Abstract
Objective:
To correlate imaging parameters from baseline MRI diffusion-weighted imaging (DWI) and fludeoxyglucose (FDG) positron emission tomography (PET)-CT with synchronous and metachronous metastases in mucinous carcinoma (MC) and non-mucinous carcinoma (NMC) rectal cancer.
Methods:
111 patients with extraperitoneal locally advanced rectal cancer, who underwent pelvic MRI, DWI and FDG PET-CT, were stratified into MC (n = 23) and NMC (n = 88). We correlated adverse morphologic features on MRI [mT4, mesorectal fascia involvement, extramural venous invasion (mEMVI), mN2] and quantitative imaging parameters [minimum apparent diffusion coefficient (ADCmin), maximum standardized uptake value, total lesion glycolysis, metabolic tumour volume, T2 weighted and DWI tumour volumes] with the presence of metastatic disease. All patients underwent pre-operative chemoradiation therapy (CRT); 100/111 patients underwent surgery after CRT and were classified as pathological complete response (PCR) and no PCR [tumour regression grade (TRG)1 vs TRG2–5] and as ypN0 and ypN1–2. Median follow-up time was 48 months. Metastases were confirmed on FDG PET-CT and contrast-enhanced multidetector CT.
Results:
The percentage of mucin measured by MRI correlates with that quantified by histology. On multivariate analysis, the synchronous metastases were correlated with mEMVI [odds ratio (OR) = 21.48, p < 0.01] and low ADCmin (OR = 0.04, p = 0.038) in NMC. The difference of metachronous recurrence between the MC group (10–90% mucin) and NMC group was significant (p < 0.01) (OR = 21.67, 95% confidence interval 3.8–120.5). Metachronous metastases were correlated with ypN2 (OR = 8.24, p = 0.01) in MC and in NMC. In NMC, mEMVI correlated with no PCR (p = 0.018) and ypN2 (p < 0.01).
Conclusion:
mEMVI could identify patients with NMC, who are at high risk of synchronous metastases. The MC group is at a high risk of developing metachronous metastases.
Advances in knowledge:
Patients at high risk of metastases are more likely to benefit from more aggressive neoadjuvant therapy.
INTRODUCTION
In patients with advanced rectal cancer, MRI is used to assess local tumour invasion and to identify patients at high risk, who require neoadjuvant therapy.1–3 Diffusion-weighted imaging (DWI) maps the diffusion of water molecules, with diffusion in malignant tissues having high signal intensity and a low apparent diffusion coefficient (ADC) and it is considered as a potential predictive value of aggressiveness.4,5
Fluorine-18 fludeoxyglucose (FDG) positron emission tomography (PET)-CT imaging is based on the theory that enhanced glucose metabolism represents a major and characteristic metabolic change in tumour cells. According to this theory, tumour cells demonstrate increased FDG uptake, which is related to the proliferative activity and aggressiveness of the tumour.6–8 A measurement of tumour FDG uptake is the standardized uptake value (SUV).9 Recently, additional PET-derived measurements of tumour uptake, namely metabolic tumour volume and total lesion glycolysis (TLG), have been used as measures of metabolic tumour burden.7,8 Mucinous carcinoma (MC) represents a subtype of rectal carcinoma; it is defined by high signal intensity in T2 weighted (T2w) imaging, which identifies mucin pools in rectal carcinomas with a high accuracy.10–14 Most studies have found that patients with MC have a poorer prognosis than those with non-mucinous (NMC) rectal cancer,15–18 although some studies found no correlation between mucinous histotype and prognosis.19,20 Recently, Hugen et al21 performed a large population-based study in which multivariate analysis showed no differences in overall survival in MC and NMC except when the tumour was located in the rectum. Similar results were previously obtained by Hyngstrom et al.22 This indicates that efforts should be made to improve the prognosis of patients with a mucinous form of rectal cancer.
Thus, the aim of this study was to correlate MRI stage, quantitative parameters and tumour volumes on T2w, DWI and PET-CT in primary MC and NMC with the incidence of synchronous and metachronous metastases, in order to identify additional potential predictive values of patients at high risk, who might benefit from more aggressive multimodal treatments.
METHODS AND MATERIALS
Patients
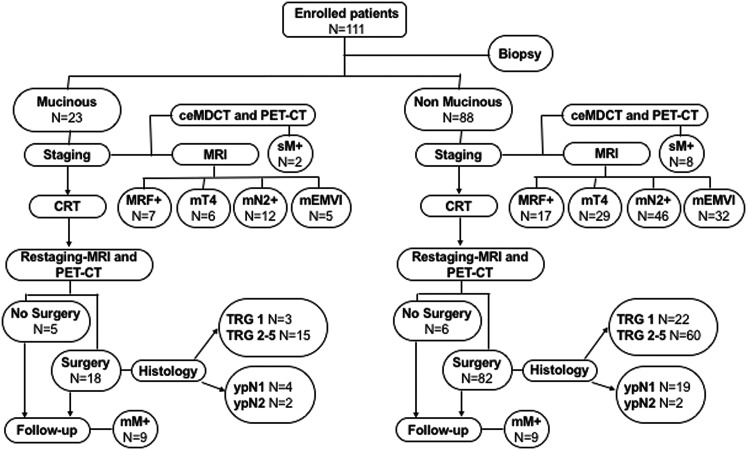
This prospective study was approved by the ethics committee at our institution, and all patients provided written informed consent. A total of 111 consecutive patients with biopsy-proven rectal carcinoma over a 5-year period (May 2009–June 2014) were staged and treated in the Department of Radiation Oncology of our university. We included patients with locally advanced extraperitoneal rectal cancer (T3–T4 any N), 5–10 cm above the anorectal ring, who had pelvic MRI and contrast-enhanced multidetector CT (ceMDCT) of the thorax and abdomen performed as part of the standard pre-operative staging and FDG PET-CT to assess its role in locally advanced rectal cancer. Our study design is shown in Figure 1.
Figure 1.
A flowchart showing our study design. ceMDCT, contrast-enhanced multidetector CT; CRT, chemoradiation therapy; mEMVI, extramural venous invasion; mM+, metachronous metastases; mN2+, pre-CRT mN2 stage; MRF+, mesorectal fascia; mT4, mT4 stage; N, number; PET, positron emission tomography; sM+, synchronous metastases; TRG, tumour regression grade; ypN, pathological nodal status after CRT.
Imaging studies and analysis
MRI
MRI was performed with a 1.5-T unit (Horizon Advantage; GE Medical Systems, Milwaukee, WI) with an eight-element pelvic phased-array surface coil. All patients were imaged in the supine position and, to reduce potential artefacts, received an enema of sonography transmission gel (60–100 cm3) to distend the rectal lumen and limit luminal air. An intramuscular antiperistaltic agent, hyoscine-N-butyl bromide (20 mg; Buscopan), was also administered. A sagittal localizing image was obtained to select transverse and coronal images with a T2w fast spin-echo sequence (Table 1).23 MRI evaluations were performed separately by two experienced radiologists, each with >10 and >5 years' experience in abdominal imaging. Table A1 lists the MRI findings that were interpreted as suggesting poor prognosis.3,24–27 MRI evaluation details [lesion volume calculation, mucin percentage calculation, regions of interest selection and minimum apparent diffusion coefficient (ADCmin) value measurement] are explained in Appendix A.
