longevity as follows:
with a standard error estimate of
where i indexes the SNPs in the GRS, wi is the parameter estimate of SNP i on the log odds of LOAD status, is the parameter estimate of SNP i on the log odds of longevity status, and si is the standard error of [23], [24].
3. Results
Characteristics from the first wave in which each individual contributed an observation are shown for the 2330 ACT sample members and 5964 HRS sample members used in our models (Table 1). Average follow-up time was 8.6 years (up to 18 years) in ACT and 4.6 years (up to 6 years) in HRS. In ACT, 1267 (54.4%) participants died during the study, whereas 705 (11.8%) died in HRS. When we limited the follow-up time in ACT to match HRS (i.e., 6 years), 561 (23.7%) participants died. Characteristics of each cohort from the CHARGE consortium have been described previously [13].
Table 1.
Baseline characteristics of the ACT and HRS samples
| Characteristics | ACT (N = 2330) | HRS (N = 5964) |
|---|---|---|
| Age, mean (SD) | 75.1 (6.56) | 74.8 (7.0) |
| Male, N (%) | 1035 (44.4) | 2616 (43.9) |
| GRS, mean (SD) | 0.0 (1.0) | 0.0 (1.0) |
| APOE ε4, N (%) | 599 (25.7) | 1534 (25.7) |
| CASI score at baseline visit, mean (SD) | 0.32 (0.71) | |
| Memory score at baseline visit, mean (SD) | 0.89 (0.47) |
Abbreviations: ACT, Adult Changes in Thought; CASI, Cognitive Abilities Screening Instrument; GRS, genetic risk score; HRS, Health and Retirement Study; SD, standard deviation.
In ACT, both the GRS (hazard ratio [HR] = 1.27; 95% confidence interval [CI]: 1.14–1.41) and APOE ε4 (HR = 1.98; 95% CI: 1.62–2.43) predicted time to dementia onset. Using Cox models, we found a significant association between higher GRS and time to death, but not between APOE ε4 and time to death (Table 2). In models adjusted for time-dependent CASI score, the association between the GRS and time to death was attenuated by more than half and was no longer significant. In addition, we found no evidence that the GRS predicted time to death when restricting analyses to either individuals with or individuals without a dementia diagnosis.
Table 2.
Hazard ratios and 95% confidence intervals for GRS and APOE ε4 status on time to death in the ACT study
| Predictors | Pooled analyses (N = 2330) |
Stratified analyses |
||||||
|---|---|---|---|---|---|---|---|---|
| Predementia diagnosis (N = 2330) |
Postdementia diagnosis (N = 526) |
|||||||
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| AD-GRS | 1.07 (1.01–1.14) | .03 | 1.03 (0.96–1.09) | .43 | 1.04 (0.97–1.12) | .25 | 0.98 (0.88–1.10) | .73 |
| APOE ε4 (yes/no any ε4 alleles) | 1.03 (0.91–1.17) | .64 | 0.88 (0.78–1.01) | <.001 | 0.87 (0.74–1.03) | .10 | 0.83 (0.67–1.03) | .09 |
| CASI score | 0.59 (0.55–0.62) | .07 | ||||||
Abbreviations: ACT, Adult Changes in Thought; AD-GRS, Alzheimer's disease genetic risk score; CASI, Cognitive Abilities Screening Instrument; CI, confidence interval; HR, hazard ratio; SD, standard deviation.
NOTE. All models were adjusted for sex, intake age and age2, wave of genetic data collection, and the first three principal components of population substructure. Models include pooled analyses with and without adjustment for time-dependent CASI score and dementia-stratified analyses.
In HRS, the association between the GRS and time to death was not statistically significant, although the 95% CI included the point estimate from the ACT model. The point estimate in HRS was attenuated by about half when the time-dependent memory score was included in the model, although the absolute change was small from a point estimate of 1.02 to 1.01 (Table 3). We found no evidence of an association between APOE ε4 and time to death.
Table 3.
