Abstract
Tumours contain multiple different cell populations, including cells derived from the bone marrow as well as cancer-associated fibroblasts and various stromal populations including the vasculature. The microenvironment of the tumour cells plays a significant role in the response of the tumour to radiation treatment. Low levels of oxygen (hypoxia) caused by the poorly organized vasculature in tumours have long been known to affect radiation response; however, other aspects of the microenvironment may also play important roles. This article reviews some of the old literature concerning tumour response to irradiation and relates this to current concepts about the role of the tumour microenvironment in tumour response to radiation treatment. Included in the discussion are the role of cancer stem cells, radiation damage to the vasculature and the potential for radiation to enhance immune activity against tumour cells. Radiation treatment can cause a significant influx of bone marrow-derived cell populations into both normal tissues and tumours. Potential roles of such cells may include enhancing vascular recovery as well as modulating immune reactivity.
INTRODUCTION
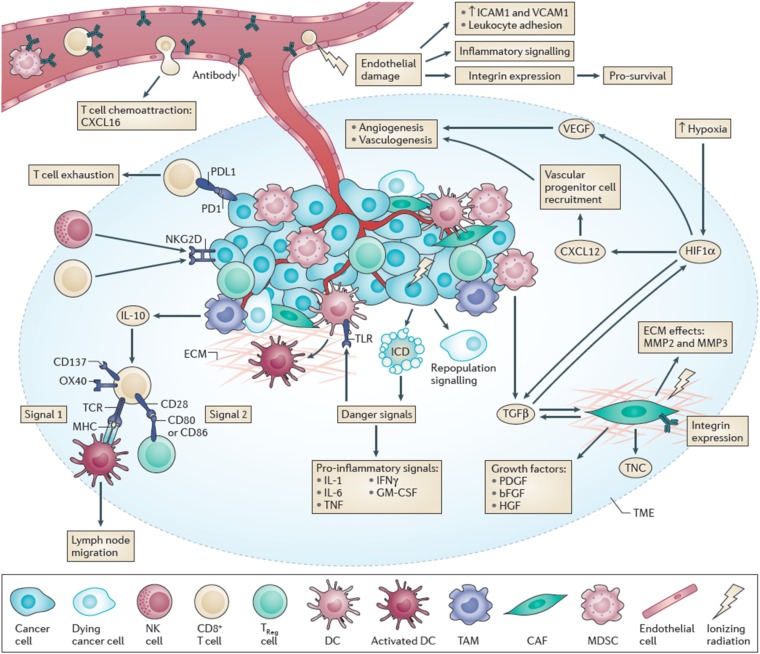
The response of tumours to radiation treatment is multifactorial and depends on features of the tumour microenvironment as well as the intrinsic sensitivity of the tumour cells themselves. Tumours contain multiple different cell populations derived from the host as well as the tumour cells. These cells include populations derived from the bone marrow (e.g. lymphocytes, macrophages/monocytes, granulocytes and dendritic cells), as well as cancer-associated fibroblasts and various stromal populations including the cells and stromal components comprising the vasculature (for an overview of the potential role of the various cell populations in the tumour microenvironment and how they may interact with radiation, see Figure 1).1 Furthermore, it is now well established that owing to their genetic instability, the tumour cells themselves may consist of multiple clonal populations that reflect the evolution of the tumour and the ability of different genetic or epigenetic alterations to promote growth within the tumour mass. However, only a fraction of the tumour cells (the stem cells) may have long-term proliferative potential and the ability to regenerate the tumour. The microenvironment of the tumour cells plays a significant role in the tumour response to radiation treatment. Low levels of oxygen (hypoxia) caused by the poorly organized vasculature in tumours have long been known to affect radiation response.2,3 However, other aspects of the microenvironment also appear to play important roles. There are increasing numbers of reports implicating the potential role of radiation in enhancing immune activity against tumour cells.4,5 There is also renewed interest in the potential role of radiation damage to the vasculature, in particular, its ability to recover following radiation treatment, so that it can support tumour regrowth. Blocking such recovery has been reported to increase the response of tumours to radiation treatment.6 Radiation treatment can cause a significant influx of bone marrow-derived cell (BMDC) populations into both normal tissues and tumours.7 Potential roles of such cells may include enhancing vascular recovery as well as modulating immune reactivity or possibly enhancing metastasis.8,9 High levels of neutrophils in the circulation and the tumour have also been associated with poor treatment outcome in cancers following irradiation.10–12 In this article, I will review some of the old literature concerning tumour response to radiation treatment and relate this to current concepts about the role of the microenvironment in tumour response to radiation treatment.
Figure 1.
Multiple cell populations in the tumour microenvironment can be affected by that environment and by irradiation. Reproduced from Barker et al1 with permission from Nature Publishing Group.
RETROSPECTIVE
Prior to the development of in vivo–in vitro clonogenic assays for mammalian cells growing in culture, studies of the response of tumours to irradiation were largely conducted using growth delay or tumour cure assays in rodents.13,14 Many of these studies were conducted using transplantable tumours given single radiation doses or a few dose fractions. These studies generally established that fairly large doses of irradiation were required to cure such tumours, unless the tumour was grown in an animal that was not “immune-compatible” or the tumour was chemically induced, in which case, much lower doses could be curable indicating the potential role of the immune system.15,16 These studies demonstrated that animals in which “immune-incompatible” tumours were grown and had been cured were largely resistant to a secondary transplant of that tumour, whereas this was not the case for tumours grown and cured in animals that were immune-compatible with the tumour involved (usually tumours which had arisen spontaneously in the inbred animal strain used for transplantation). These findings led to the view that chemically induced tumours were not very relevant models for assessing tumour response to radiation treatment and that such studies were better conducted with spontaneously arising tumours grown or transplanted into immune-compatible inbred hosts, which were generally found to show very limited evidence of immune reactivity.17,18
RADIATION RESPONSE OF TUMOUR CELLS
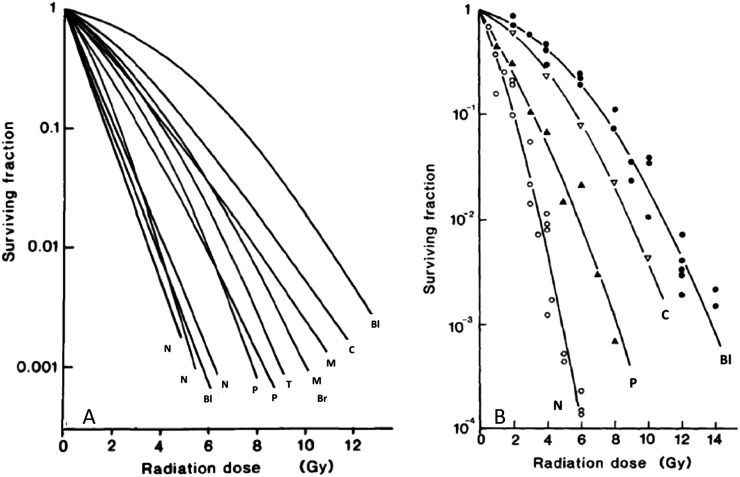
Following the development of in vivo–in vitro clonogenic assays and the development of cell lines derived directly from tumours, response of tumours focused on the radiosensitivity of the tumour cell population. Malignant cell populations in culture were found to have quite consistently shaped survival curves with a shoulder followed by a decline that could be reasonably well fitted by an exponential, although various different models have been proposed to fit such curves.19–22 The current model of choice is the linear-quadratic model [S = exp − (αD + βD2)], and the survival data shown in Figure 2(b) are fitted with this model. In general terms, the alpha parameter defines the slope of the initial part of the curve and the beta parameter defines the curvature of the curve. A derivation from this model has been widely used to compare response of tumours and normal tissues to different fractionated treatments, over a range of doses, based on estimates of the alpha/beta ratio.23
Figure 2.
(a, b) Cell survival curves for various different human tumour cell lines treated with a single dose of radiation. (b) Data are fitted with the linear-quadratic model of cell survival. Br, breast; Bl, bladder; C, cervix; M, melanoma; N, neuroblastoma; P, pancreas; T, teratoma. Modified from Steel148 and Steel et al149 with permission from Elsevier.