Table 1.
MRI protocol
| Sequences | Description |
|---|---|
| Coronal and sagittal T2w fast SE sequences | Repetition time/echo time: 2500–5000 ms/100 ms |
| Matrix: 256 × 256 | |
| Echo train length: 16 | |
| Acquired signals: 4 | |
| FOV: 24 cm | |
| Slice thickness: 4 mm | |
| Intersection gap: 1 mm | |
| Oblique HR images | Repetition time/echo time: 2500–5000 ms/100 ms |
| Matrix: 256 × 256 | |
| Echo train length: 16 | |
| Acquired signals: 4 | |
| Plane: orthogonal to the tumour | |
| FOV: 18 cm | |
| Slice thickness: 3 mm | |
| No intersection gap | |
| Axial T2w fast SE sequences | Repetition time/echo time: 2500–5000 ms/100 ms |
| Matrix: 256 × 256 | |
| Echo train length: 16 | |
| Acquired signals: 4 | |
| FOV: 34–40 cm | |
| Slice thickness: 4 mm | |
| Intersection gap: 0.5 mm | |
| Axial DW images (b-values: 0 and 1000 s mm−2) | Single-shot/echoplanar imaging |
| Echo time/repetition time: minimum/>8000 ms | |
| Signal averages: 8 | |
| FOV: 34–40 cm | |
| Matrix: 128 × 128 | |
| Slice thickness: 4 mm | |
| Slice spacing: 0.5 mm |
DW, diffusion-weighted; FOV, field of view; HR, high-resolution; SE, spin-echo; T2w, T2 weighted.
Axial T2w images were obtained using the same parameters as those for axial DW images; our choice of the FOV was a compromise between voxel size, volume coverage and signal-to-noise ratio, with a maximum FOV of 40 cm. Smaller FOVs (34–40 cm) were also used, depending on the patient characteristics. The total imaging time was 25 min.
Multidetector CT
The ceMDCT scans were performed on a GE 64-slice VCT scanner (120 kV, Smart mAs 100–600, collimation 1.25 mm). A total of 120–150 ml of i.v. contrast was administered at 3 ml per second, and images were acquired in the venous phase. This is the standard ceMDCT protocol used for pre-operative staging of rectal cancer at our institution.
Fludeoxyglucose positron emission tomography CT
All PET-CT scans were performed using a GEMINI GXL PET-CT scanner (Philips Medical Systems, OH) combining a PIXELAR detector with high-resolution gadolinium oxyorthosilicate crystal technology and a 16-slice CT scanner. Two experienced nuclear medicine physicians, each with >10 and >5 years' experience, analyzed the PET-CT images and a consensus was reached for all patients. PET-CT acquisition protocol and PET-CT analysis details are explained in Appendix B.
For the purpose of this study, distant metastases were confirmed by both FDG PET-CT and ceMDCT.
Pre-operative chemoradiation therapy
External beam radiotherapy was delivered with a three-dimensional technique to a total dose of 55 Gy in 5 weeks: all patients received 45 Gy with a conventional fractionation of 1.8 Gy a day on the pelvic lymph nodes; a concomitant boost dose of 10 Gy was delivered on the tumour and the corresponding mesorectum, with a fractionation of 1 Gy delivered twice a week immediately after the daily dose on the pelvis.
Concomitant chemotherapy changed according to tumour stage (Table A2).
Adjuvant chemotherapy with Folinic acid, 5-Fluorouracil and Oxaliplatin-4 (FOLFOX-4) was delivered in all patients with mT3 mesorectal fascia, mT4 and metastasis.
Restaging
All patients underwent pelvic MRI and FDG PET-CT at least 8 weeks after chemoradiation therapy (CRT), 100 of them shortly before surgery; in patients with metastasis, ceMDCT of the thorax and abdomen was performed.
Histological analysis, surgery and pathological analysis
Histological sections from biopsy were evaluated by a pathologist with more than 20 years' experience.
The sections were stained using haematoxylin and eosin, periodic acid–Schiff and periodic acid–Schiff–diastase stains. The mucin content was estimated by light microscope observation and defined as the percentage of mucin (from 0 to 100%) relative to the total area on transverse section slides stained with periodic acid–Schiff and periodic acid–Schiff–diastase. The neoplastic cellular component was estimated on slides stained with haematoxylin and eosin.
The standard surgical procedure was the total mesorectal excision at least 8 weeks after the end of CRT.
The assessment of tumour response to pre-operative CRT was performed according to the tumour regression grade (TRG) score proposed by Mandard et al28 (Table A3). Based on the TRG, the patients were grouped into patients with pathological complete response (PCR) (TRG1) and patients with no PCR (TRG2–5). In the surgical specimens after CRT, the pathological nodal status (ypN) was classified as follows: node negative (ypN0) and node positive (ypN1, from one to three nodes; and ypN2, four or more nodes).
Follow-up
The ceMDCT scans were performed every 6 months; FDG PET-CT was performed to confirm metastatic disease.
Statistical analysis
The relationships among the imaging data, imaging adverse features and pathological analysis response evaluation were assessed using the Mann-Whitney–Wilcoxon two-sample test and the χ2 test. Correlations among continuous variables were evaluated using Spearman test.
Bland–Altman 95% limits of agreement were used to evaluate the agreement between MRI and histology in the % of mucin.
Univariate analysis was performed to test the association between the possible explicative variables and the metastatic disease.
Metachronous metastases recurrence between NMC and MC groups was evaluated using Kaplan–Meier survival analysis. The equality of survivor functions across the groups was compared using the log-rank test. Furthermore, the association of imaging data with the metastatic disease was assessed through a multivariate logistic model. Two different models were created using a stepwise approach: the first model tested the association between the dependent variable “synchronous metastatic disease” and all the possible explicative variables regarding imaging data; the second one assessed the association between the dependent variable “metachronous metastatic disease” and all the possible explicative variables regarding imaging and pathological data in all patients without synchronous metastatic disease. Both the models were adjusted for potential confounders (age and sex). Explicative variables with p-value >0.20 were removed by backward selection. Interactions between the retained variables were tested and included in the final model if significant (p-value < 0.10). A p-value < 0.05 was considered statistically significant. All analyses were performed using IC STATA 14 software for Mac.
RESULTS
Characteristics of the study population
In this study, 23 patients (mean age 63 years; range, 38–81 years) had MC and 88 patients (mean age, 65 years; range, 28–83 years) had NMC. All tumours were locally advanced T3 or T4 stage tumours based on baseline (pre-CRT) MRI (mT3 or mT4, respectively) and were classified as node positive (mN1 or mN2) or node negative (mN−). The characteristics of the study population are shown in Table 2. MCs were more frequent in male patients (p = 0.01). There were no significant differences in clinical stages for MC vs NMC.
