Abstract
Animal modelling is essential to the study of radiobiology and the advancement of clinical radiation oncology by providing preclinical data. Mouse models in particular have been highly utilized in the study of both tumour and normal tissue radiobiology because of their cost effectiveness and versatility. Technology has significantly advanced in preclinical radiation techniques to allow highly conformal image-guided irradiation of small animals in an effort to mimic human treatment capabilities. However, the biological and physical limitations of animal modelling should be recognized and considered when interpreting preclinical radiotherapy (RT) studies. Murine tumour and normal tissue radioresponse has been shown to vary from human cellular and molecular pathways. Small animal irradiation techniques utilize different anatomical boundaries and may have different physical properties than human RT. This review addresses the difference between the human condition and mouse models and discusses possible strategies for future refinement of murine models of cancer and radiation for the benefit of both basic radiobiology and clinical translation.
INTRODUCTION
The study of radiation biology has been much advanced through the use of animal models, with many basic tenets of the field derived from animal experiments. A well-known example is the use of rams by Regaud and Nogier1 to investigate the ability of fractionation to spare normal tissue. Experiments in preclinical models have been used for decades with the premise that they will give scientists and clinicians more insight in disease processes and assist in the development of efficient and effective strategies to prevent, cure and mitigate cancer and radiation effects. Preclinical models are also useful to test novel drugs and combination therapies prior to the expense and risk of human study.2,3
Animal models may not only prevent the exposure of humans to harmful substances, they have proven essential to dissect molecular and physiological mechanisms of radiation response. We continue to discover new pathways and molecules that govern cancer growth and the interaction between cancer cell and intervention. In the field of medicine, the use of knock-out mice has given unprecedented information in molecular biology that has led to more specific diagnostic tools and the identification of many targets for drugs.4 These “mechanistic” experiments are an essential way to move from in vitro experiments, including more recent spheroid, organoid and organ-on-chip models to human trials. The use of mice in studying tumour response to potential therapies has become a typical part of an oncology pipeline. Recent consensus work to promote oncologic discovery has championed the evaluation of novel drug–radiation combinations,5 and such preclinical work will predominantly use mice in preclinical testing.
Mouse studies of radiation effects on tumour biology have increased the field knowledge about the effect on the tumour microenvironment, including cytokine and immune cell response to radiation which affects the overall tumour response.6 Modelling tumour and normal tissue biology has grown beyond evaluating the tumour response of an isolated malignant human cell, but considers the supporting vascular supply, host immune system and infiltrating non-malignant cells. Thus, an in vivo mouse radiation model is limited by dissimilarities between rodent and human microenvironments. Likewise, as human radiation treatments have become more complex, modelling conformal therapies becomes a concern. Table 1 summarizes considerations with small animal radiotherapy (RT) models.
Table 1.
Considerations with small animal radiotherapy models
| Concern | Explanation |
|---|---|
| Biological | |
| Treatment naïve animals | Mutational stress caused by heavily pre-treated human cancer |
| Immune status | Immune system part of radiation response |
| Inherent biology differences | Molecular biology differences between mouse and human |
| Physical | |
| Field size | Dose accuracy |
| Similarity to human anatomical borders | |
| Radiation dose/fractionation/dose rate | Radiobiology differences |
| Irradiator energy differences | kV planning software for accurate dose prediction |
| Animal setup | OAR |
| Anaesthesia: air mix | |
| Radiation QA procedures | Frequency of testing |
kV, kilovoltage; OAR, organs at risk; QA, quality assurance.