It should be noted that survival following radiation treatment is specifically defined in terms of the retention of proliferative capacity of the cells (i.e. as the ability of an individual cell to regenerate a colony of a certain size—usually 50+ cells) and does not relate to whether the cell actually dies and is lost from the population as a whole. This was consistent with observations that although some cells would undergo apoptosis following radiation treatment (depending to some extent on tissue type), many cells would often attempt cell division a few times before ultimately dying (of mitotic catastrophe) and being lost from the culture (or tumour) or undergoing senescence and ceasing proliferation. Thus, it was argued that the rate of regression of tumours following irradiation treatment largely reflected the underlying proliferative rate (fraction of cells in the proliferative cycle or the labelling index) of the tumour cell population. This in turn led to the view that the rate of regression of the tumour did not necessarily reflect the actual level of loss of cell survival due to the treatment and that, in tumour-regrowth assays, it was necessary to allow the tumour to regrow to larger than treatment size in order to determine the true response to the treatment in the context of surviving cells.24
It was established that the shoulder of the survival curve reflected the ability of the cells to repair radiation damage25 and the observed differences in survival curves for cells in different phases of the cell cycle were also largely thought to relate to different abilities of the cells in these various phases to repair radiation damage.26 Subsequent studies have also indicated that at doses <0.5 Gy, some cell populations may have a “hypersensitivity” to irradiation, which is thought to relate to reduced repair capacity following these low doses, and that, at slightly higher doses (>1 Gy), there is enhanced repair of this damage and that this may be related to cell cycle status.27,28
Repair of radiation damage associated with the shoulder of the survival curve has been modelled with various combinations of exponential functions but appears to be reasonably well fitted using a biexponential with rapid repair having an approximate half-time of about 0.3–0.5 h and slower repair with an approximate half-time of 4–5 h.29 However, similar to the extent of repair, these half-times can be variable for different cells with values ranging by about a factor of 2 around these values and the longer repair times may be more relevant to late responding normal tissues than most tumours.30 An important consequence of repair is that the efficacy of radiation treatment depends on the dose rate, particularly when this is below a value of about 0.5–1 Gy min−1 with treatment generally becoming less effective below this value. This is true for both tumour cells and normal tissues and the extent of the effect depends on the repair capacity of the relevant cell populations. Thus, for cells with low repair capacity, such as bone-marrow-derived populations or tumours such as neuroblastoma, the effect is small, whereas for tissues or tumours whose cells have high repair capacity such the lung and gastrointestinal tract, it can be a large effect.31 Although the instantaneous dose rate is important, for treatments using multiple beam protocols (such as intensity-modulated radiation therapy), the effective dose rate per treatment session also needs to be considered, and modelling suggests that treatments which exceed about 15–30 min may have reduced treatment efficacy.29,32,33
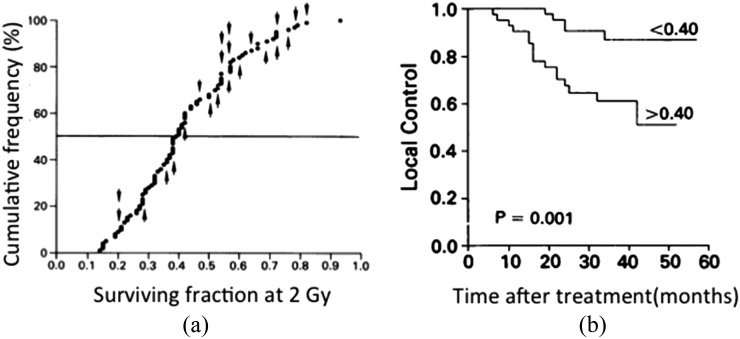
Studies of the radiation sensitivity of human tumour cell lines derived from different tumour types demonstrated that there were significant differences in the sensitivity of cells from different tumours (Figure 2). These effects were observed in relation to both the slopes and, particularly, the size of the shoulders of the survival curves for different cell lines, although in no case was it observed that cells were absolutely resistant to radiation. The analysis of the survival curves of cells from different types of tumours led to the view that differences in the shoulder region of the survival curve (i.e. at a dose of 2 Gy) reflected the in vivo radioresponse of the different types of tumours in patients, given “standard” fractionated radiation treatment (i.e. ∼2 Gy fractions), but that this was not generally true for the slope of the survival curve (Table 1).34,35 This concept was followed up by a number of different groups, notably West et al,36,37 who adapted in vitro assays to assess the radiation sensitivity of tumour cells derived directly from biopsies of cervical cancers in females due to undergo radiation treatment. The results of these studies indicated that the survival following a dose of 2 Gy correlated with the treatment outcome of the patients from whose tumour the cells were derived, indicating the importance of radiation sensitivity of the tumour cells for tumour response to irradiation (Figure 3). Subsequent studies reported similar effects for head and neck cancers, but other studies did not show significant effects.38 Owing to the difficulty of performing the technique, the time necessary to obtain the result and the fact that for a significant fraction of patient's tumours no data could be obtained, this approach to personalizing treatment based on the individual radiosensitivity of cancer cells in individual patients was not pursued further. However, the data are important both in the context of the studies of cancer stem cells (CSCs) (see Cancer Stem Cells section) and in relation to the increasing use of high dose treatment strategies [stereotactic body radiotherapy (SBRT)] for which response in the shoulder region of the survival curve is of limited relevance.
Table 1.
Values of the surviving fraction at 2 Gy for human tumour cell lines
| Tumour cell type | Number of lines | Mean survival at 2 Gy (range) | |
|---|---|---|---|
| 1. | Lymphoma Neuroblastoma Myeloma Small-cell lung cancer Medulloblastoma |
14 | 0.20 (0.08–0.37) |
| 2. | Breast cancer Squamous-cell cancer Pancreatic cancer Colorectal cancer Non-small-cell lung cancer |
12 | 0.43 (0.14–0.75) |
| 3. | Melanoma Osteosarcoma Glioblastoma Hypernephroma |
25 | 0.52 (0.20–0.86) |
Tumours are grouped 1–3 in approximate order of their likelihood of tumour control by radiation. Modified from Deacon et al.34
Figure 3.
(a) Surviving fraction at 2 Gy (SF2 value) for primary cells derived from 88 different cervical cancers and (b) Kaplan–Meier plot of local control for those cancers given radiation treatment based on the SF2 value being above or below the median value (0.4). Reproduced from West et al36 with permission from Nature Publishing Group.
More recent approaches have involved using genetic analysis of multiple cell lines in attempts to identify genetic signatures which predict radiation sensitivity above and beyond the known effects of mutations in the ataxia telangiectasia mutated (Atm) and other DNA repair genes.39,40 This approach has defined a variety of genes that may be involved in radiosensitivity of different tumour cell populations, but there are various limitations to such studies, notably the use of high throughput analyses of radiation response of the cells, which limits the ability of the studies to examine genetic factors separately affecting the low-dose vs the high-dose region of the survival curve. Other concerns with such studies can relate to the potential selection bias associated with the growth of cells in vitro (if cell lines are used in the study) or the potential role of heterogeneity and multiple clones within tumours (if biopsies are used in the study). Studies to identify genetic factors affecting normal tissue response to irradiation are also under way based on analyses of patients who have experienced serious side effects of radiation treatment,41,42 and these studies may also be informative concerning genetic factors affecting radiation response of specific tumour cell populations. To date such analyses have not translated into significant attempts to personalize radiation therapy in the clinic.
ROLE OF HYPOXIA
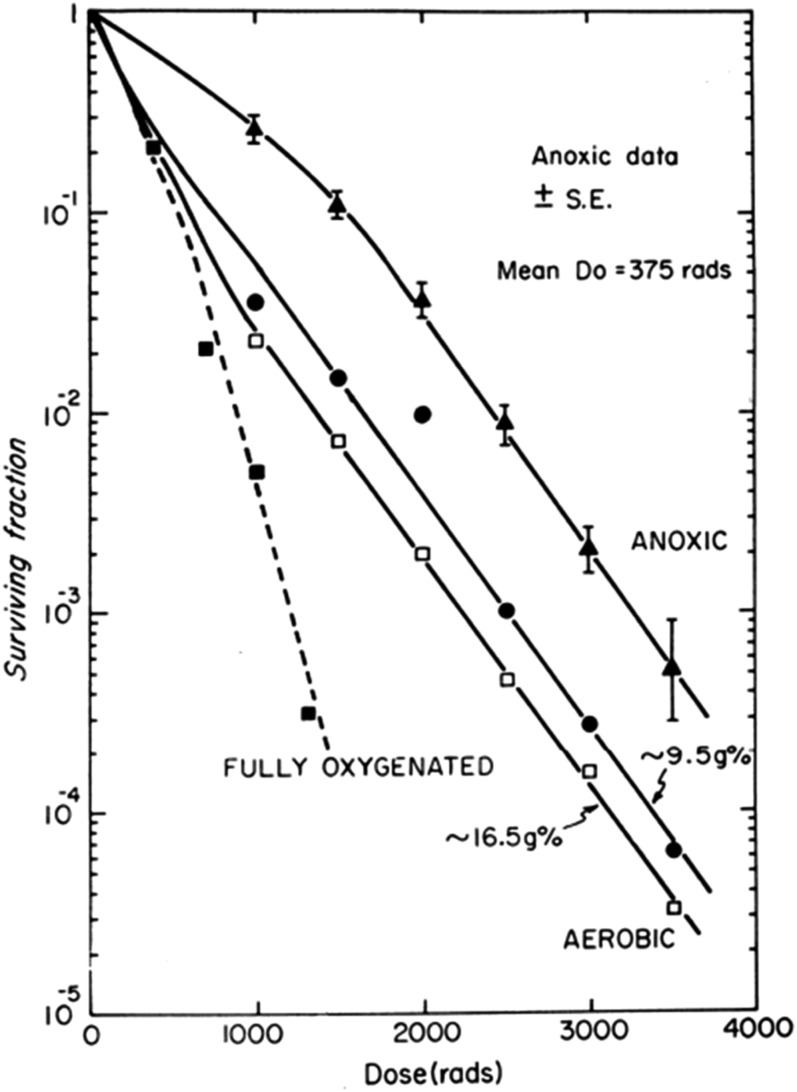
The further development of in vivo/in vitro assays to allow malignant cells taken directly from tumours to be grown in culture allowed studies of the radiosensitivity of malignant cells irradiated in situ in the tumour growing in a host animal. These studies confirmed earlier data from in vivo growth delay or tumour cure assays that tumours contained a fraction of viable cells at low oxygen levels (hypoxic) that were more resistant to irradiation than well-oxygenated cell populations (Figure 4).43,44 These observations were a clear demonstration of the role of the microenvironment of the tumour cells on their response to irradiation, since it was established that resistance was directly due to the lack of oxygen during radiation treatment. Detailed studies demonstrated that the effect was largely due to direct interaction of oxygen with radiation-induced radicals on the DNA-preventing rapid chemical repair of the radical by antioxidant molecules such as sulphydryls (−SH0).45 The level of oxygen required for an observed decrease in radiation response was less than about 10 mmHg, but full resistance was not observed until oxygen levels were below approximately 0.5 mmHg. Studies indicated that the levels of free sulfhydryls (e.g. glutathione, cysteine etc.) in the cells can modify this range; a factor that may be different for cells in tumours vs those growing in culture.46,47 An important aspect of hypoxia in tumours was the finding that it was quite dynamic and that during the course of fractionated radiation treatment, hypoxic cells that had survived one fraction could become “reoxygenated” and hence become more sensitive to a subsequent fraction. This was a widely observed phenomenon in both transplanted and spontaneously arising tumours.48–50 It was subsequently shown that tumours usually contain regions subject to fluctuating blood flow,51,52 leading to the concept that tumour cells may be exposed to either chronic or acute (cycling) hypoxic environments and that observed reoxygenation could be partially explained by such cycling hypoxia. Chronic hypoxia is largely thought to occur as a result of oxygen diffusion limitations, but potentially, it may also result from the presence of unusually long vessels or slow blood flow when the oxygen capacity of the blood may be exhausted along the length of the vessel.53,54
Figure 4.