Table 2.
Characteristics of the study population
| Patient demographic and clinical characteristics |
|||
|---|---|---|---|
| MC (n = 23) | NMC (n = 88) | p-value | |
| Mean age (years) (SD) | 62.7 (12.3) | 65.4 (9.4) | 0.261 |
| Male (%) | 87 | 59 | 0.01 |
| Tumour height (%) | |||
| Lower rectum | 17 (73.9) | 53 (60.2) | 0.72 |
| Middle rectum | 6 (26.1) | 35 (39.8) | |
| mT stage (%) | |||
| T3 | 10 (43.5) | 42 (47.7) | 0.668 |
| T3 MRF+ | 7 (30.4) | 17 (19.3) | |
| T4 | 6 (26.1) | 29 (33) | |
| mN stage (%) | |||
| N0 | 1 (4.3) | 3 (3.4) | 0.993 |
| N1 | 10 (43.5) | 39 (44.3) | |
| N2 | 12 (52.2) | 46 (52.3) | |
| mEMVI+ (%) | 5 (21.7) | 32 (36.4) | 0.185 |
| sM+ (%) | 2 (8.7) | 8 (9.1) | 0.953 |
| mM+ (%) | 9 (39.1) | 9 (10.2) | <0.01 |
MC, mucinous carcinoma; mEMVI, extramural venous invasion; mM+, metachronous metastases; MRF, mesorectal fascia; N, number; NMC, non-mucinous carcinoma; SD standard deviation; sM+, synchronous metastases.
The position of 70 tumours was low rectal, i.e. the distal extent of the tumour was 5 cm above the anorectal ring. The remaining 41 tumours were mid-rectal, i.e. 5–10 cm above the anorectal ring. 10 patients had synchronous metastatic lesions (lung metastases in 1 patient, liver metastases in 8 patients and liver and lung metastases in 1 patient). 18 patients had metachronous metastatic lesions (lung metastases in 10 patients, liver metastases in 4 patients, liver and lung metastases in 2 patients, adrenal metastases in 1 patient and peritoneal metastases in 1 patient).
Agreement between MRI and histology in the percentage of mucin evaluation
We found a significant correlation between the percentage of mucin as defined by MRI and the percentage of mucin as evaluated by histology in MC (p < 0.001, r = 0.854). In the Bland–Altman plot (Figure 2), all of the values except one (Figure 3) were included within the range of 1.96 standard deviations of the difference, indicating significant agreement between MRI and histology. The mean difference was 12.9 [95% confidence interval (CI) 6.9–18.9] and the 95% limits of agreement were from −14.7 to 40.5.
Figure 2.
Agreement between MRI and histology in the % of mucin (Bland–Altman plot): the mean % of mucin values between MRI and histology is shown in the x-axis and the difference between these values is shown in the y-axis. There was one patient (round circle) who showed 70% mucin on MRI but no evidence of mucin by histology.
Figure 3.
A 62-year-old male with advanced mucinous mid-rectal cancer: (a) axial and (b) sagittal T2 weighted (T2w) MR images are showing a huge tumour with a prevalent mucinous component (70% of the T2w total tumour volume), which is evident on the right side and anteriorly as areas of high signal intensity that reach the mesorectal fascia (lower arrow in a), right seminal vesicle (upper arrow in a) and the bladder (arrow in b). The white asterisks in b are highlighting the mucinous invasion of the bladder as a highly hyperintense thickened wall; this was confirmed by cystoscopy. (c) An axial T2w MR image is showing the superficial intraluminal non-mucinous component as a moderately hyperintense area (arrowhead in c) that is perfectly superimposable on the axial diffusion-weighted image with a b-value of 1000 s mm−2 as an area of high signal intensity (arrowhead in bottom left corner in c). A photomicrograph (original magnification, ×10; haematoxylin and eosin staining) after biopsy is showing the proliferation of a complex glandular structure without evidence of mucin pools (bottom right corner in c). (d) A sagittal fused positron emission tomography CT image is showing intense and homogeneous tumoral fludeoxyglucose uptake (maximum standardized uptake volume, 47.00; metabolic tumour volume, 287 cm3; total lesion glycolysis, 2296) in the bladder (black asterisk).
Value of the MRI and positron emission tomography CT-derived parameters in MC and NMC
Table 3 summarizes the ADCmin, maximum standardized uptake value (SUVmax), T2w, DWI tumour volumes, metabolic tumour volume and TLG values in NMC and MC. The ADCmin was significantly greater in MC than in NMC (mean value: 1.17 vs 1.03 × 10−3 mm2 s−1; p = 0.005) and correlated with the percentage of mucin as calculated by MRI (r = 0.430). The T2w volume was significantly greater in MC than in NMC (median value: 33.52 vs 16.67 cm3; p = 0.0017). In contrast, the SUVmax and TLG values in MC were not significantly different from those in NMC.
Table 3.
Values of the most important MRI and positron emission tomography CT parameters in mucinous carcinoma (MC) and non-mucinous carcinoma (NMC) of the rectum
| Imaging data | MC (n = 23) | NMC (n = 88) | p-value |
|---|---|---|---|
| ADCmin | |||
| Median value (10−3 mm2 s−1) | 1.2 | 1.0 | 0.005 |
| Interquartile 1–3 (10−3 mm2 s−1) | 1.0–1.4 | 0.9–1.2 | |
| Range (10−3 mm2 s−1) | 0.3–1.6 | 0.51–1.53 | |
| SUVmax | |||
| Median value | 11.9 | 15.1 | 0.182 |
| Interquartile 1–3 | 9.2–16.6 | 11.6–19.4 | |
| Range | 5.7–47.1 | 7.5–46.1 | |
| TLG | |||
| Median value | 297.6 | 200.7 | 0.344 |
| Interquartile 1–3 | 124.4–605.8 | 118.5–386.2 | |
| Range | 25.8–2500.6 | 24.7–2174.1 | |
| T2w volume | |||
| Median value (cm3) | 33.52 | 16.67 | 0.0017 |
| Interquartile 1–3 (cm3) | 18.25–53.73 | 9.63–29.2 | |
| Range (cm3) | 2–181.22 | 2.1–200 | |
| DWI volume | |||
| Median value (cm3) | 17.40 | 14.61 | 0.155 |
| Interquartile 1–3 (cm3) | 11.85–34.03 | 9.35–27.24 | |
| Range (cm3) | 2.20–87.42 | 0.39–210.70 | |
| MTV | |||
| Median value (cm3) | 43.14 | 29.70 | 0.08 |
| Interquartile 1–3 (cm3) | 22.82–77.57 | 17.98–46.25 | |
| Range (cm3) | 6–287 | 3–310 | |
ADCmin, minimum apparent diffusion coefficient; DWI, diffusion-weighted imaging; MC, mucinous carcinoma; MTV, metabolic tumour volume; N, number; NMC, non-mucinous carcinoma; SUVmax, maximum standardized uptake value; T2w, T2 weighted; TLG, total lesion glycolysis.