Hazard ratios and 95% confidence intervals for GRS and APOE ε4 status on time to death in the HRS study (N = 5964), with and without adjustment for time-dependent memory score
| Predictors | Model 1 |
P-value | Model 2 |
P-value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| AD-GRS | 1.02 (0.95–1.10) | .64 | 1.01 (0.94–1.08) | .86 |
| APOE ε4 (yes/no any ε4 alleles) | 1.08 (0.91–1.27) | .41 | 1.00 (0.85–1.19) | .96 |
| Memory score | 0.46 (0.38–0.56) | <.001 |
Abbreviations: AD-GRS, Alzheimer's disease genetic risk score; CI, confidence interval; HR, hazard ratio; HRS, Health and Retirement Study.
NOTE. Both models were adjusted for sex, intake age and age2, wave of genetic data collection, and the first six principal components of population substructure.
In the meta-analysis of the ACT and HRS results from the models unadjusted for cognitive performance, there was a significant association between the GRS and time to death (Table 4). In the meta-analysis of the models adjusted for time-dependent cognitive performance, we found that the point estimate was attenuated by about 60% and was no longer statistically significant. Using GWAS summary statistics for longevity from the CHARGE [13] and for LOAD from the Lambert et al. meta-analysis [1], we found an OR of 0.64 (95% CI: 0.41–1.00), suggesting the GRS reduced chances of living past age 90 years.
Table 4.
GRS survival meta-analysis and longevity case/control analysis
| Studies | N | Model 1 |
Model 2 |
||
|---|---|---|---|---|---|
| Effect size∗ (95% CI) | P-value | Effect size∗ (95% CI) | P-value | ||
| Survival meta-analysis (ACT and HRS) | 7294 | 1.05 (1.00–1.10) | .04 | 1.02 (0.97–1.07) | .47 |
| Longevity case/control† (CHARGE) | 9793 | 0.64 (0.41–1.00) | .05 | ||
Abbreviations: ACT, Adult Changes in Thought; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CI, confidence interval; GRS, genetic risk score; HR, hazard ratio; HRS, Health and Retirement Study; OR, odds ratio.
NOTE. Model 2, but not Model 1, was adjusted for time-dependent cognitive performance.
HRs for survival models and OR for case/control model. Note HRs and ORs are not on the same scale and cannot be directly compared.
Controls (died between the ages of 55 and 80 years) are the reference group.
4. Discussion
In the ACT study, we found that a GRS based on 21 non-APOE loci that achieved genome-wide significance in a large meta-analysis of LOAD incidence [1] predicted reduced time to death, and this association was largely explained by cognitive function or dementia incidence. Although the GRS was nonsignificantly associated with higher mortality in the HRS sample, adjusting for cognitive performance attenuated the association to a similar magnitude, as in ACT, albeit the absolute change was quite small. In addition, the meta-analysis of ACT and HRS supported an association between the GRS and time to death and an attenuation of that association with adjustment for time-dependent cognitive performance. As confirmation of the unadjusted result, we found that the GRS predicted reduced odds of surviving to age ≥90 years (compared to dying between the ages of 55 and 80 years) in participants in the CHARGE Consortium.
We investigated whether the GRS was related to mortality because APOE ε4, the largest risk allele for LOAD, has consistently been identified as a risk factor for death in the GWAS of longevity [2], [3], [4]. The heritability of longevity has been estimated to be approximately 20% to 30%; however, as with many complex traits, genetic risk factors for longevity identified through GWAS only account for a small portion of the predicted heritability, suggesting other loci also contribute [25]. Our findings suggest that LOAD risk loci other than APOE ε4 may contribute to the heritability of longevity, most likely via their influence on dementia incidence.