Tumour cell response to irradiation when cells are removed from the tumour for analysis of clonogenic capacity immediately after irradiation. The figure demonstrates: (1) the effect of making the tumour hypoxic immediately before irradiation (anoxic), (2) the effect of different levels of haemoglobin in the blood of mice on the fraction of hypoxic cells in tumours in air-breathing animals. Survival curve for fully oxic cells is shown for comparison. Reproduced from Hill et al44 with permission from British Institute of Radiology. Do, slope of the survival curve; SE, standard error.
Follow-up on the effect of hypoxia on tumour growth and response to treatment has continued to the present day as more and more knowledge has been accumulated about the effects of different levels of hypoxia on cellular metabolic function, and it is increasingly clear that other aspects of the effects of hypoxia may also play an important role in the response of tumours to treatment.55 Relevant to radiation response are observations that the extent of repair of radiation damage in hypoxic cells depends on the length of their exposure to hypoxia both before and after irradiation, such that chronically hypoxic cells may have a lesser degree of resistance to radiation (due to reduced repair capacity) than acutely (cycling) hypoxic cells.56 Early studies had also suggested such an effect using tumour cure assays.57 Furthermore, cells may have limited ability to maintain viability long term under chronic hypoxia, depending on such factors as their ability to induce mechanisms of survival under low-energy conditions, such as autophagy.58 Thus, some chronically hypoxic cells may not be able to induce tumour regrowth even if they could survive the initial radiation treatment. The possibility that hypoxic cells may die in vivo following doses of radiation but be able to survive if reoxygenated was proposed quite early but was quite variable in different studies.59–61 Recent studies have suggested that this may be an important factor in the response of tumours to SBRT.62 These observations are consistent with the concept that acutely hypoxic cells and/or the larger number of cells that are partially resistant to irradiation due to being at intermediate oxygen levels in tumours may in fact represent a more important population of cells relevant to tumour control effected by standard fractionated radiation therapy rather than the most hypoxic cell populations.63
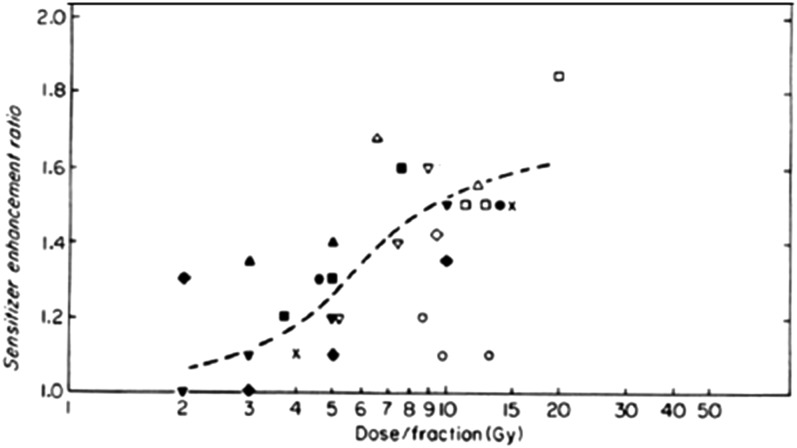
However, despite the overwhelming data that many tumours contain hypoxic cells and the findings that levels of hypoxia in various tumour types are predictive of treatment outcome, approaches to addressing this issue in radiation therapy treatment of patients have had only limited success. Initial studies using hyperbaric oxygen breathing or drugs which could specifically radiosensitize hypoxic cells showed some positive effects, and a meta-analysis of many of these early trials did show a significant gain overall.64 However, a number of clinical studies testing the potential benefit of drugs that were directly cytotoxic to hypoxic cells have met with limited success when combined with radiation.65 Partly, this may be due to the process of reoxygenation (and cycling hypoxia) occurring between treatment fractions, since pre-clinical studies did generally demonstrate that the degree of sensitization achieved with the radiosensitizer misonidazole declined to small values in the range of fraction sizes (1.5–2.5 Gy) generally used in radiation therapy (Figure 5).66 However, another factor is likely to be the heterogeneity in the levels of hypoxia in different tumours. None of the studies of hypoxic radiosensitizers or hypoxic cytotoxins have involved measurements of levels of hypoxia in the tumours of all the patients included in the studies, thus it was impossible to identify if the patients with the most hypoxic tumours did in fact benefit from the drug treatment. This is particularly relevant since small subpopulations in these studies, in whose tumours' hypoxia was measured, did demonstrate benefit in the most hypoxic tumours,67 and a derived genetic signature associated with hypoxia-induced genes did show a correlation of the most hypoxic tumours with benefit from combination of a radiosensitizing drug (nimorazole) with radiation treatment.68 The combination of robust techniques for imaging hypoxia prior to and during treatment with some form of genetic analysis would be consistent with current moves towards more personalized therapy. The supplementation of such genetic signatures with genes associated with the ability of cells to survive hypoxic stress or those that are associated with the environment of an hypoxic stem cell niche could potentially be beneficial but will need to be tested in future studies.69
Figure 5.
The sensitizer enhancement ratio obtained when different animal tumours were treated with fractionated radiation doses in the presence or absence of misonidazole is plotted as a function of the dose per fraction. The different symbols represent data from different experiments. The broken line is drawn to represent the trend of the data. Modified from Hill66 with permission from Elsevier.
CANCER STEM CELLS
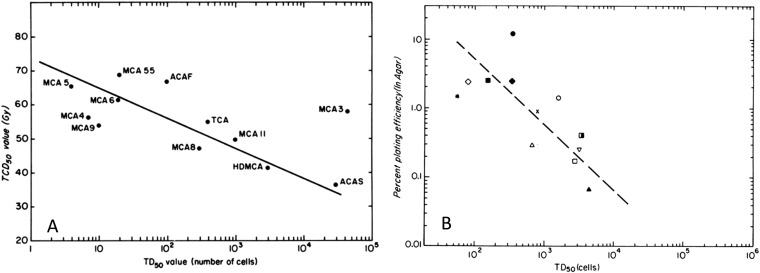
Recent studies have highlighted the potential importance of stem cell populations in tumours—CSCs.70,71 Such cells (CSCs) are argued to be ultimately responsible for maintaining tumour growth and for recurrence following therapy. They thus represent the critical population for predicting treatment outcome, and their number and radiosensitivity are important for tumour control by radiation therapy. The numbers of CSCs in tumours have been reported to vary widely, and it has been demonstrated in animal models that the number of (putative) stem cells correlates with the single radiation dose required for tumour control (Figure 6).72,73 Similar results have been reported for experimental studies in animal models using fractionated radiation treatment74 and the expression of the stem-cell-related marker CD44 has been reported to correlate with local control in early laryngeal cancers treated with radiation.75 Interestingly, in the studies of West et al76 discussed above, the plating efficiency (PE) of the cells from cervical cancers was not found to be as good a predictor of patient outcome as the SF2 value determined in vitro. However, in vitro assays may not reflect well the long-term potential for proliferation required of CSCs. In studies of cells derived from spontaneous mouse mammary tumours, there was a correlation of PE in vitro with the number of cells required to be injected to successfully achieve 50% transplant of the tumours (TD50 value), which is regarded as the gold standard for assessing CSCs, but the in vitro PE was greater than a factor of 10 higher than the in vivo TD50 value (Figure 7).72 This is consistent with the data for murine bone marrow, for which there are cells that can form colonies in vitro as well as cells that can be classified as intermediate-term and long-term stem cells in vivo.77,78
Figure 6.
Relationship between tumour “stem-like” cells and tumour control for a group of first passage mouse mammary tumours in C3H mice. (a) The relationship between the single radiation dose given under hypoxic conditions required to cure 50% of the tumours (TCD50 value) and the number of cells required to be injected to generate growth of tumours in 50% of injection sites (TD50 value). The slope of the line is consistent with the radiation sensitivity of hypoxic cells. (b) The relationship between the in vitro plating efficiency and the TD50 value for the same group of tumours. Modified from Hill and Milas72 with permission from Elsevier.
Figure 7.