POOR TRANSLATION OF CURRENT PRECLINICAL MODELS INTO CLINICAL TRIALS
The predominant animal model used in radiation oncology research is the mouse—because of low cost, easy handling and ability to manipulate the phenotype and genotype. However, the vast majority of studies noting promising therapies are not replicated in humans.7 It is clear that we are facing a qualitative as well as a quantitative problem in translating preclinical work to successful standard treatments. A systematic review showed that the methodological quality of 76 animal trials for a variety of conditions including cancer with a median citation of 886 was below quality standards for clinical studies.8 Not a single report fulfilled all quality criteria on dose response, “clinical” outcomes, long-term outcomes, disease spectrum, physiological monitoring, safety outcome, optimal time window, blinding, adjustment for multiplicity and randomization. Of these 76 studies, only 28 (37%) studies were replicated in human randomized trials. 14 of the 28 (50%) studies were contradicted by randomized trials. A large portion of the reviewed animal preclinical studies (34 of 76 or 45% studies) remains untested. No study attribute was predictive for translation to humans, except studies incorporating dose–response relationships for treatment interventions (mostly drugs) (overall survival 3.3; 95% confidence interval 1.1–10.1). The median time to replication was 7 years (range, 1–15 years). Eight replicated interventions were subsequently approved for use. Neither in cancer, nor in non-malignant conditions such as inflammatory disease or stroke, the majority of preclinical findings could be reproduced in subsequent trials in humans.4,9 Several studies have noted this gap in successfully translating preclinical findings into positive findings in human studies.6,10–12 One such study by Denayer et al12 describes strategies to increase the productivity of preclinical research, including back translation of unexpected clinical findings to improve animal modelling, inclusion of “clinical-trial like” end points (survival, quality of life) and model humanization. Another highlights the need to match end points to the clinical goal and potential mechanism of combined radiation and drug combination, as well as the importance of standardization in reporting.11
LIMITATIONS OF MOUSE TUMOUR MODELS
There is no doubt that experimental tumour models have contributed significantly in the understanding of molecular and other mechanisms of cancer development and treatment. Nevertheless, it has also been increasingly acknowledged that some fundamental shortcomings of preclinical models preclude the direct translation of these results to humans.
Classical xenografts still allow a rapid analysis of a hypothesis for there is an unlimited source of tumour cell lines available. However, when preclinical tumours are established from cultured cell lines, the heterogeneity of human cancers is lost to a great extent.13 Tumour cell lines have been grown in vitro for many years and hence may have altered characteristics compared with de novo tumours. In vitro culturing of cancer cells may introduce additional stress that lead to changes in the genotype and the phenotype of tumour cells.14–16 In addition, in these xenografts, the human microenvironment is not reproduced.
In an attempt to produce more realistic tumour models, patient-derived xenograft (PDX) models have been developed. Fresh cancer tissue is implanted orthotopically, subcutaneously or under the renal capsule of immunodeficient mice. Histologically, these PDX models show a similar architecture and stroma as the original tumour.10,17,18 The intratumour heterogeneity is preserved, such as chromosomal copy numbers, single-nucleotide polymorphisms and gene expression profiles. The orthotopic implantation may better mimic the human environment in which tumours originated and progressed. These orthotopic PDX models can be used to predict the drug response of the patient tumour.19,20 However, orthotopic implantation is technically difficult and the processing of the surgical fragments cumbersome. Obviously, a single human tumour is only a limited source of material. PDX models have the disadvantage that the immune component and the vasculature of the tumour is still of host (mouse) origin and hence do not perfectly reflect the human situation.
Genetically engineered mouse models (GEMMs) display specific mutations in somatic cells that underlie human cancer, such as a p53 mutation in lung cells leading to non-small-cell lung cancer.21,22 This allows study of defined mutations in the development and progression of tumours. The tumour microenvironment is representative of the cancer and it is possible to study the specific mutations in mice strains with a variety of genetic backgrounds. At the moment, it is possible to study simultaneously only a limited number of genes that are usually not representative of the full heterogeneity of the tumour. The development of the tumour may be slow and variable. Again, the tumour and its microenvironment are of murine origin and so do not fully mimic the human situation. Lastly, the cost of these GEMMs may be limiting.
Humanized in vivo models have been produced by engrafting a functional human immune system in a small animal.14 The most popular model is the bone marrow, liver, thymus model, which is established by subrenal capsule implantation of fetal liver and thymus fragments and i.v. injection of autologous haematopoietic stem cells derived from the same fetal liver donor. This model allows the most robust human immune system engraftment. When implanting tumours of individual patients and transplanting the immune system of the same patient in mice, it is hoped that the resulting tumours would nearly completely resemble the human donor. It remains an open question what the advantages and the disadvantages of these models are, and improvements are still being made, e.g. by reducing the mouse innate immunity, enhancing the human innate immunity and enhancing the human adaptive immunity, to generate class switching and immunoglobulin G antibody production.