Correlations between the imaging data with synchronous metastases
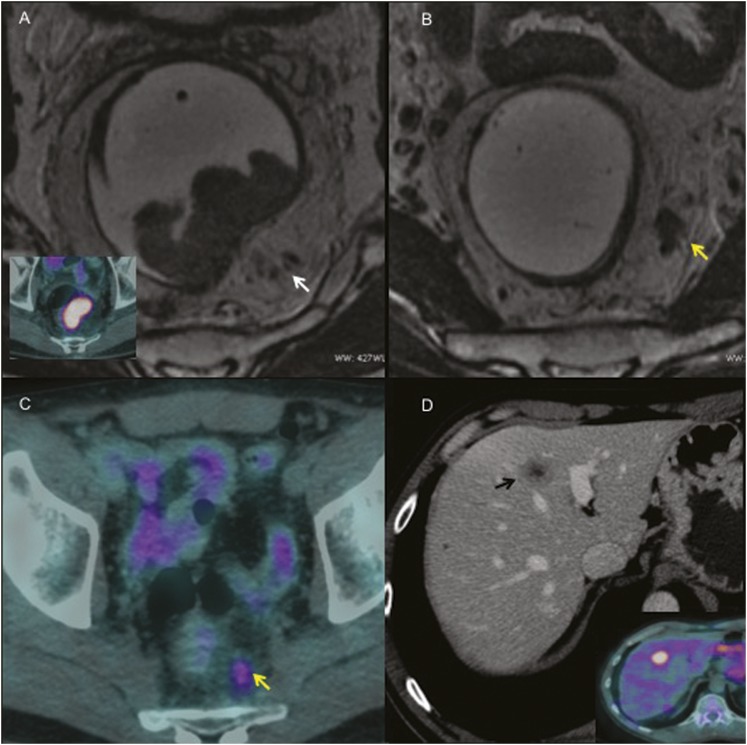
In NMC, the ADCmin was significantly lower in patients with synchronous metastases than that in patients without metastases (mean value: 0.90 vs 1.05 × 10−3 mm2 s−1; p = 0.04); there was a consistently higher significant correlation between extramural venous invasion (mEMVI) and synchronous metastatic disease in NMC (p < 0.01) (Figure 4).
Figure 4.
A 58-year-old male with non-mucinous mid-rectal cancer: (a, b) on the high-resolution axial T2 weighted (T2w) MR images, an intermediate signal endoluminal mass can be seen on the posterior rectal wall. An axial fused positron emission tomography (PET)-CT image is showing intense tumoral fludeoxyglucose (FDG) uptake (bottom left corner in a). The serpiginous extension of the tumour signal within a vascular structure (tubular structure containing signal void on T2w images that are continuing on adjacent slices), which is indicating extramural vascular invasion (arrow in a), can be noted. The yellow arrow in b is indicating nodular invasion along an irregular vessel in the mesorectum. (c) An axial fused PET-CT image is showing intense tumoral FDG uptake, which is associated with the increased metabolic activity of extramural vascular invasion (arrow in c). (d) The contrast-enhanced CT scan, portal phase, is showing a heterogeneous hypodense metastatic lesion in the left liver with central necrotic foci (black arrow in d); increased FDG uptake is evident (bottom right corner in d).
No correlations were found between any of the baseline imaging-based adverse features with synchronous metastatic disease in MC.
Pathological analysis response distribution after chemoradiation therapy
At least 8 weeks after the end of CRT, 100 of the 111 patients underwent total mesorectal excision (18 patients with MC and 82 patients with NMC). No statistical difference was found between the two groups in tumour response and in ypN status. The distribution of pathological analysis response in NMC and MC is shown in Table 4.
Table 4.
Pathological analysis response distribution after pre-operative chemoradiation therapy
| Pathological analysis response distribution |
|||
|---|---|---|---|
| MC (n = 18) | NMC (n = 82) | p-value | |
| PCR (TRG1) (%) | 3 (17) | 22 (27) | 0.367 |
| No PCR (TRG2–4) (%) | 15 (83) | 60 (73) | |
| ypN stage | |||
| ypN0 (%) | 12 (67) | 61 (74) | 0.148 |
| ypN1 (%) | 4 (22) | 19 (23) | |
| ypN2 (%) | 2 (11) | 2 (3) | |
MC, mucinous; n, number; NMC, non-mucinous carcinoma; PCR, pathological complete response; TRG, tumour regression grade; ypN, pathological nodal status.
11 of the 111 patients did not undergo surgery because of diffuse metastatic disease.
Correlations between the baseline imaging data with pathological analysis response evaluation
In NMC, the presence of mEMVI in pre-treatment MRI examination showed a statistically significant correlation with no PCR (TRG2−, p = 0.018) and pathological post-CRT ypN2 (p < 0.01).
No correlations were found between any of the baseline imaging-based adverse features with pathological analysis response evaluation in MC.
Distant metachronous recurrence at follow-up
Follow-up was available for 98 of the 101 patients without synchronous metastatic disease; of the 3 patients without follow-up, 2 patients (1 patient with NMC and 1 patient with MC) died and 1 patient (NMC) was lost immediately after diagnosis (follow-up time of 0 months). Median follow-up time was 48 months (range 4–82 months); median follow-up time was significantly higher in the NMC group (mean value: 51 vs 38 months; p < 0.001). 20 patients (18 patients with NMC and 2 patients with MC; p = 0.2) were lost during follow-up, after a median follow-up time of 46 months. Only 3 patients (2 patients with NMC and 1 patient with MC) showed local relapse at the time of analysis, being the statistical analysis not appropriate. 9/21 patients with MC and 9/80 patients with NMC (43% and 11%, respectively) showed distant metachronous recurrence. There was a significantly higher proportion of patients with metachronous metastatic disease in the MC group (p < 0.01), independently of the percentage of mucin (mean value: 46.9 vs 41.4%; p = 0.38).
Kaplan–Meyer analysis also shows significantly higher and early probability of metachronous metastasis recurrence in the MC group (Figure 5). The incidence rate of metachronous metastasis was 0.012 months/person for MC and 0.0023 months/person for NMC, and the incidence rate ratio was 5.23 (95% CI = 1.84–14.87; p < 0.01).
Figure 5.
Kaplan-Meyer survival curves of metachronous metastases recurrence in mucinous cancer (MC) and non-mucinous cancer (NMC). The analysis shows a worse prognosis in the MC group (steeper gradiant curve).
Univariate analysis
Univariate analysis showed that synchronous metastatic disease was significantly correlated with mEMVI [odds ratio (OR) = 9.931, p = 0.005] (Table 5). On univariate analysis, distant metachronous metastases were significantly correlated with MC (OR = 5.916; p = 0.002) and post-CRT ypN2 (OR = 2.535; p = 0.042) (Table 6).