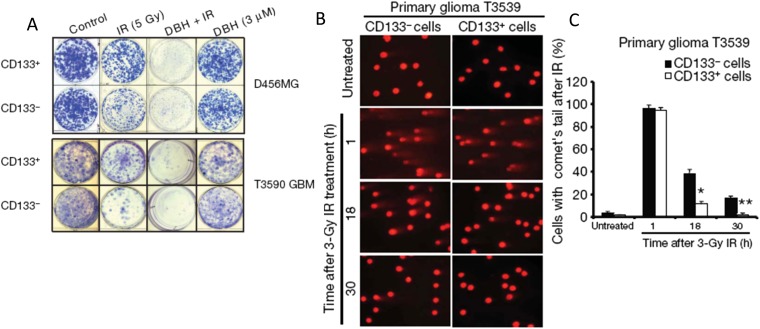
Reduced radiation sensitivity of stem cells. (a) Plating of CD133+ (stem cells) or CD133− (progenitor cells) glioblastoma cells irradiated or not with 5 Gy and treated or not with debromohymenialdisine (DBH), a CHK1/2 inhibitor. The CD133+ cells show higher plating after 5 Gy than the CD133− cells. (b) Alkaline comet assays of CD133+ or CD133− cells from one of the cell lines at various times after irradiation (IR) with 3 Gy. (c) The quantification of the number of cells retaining comet tails (i.e. those that have not completed DNA repair) at various times after irradiation. Reproduced from Bao et al80 with permission from Nature Publishing Group.
There are several data sets which support a higher radioresistance of CSCs than their progenitor cells in tumours.73,79 In extensive experiments, an increase of the ex vivo fraction of CD133+ cells, confirmed as CSC by transplantation assays, was observed after in vivo irradiation of glioma xenografts, and CD133+ cells were found to be more radiation resistant in vitro (Figure 7).80 There was preferential activation of DNA damage checkpoints in CD133+ vs CD133− cells. Another resistance mechanism may be related to higher levels of antioxidant molecules to deal with reactive oxygen species (ROS), a critical mediator of radiation damage in cells.81 Compared with progenitor cells, breast CSCs have been shown in vitro to contain a lower level of ROS with higher expression of genes involved in ROS scavenging. Moreover, the initially higher post-irradiation clonogenic cell survival of breast CSC can be altered by pharmacological modulation of the ROS levels. However, a higher intrinsic radioresistance of CSC cannot be regarded as a general phenomenon, since heterogeneity seems to exist between individual tumours of the same histology.82 A link between hypoxia and putative stem cells has also been shown by an increase in the fraction of CD133+ cells in brain tumour cells exposed to hypoxia in vitro,83,84 and the preferential expression of hypoxia induced factor (HIF)-2α- and HIF-regulated genes in glioma stem cells.85 Specific microenvironmental factors such as cell–cell interactions and genetically regulated cellular signals may also be important determinants for stem cell maintenance and survival. Different kinds of “niches” have been described that contain a higher fraction of CSCs or maintain the stem-like phenotype of tumour cells, including a hypoxic niche.86,87 A hypoxic niche for CSCs would be expected to affect their relative radiosensitivity, but if the level of hypoxia is consistent with increased levels of HIF-1α and HIF-2α (approximately <10–20 mmHg) rather than the levels required for full hypoxia-induced radioresistance (<1–5 mmHg),88 it may only cause a small increase in radioresistance. However, as noted above, oxygen sensitization relates to a competition between oxygen and free sulfhydryls to interact with the radiation-induced radical sites on damaged DNA,47 thus higher levels of SH-containing antioxidant molecules such as glutathione or cysteine in CSCs might enhance the protection associated with intermediate levels of hypoxia.
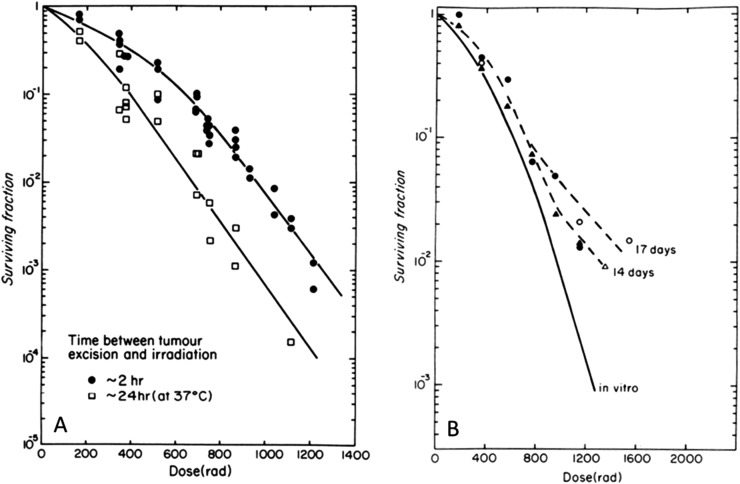
Another aspect of the issue of radiation sensitivity of CSCs is the finding that allowing tumour cells to grow in three-dimensional cultures or “spheroids” can enhance the fraction of them which express the phenotype of stem cells.89,90 This is of interest in the context that such growth has also been noted to modify the radiation sensitivity of cells (independent of oxygen effects). Early work by Durand and Sutherland91 found that cells from spheroids had an increased shoulder on their radiation survival curve and that this reflected increased repair capacity. This was demonstrated to decay away over a period of about 24 h when the cells were separated into single cells.92 A similar effect was observed in vivo with cells growing as small colonies in the lung after they were removed and assayed for radiosensitivity as a single-cell suspension (Figure 8).93 More recently, increased radiation resistance has been reported for cells growing in three-dimensional culture or on various extracellular matrix substrates. This resistance has been related to the changed expression of certain cell surface molecules such as integrins and focal adhesion genes, which are involved in cell–cell and cell–matrix interactions.94–97 An important difference here may be the usual absence of serum in the medium used for stem cell studies in spheroids, but it is unclear whether the change in radiation sensitivity associated with growth in spheroids is directly related to the “stemness” of the cells or is a parallel phenomenon.
Figure 8.
Increased shoulder on the survival curve for cells growing in contact in vivo. (a) The effect of holding cells obtained from an intramuscular tumour (KHT sarcoma) in vitro for 2 or 24 h before irradiation. The data points are from analyses of cells from individual groups of tumours. (b) Results for irradiation of the cells as small colonies growing in the lung compared with a survival curve for irradiation of the same cells growing in vitro. The tails on these curves show the development of hypoxia in these small lung colonies. The individual points represent analysis of different groups of lungs. Reproduced from Hill et al93 with permission from Radiation Research.
A further concern with studies of CSCs is the increasing evidence for plasticity of the CSC phenotype and the concept that early progenitor cells may be able to regain stem cell properties, thereby increasing the effective number of CSCs in the tumour.98 Particularly important in the context of radiation treatment is that back differentiation of progenitor cells into stem cells may be induced by radiation, although the extent of this phenomenon is currently uncertain.99–102 However, comparisons of the radiation dose required to cure xenografted tumour cell lines in immune-deprived mice using single doses vs fractionated treatments have given a reasonably good correlation, suggesting that this effect may be limited.74 Further studies are needed in this area, particularly with tumours directly from patients and early patient-derived xenograft (PDX) models. Studies of murine bone marrow stem cells have demonstrated stem cell populations with intermediate-term or long-term ability to repopulate the marrow,78 but little is known about whether such populations may exist in tumours. The potential role of genetic heterogeneity and genetic instability also needs to be considered, since this could relate to selection of radiation-resistant cell populations following treatment.
RADIATION-INDUCED DAMAGE TO VASCULATURE
The potential role of radiation damage to vasculature in relation to tumour response was presaged by early studies examining what was called the “tumour bed effect”. The essence of these observations was that transplanting a tumour into a site in an animal that had been previously irradiated resulted in a requirement for larger numbers of cells for transplantation and slower growth of the subsequent tumour than was observed when the same type and number of tumour cells were transplanted into a non-irradiated site.103 These observations were also consistent with early reports that tumours regrowing after large doses of irradiation had an initial phase of slower growth before increasing14 or that regrowing tumours following radiation treatments often grow more slowly than untreated tumours. This latter observation led to recommendations that such a difference in growth rate could be built into analysis of growth delay studies by calculating the delay in terms of the number of tumour growth doublings rather than the absolute delay time. Importantly, these effects were largely observed following quite large doses of irradiation (10–20 Gy+) and limited tumour-bed effects were observed following lower doses. These observations suggest that vascular damage could impact the ability of tumours to regrow after irradiation and are consistent with the more recent data on the effect of radiation-induced apoptosis in endothelial cells on tumour response.104 Doses in excess of about 8–10 Gy are needed to induce this effect.105
The concept that damage to tumour vasculature affects tumour response was tested in a study that examined the single doses of radiation required to control a series of transplantable tumours growing in either nude or severe combined immunodeficiency (SCID) mice.106 Since the scid mutation makes the cells of SCID mice more radiation sensitive, it was argued that the vasculature of tumours growing in these mice should be more sensitive than those growing in nude mice and, consequently, that tumours growing in SCID mice should require a lower dose for cure than those growing in nude mice. This was not found to be the case, thus it was concluded that damage to vasculature did not play a significant role in tumour response to irradiation. Similar results have also been reported recently for cure of a rodent tumour model in which endothelial cells were sensitized by a mutation in the Atm gene (which affects DNA repair).107 However, in this study, it was found that radiation-induced growth delay was enhanced in the tumours with the more sensitive endothelial cells, suggesting that increased damage to vasculature could affect the ability of the tumour to regrow over time, although not the dose required for cure. Various possible mechanisms of vascular repair that could occur over time include the presence of circulating endothelial precursor cells, observations that certain tumour cells can transdifferentiate into endothelial cells and the findings that there is an influx of BMDC populations into irradiated tissue that can potentially help to support the vasculature during recovery.7 Studies using a window-chamber model have delineated the effects of large doses of radiation on depleting vascular function, particularly the microvasculature, in tumours and demonstrated the recovery of function a number of days later.108–110
Following the work of Folkman111 on the essential role of angiogenesis in tumour growth, there has been the development of agents that target vasculature, both in terms of blocking angiogenesis and by direct disruption of tumour vasculature.112 A number of antiangiogenic agents are approved for clinical application,113 and some of these agents have been combined with radiation treatment. To date, these studies have shown quite limited benefits,114 and it is hypothesized that this may be due to multiple angiogenesis pathways and/or differential ways in which tumour vasculature may recover following radiation treatment, primarily by angiogenesis or vasculogenesis. A major difference between these two pathways is new vessels sprouting from existing vessels (angiogenesis) vs the influx of cells from other parts of the body or bone marrow, which can build or rebuild vessels (vasculogenesis).115 Various vascular disrupting agents are also in clinical development,116 and pre-clinical studies have suggested that these agents have potential to be combined with radiation, but apart from anecdotal observations in early stage trials, it is unclear if these will prove to be any more beneficial than antiangiogenic agents.