CONTRIBUTION OF RADIATION TECHNIQUE TO MODEL RESPONSE
In addition to the difficulties in matching human biology to that of the mouse, we must recognize that the radiation treatments used in the mouse are often quite different than their human counterparts.23 Just as drug doses are often much higher in preclinical studies, radiation studies are often single fraction or severely hypofractionated. It is known that different fractionation schedules and doses change the hypoxia fraction and repopulation of tumour cells.24 Even treatment setup can be a factor, as irradiation under anaesthesia has been shown to have some effects on the alpha/beta ratio, again likely through radiosensitization of the anaesthetic: oxygen mixture.25 Consequently, it is now more common to use a mixture of air and anaesthetic to prevent this manipulation of tumour biology. Injectable anaesthesia has also been shown to affect radiation response in mice, potentially related to energy metabolism and hypothermia in the anaesthetized animal.26
The physical properties of the irradiators can also produce biologically relevant differences in the mice compared with human irradiators. Laboratory irradiation until recently typically used kilovoltage or radioisotope irradiators (e.g. caesium-137), with simplistic irradiation techniques compared with today's human radiation therapy equipment. Traditional cabinet-type irradiators have a fixed radiation source and minimal beam-modulating capabilities, often in the form of simple static shielding techniques.27 With these irradiators, an excess of normal tissue is included in targeted fields and only uniform dose distributions can be administered to the target structures. While targeting of a flank tumour or bilateral lungs is feasible with this equipment, the study of hippocampal28 or penile bulb radiation injury29 requires a much more elaborate technique. Modern advances in small animal irradiators have allowed partial animal irradiation,30 image guidance,31 arc therapy32 and even respiratory gating33 in small animal RT. Figure 1 offers an example of a small animal irradiator capable of highly conformal treatment and on-board imaging, including bioluminescence. While these technical feats bring us closer to the conformality and targeting of human treatment, we must remember that the small animal irradiators have key differences in physical properties with their linear accelerator cousins.
Figure 1.
A typical modern small animal irradiator imaging system (XRAD-SmART; PXi, Branford, CT) consisting of a heavy-duty X-ray tube equipped with precision collimators, a precision automated animal stage, a high-resolution X-ray imaging panel mounted opposite the X-ray tube and an optical camera for bioluminescence measurements.
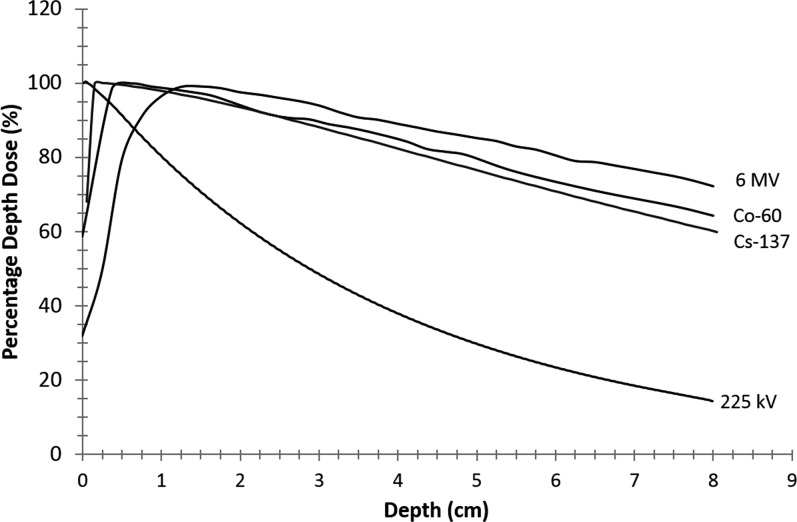
Research irradiators housed in laboratory settings use lower photon energy than clinical equipment, whether kilovoltage (225 kV) or caesium-137 (662 keV), while modern linear accelerators deliver photon beams with energies between 4 and 25 MV. Lower energies have a less build-up effect, delivering 100% dose at the skin with steeper absorption in the tissue (Figure 2). In addition, the photoelectric absorption is greater at lower energies. This differential can cause greater dose heterogeneity between low and high atomic number media and requires that accurate treatment planning use dose calculation algorithms specifically designed for the energy treated. Most modern image-guided precision irradiators use 225-kV photons, since these are a good compromise between a not too steep dose fall-off with depth and very sharp beam penumbras, allowing optimal sparing of sensitive tissues. In addition to differences in radiation dose distributions, the lower energy kilovoltage radiation employed in modern animal irradiators may also cause an increase in relative biological effectiveness (i.e. radiation quality) compared with megavoltage photons employed in RT.34
Figure 2.