Table 5.
Univariate analysis for synchronous metastatic disease
| Possible explicative variables | OR | p-value | 95% CI |
|---|---|---|---|
| Sex | 2.312 | 0.305 | 0.466–11.470 |
| Age | 0.997 | 0.938 | 0.936–1.062 |
| Mucinous | 0.952 | 0.953 | 0.188–4.823 |
| mT4 | 0.564 | 0.485 | 0.113–2.813 |
| mN2 | 1.413 | 0.608 | 0.376–5.312 |
| MRF+ | 1.153 | 0.833 | 0.305–4.352 |
| mEMVI | 9.931 | 0.005 | 1.988–49.600 |
| ADCmin (10−3) | 0.634 | 0.070 | 0.003–1.251 |
| DWI volume | 0.990 | 0.509 | 0.961–1.019 |
| T2w volume | 0.994 | 0.587 | 0.972–1.015 |
| MTV | 0.997 | 0.708 | 0.983–1.011 |
| TLG | 0.999 | 0.851 | 0.999–1.000 |
| SUVmax | 1.001 | 0.949 | 0.957–1.048 |
ADCmin, minimum apparent diffusion coefficient; CI, confidence interval; DWI, diffusion-weighted imaging; mEMVI, extramural venous invasion; MRF, mesorectal fascia; MTV, metabolic tumour volume; OR, odds ratio; SUVmax, maximum standardized uptake value; T2w, T2 weighted; TLG, total lesion glycolysis.
Table 6.
Univariate analysis for metachronous metastatic disease
| Possible explicative variables | OR | p-value | 95% CI |
|---|---|---|---|
| Sex | 1.192 | 0.749 | 0.406–3.497 |
| Age | 1.004 | 0.866 | 0.953–1.057 |
| Mucinous | 5.916 | 0.002 | 1.953–17.919 |
| mT4 | 0.843 | 0.768 | 0.272–2.612 |
| mN2 | 1.220 | 0.703 | 0.438–3.399 |
| MRF+ | 2.592 | 0.072 | 0.919–7.312 |
| mEMVI | 0.945 | 0.923 | 0.303–2.942 |
| ADCmin (10−3) | 6.538 | 0.129 | 0.579–73.754 |
| DWI volume | 0.990 | 0.362 | 0.969–1.011 |
| T2w volume | 1.001 | 0.763 | 0.990–1.013 |
| MTV | 0.996 | 0.519 | 0.985–1.007 |
| TLG | 0.999 | 0.252 | 0.998–1.000 |
| SUVmax | 0.974 | 0.250 | 0.933–1.018 |
| PCR | 1.153 | 0.808 | 0.363–3.661 |
| ypN (1,2) | 2.535 | 0.042 | 1.032–6.227 |
ADCmin, minimum apparent diffusion coefficient; CI, confidence interval; DWI, diffusion-weighted imaging; mEMVI, extramural venous invasion; MRF, mesorectal fascia; MTV, metabolic tumour volume; OR, odds ratio; PCR, pathological complete response; SUVmax, maximum standardized uptake value; T2w, T2 weighted; TLG, total lesion glycolysis; ypN, pathological nodal status.
Multiple regression analysis
In NMC, multiple regression analysis showed that the synchronous metastatic disease was significantly correlated with mEMVI (OR = 21.48; p < 0.01) and low ADCmin (OR = 0.04; p = 0.038) (Table 7).
Table 7.
Multivariate logistic regression for synchronous metastatic disease
| Pseudo R2 = 0.28 | OR | p-value | 95% CI |
|---|---|---|---|
| Mucinous | 3.92 | 0.190 | 0.507–30.347 |
| mEMVI | 21.48 | 0.002 | 3.153–146.319 |
| ADCmin (10−3) | 0.04 | 0.038 | 0.002–0.830 |
| DWI volume | 0.96 | 0.056 | 0.911–1.001 |
ADCmin, minimum apparent diffusion coefficient; CI, confidence interval; DWI, diffusion-weighted imaging; mEMVI, extramural venous invasion; OR, odds ratio.
The difference in rates of distant recurrence between the MC group and NMC group was highly significant (p < 0.01); the OR for metachronous metastases in MC compared with NMC was 21.67 (95% CI 3.8–120.5). On multivariate analysis, distant metachronous metastases were significantly correlated with post-CRT ypN2 (OR = 8.24; p = 0.01) in MC and NMC (Table 8).
Table 8.
Multivariate logistic regression for metachronous metastatic disease
| Pseudo R2 = 0.27 | OR | p-value | 95% CI |
|---|---|---|---|
| Mucinous | 21.67 | 0.00 | 3.893–120.572 |
| ypN (1,2) | 8.24 | 0.01 | 1.650–41.104 |
| PCR | 4.97 | 0.074 | 0.856–28.906 |
| DWI volume | 0.97 | 0.16 | 0.932–1.012 |
CI, confidence interval; DWI, diffusion-weighted imaging; OR, odds ratio; PCR, pathological complete response; ypN stage, pathological nodal status.
DISCUSSION
As we enter an era of personalized medicine in which therapies are stratified according to the risk of local or distant recurrence, there is an increasing need for pre-operative imaging methods that can non-invasively stratify patients at high risk who could benefit from more aggressive multimodal treatments.3 For extraperitoneal rectal tumours, 5–10 cm above the anal verge, no clear evidence of the superiority of combination chemotherapy existed; thus, the pre-operative identification of patients at high risk of metastatic disease may potentiate clinical trials of neoadjuvant chemoradiotherapy regimes.29,30 MRI is the most accurate imaging modality for assessing the T stage and mesorectal fascia, which are the most important risk factors for local recurrence, and MRI can contribute additional staging information about gross vascular invasion that is not necessarily recognized histologically but is associated with poor survival.25,31,32 Recent reports have shown that mEMVI can be readily identified on MRI and may be superior to routine histopathological analysis.27,33
The SUVmax reflects the higher tumour metabolism, and the ADC reflects the higher cellularity. Accordingly, these represent two facets of tumour biology, and both parameters are used for predicting prognosis.4,5,9,34 Curvo-Semedo et al5 demonstrated that lower ADC values were associated with a more aggressive tumour profile in 50 rectal cancers. In our experience, mEMVI and low ADCmin values were significantly correlated with synchronous M stage in NMC.