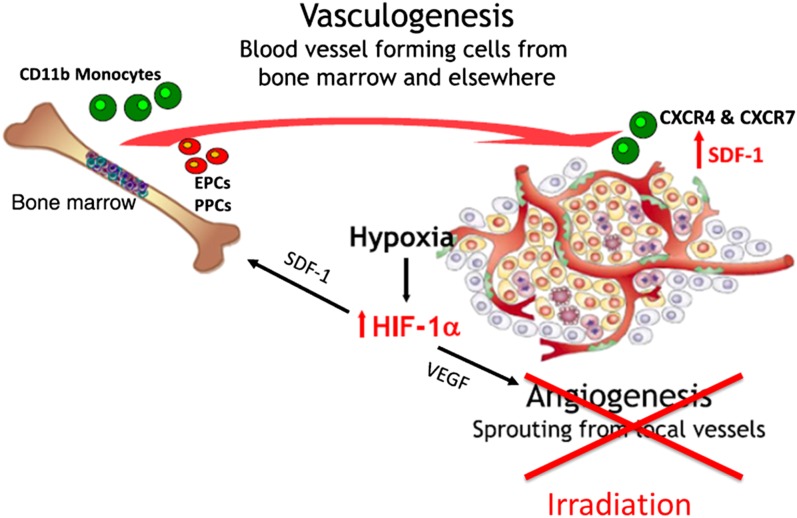
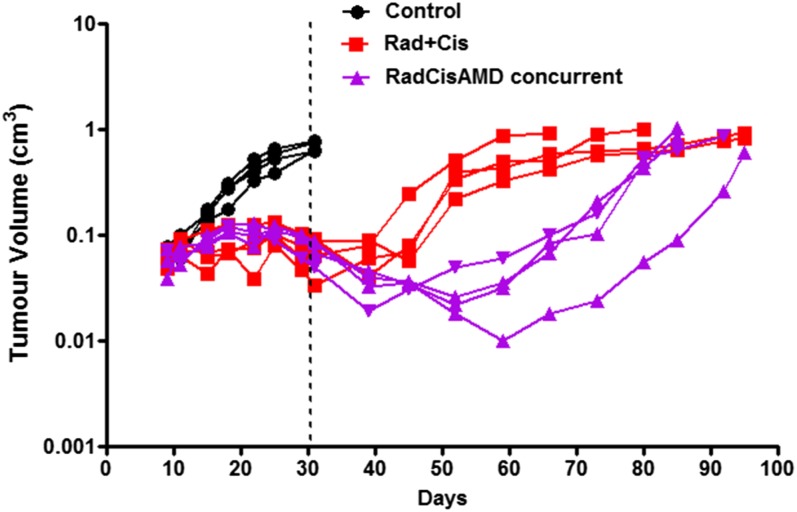
Recently, blocking vasculogenesis has been postulated to be important for exploiting the damaging effects of radiation on the vasculature.6 This has arisen partly from the view that angiogenesis is largely an in-field phenomenon, and consequently, since it should be impaired by the radiation treatment, it is likely vasculogenesis that is responsible for vascular repair following radiation treatment (Figure 9). This is consistent with the influx of BMDC that occurs in irradiated tissues and the various findings suggesting that BMDC may play a role in alleviating vascular damage. Such cells have been observed to associate with the outside of vessels in irradiated tissues in a manner similar to pericytes, and it is been hypothesized that this helps to maintain the structure of the vessels.7 In fact it has been recently reported that mesenchymal stem cells from the bone marrow can differentiate and act as progentors of pericytes (PPCs) in irradiated tumours.117 Together with the presence of endothelial precursor cells (EPCs) in the circulation (whether these cells derive from the marrow is controversial), this influx of cells may help to stabilize and repair irradiated vessels. The influx of cells from the marrow has been demonstrated to be related to the production of various chemokines including C-X-C motif chemokine 12 [CXCL12; also known as stromal cell derived factor 1 (SDF-1)] and blocking the interaction of this chemokine with its receptors C-X-C chemokine receptor type 4 (CXCR4) or C-X-C receptor type 7 (CXCR7) has been reported to enhance tumour (glioblastoma) response to irradiation.6 We have obtained similar results using ME180 cervical cancer xenografts growing orthotopically in mice, where it was found that blocking the interaction of CXCR4 with CXCL12 using AMD3100 (Plerixafor) increased the growth delay induced in the tumours by radiation plus cisplatinum treatment (Figure 10). Plerixafor is a clinically available drug that helps to mobilize bone marrow stem cells into the circulation. Similar increases in tumour radiation response have also been obtained by blocking HIF-1/2a expression, which is one factor that can cause the upregulation of CXCL12 levels in irradiated tumours.118
Figure 9.
Model for repair of vascular damage following irradiation. Inactivation of angiogenesis by a large dose of irradiation causes the tumour vasculature to rely on vasculogenesis for repair prior to tumour regrowth. Modified from Brown6 with permission from British Institute of Radiology.
Figure 10.
Tumour response curves for an orthotopic cervix Ca xenograft (ME180) treated with fractionated irradiation (15 × 2 Gy given over 3 weeks) + cisplatinum (4 mg kg−1 given at the beginning of every week) (Rad + Cis) or Rad + Cis + AMD 3100 (Plerixafor) given throughout the course of treatment by infusion pump.
OTHER AREAS OF CURRENT INTEREST
One area that is exciting particular current interest is the combination of radiation therapy with immunotherapy. Attempts to enhance the immune reactivity of tumours have been investigated for many years in experimental models,119 at least in part due to the occasional observations of abscopal effects of radiation in the clinic.120 This work also built initially on studies with chemically induced tumours, which showed evidence of an ability of the immune system to act against such tumours. Our improved understanding of the evolution of the interaction of the immune system with malignant cell populations with the concept of “immunoediting” in three phases—elimination, equilibrium and escape—during tumour development and growth has led to a variety of different approaches, including inhibition of T-regulatory cell activity, cytokine-based enhancement of T-cell activity, myeloid cell activators, checkpoint blockade and various therapeutic vaccines.5,121,122 It is proposed that increased exposure of immune effector cells to tumour antigens due to the death of tumour cells following radiation treatment has the potential to enhance immune reactivity. A number of pre-clinical studies have reported improved response of radiation-treated tumours and in some cases of metastatic lesions not given radiation treatment.16 The recent success of the use of immune checkpoint blockade in certain human cancers has raised hopes that such an approach may be further enhanced by radiation therapy and a number of clinical trials are under way to investigate various aspects of this idea.122,123 In the context of radiation treatment, studies of abscopal effects suggest that the size of the radiation dose may be important for such effects and that standard fractionation schedules (2 Gy) may be less effective than larger doses per fraction (6–8 Gy) and that these may be superior to bigger single doses (20 Gy). The early data also suggest that giving checkpoint inhibitors concurrently with radiation is more effective than giving them after the end of the radiation treatment, but further studies are needed to enhance our understanding in these areas.124,125 A separate but related issue is the induction of bystander effects in which irradiated cells may produce molecules that can induce damage in closely located cells that are not directly irradiated.126 The role of such effects in standard radiation therapy is currently unclear, but they may play a role in radionuclide therapy.127
Another area of current interest is the use of SBRT treatments involving one or a few large fractions. Such treatments have largely been made possible by the improved ability to target radiation treatment and have met with significant success particularly in treatment of small lung cancer lesions.128,129 The success of such treatments is at least partially due to the fact that much larger effective doses (based on linear-quadratic modelling) can be delivered than was previously possible, without causing serious normal tissue side effects, and it is unclear if other factors play a significantly different role than for standard fractionation.130 One possible factor of importance may be the role of hypoxia since pre-clinical studies have suggested that the extent of reoxygenation is related to the degree of fractionation as noted above. Whether SBRT schedules may be more appropriate for combination with hypoxia cell sensitizers or hypoxic cytotoxins remains to be determined. As noted above, any such studies will need to consider the level of hypoxia in the tumours and select suitable patients based on measurements of this level.