Although most genetic studies of longevity have used a case-control design, comparing long-lived “cases” with younger “controls”, a few studies have used survival analysis in a community-based cohort. These survival studies have allowed for investigation of whether dementia status mediates the effect of APOE ε4 on longevity, but results have been inconsistent. Two smaller studies showed that dementia mediates the relationship [5], [6], whereas a larger meta-analysis of several cohorts did not show evidence of mediation [26]. APOE ε4 has also been implicated in risk for other disease processes including cardiovascular disease [10], so it may influence longevity via mechanism(s) other than dementia. In our study, we found an estimated effect of the GRS on mortality, but we did not find statistically significant associations between APOE ε4 and mortality in either ACT or HRS. The lack of association with APOE ε4 may reflect the older age of the ACT and HRS samples considered here, as carriers may not have been included because they were already impaired by age 65 years. Of note, the HRS begins enrollment at age 50 years, and when we included all genotyped participants (rather than just those age >65 to mimic ACT), APOE ε4 still was not significantly associated with mortality. Another possibility is that we were simply underpowered to detect an association. Although CIs describing the association between APOE ε4 and mortality in ACT (0.91, 1.17) and in HRS (0.91, 1.27) were consistent with increases in mortality, the previous longevity studies reported point estimates larger than the upper bounds of these CIs. A third possibility is true heterogeneity of effects of APOE across populations, for reasons that remain to be discovered.
The estimated effect of the GRS on mortality was attenuated by adjusting for cognition, providing evidence that the GRS influences mortality primarily through cognition (Fig. 1, arrows 1–3, 5). Collider stratification bias, a type of survival bias that can occur when stratifying on a variable in a causal system that is a shared effect of more than one cause [27], is recognized as an important potential bias in genetic studies of LOAD but has rarely been formally assessed. Because genes can influence mortality through more than one mechanism (pleiotropy), genetic studies of late-life health outcomes, which by default stratify on survival (i.e., only those who have survived can be studied), may suffer from collider stratification bias [8]. This study provides evidence that collider stratification bias is less likely to be a large concern in genetic studies of LOAD. Because most of the effect of the GRS on mortality was through cognition (Fig. 1, arrows 1–3, 5) and not an alternative pathway (Fig. 1, arrow 4), there is less likely to be substantial collider stratification bias.
When we limited our survival analysis to those with a dementia diagnosis, the effect of the GRS on mortality was attenuated (from an HR of 1.07 to 0.98), suggesting that the risk score is not associated with differential mortality among patients with dementia. Although the genetic profile increases risk of developing dementia, and mortality is known to be higher in dementia patients, patients with dementia who carry high-risk genetic profiles do not have higher mortality risk than patients with dementia without high-risk genetic profiles. This is consistent with a genome-wide survival analysis of mortality among LOAD cases that found that the top nominally significant loci (none achieved genome-wide significance) were not the loci identified in LOAD case-control GWAS [28]. The estimated effect of the GRS on mortality among people without a diagnosis of dementia (some of whom likely had undiagnosed disease) was about half as large as the unstratified estimate (from an HR of 1.07 to 1.04) and not statistically significant. Although larger data sets are necessary to more precisely quantify the effect of the GRS on mortality in dementia-free individuals, this result suggests that a fraction of the mortality risk is related to cognitive deterioration before diagnosis of dementia (Fig. 1, arrows 1 and 5).
One of the strengths of our study is the use of a GRS, which is a valuable tool for assessing the cumulative predictive capacity of genetic variation. We derived the GRS based on variants that achieved genome-wide significance in the largest LOAD meta-analysis to date (25,580 cases and 48,466 controls) [1]. We weighted each variant's contribution to the GRS based on its effect size in the GWAS, an approach that has been shown to improve power to detect an association compared with an unweighted approach [24]. Another strength is that we were able to show suggestive association of the GRS with longevity using two different approaches—a survival analysis in two epidemiological cohorts and a case-control approach comparing long-lived cases and younger controls. An epidemiological cohort is the ideal setting to carry out unbiased evaluations of longitudinal relationships [29]. Here, it allowed us to investigate the mechanism by which the GRS affects mortality.