Mucinous tumours may fall into one of three categories. There are those tumours which are diagnosed as mucinous prior to any treatment and remain mucinous throughout their course; they carry an overall worse prognosis compared with adenocarcinoma of the same stage.35,36
A second group of tumours exist, which become mucinous during treatment; Nagtegaal et al37 found that those tumours that had become mucinous following short-course radiotherapy had a better prognosis than those tumours that were mucinous prior to treatment. The final group of mucinous tumours are those that demonstrate acellular mucin on histological analysis of post-treatment specimens. The acellular mucin had no impact on recurrence-free survival.38 One explanation offered by the College of American Pathologists is that mucinous change is a feature of response to pre-operative treatment.35 In this study, we analyzed MC prior to any treatment; discussion on the post-treatment mucinous change is beyond the scope of this study. It is important that radiologists be able to recognize MC on MRI. Yu et al13 demonstrated that MRI is diagnostically superior to biopsy in the pre-operative detection of mucinous rectal cancer. The current accepted definition of MC, initially proposed by Jass et al,10 is based on the presence of a minimum of 50% of mucin to tumour volume. Previous studies have used varying percentages of mucin to define these tumours.39,40 In this study, we included all tumours that appeared to have mucin by pre-treatment MRI and we found a significant correlation between the percentage of mucin as defined by MRI and the percentage of mucin as evaluated by histology. Symonds and Vickery41 reviewed the pathological characteristics of mucinous tumours and proposed that the spread of mucin separated the muscle bundles but upon reaching the perirectal fat showed pooling. This is similar to the pressure theory put forward by Sugarbaker,42 whereby the mucin exerts a pressure effect on the bowel wall structure, thus disseminating cancer cells that are taken up into lymphatics. This may explain the perceived aggressiveness of these tumours. The more aggressive behaviour of mucinous rectal tumours, rather than colonic tumours, may simply be due to anatomical location, and the mechanism of spread described above has a more immediate effect in the narrow confines of the pelvis.
In this study, we found a significantly higher proportion of patients with metachronous metastases in MC than in NMC according to previous results of Hugen et al,21 who demonstrated a worse overall survival of MC located in the rectum. Interestingly, in our experience, the consistently higher proportion of distant recurrence was independent of percentage of mucin in the rectal cancer (i.e. carcinomas that are 10–90% mucin). To our knowledge, this is the first study which demonstrated no significant difference in distant recurrence between MC with 50% or less of intratumoral mucin. Interestingly, the largest analysis in patients with MC concerning adjuvant chemotherapy to date demonstrated a similar survival for patients with NMC and MC who underwent adjuvant chemotherapy;3 thus, these patients, who are at risk of developing metachronous metastases, are more likely to benefit from more aggressive chemotherapy.
In agreement with the results of previous studies of tumours of various organs,43,44 in this study, the ADCmin values were significantly greater in MC than that in NMC. MCs have a large amount of extracellular mucin; this results in higher ADC values. Several studies have reported that gastrointestinal mucinous adenocarcinoma may have a low FDG uptake. Berger et al7 reported that the sensitivity of FDG PET in detecting colonrectal cancers was only 59%. However, our results showed a much higher sensitivity in rectal cancer, as all MCs showed increased FDG uptake and there was no significant difference in tumour SUVmax and TLG between MC and NMC. The molecular mechanism underlying the detection of colonrectal cancers by FDG PET-CT has largely shown that the specific biological characteristics of a given tumour, such as cell density, tumour size, deep invasion and hypoxia, determine its glucose metabolism.45–47 Therefore, in our rectal cancer series, the large tumour size as well as the more aggressive behaviour and pattern of the spread of MC could explain the increased FDG uptake that we observed.
The current standard method for discriminating responders from non-responders to pre-operative CRT and tumour restaging by histology is histopathological analysis.
In our study, tumour volume evaluated in T2w sequences was significantly higher in MC than in NMC, but a non-significant difference was demonstrated between patients with and without PCR in the MC group (17% and 83%) and NMC group (27% and 73%); oppositely, in NMC, the presence of mEMVI in pre-treatment MRI showed a statistically significant correlation with no PCR (TRG2–5) and pathological post-CRT ypN2stage; thus, according to previous results, mEMVI is a poor predicting factor of response after CRT.25,27,31–33 In accordance with many studies,30,48,49 our analysis shows that post-CRT ypN2 stage was significantly correlated with a higher risk of developing metachronous metastases (OR = 8.24, p = 0.01) in MC and NMC.
Our study has limitations. First, there were relatively limited number of MC cases owing to the different incidence of MC vs NMC; MCs are believed to account for 15% of advanced colorectal carcinomas. The second limitation was the lack of correlations with restaging imaging parameters; however, this issue is beyond the scope of this study.
CONCLUSION
In conclusion, in our experience, the use of mEMVI could identify patients with NMC who are at high risk of synchronous distant metastases. All patients with MCs located in the extraperitoneal rectum (i.e. carcinomas that are 10–90% mucin) are at high risk of developing metachronous metastatic disease. These groups may benefit from more aggressive neoadjuvant therapy, including induction chemotherapy.
Appendix A
The lesion volumes were calculated by manually tracing the tumour contours with a cursor and summing each of the cross-sectional volumes (multiplying cross-sectional area by section thickness) for the entire lesion at a workstation (Advantage Workstation 4.4; GE Medical Systems). Tumours that showed high signal intensity in T2w imaging, i.e. signals that were higher than those of the surrounding fat, were considered mucinous. The proportion of mucinous lesions (%) within a tumour was calculated by determining lesion and tumour volumes by manually tracing the mucinous lesion and the whole tumour separately and then dividing the mucinous volume by the total tumour volume × 100.
For DWI analysis, the data were analyzed with the Functool dynamic analysis tool on a workstation. ADCs were calculated for each pixel of the image and displayed as a parametric map. For quantitative analysis, regions of interest (ROIs) were drawn manually on the ADC maps and matched to the corresponding axial T2w images. We contoured ROIs over the whole tumour, section by section (using 4-mm-thick sections). The ADCmin value for the whole tumour was calculated as the minimum value of 4–6 measurements. In cases where the difference between measurements was ≤10%, ADC values were averaged between the two observers for further diagnostic analyses. In cases where the difference between measurements exceeded 10%, another series of measurements were performed and a consensus was reached. In the same reading session, qualitative analysis of DWI was performed by two radiologists. High signal intensity on DWI that corresponded with low signal intensity on the ADC map was interpreted as indicating a tumour. We manually contoured these suspicious areas to calculate the DWI tumour volume (multiplying the cross-sectional area by section thickness and then summing each of the cross-sectional volumes). ROI size was chosen to include as much of the hyperintense tumour as possible. The reference standard for signal intensity was the signal intensity of the prostate or small bowel on DWI (b-value, 1000 s/mm2).
Appendix B
Whole-body PET-CT imaging was obtained 60–70 min after FDG injection (222–333 MBq). All patients fasted for at least 6 h prior to FDG injection, which was followed by an injection of 500 ml of physiologic saline. The blood glucose level was measured systematically before FDG injection and was <160 mg/dl in all patients. Immediately before the start of the scan, all patients were asked to void; bladder catheters or diuretics were not used. The CT acquisition protocol included a “low-dose” CT (50 mAs, 140 kV; 3-mm slice thickness, 3-mm slice increment) from the base of the skull to mid-thigh for attenuation correction and anatomical localization. All PET scans were acquired in three-dimensional mode with an acquisition time of 3 min per bed position. PET data were reconstructed by iterative algorithms (3D-RAMLA) and corrected for dead time, decay, random events and attenuation.