FUTURE STUDIES
The recent decision by the National Cancer Institute/National Institutes of Health to retire the NCI60 panel of cell lines, long used to test new drugs, has highlighted concerns that drug testing using long-term cell lines has limited predictability for efficacy of such drugs in clinical application.131–133 These articles have argued that evaluating new targets using established cell lines is limited by the often poor correlation between responsiveness observed in cell lines vs that elicited in the patient. It is argued that PDXs generated from fresh tumour specimens are likely to recapitulate better the diversity of cancers and are more reflective of the histopathology and properties of the original tumour. There is also increasing evidence that PDXs can recapitulate treatment responses of individual parental tumours.134,135 Although these concerns were focused on drug response, rather than radiation response, it is very likely that they are relevant to studies of radiation effects in tumours.136 This may be particularly so in modern radio(chemo)therapy with the widespread use of drugs in combination with radiation. These issues may also be a concern for studies trying to identify genetic changes in which large numbers of cancer cell lines are examined for correlations between genetic or epigenetic abnormalities associated with radiation response.40 The findings of such studies will need careful follow-up in appropriate models before they can be used for personalizing radiation treatment strategies. Recent studies in primary tumours have, however, identified genomic instability as a driver of disease aggressiveness and treatment failure in both surgical and radiation treatment of prostate cancer.137–139 Furthermore, it appears that patient-specific measurements of tumour hypoxia and genomic instability provide complementary information about clinical outcome, with hypoxia being more or less important in some genomic subgroups than in others,138 emphasizing the role of the tumour microenvironment.
A further issue concerning suitable tumours for study relates to the site of transplantation of the tumours. Subcutaneous or intramuscular transplantation of tumours on a body extremity has long been the preferred site for experimental radiation studies in rodents due to simplicity and easy of access for treatment without excessive normal tissue exposure; however, it may not be the optimum site. Increasingly, orthotopic transplantation is preferred since tumours growing subcutaneously have clearly different properties such as reduced metastases, increased encapsulation and, in many cases, increased levels of necrosis as they grow, suggesting differences in vascular function.140 Modern small animal irradiators, which provide small defined focused beams and an ability to target the tumour from different angles have reduced concerns about normal tissue exposure and have largely obviated the need to use subcutaneous transplantation sites.141–144 These current technologies are allowing investigation of treatment strategies not previously possible in small animals. They provide opportunities to test different approaches with the attending possible differences in the critical parameters affecting tumour treatment response, particularly those related with the microenvironment. These technologies also make it easier to address one downside to using PDX model, i.e. the need to use immune-deprived animals, which limits the study of any immune interactions with the radiation treatment. The availability of many genetically modified mouse models of different cancers or other spontaneously arising rodent tumours can help to address this concern, since they can be more easily treated in the site of origin, either as the primary tumour or following orthotopic transplantation in the same context as PDX models.136,145,146
In summary, the emerging evidence of the multiple factors that may affect radiation response impacts the ability to select suitable tumours for personalized changes in radiation therapy treatment. Such selection needs to encompass the microenvironmental factors associated with tumour growth and response to treatment, as well as specific aspects of the radiation sensitivity of the tumour (stem) cells, related to aetiological, genomic and epigenomic factors. The increasing long-term survival of patients with cancer has prompted greater concern about the side effects of radiation/chemotherapy treatments raising the issue of any associated changes on normal tissue response, since these are a critical component of the therapeutic ratio. Such issues may be partially addressed by improvements in the accuracy of precision radiotherapy and particularly the use of particle therapy (currently protons or carbon ions), which has the potential to reduce the volume of normal tissue exposed as well as exploiting the higher relative biological effectiveness associated with these radiations.147 However, parallel studies of critical normal tissue responses will remain necessary as new drugs are introduced in combination with radiation treatment as part of personalized therapy.
Acknowledgments
ACKNOWLEDGMENTS
The author acknowledges many discussions with colleagues at the Princess Margaret Cancer Centre and, particularly, Dr N Chaudary, Dr M Milosevic and Dr RM Sutherland for reading and commenting on the manuscript. The research of the author is funded by the Terry Fox Foundation.
CONFLICTS OF INTEREST
The author is part of a team involved in the development of a small animal irradiator (XRad 225Cx) and receives a royalty from the company (Precision X-ray) marketing the device.
REFERENCES
- 1.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015; 15: 409–25. doi: https://doi.org/10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray LH. Radiobiologic basis of oxygen as a modifying factor in radiation therapy. Am J Roentgenol Radium Ther Nucl Med 1961; 85: 803–15. [PubMed] [Google Scholar]
- 3.Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 2001; 18: 243–59. doi: https://doi.org/10.1385/MO:18:4:243 [DOI] [PubMed] [Google Scholar]
- 4.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013; 105: 256–65. doi: https://doi.org/10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16: e498–509. doi: https://doi.org/10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 6.Brown JM. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br J Radiol 2014; 87: 20130686. doi: https://doi.org/10.1259/bjr.20130686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrell K, Singh S, Jalali S, Hill RP, Zadeh G. VEGF regulates region-specific localization of perivascular bone marrow-derived cells in glioblastoma. Cancer Res 2014; 74: 3727–39. doi: https://doi.org/10.1158/0008-5472.CAN-13-3119 [DOI] [PubMed] [Google Scholar]
- 8.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009; 15: 35–44. doi: https://doi.org/10.1016/j.ccr.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chafe SC, Lou Y, Sceneay J, Vallejo M, Hamilton MJ, McDonald PC, et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res 2015; 75: 996–1008. doi: https://doi.org/10.1158/0008-5472.CAN-14-3000 [DOI] [PubMed] [Google Scholar]
- 10.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: dju124. doi: https://doi.org/10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 11.Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, et al. Pretreatment neutrophil: lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 2012; 32: 1555–61. [PubMed] [Google Scholar]
- 12.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16: 431–46. doi: https://doi.org/10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 13.Suit HD, Shalek RJ. Response of spontaneous mammary carcinoma of the C3H mouse to X irradiation given under conditions of local tissue anoxia. J Natl Cancer Inst 1963; 31: 497–509. [PubMed] [Google Scholar]
- 14.Thomlinson RH, Craddock EA. The gross response of an experimental tumour to single doses of x-rays. Br J Cancer 1967; 21: 108–23. doi: https://doi.org/10.1038/bjc.1967.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suit HD, Sedlacek RS, Silobrcic V, Linggood RM. Radiation therapy and Corynebacterium parvum in the treatment of murine tumors. Cancer 1976; 37: 2573–9. doi: https://doi.org/10.1002/1097-0142(197606)37:6<2573::AID-CNCR2820370602>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- 16.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 1979; 63: 1229–35. [PubMed] [Google Scholar]
- 17.Hewitt HB. A critical examination of the foundations of immunotherapy for cancer. Clin Radiol 1979; 30: 361–9. doi: https://doi.org/10.1016/S0009-9260(79)80209-3 [DOI] [PubMed] [Google Scholar]
- 18.Hewitt HB. Second point: animal tumor models and their relevance to human tumor immunology. J Biol Response Mod 1983; 2: 210–6. [PubMed] [Google Scholar]
- 19.Curtis SB. Lethal and potentially lethal lesions induced by radiation—a unified repair model. Radiat Res 1986; 106: 252–70. doi: https://doi.org/10.2307/3576798 [PubMed] [Google Scholar]
- 20.Douglas BG, Fowler JF. The effect of multiple small doses of X rays on skin reactions in the mouse and a basic interpretation. 1976. Radiat Res 2012; 178: AV125–38. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert CW, Hendry JH, Major D. The approximation in the formulation for survival S = exp−(alpha D + beta D2). Int J Radiat Biol Relat Stud Phys Chem Med 1980; 37: 469–71. doi: https://doi.org/10.1080/09553008014550571 [DOI] [PubMed] [Google Scholar]
- 22.Tannock IF, Hill RP, Bristow RG, Harrington L, eds. The basic science of oncology. 5th edn. New York: McGraw-Hill; 2013. [Google Scholar]
- 23.Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys 2013; 85: 89–94. doi: https://doi.org/10.1016/j.ijrobp.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 1999; 59: 1391–9. [PubMed] [Google Scholar]
- 25.Elkind MM, Sutton H. Radiation response of mammalian cells grown in culture. 1. Repair of X-ray damage in surviving Chinese hamster cells. Radiat Res 1960; 13: 556–93. doi: https://doi.org/10.2307/3570945 [PubMed] [Google Scholar]