Variation in the percentage of dose delivered by tissue depth. Higher energy results in lower dose at the skin surface and less attenuation at depth.
Field size, penumbra, inclusion of normal tissue and differences in radiation quality must be carefully considered when using an animal to model radiation effects. Unintentional vascular, marrow or other critical organ inclusion can result in response that may not accurately mimic human response because of the interplay between all these components of radiation injury. Very small field sizes become a potential concern, as the accuracy of dose prediction decreases with field sizes <2 mm.35,36
In addition, we should consider that human radiation therapy machines undergo extensive quality assurance (QA) to ensure that every treatment is within 5% accuracy. Detailed annual, monthly and daily QA is performed to meet this standard. Animal irradiators have less guidance for the frequency and type of QA performed, with the result that delivery of radiation may not be as accurate as reported.37 With the advent of partial irradiation techniques for animal models, targeting also becomes a necessary part of QA but is not standardized, leaving each laboratory to determine what level of testing is sufficient for the experiment at hand.
MICE AS HOSTS—REASONS FOR THE “TRANSLATIONAL GAP”
An obvious reason for the disparity between preclinical experiments and the subsequent implementation in the clinic is the biologic difference between mice and humans. As an example that is instructive for immunology, haematopoiesis in the mouse spleen is active into adulthood, whereas in humans, this ends before birth.38–40 The use of splenic lymphocytes as is standard in preclinical research should be interpreted in this context. In the context of targeted therapy, it is important to know that targets such as fms-related tyrosine kinase-3 (flt-3) and toll-like receptors are distributed and regulated differently in mice compared with humans. Haematopoietic stem cells are flt-3 negative in mice and flt-3 positive in males; toll-like receptor-3 is induced by lipopolysaccharide in mice, whereas this is not the case in humans and toll-like receptor-9 is expressed on all myeloid cells in mice.
Acute inflammatory stress from different causes results in highly similar genomic responses in humans, whereas the responses in corresponding mouse models correlate poorly with the human situation.41 The correlation between human and mice burn, endotoxemia and trauma was only 0.08, 0.05 and 0.00, respectively. HLA class II histocompatibility antigen: DR alpha (HLA-DRA) was upregulated in mouse burn and trauma, but not in endotoxemia, whereas HLA-DRA was mostly more than two orders of magnitude downregulated in humans in all three inflammatory conditions.
Moreover, tumour cell lines have been grown in vitro for many years and hence may have altered characteristics compared with de novo tumours. These tumour cells are implanted subcutaneously in mice and tend to grow rapidly and thus do not mimic the much slower doubling times of most human cancers. This faster tumour growth may lead to a higher sensitivity for most chemotherapy drugs and hence erroneous conclusions. Moreover, ectopic-implanted tumours may respond differently to treatment compared with tumours grown in an orthotopic site. Metastases frequently show other responses than primary tumours in patients, and it is only recently that these effects could be mimicked in GEMMs. Tumour-bearing mice are often treated with drug doses, or with pharmacokinetics, that are not relevant to humans.
An often neglected reason for the translational gap is that nearly all preclinical models have not used tumours that were pre-exposed to another therapy, whereas in many Phase I and Phase II clinical trials, only patients who show tumour progression after one or more systemic treatments are included.
Specifically for radiation therapy, most preclinical series deal with single-fraction irradiation of subcutaneous tumours without any imaging. In normal tissue research, generally very large volumes are treated with radiation dose distributions that are by no means representative o the real clinical world, where typically only small volumes of organs at risk receive high doses, whereas large volumes get low doses. The strong dose–volume dependency of radiation effects thus makes most preclinical radiation dose–volume relations quite unreliable.
ADVANCES IN MOUSE MODELS—HOW TO FILL THE “TRANSLATIONAL GAP”?