Important limitations of this study include the possibility that people with prodromal LOAD were at higher mortality risk before diagnosis, as suggested by the nonsignificant but slightly elevated mortality risk associated with the GRS among people without a dementia diagnosis in ACT. This is partially mitigated by the ACT study practice of decreasing the time between visits from 2 years to 1 year for participants with impaired CASI scores who do not meet criteria for dementia, so that they can be followed more closely. Another limitation is that we did not replicate in HRS the association of the GRS with mortality that we observed in ACT. However, the HRS genetic cohort had accumulated a relatively small number of deaths at the time of this analysis, leading to wide CIs; the HRS and ACT results were consistent based on the CIs. In addition, the CHARGE case-control analysis provided confirmation (if not true replication) of the ACT finding. The CHARGE consortium longevity case-control analysis included 785 HRS participants (out of 9793 total participants), many of whom were likely included in our HRS survival analysis. Unfortunately, it was not possible to conduct CHARGE analyses without these individuals. A further limitation is that we could only examine genetic variants that have been shown to predict LOAD; to the extent that survivor bias is severely affecting LOAD GWAS, some important genetic risk factors for LOAD may not yet have been identified. There is no straightforward solution to this problem, but it suggests the potential value of evaluating sensitivity to survival bias in the primary GWAS. Finally, we note that the lack of evidence for a pathway from the GRS to mortality that does not go through cognitive decline is not necessarily evidence for the absence of the pathway. Rather, we may not have power to detect a small effect.
In conclusion, we found that a non-APOE LOAD GRS is associated with mortality and that this relationship may be mediated by cognitive changes before dementia diagnosis. Our study identifies new genetic risk factors for longevity and provides insight into the mechanism by which they may act. It also provides evidence that collider stratification bias is unlikely to be a large concern in genetic studies of LOAD. Future studies that are better powered should investigate the role of LOAD genetic risk factors on mortality among nondemented older individuals.
Research in Context.
-
1.
Systematic review: We searched PubMed to identify relevant publications. References that contextualize the hypothesis, methodology, and findings are cited. To our knowledge, no previous study has analyzed the relationship between the cumulative effect of non-APOE late-onset Alzheimer's disease (LOAD) genetic risk variants and mortality.
-
2.
Interpretation: Our findings suggest new genetic risk loci for longevity, add insight into how LOAD risk loci affect dementia incidence and disease course, and provide reassurance that the effect estimates of these loci on LOAD incidence do not suffer from selection bias.
-
3.
Future directions: We find that a fraction of the mortality risk from LOAD risk variants is related to cognitive deterioration before the diagnosis of dementia, but larger data sets are necessary to more precisely quantify this effect in dementia-free individuals.
Acknowledgments
This work was supported by the National Institutes of Health (R13 AG030995, K99 AG047963, F31 HL112613, T32 MH017119, R00 LM011384, K23 AG046377, U01 AG006781, P50 AG05136, P30 AG13846) and by the Yerby Postdoctoral Fellowship Program.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.07.002.
Supplementary data
References
- 1.Lambert J.C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deelen J., Beekman M., Uh H.W., Helmer Q., Kuningas M., Christiansen L. Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 2011;10:686–698. doi: 10.1111/j.1474-9726.2011.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nebel A., Kleindorp R., Caliebe A., Nothnagel M., Blanché H., Junge O. A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech Ageing Dev. 2011;132:324–330. doi: 10.1016/j.mad.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Sebastiani P., Solovieff N., Dewan A.T., Walsh K.M., Puca A., Hartley S.W. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7:e29848. doi: 10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayden K.M., Zandi P.P., Lyketsos C.G., Tschanz J.T., Norton M.C., Khachaturian A.S. Apolipoprotein E genotype and mortality: findings from the Cache County Study. J Am Geriatr Soc. 2005;53:935–942. doi: 10.1111/j.1532-5415.2005.53301.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosvall L., Rizzuto D., Wang H.-X., Winblad B., Graff C., Fratiglioni L. APOE-related mortality: effect of dementia, cardiovascular disease and gender. Neurobiol Aging. 2009;30:1545–1551. doi: 10.1016/j.neurobiolaging.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Alzheimer's Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Hernán M.A., Hernández-Díaz S., Robins J.M. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 9.Glymour M.M. Invited commentary: when bad genes look good–APOE*E4, cognitive decline, and diagnostic thresholds. Am J Epidemiol. 2007;165:1239–1246. doi: 10.1093/aje/kwm092. author reply 1247. [DOI] [PubMed] [Google Scholar]