- 26.Sinclair WK. Cyclic X-ray responses in mammalian cells in vitro. 1968. Radiat Res 2012; 178: AV112–24. [DOI] [PubMed] [Google Scholar]
- 27.Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 2001; 49: 379–89. doi: https://doi.org/10.1016/S0360-3016(00)01471-1 [DOI] [PubMed] [Google Scholar]
- 28.Krueger SA, Wilson GD, Piasentin E, Joiner MC, Marples B. The effects of G2-phase enrichment and checkpoint abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol Biol Phys 2010; 77: 1509–17. doi: https://doi.org/10.1016/j.ijrobp.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery. Int J Radiat Oncol Biol Phys 2004; 59: 242–9. doi: https://doi.org/10.1016/j.ijrobp.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 30.Steel GG. Recovery kinetics deduced from continuous low dose-rate experiments. Radiother Oncol 1989; 14: 337–43. doi: https://doi.org/10.1016/0167-8140(89)90146-1 [DOI] [PubMed] [Google Scholar]
- 31.Steel GG, Down JD, Peacock JH, Stephens TC. Dose-rate effects and the repair of radiation damage. Radiother Oncol 1986; 5: 321–31. [DOI] [PubMed] [Google Scholar]
- 32.Mu X, Löfroth PO, Karlsson M, Zackrisson B. The effect of fraction time in intensity modulated radiotherapy: theoretical and experimental evaluation of an optimisation problem. Radiother Oncol 2003; 68: 181–7. doi: https://doi.org/10.1016/S0167-8140(03)00165-8 [DOI] [PubMed] [Google Scholar]
- 33.Wang JZ, Li XA, D'Souza WD, Stewart RD. Impact of prolonged fraction delivery times on tumor control: a note of caution for intensity-modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys 2003; 57: 543–52. doi: https://doi.org/10.1016/S0360-3016(03)00499-1 [DOI] [PubMed] [Google Scholar]
- 34.Deacon J, Peckham MJ, Steel GG. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother Oncol 1984; 2: 317–23. doi: https://doi.org/10.1016/S0167-8140(84)80074-2 [DOI] [PubMed] [Google Scholar]
- 35.Fertil B, Malaise EP. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys 1985; 11: 1699–707. doi: https://doi.org/10.1016/0360-3016(85)90223-8 [DOI] [PubMed] [Google Scholar]
- 36.West CM, Davidson SE, Roberts SA, Hunter RD. Intrinsic radiosensitivity and prediction of patient response to radiotherapy for carcinoma of the cervix. Br J Cancer 1993; 68: 819–23. doi: https://doi.org/10.1038/bjc.1993.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West CM, Davidson SE, Hunter RD. Evaluation of surviving fraction at 2 Gy as a potential prognostic factor for the radiotherapy of carcinoma of the cervix. Int J Radiat Biol 1989; 56: 761–5. doi: https://doi.org/10.1080/09553008914552011 [DOI] [PubMed] [Google Scholar]
- 38.Levine EL, Renehan A, Gossiel R, Davidson SE, Roberts SA, Chadwick C, et al. Apoptosis, intrinsic radiosensitivity and prediction of radiotherapy response in cervical carcinoma. Radiother Oncol 1995; 37: 1–9. doi: https://doi.org/10.1016/0167-8140(95)01622-N [DOI] [PubMed] [Google Scholar]
- 39.Yard B, Chie EK, Adams DJ, Peacock C, Abazeed ME. Radiotherapy in the era of precision medicine. Semin Radiat Oncol 2015; 25: 227–36. doi: https://doi.org/10.1016/j.semradonc.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 40.Yard BD, Adams DJ, Chie EK, Tamayo P, Battaglia JS, Gopal P, et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun 2016; 7: 11428. doi: https://doi.org/10.1038/ncomms11428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerns SL, Kundu S, Oh JH, Singhal SK, Janelsins M, Travis LB, et al. The prediction of radiotherapy toxicity using single nucleotide polymorphism-based models: a step toward prevention. Semin Radiat Oncol 2015; 25: 281–91. doi: https://doi.org/10.1016/j.semradonc.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerns SL, West CM, Andreassen CN, Barnett GC, Bentzen SM, Burnet NG, et al. Radiogenomics: the search for genetic predictors of radiotherapy response. Future Oncol 2014; 10: 2391–406. doi: https://doi.org/10.2217/fon.14.173 [DOI] [PubMed] [Google Scholar]
- 43.Powers WE, Tolmach LJ. Demonstration of an anoxic component in a mouse tumor-cell population by in vivo assay of survival following irradiation. Radiology 1964; 83: 328–36. doi: https://doi.org/10.1148/83.2.328 [DOI] [PubMed] [Google Scholar]
- 44.Hill RP, Bush RS, Yeung P. The effect of anaemia on the fraction of hypoxic cells in an experimental tumour. Br J Radiol 1971; 44: 299–304. doi: https://doi.org/10.1259/0007-1285-44-520-299 [DOI] [PubMed] [Google Scholar]
- 45.Shenoy MA, Asquith JC, Adams GE, Micheal BD, Watts ME. Time-resolved oxygen effects in irradiated bacteria and mammalian cells: a rapid-mix study. Radiat Res 1975; 62: 498–512. doi: https://doi.org/10.2307/3574143 [PubMed] [Google Scholar]
- 46.Koch CJ, Evans SM. Cysteine concentrations in rodent tumors: unexpectedly high values may cause therapy resistance. Int J Cancer 1996; 67: 661–7. doi: https://doi.org/10.1002/(SICI)1097-0215(19960904)67:5<661::AID-IJC12>3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 47.Horan AD, Koch CJ. The K(m) for radiosensitization of human tumor cells by oxygen is much greater than 3 mmHg and is further increased by elevated levels of cysteine. Radiat Res 2001; 156: 388–98. doi: https://doi.org/10.1667/0033-7587(2001)156[0388:TKMFRO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 48.Van Putten LM, Kallman RF. Oxygenation status of a transplantable tumor during fractionated radiation therapy. J Natl Cancer Inst 1968; 40: 441–51. [PubMed] [Google Scholar]
- 49.Hawkes MJ, Hill RP, Lindop PJ, Ellis RE, Rotblat JR. The response of C3H mammary tumours to irradiation in single and fractionated doses. Br J Radiol 1968; 41: 134–41. doi: https://doi.org/10.1259/0007-1285-41-482-134 [DOI] [PubMed] [Google Scholar]
- 50.Howes AE, Page A, Fowler JF. The effect of single and fractionated doses of x rays on the effective proportion of hypoxic cells in C3H mouse mammary tumours. Br J Radiol 1972; 45: 250–6. doi: https://doi.org/10.1259/0007-1285-45-532-250 [DOI] [PubMed] [Google Scholar]
- 51.Dewhirst MW, Kimura H, Rehmus SW, Braun RD, Papahadjopoulos D, Hong K, et al. Microvascular studies on the origins of perfusion-limited hypoxia. Br J Cancer Suppl 1996; 27: S247–51. [PMC free article] [PubMed] [Google Scholar]
- 52.Brurberg KG, Gaustad JV, Mollatt CS, Rofstad EK. Temporal heterogeneity in blood supply in human tumor xenografts. Neoplasia 2008; 10: 727–35. doi: https://doi.org/10.1593/neo.08388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998; 58: 1408–16. [PubMed] [Google Scholar]
- 54.Koch CJ, Jenkins WT, Jenkins KW, Yang XY, Shuman AL, Pickup S, et al. Mechanisms of blood flow and hypoxia production in rat 9L-epigastric tumors. Tumor Microenviron Ther 2013; 1: 1–13. doi: https://doi.org/10.2478/tumor-2012-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and predicting radiation response. Semin Radiat Oncol 2015; 25: 260–72. doi: https://doi.org/10.1016/j.semradonc.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 56.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. doi: https://doi.org/10.1038/nrc2344 [DOI] [PubMed] [Google Scholar]
- 57.Urano M, Suit HD. Experimental evaluation of tumor bed effect for C3H mouse mammary carcinoma and for C3H mouse fibrosarcoma. Radiat Res 1971; 45: 41–9. doi: https://doi.org/10.2307/3573078 [PubMed] [Google Scholar]
- 58.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 2008; 8: 851–64. doi: https://doi.org/10.1038/nrc2501 [DOI] [PubMed] [Google Scholar]
- 59.Hill RP. Radiation-induced changes in the in vivo growth rate of KHT sarcoma cells: implications for the comparison of growth delay and cell survival. Radiat Res 1980; 83: 99–108. doi: https://doi.org/10.2307/3575262 [PubMed] [Google Scholar]
- 60.Hermens AF, Barendsen GW. The proliferative status and clonogenic capacity of tumour cells in a transplantable rhabdomyosarcoma of the rat before and after irradiation with 800 rad of X-rays. Cell Tissue Kinet 1978; 11: 83–100. [DOI] [PubMed] [Google Scholar]
- 61.McNally NJ. Recovery from sub-lethal damage by hypoxic tumour cells in vivo. Br J Radiol 1972; 45: 116–20. doi: https://doi.org/10.1259/0007-1285-45-530-116 [DOI] [PubMed] [Google Scholar]
- 62.Song CW, Lee YJ, Griffin RJ, Park I, Koonce NA, Hui S, et al. Indirect tumor cell death after high-dose hypofractionated irradiation: implications for stereotactic body radiation therapy and stereotactic radiation surgery. Int J Radiat Oncol Biol Phys 2015; 93: 166–72. doi: https://doi.org/10.1016/j.ijrobp.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res 1997; 147: 541–50. doi: https://doi.org/10.2307/3579620 [PubMed] [Google Scholar]
- 64.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol 2007; 25: 4066–74. doi: https://doi.org/10.1200/JCO.2007.12.7878 [DOI] [PubMed] [Google Scholar]
- 65.Dhani N, Fyles A, Hedley D, Milosevic M. The clinical significance of hypoxia in human cancers. Semin Nucl Med 2015; 45: 110–21. doi: https://doi.org/10.1053/j.semnuclmed.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 66.Hill RP. Sensitizers and radiation dose fractionation: results and interpretations. Int J Radiat Oncol Biol Phys 1986; 12: 1049–54. doi: https://doi.org/10.1016/0360-3016(86)90223-3 [DOI] [PubMed] [Google Scholar]
- 67.Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006; 24: 2098–104. doi: https://doi.org/10.1200/JCO.2005.05.2878 [DOI] [PubMed] [Google Scholar]
- 68.Toustrup K, Sørensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J, et al. ; Danish Head and Neck Cancer Group (DAHANCA). Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 2012; 102: 122–9. doi: https://doi.org/10.1016/j.radonc.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 69.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 2010; 120: 127–41. doi: https://doi.org/10.1172/JCI40027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006; 66: 9339–44. doi: https://doi.org/10.1158/0008-5472.CAN-06-3126 [DOI] [PubMed] [Google Scholar]