An expert panel that convened in 2011 evaluated radiation oncology trials with negative results. This workshop “Lessons Learned from Radiation Oncology Trials”42 concluded that preclinical studies must conduct at least in vitro clonogenic assay and contact the Radiation Research Program at the US National Cancer Institute (NCI), which is coordinating the preclinical and clinical studies for multiple targeted agents combined with RT in panels of human cancer cells, before embarking on combinatorial therapies and generate in vivo data using different human cancer xenograft models. Orthotopic models are encouraged. One example study of effectively utilizing small animal-targeted irradiation in an orthotopic model is that of Yahyanejad et al,43 who used U87MG glioblastoma cells orthotopically injected into the mouse brain. After CT-documented growth, tumours were irradiated using a partial brain technique conformal to the tumour. Comparison of sham, temozolomide and radiation combination therapy showed the efficacy of this model in reproducing the synergy of combination temozolomide and radiation.
Biomarkers should be an integral part of preclinical research in order to develop and validate tumour microenvironment-predictive biomarkers as well as for sensitivity to molecular-targeted therapies. “Clinically-ready” pharmacodynamic read-outs and robust imaging methods for tumour identification, segmentation, and characterization should be used across institutions.
Moreover, the recent improvements in animal models provide unique new, although expensive, opportunities.4 Both specific and multiple novel mouse models as well as the read-out methods are better but cost more than the simple models that were used in the past. The knowledge of the genetic background has led to the standardization and deep characterization of traditional strains and the generation of genetically diverse inbred and outbred strains. It is now possible to incorporate environmental factors such as diet, physical activity and microbiome in the models. Computational modelling allows getting an insight into gene–gene interactions and gene–environment interactions. Genome engineering and deep phenotyping is now reality.
In our view, there should be a direct bilateral link in preclinical models to the molecular characteristics of the human condition and that of the appropriate mouse strain. As an example, in a preclinical model of dendritic vaccination in a mouse glioblastoma model, the ratio between the T-lymphocyte subtypes type 1 helper (TH1), regulatory (Treg) and type 17 helper (TH17) were significantly related to survival.44 In human glioblastoma, only the TH17-associated metagene was related to the prognosis. In order to increase the translation ability of the preclinical model to males, only effects on TH17 should be considered relevant and optimized instead of obscuring the preclinical model by including non-relevant parameters for the human condition (Table 1).
ADVANCES IN SMALL ANIMAL IRRADIATION
The best strategy for the use of small animal irradiators is to have a thoughtful QA plan performed on a routine basis and to perform spot dosimetry as needed for technically complex plans. Multiple examples exist in the literature of QA regimens for initial commissioning and routine maintenance.45–47 Key elements are QA testing for dose rate constancy, imaging quality and targeting localizations that are performed on a regular basis. More advanced dosimetry techniques for small animal research may be based on using an imaging panel, in analogy with portal dosimetry in RT.48,49 Recently, some advances were reported on motion management33 and beam gating,50 as well as progress in imaging, e.g. bioluminescence,51,52 optical imaging and dual-energy CT.53 These techniques, fully integrated in the novel precision irradiation cabinets, will be required to allow sophisticated radiation research that can be translated to the human level.
CONCLUSION
Obviously, the ideal mouse model does not exist. All models have drawbacks and never reflect the full range of complexity of the tumour and the host of patients. However, successful translation (which is different from fundamental studies) will be in our view best secured when features that are appropriate for a certain research question in patients are selected, e.g. genetic response of an organ to radiation. It should be investigated which mouse model (tumour or normal tissue) displays similar features as the ones in humans for that particular feature. Preclinical studies should be performed in these models with a loop to the patient and back.4,33 This can be performed by considering the biology which needs to be represented in the model, tailoring the physics of the irradiation as close to the human situation as possible and acknowledging the limitations that cannot be overcome. We recommend the generation of international guidelines to create standards in performing and reporting preclinical radiation studies.
Acknowledgments
ACKNOWLEDGMENTS
We gratefully acknowledge S van Hoof of MAASTRO Clinic for providing the depth dose curve for caesium-137.
Contributor Information
Bridget F Koontz, Email: bridget.koontz@duke.edu.
Frank Verhaegen, Email: frank.verhaegen@maastro.nl.
Dirk De Ruysscher, Email: dirk.deruysscher@maastro.nl.