- 10.Lahoz C., Schaefer E.J., Cupples L.A., Wilson P.W., Levy D., Osgood D. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154:529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 11.Mayeda E.R., Tchetgen Tchetgen E.J., Power M.C., Weuve J., Jacqmin-Gadda H., Marden J.R. A Simulation Platform for Quantifying Survival Bias: An Application to Research on Determinants of Cognitive Decline. Am J Epidemiol. 2016;184:378–387. doi: 10.1093/aje/kwv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnega A., Faul J.D., Ofstedal M.B., Langa K.M., Phillips J.W.R., Weir D.R. Cohort profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broer L., Buchman A.S., Deelen J., Evans D.S., Faul J.D., Lunetta K.L. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J Gerontol A Biol Sci Med Sci. 2015;70:110–118. doi: 10.1093/gerona/glu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee S., Walter S., Kauwe J.S.K., Saykin A.J., Bennett D.A., Larson E.B. Genetically predicted body mass index and Alzheimer's disease–related phenotypes in three large samples: Mendelian randomization analyses. Alzheimers Dement. 2015;11:1439–1451. doi: 10.1016/j.jalz.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weir D.R., Kardia S., Langa K.M., Faul J.D., Sun Y.V., Mayeux R. Institute for Social Research, University of Michigan; Ann Arbor, MI: 2010. Health and Retirement Study. Genome Wide Association Study Overview. [Google Scholar]
- 16.Johnson A.D., Handsaker R.E., Pulit S.L., Nizzari M.M., O'Donnell C.J., Bakker D. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng E.L., Hasegawa K., Homma A., Imai Y., Larson E., Graves A. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 18.Crane P.K., van Belle G., Larson E.B. Test bias in a cognitive test: differential item functioning in the CASI. Stat Med. 2004;23:241–256. doi: 10.1002/sim.1713. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q., Tchetgen Tchetgen E.J., Osypuk T.L., White K., Mujahid M., Maria Glymour M. Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer Dis Assoc Disord. 2013;27:207–212. doi: 10.1097/WAD.0b013e31826cfe90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Association AP . 4th ed. American Psychiatric Publishing, Inc.; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition. [Google Scholar]
- 21.Marden J.R., Walter S., Tchetgen Tchetgen E.J., Kawachi I., Glymour M.M. Validation of a polygenic risk score for dementia in black and white individuals. Brain Behav. 2014;4:687–697. doi: 10.1002/brb3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stata Statistical Software. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 23.Dastani Z., Hivert M.F., Timpson N., Perry J.R.B., Yuan X., Scott R.A. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic Traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess S., Thompson S.G. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol. 2013;42:1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herskind A.M., McGue M., Holm N.V., Sørensen T.I., Harvald B., Vaupel J.W. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 26.Kulminski A.M., Arbeev K.G., Culminskaya I., Arbeeva L., Ukraintseva S.V., Stallard E. Age, gender, and cancer but not neurodegenerative and cardiovascular diseases strongly modulate systemic effect of the Apolipoprotein E4 allele on lifespan. PLoS Genet. 2014;10:e1004141. doi: 10.1371/journal.pgen.1004141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitcomb B.W., Schisterman E.F., Perkins N.J., Platt R.W. Quantification of collider-stratification bias and the birthweight paradox. Paediatr Perinat Epidemiol. 2009;23:394–402. doi: 10.1111/j.1365-3016.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Lopez O., Sweet R.A., Becker J.T., DeKosky S.T., Barmada M.M. Genetic determinants of survival in patients with Alzheimer's disease. J Alzheimers Dis. 2015;45:651–658. doi: 10.3233/JAD-142442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.