PET-CT images were displayed (Fusion Viewer V2.0 by Philips Medical Systems) as rotating maximum intensity projections and as transverse, sagittal and coronal plane images. Tumour lesions were identified as areas of abnormal FDG uptake, and SUV was measured by placing a volume of interest (VOI) over the lesion, taking care to avoid other sources of physiological or non-specific uptake. For semi-quantitative analysis, the SUVmax was calculated using the maximum activity within the primary tumour normalized to the injected dose and the patient body weight. MTV was calculated using a fixed background SUV cut-off of 2.5. The mean standardized uptake value (SUVmean) was then determined as the average of the SUVs in all voxels within the defined tumour volume. TLG was calculated by multiplying the SUVmean of the primary tumour by the MTV.
Table A1.
MRI findings suggesting poor prognosis in rectal cancer
| MRI findings suggesting poor prognosis | |
|---|---|
| MRF involvement | Tumour <1 mm to the MRF |
| T4 stage | T4a peritoneal infiltration |
| T4b low encroachment of rectal cancer into the intersphincteric plane or levators and tumour infiltration into an adjacent structure or viscus | |
| mEMVI | Tumour signal intensity shows an expanding nodule within a grossly expanded and irregular vascular structure |
| N2 stage | Four or more suspicious nodes based on MRI morphological criteria |
Table A2.
Concomitant chemotherapy protocol
| Tumour stage concomitant chemotherapy protocol | |
|---|---|
| mT3 MRF− | 1650 mg m−2 of capecitabina, 7 days a week for the entire RT |
| mT3 MRF+ mT4 |
1650 mg m−2 of capecitabina, 7 days a week for the entire RT and five doses of oxaliplatin 60 mg m−2 once a week, for 5 weeks |
| sM+ | 1650 mg m−2, 7 days a week for the entire RT and five doses of oxaliplatin 60 mg m−2 once a week, for 5 weeks plus three cycles of chemotherapy in the waiting time between the end of RT and surgery, with the FOLFOX-4 regimen |
RT, radiotherapy treatment.
Table A3.
TRG score proposed by Mandard et al28
| TRG score | |
|---|---|
| TRG 1 | Complete tumour response |
| TRG 2 | Residual cancer cells scattered through fibrosis |
| TRG 3 | Increased number of residual cancer cells with predominant fibrosis |
| TRG 4 | Residual cancer outgrowing fibrosis |
| TRG 5 | No regressive changes within the tumour |
Contributor Information
Brunella Barbaro, Email: bbarbaro@rm.unicatt.it.
Lucia Leccisotti, Email: lucialeccisotti@yahoo.it.
Fabio M Vecchio, Email: fmvecchio@rm.unicatt.it.
Marialuisa Di Matteo, Email: marialuisa.dimatteo@libero.it.
Teresa Serra, Email: teresa.serra6@gmail.com.
Marco Salsano, Email: marco.salsano@hotmail.it.
Andrea Poscia, Email: andreaposcia@yahoo.com.
Claudio Coco, Email: coco_claudio@rm.unicatt.it.
Roberto Persiani, Email: rpersiani@rm.unicatt.it.
Sergio Alfieri, Email: s.alfieri@rm.unicatt.it.
Maria Antonietta Gambacorta, Email: nettagambacorta@gmail.com.
Vincenzo Valentini, Email: vvalentini.it@gmail.com.
Alessandro Giordano, Email: a.giordano@rm.unicatt.it.
Lorenzo Bonomo, Email: lbonomo@rm.unicatt.it.
REFERENCES
- 1.MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 2007; 243: 132–9. [DOI] [PubMed] [Google Scholar]
- 2.Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2013; 23: 2522–31. doi: https://doi.org/10.1007/s00330-013-2864-4 [DOI] [PubMed] [Google Scholar]
- 3.Hunter CJ, Garant A, Vuong T, Artho G, Lisbona R, Tekkis P, et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol 2012; 19: 1199–205. doi: https://doi.org/10.1245/s10434-011-2036-1 [DOI] [PubMed] [Google Scholar]
- 4.Meng X, Li H, Kong L, Zhao X, Huang Z, Zhao H, et al. MRI in rectal cancer: correlations between MRI features and molecular markers Ki-67, HIF-1α, and VEGF. J Magn Reson Imaging 2016; 44: 594–600. doi: https://doi.org/10.1002/jmri.25195. [DOI] [PubMed] [Google Scholar]
- 5.Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 2012; 35: 1365–71. doi: https://doi.org/10.1002/jmri.23589 [DOI] [PubMed] [Google Scholar]
- 6.Avril N. GLUT1 expression in tissue and (18)F-FDG uptake. J Nucl Med 2004; 45: 930–2. [PubMed] [Google Scholar]
- 7.Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol 2000; 174: 1005–8. doi: https://doi.org/10.2214/ajr.174.4.1741005 [DOI] [PubMed] [Google Scholar]
- 8.Whiteford MH, Whiteford HM, Yee LF, Ogunbiyi OA, Dehdashti F, Siegel BA, et al. Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. Dis Colon Rectum 2000; 43: 759–67; discussion 767–70. doi: https://doi.org/10.1007/BF02238010 [DOI] [PubMed] [Google Scholar]
- 9.Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med 2004; 45: 1431–4. [PubMed] [Google Scholar]
- 10.Jass JR, Sobin LH, Watanabe H. The world health organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer 1990; 66: 2162–7. doi: https://doi.org/10.1002/1097-0142(19901115)66:10<2162::AID-CNCR2820661020>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Park JS, Park SI, Kim NK, Kim JH, Moon HJ, et al. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr 2003; 27: 48–55. doi: https://doi.org/10.1097/00004728-200301000-00010 [DOI] [PubMed] [Google Scholar]
- 12.Oberholzer K, Menig M, Kreft A, Schneider A, Junginger T, Heintz A, et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys 2012; 82: 842–8. doi: https://doi.org/10.1016/j.ijrobp.2010.08.057 [DOI] [PubMed] [Google Scholar]
- 13.Yu SK, Chand M, Tait DM, Brown G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer 2014; 50: 920–7. doi: https://doi.org/10.1016/j.ejca.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 14.Chand M, Yu S, Swift RI, Brown G. Mucinous carcinoma of the rectum: a distinct clinicopathological entity. Tech Coloproctol 2014; 18: 335–44. doi: https://doi.org/10.1007/s10151-013-1099-3 [DOI] [PubMed] [Google Scholar]
- 15.Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, et al. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum 2003; 46: 160–7. doi: https://doi.org/10.1007/s10350-004-6518-0 [DOI] [PubMed] [Google Scholar]
- 16.Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005; 48: 1161–8. doi: https://doi.org/10.1007/s10350-004-0932-1 [DOI] [PubMed] [Google Scholar]
- 17.Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol 2000; 75: 103–7. doi: https://doi.org/10.1002/1096-9098(200010)75:2<103::AID-JSO6>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- 18.Adell R, Marcote E, Segarra MA, Pellicer V, Gamón R, Bayón AM, et al. Is mucinous colorectal adenocarcinoma a distinct entity? [In Spanish.] Gastroenterol Hepatol 2002; 25: 534–40. [PubMed] [Google Scholar]