CONFLICTS OF INTEREST
FV is the founder of SmART Scientific Solutions BV.
FUNDING
BK—research funding: Janssen Pharmaceuticals, Advisory Board: Blue Earth Diagnostics (current), GenomeDx Biosciences (past); DDR—research funding: Boehringer, Advisory Board: Merck Serono (current), GSK (past).
REFERENCES
- 1.Regaud C, Nogier T. Stérilisation reontgénienne, totale et définitive, sans radiodermite, des testicules du bélier adulte. Conditions de sa résalisation. C R Soc Biol 1911; 70: 202–3. [Google Scholar]
- 2.Shear MJ, Hartwell JL, Peters VB. Some aspects of a joint institutional research program on chemotherapy of cancer: current laboratory and clinical experiments with bacterial polysaccharide and with synthetic organic compounds. In: Moulton FR, ed. Approaches to tumor chemotherapy. Washington, DC: American Association for the Advancement of Science; 1947. pp. 236–84. [Google Scholar]
- 3.Skipper HE, Schabel FR, Jr, Wilcox WS. Experimental evaluation of potential anticancer agents. XII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep 1964; 35: 1–111. [PubMed] [Google Scholar]
- 4.Kerbel RS. A decade of experience in developing preclinical models of advanced- or early-stage spontaneous metastasis to study antiangiogenic drugs, metronomic chemotherapy, and the tumor microenvironment. Cancer J 2015; 21: 274–83. doi: https://doi.org/10.1097/PPO.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 5.Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al. ; NCRI CTRad Academia-Pharma Joint Working Group. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol 2016. doi: https://doi.org/10.1038/nrclinonc.2016.79 [DOI] [PubMed] [Google Scholar]
- 6.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006; 6: 702–13. [DOI] [PubMed] [Google Scholar]
- 7.Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nat Med 2015; 21: 431–9. doi: https://doi.org/10.1038/nm.3853 [DOI] [PubMed] [Google Scholar]
- 8.Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA 2006; 296: 1731–2. doi: https://doi.org/10.1001/jama.296.14.1731 [DOI] [PubMed] [Google Scholar]
- 9.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Med 2010; 7: e1000245. doi: https://doi.org/10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014; 6: 114–18. [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman CN, Higgins GS, Brown JM, Baumann M, Kirsch DG, Willers H, et al. Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res 2016; 22: 3138–47. doi: https://doi.org/10.1158/1078-0432.CCR-16-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denayer T, Stohr T, Van Roy M. Animal models in translational medicine: validation and prediction. New Horiz Transl Med 2014; 2: 5–11. doi: https://doi.org/10.1016/j.nhtm.2014.08.001 [Google Scholar]
- 13.Briske-Anderson MJ, Finley JW, Newman SM. The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc Soc Exp Biol Med 1997; 214: 248–57. [DOI] [PubMed] [Google Scholar]
- 14.Wenger SL, Senft JR, Sargent LM, Bamezai R, Bairwa N, Grant SG. Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci Rep 2004; 24: 631–9. doi: https://doi.org/10.1007/s10540-005-2797-5 [DOI] [PubMed] [Google Scholar]
- 15.Neri A, Nicolson GL. Phenotypic drift of metastatic and cell-surface properties of mammary adenocarcinoma cell clones during growth in vitro. Int J Cancer 1981; 28: 731–8. doi: https://doi.org/10.1002/ijc.2910280612 [DOI] [PubMed] [Google Scholar]
- 16.Garber K. From human to mouse and back: “tumorgraft” models surge in popularity. J Natl Cancer Inst 2009; 101: 6–8. doi: https://doi.org/10.1093/jnci/djn481 [DOI] [PubMed] [Google Scholar]
- 17.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 2012; 9: 338–50. doi: https://doi.org/10.1038/nrclinonc.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SY, Lin D, Gout PW, Collins CC, Xu Y, Wang Y. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev 2014; 79: 222–37. doi: https://doi.org/10.1016/j.addr.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 19.Roscilli G, De Vitis C, Ferrara FF, Noto A, Cherubini E, Ricci A, et al. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity. J Transl Med 2016; 14: 61. doi: https://doi.org/10.1186/s12967-016-0816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med 2015; 21: 1318–25. doi: https://doi.org/10.1038/nm.3954 [DOI] [PubMed] [Google Scholar]
- 21.Gazdar AF, Hirsch FR, Minna JD. From mice to men and back: an assessment of preclinical model systems for the study of lung cancers. J Thorac Oncol 2016; 11: 287–99. doi: https://doi.org/10.1016/j.jtho.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 2015; 163: 39–53. doi: https://doi.org/10.1016/j.cell.2015.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhaegen F, Granton P, Tryggestad E. Topical review: small animal radiotherapy research platforms. Phys Med Biol 2011; 56: R55–83. doi: https://doi.org/10.1088/0031-9155/56/12/R01 [DOI] [PubMed] [Google Scholar]
- 24.Kitakabu Y, Shibamoto Y, Sasai K, Ono K, Abe M. Variations of the hypoxic fraction in the SCC VII tumors after single dose and during fractionated radiation therapy: assessment without anesthesia or physical restraint of mice. Int J Radiat Oncol Biol Phys 1991; 20: 709–14. doi: https://doi.org/10.1016/0360-3016(91)90013-T [DOI] [PubMed] [Google Scholar]
- 25.Stüben G, Landuyt W, van der Schueren E, van der Kogel AJ. Different immobilization procedures during irradiation influence the estimation of alpha/beta ratios in mouse lip mucosa. Strahlenther Onkol 1993; 169: 678–83. [PubMed] [Google Scholar]
- 26.Suit HD, Sedlacek RS, Silver G, Dosoretz D. Pentobarbital anesthesia and the response of tumor and normal tissue in the C3Hf/sed mouse to radiation. Radiat Res 1985; 104: 47–65. doi: https://doi.org/10.2307/3576776 [PubMed] [Google Scholar]
- 27.Tillner F, Thute P, Bütof R, Krause M, Enghardt W. Pre-clinical research in small animals using radiotherapy technology—a bidirectional translational approach. Z Med Phys 2014; 24: 335–51. doi: https://doi.org/10.1016/j.zemedi.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 28.Cramer CK, Yoon SW, Reinsvold M, Joo KM, Norris H, Hood RC, et al. Treatment planning and delivery of whole brain irradiation with hippocampal avoidance in rats. PLoS One 2015; 10: e0143208. doi: https://doi.org/10.1371/journal.pone.0143208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koontz BF, Yan H, Kimura M, Vujaskovic Z, Donatucci C, Yin FF. Feasibility study of an intensity-modulated radiation model for the study of erectile dysfunction. J Sex Med 2011; 8: 411–18. doi: https://doi.org/10.1111/j.1743-6109.2010.02125.x [DOI] [PubMed] [Google Scholar]
- 30.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, et al. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013; 13: 179. doi: https://doi.org/10.1186/1471-2407-13-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CL, Min H, Befera N, Clark D, Qi Y, Das S, et al. Assessing cardiac injury in mice with dual energy-microCT, 4D-microCT, and microSPECT imaging after partial heart irradiation. Int J Radiat Oncol Biol Phys 2014; 88: 686–93. doi: https://doi.org/10.1016/j.ijrobp.2013.11.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bache ST, Juang T, Belley MD, Koontz BF, Adamovics J, Yoshizumi TT, et al. Investigating the accuracy of microstereotactic-body-radiotherapy utilizing anatomically accurate 3D printed rodent-morphic dosimeters. Med Phys 2015; 42: 846–55. doi: https://doi.org/10.1118/1.4905489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Heyden B, van Hoof S, Schyns L, Verhaegen F. The influence of respiratory motion on dose delivery in a mouse lung tumor irradiation using the 4D MOBY phantom. Br J Radiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikjoo H, Lindborg L. RBE of low energy electrons and photons. Phys Med Biol 2010; 55: R65–109. doi: https://doi.org/10.1088/0031-9155/55/10/R01 [DOI] [PubMed] [Google Scholar]
- 35.Granton P, Verhaegen F. On the use of an analytic source model for dose calculations in image-guided small animal radiotherapy. Phys Med Biol 2013; 58: 3377–95. doi: https://doi.org/10.1088/0031-9155/58/10/3377 [DOI] [PubMed] [Google Scholar]
- 36.van Hoof SJ, Granton PV, Verhaegen F. Development and validation of a treatment planning system for small animal radiotherapy: SMART-plan. Radiother Oncol 2013; 109: 361–6. doi: https://doi.org/10.1016/j.radonc.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 37.Lindsay PE, Granton PV, Gasparini A, Jelveh S, Clarkson R, van Hoof S, et al. Multi-institutional dosimetric and geometric commissioning of image-guided small animal irradiators. Med Phys 2014; 41: 031714. doi: https://doi.org/10.1118/1.4866215 [DOI] [PubMed] [Google Scholar]