- 19.Consorti F, Lorenzotti A, Midiri G, Di Paola M. Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case-control study. J Surg Oncol 2000; 73: 70–4. doi: https://doi.org/10.1002/(SICI)1096-9098(200002)73:2<70::AID-JSO3>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 20.Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol 2009; 34: 1109–15. doi: https://doi.org/10.3892/ijo_00000238 [DOI] [PubMed] [Google Scholar]
- 21.Hugen N, Verhoeven RH, Radema SA, de Hingh IH, Pruijt JF, Nagtegaal ID, et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol 2013; 24: 2819–24. doi: https://doi.org/10.1093/annonc/mdt378 [DOI] [PubMed] [Google Scholar]
- 22.Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012; 19: 2814–21. doi: https://doi.org/10.1245/s10434-012-2321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbaro B, Vitale R, Leccisotti L, Vecchio FM, Santoro L, Valentini V, et al. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics 2010; 30: 699–716. doi: https://doi.org/10.1148/rg.303095085 [DOI] [PubMed] [Google Scholar]
- 24.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227: 371–7. doi: https://doi.org/10.1148/radiol.2272011747 [DOI] [PubMed] [Google Scholar]
- 25.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol 2008; 191: 1517–22. doi: https://doi.org/10.2214/AJR.08.1298 [DOI] [PubMed] [Google Scholar]
- 26.Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 2009; 250: 730–9. doi: https://doi.org/10.1148/radiol.2503080310 [DOI] [PubMed] [Google Scholar]
- 27.Chand M, Swift RI, Tekkis PP, Chau I, Brown G. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer 2014; 110: 19–25. doi: https://doi.org/10.1038/bjc.2013.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–6. doi: https://doi.org/10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- 29.Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015; 16: 200–7. doi: https://doi.org/10.1016/S1470-2045(14)71199-4 [DOI] [PubMed] [Google Scholar]
- 30.Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 2011; 29: 3163–72. doi: https://doi.org/10.1200/JCO.2010.33.1595 [DOI] [PubMed] [Google Scholar]
- 31.Chand M, Palmer T, Blomqvist L, Nagtegaal I, West N, Brown G. Evidence for radiological and histopathological prognostic importance of detecting extramural venous invasion in rectal cancer: recommendations for radiology and histopathology reporting. Colorectal Dis 2015; 17: 468–73. doi: https://doi.org/10.1111/codi.12920 [DOI] [PubMed] [Google Scholar]
- 32.Yu SK, Tait D, Chau I, Brown G. MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy—implications for induction chemotherapy? Int J Radiat Oncol Biol Phys 2013; 87: 505–11. doi: https://doi.org/10.1016/j.ijrobp.2013.06.2052 [DOI] [PubMed] [Google Scholar]
- 33.Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol 2016; 22: 1721–6. doi: https://doi.org/10.3748/wjg.v22.i4.1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbaro B, Vitale R, Valentini V, Illuminati S, Vecchio FM, Rizzo G, et al. Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012; 83: 594–9. doi: https://doi.org/10.1016/j.ijrobp.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 35.Bouzourene H, Bosman FT, Matter M, Coucke P. Predictive factors in locally advanced rectal cancer treated with preoperative hyperfractionated and accelerated radiotherapy. Hum Pathol 2003; 34: 541–8. doi: https://doi.org/10.1016/S0046-8177(03)00176-X [DOI] [PubMed] [Google Scholar]
- 36.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American pathologists consensus statement 1999. Arch Pathol Lab Med 2000; 124: 979–94. [DOI] [PubMed] [Google Scholar]
- 37.Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol 2004; 204: 183–92. doi: https://doi.org/10.1002/path.1621 [DOI] [PubMed] [Google Scholar]
- 38.Shia J, McManus M, Guillem JG, Leibold T, Zhou Q, Tang LH, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol 2011; 35: 127–34. doi: https://doi.org/10.1097/PAS.0b013e318200cf78 [DOI] [PubMed] [Google Scholar]
- 39.Pihl E, Nairn RC, Hughes ES, Cuthbertson AM, Rollo AJ. Mucinous colorectal carcinoma: immunopathology and prognosis. Pathology 1980; 12: 439–47. doi: https://doi.org/10.3109/00313028009077107 [DOI] [PubMed] [Google Scholar]
- 40.Umpleby HC, Ranson DL, Williamson RC. Peculiarities of mucinous colorectal carcinoma. Br J Surg 1985; 72: 715–18. doi: https://doi.org/10.1002/bjs.1800720915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer 1976; 37: 1891–900. doi: https://doi.org/10.1002/1097-0142(197604)37:4<1891::AID-CNCR2820370439>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- 42.Sugarbaker PH. Mucinous colorectal carcinoma. J Surg Oncol 2001; 77: 282–3. doi: https://doi.org/10.1002/jso.1111 [DOI] [PubMed] [Google Scholar]
- 43.Woodhams R, Kakita S, Hata H, Iwabuchi K, Umeoka S, Mountford CE, et al. Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol 2009; 193: 260–6. doi: https://doi.org/10.2214/AJR.08.1670 [DOI] [PubMed] [Google Scholar]
- 44.Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Measurement of the apparent diffusion coefficient in intraductal mucin-producing tumor of the pancreas by diffusion-weighted echo-planar MR imaging. Abdom Imaging 2002; 27: 82–7. doi: https://doi.org/10.1007/s00261-001-0045-4 [DOI] [PubMed] [Google Scholar]
- 45.Izuishi K, Yamamoto Y, Sano T, Takebayashi R, Nishiyama Y, Mori H, et al. Molecular mechanism underlying the detection of colorectal cancer by 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography. J Gastrointest Surg 2012; 16: 394–400. doi: https://doi.org/10.1007/s11605-011-1727-z [DOI] [PubMed] [Google Scholar]
- 46.Furudoi A, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, et al. Clinical significance of human erythrocyte glucose transporter 1 expression at the deepest invasive site of advanced colorectal carcinoma. Oncology 2001; 60: 162–9. doi: https://doi.org/10.1159/000055314 [DOI] [PubMed] [Google Scholar]
- 47.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res 2003; 63: 1138–43. [PubMed] [Google Scholar]
- 48.Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005; 62: 752–60. doi: https://doi.org/10.1016/j.ijrobp.2004.11.017 [DOI] [PubMed] [Google Scholar]
- 49.Fietkau R, Barten M, Klautke G, Klar E, Ludwig K, Thomas H, et al. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum 2006; 49: 1284–92. doi: https://doi.org/10.1007/s10350-006-0570-x [DOI] [PubMed] [Google Scholar]