- 38.O'Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, Sun CC, et al. Practical murine hematopathology: a comparative review and implications for research. Comp Med 2015; 65: 96–113. [PMC free article] [PubMed] [Google Scholar]
- 39.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 2014; 34: 433–54. [PubMed] [Google Scholar]
- 40.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004; 172: 2731–8. doi: https://doi.org/10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- 41.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110: 3507–12. doi: https://doi.org/10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu FF, Okunieff P, Bernhard EJ, Stone HB, Yoo S, Coleman CN, et al. ; Workshop Participants. Lessons learned from radiation oncology trials. Clin Cancer Res 2013; 19: 6089–100. doi: https://doi.org/10.1158/1078-0432.CCR-13-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yahyanejad S, van Hoof S, Theys J, Barbeau L, Granton P, Paesmans K, et al. An image guided small animal radiation therapy platform (SMART) to monitor glioblastoma progression and therapy response. Radiother Oncol 2015; 116: 467–72. doi: https://doi.org/10.1016/j.radonc.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 44.Vandenberk L, Garg AD, Verschuere T, Koks C, Belmans J, Beullens M, et al. Irradiation of necrotic cancer cells, employed for pulsing dendritic cells (DCs), potentiates DC vaccine-induced antitumor immunity against high-grade glioma. Oncoimmunology 2015; 5: e1083669. doi: https://doi.org/10.1080/2162402X.2015.1083669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jermoumi M, Korideck H, Bhagwat M, Zygmanski P, Makrigiogos GM, Berbeco RI, et al. Comprehensive quality assurance phantom for the small animal radiation research platform (SARRP). Phys Med 2015; 31: 529–35. doi: https://doi.org/10.1016/j.ejmp.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong J, Chen Q, Febo R, Yang J, Pham H, Xiong JP, et al. Adaptation, commissioning, and evaluation of a 3D treatment planning system for high-resolution small-animal irradiation. Technol Cancer Res Treat 2016; 15: 460–71. doi: https://doi.org/10.1177/1533034615584522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brodin NP, Guha C, Tomé WA. Proposal for a simple and efficient monthly quality management program assessing the consistency of robotic image-guided small animal radiation systems. Health Phys 2015; 109(Suppl. 3): S190–9. doi: https://doi.org/10.1097/HP.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granton PV, Podesta M, Landry G, Nijsten S, Bootsma G, Verhaegen F. A combined dose calculation and verification method for a small animal precision irradiator based on onboard imaging. Med Phys 2012; 39: 4155–66. doi: https://doi.org/10.1118/1.4725710 [DOI] [PubMed] [Google Scholar]
- 49.van Elmpt W, McDermott L, Nijsten S, Wendling M, Lambin P, Mijnheer B. A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother Oncol 2008; 88: 289–309. doi: https://doi.org/10.1016/j.radonc.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 50.Hill M, Vojnovic B. Breathing gated irradiation using an image guided small animal irradiator: development and implementation. Br J Radiol 2016. [DOI] [PubMed] [Google Scholar]
- 51.Weersink RA, Ansell S, Wang A, Wilson G, Shah D, Lindsay PE, et al. Integration of optical imaging with a small animal irradiator. Med Phys 2014; 41: 102701. doi: https://doi.org/10.1118/1.4894730 [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Wang KK, Yu J, Eslami S, Iordachita I, Reyes J, et al. Bioluminescence tomography-guided radiation therapy for preclinical research. Int J Radiat Oncol Biol Phys 2016; 94: 1144–53. doi: https://doi.org/10.1016/j.ijrobp.2015.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schyns L, Almeida I, van Hoof S, Descamps B, Vanhove C, Landry G, et al. Optimizing dual energy cone beam CT protocols for preclinical imaging and radiation research. Br J Radiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]