- 71.O'Brien CA, Kreso A, Dick JE. Cancer stem cells in solid tumors: an overview. Semin Radiat Oncol 2009; 19: 71–7. doi: https://doi.org/10.1016/j.semradonc.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 72.Hill RP, Milas L. The proportion of stem cells in murine tumors. Int J Radiat Oncol Biol Phys 1989; 16: 513–8. doi: https://doi.org/10.1016/0360-3016(89)90353-2 [DOI] [PubMed] [Google Scholar]
- 73.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 2008; 8: 545–54. doi: https://doi.org/10.1038/nrc2419 [DOI] [PubMed] [Google Scholar]
- 74.Yaromina A, Krause M, Thames H, Rosner A, Krause M, Hessel F, et al. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother Oncol 2007; 83: 304–10. doi: https://doi.org/10.1016/j.radonc.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 75.Baumann M, Krause M. CD44: a cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin Cancer Res 2010; 16: 5091–3. doi: https://doi.org/10.1158/1078-0432.CCR-10-2244 [DOI] [PubMed] [Google Scholar]
- 76.West CM, Davidson SE, Pool C, James RD, Schofield PF. Lack of a relationship between colony-forming efficiency and surviving fraction at 2 Gy. Radiat Res 1991; 126: 260–3. doi: https://doi.org/10.2307/3577827 [PubMed] [Google Scholar]
- 77.Benveniste P, Cantin C, Hyam D, Iscove NN. Hematopoietic stem cells engraft in mice with absolute efficiency. Nat Immunol 2003; 4: 708–13. doi: https://doi.org/10.1038/ni940 [DOI] [PubMed] [Google Scholar]
- 78.Frelin C, Herrington R, Janmohamed S, Barbara M, Tran G, Paige CJ, et al. GATA-3 regulates the self-renewal of long-term hematopoietic stem cells. Nat Immunol 2013; 14: 1037–44. doi: https://doi.org/10.1038/ni.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol 2014; 90: 615–21. doi: https://doi.org/10.3109/09553002.2014.892227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–60. doi: https://doi.org/10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 81.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009; 458: 780–3. doi: https://doi.org/10.1038/nature07733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zielske SP, Spalding AC, Wicha MS, Lawrence TS. Ablation of breast cancer stem cells with radiation. Transl Oncol 2011; 4: 227–33. doi: https://doi.org/10.1593/tlo.10247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133− cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys 2007; 67: 1–5. doi: https://doi.org/10.1016/j.ijrobp.2006.09.037 [DOI] [PubMed] [Google Scholar]
- 84.Platet N, Liu SY, Atifi ME, Oliver L, Vallette FM, Berger F, et al. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett 2007; 258: 286–90. doi: https://doi.org/10.1016/j.canlet.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 85.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009; 15: 501–13. doi: https://doi.org/10.1016/j.ccr.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 2009; 8: 3274–84. doi: https://doi.org/10.4161/cc.8.20.9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peitzsch C, Perrin R, Hill RP, Dubrovska A, Kurth I. Hypoxia as a biomarker for radioresistant cancer stem cells. Int J Radiat Biol 2014; 90: 636–52. doi: https://doi.org/10.3109/09553002.2014.916841 [DOI] [PubMed] [Google Scholar]
- 88.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11: 393–410. doi: https://doi.org/10.1038/nrc3064 [DOI] [PubMed] [Google Scholar]
- 89.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 2006; 98: 1777–85. doi: https://doi.org/10.1093/jnci/djj495 [DOI] [PubMed] [Google Scholar]
- 90.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells 2010; 28: 639–48. doi: https://doi.org/10.1002/stem.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Durand RE, Sutherland RM. Effects of intercellular contact on repair of radiation damage. Exp Cell Res 1972; 71: 75–80. doi: https://doi.org/10.1016/0014-4827(72)90265-0 [DOI] [PubMed] [Google Scholar]
- 92.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 1988; 240: 177–84. doi: https://doi.org/10.1126/science.2451290 [DOI] [PubMed] [Google Scholar]
- 93.Hill RP, Ng R, Warren BF, Bush RS. The effect of intercellular contact on the radiation sensitivity of KHT sarcoma cells. Radiat Res 1979; 77: 182–92. doi: https://doi.org/10.2307/3575087 [PubMed] [Google Scholar]
- 94.Kwok TT, Sutherland RM. The influence of cell-cell contact on radiosensitivity of human squamous carcinoma cells. Radiat Res 1991; 126: 52–7. doi: https://doi.org/10.2307/3578170 [PubMed] [Google Scholar]
- 95.Cordes N, Meineke V. Integrin signalling and the cellular response to ionizing radiation. J Mol Histol 2004; 35: 327–37. doi: https://doi.org/10.1023/B:HIJO.0000032364.43566.3a [DOI] [PubMed] [Google Scholar]
- 96.Hehlgans S, Eke I, Storch K, Haase M, Baretton GB, Cordes N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother Oncol 2009; 92: 362–70. doi: https://doi.org/10.1016/j.radonc.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 97.Eke I, Cordes N. Focal adhesion signaling and therapy resistance in cancer. Semin Cancer Biol 2015; 31: 65–75. doi: https://doi.org/10.1016/j.semcancer.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 98.Brooks MD, Burness ML, Wicha MS. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell 2015; 17: 260–71. doi: https://doi.org/10.1016/j.stem.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vlashi E, Pajonk F. Cancer stem cells, cancer cell plasticity and radiation therapy. Semin Cancer Biol 2015; 31: 28–35. doi: https://doi.org/10.1016/j.semcancer.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vlashi E, Chen AM, Boyrie S, Yu G, Nguyen A, Brower PA, et al. Radiation-induced dedifferentiation of head and neck cancer cells into cancer stem cells depends on human papillomavirus status. Int J Radiat Oncol Biol Phys 2016; 94: 1198–206. doi: https://doi.org/10.1016/j.ijrobp.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peitzsch C, Cojoc M, Hein L, Kurth I, Mäbert K, Trautmann F, et al. An epigenetic reprogramming strategy to resensitize radioresistant prostate cancer cells. Cancer Res 2016; 76: 2637–51. doi: https://doi.org/10.1158/0008-5472.CAN-15-2116 [DOI] [PubMed] [Google Scholar]
- 102.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells 2012; 30: 833–44. doi: https://doi.org/10.1002/stem.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milas L, Hunter N, Peters LJ. The tumor bed effect: dependence of tumor take, growth rate, and metastasis on the time interval between irradiation and tumor cell transplantation. Int J Radiat Oncol Biol Phys 1987; 13: 379–83. doi: https://doi.org/10.1016/0360-3016(87)90012-5 [DOI] [PubMed] [Google Scholar]
- 104.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. doi: https://doi.org/10.1126/science.1082504 [DOI] [PubMed] [Google Scholar]
- 105.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003; 22: 5897–906. doi: https://doi.org/10.1038/sj.onc.1206702 [DOI] [PubMed] [Google Scholar]
- 106.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst 1993; 85: 988–93. doi: https://doi.org/10.1093/jnci/85.12.988 [DOI] [PubMed] [Google Scholar]
- 107.Moding EJ, Castle KD, Perez BA, Oh P, Min HD, Norris H, et al. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci Transl Med 2015; 7: 278ra34. doi: https://doi.org/10.1126/scitranslmed.aaa4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maeda A, Leung MK, Conroy L, Chen Y, Bu J, Lindsay PE, et al. In vivo optical imaging of tumor and microvascular response to ionizing radiation. PLoS One 2012; 7: e42133. doi: https://doi.org/10.1371/journal.pone.0042133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maeda A, Kulbatski I, DaCosta RS. Emerging applications for optically enabled intravital microscopic imaging in radiobiology. Mol Imaging 2015; 14: 452–74. [PubMed] [Google Scholar]
- 110.Dewhirst MW, Gustafson C, Gross JF, Tso CY. Temporal effects of 5.0 Gy radiation in healing subcutaneous microvasculature of a dorsal flap window chamber. Radiat Res 1987; 112: 581–91. doi: https://doi.org/10.2307/3577110 [PubMed] [Google Scholar]
- 111.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71. [PubMed] [Google Scholar]
- 112.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 2005; 5: 423–35. doi: https://doi.org/10.1038/nrc1628 [DOI] [PubMed] [Google Scholar]
- 113.Siemann DW, Horsman MR. Targeting the tumor vasculature: a strategy to improve radiation therapy. Expert Rev Anticancer Ther 2004; 4: 321–7. doi: https://doi.org/10.1586/14737140.4.2.321 [DOI] [PubMed] [Google Scholar]
- 114.Siemann DW, Chaplin DJ, Walicke PA. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opin Investig Drugs 2009; 18: 189–97. doi: https://doi.org/10.1517/13543780802691068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010; 120: 694–705. doi: https://doi.org/10.1172/JCI40283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patterson DM, Zweifel M, Middleton MR, Price PM, Folkes LK, Stratford MR, et al. Phase I clinical and pharmacokinetic evaluation of the vascular-disrupting agent OXi4503 in patients with advanced solid tumors. Clin Cancer Res 2012; 18: 1415–25. doi: https://doi.org/10.1158/1078-0432.CCR-11-2414 [DOI] [PubMed] [Google Scholar]
- 117.Wang HH, Cui YL, Zaorsky NG, Lan J, Deng L, Zeng XL, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett 2016; 375: 349–59. doi: https://doi.org/10.1016/j.canlet.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 118.Coleman CN, Lawrence TS, Kirsch DG. Enhancing the efficacy of radiation therapy: premises, promises, and practicality. J Clin Oncol 2014; 32: 2832–5. doi: https://doi.org/10.1200/JCO.2014.57.3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Milas L. Effects of C. parvum on radiation response of murine tumors. Dev Biol Stand 1977; 38: 301–6. [PubMed] [Google Scholar]
- 120.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015; 356: 82–90. doi: https://doi.org/10.1016/j.canlet.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 121.Demaria S, Formenti SC. Radiotherapy effects on anti-tumor immunity: implications for cancer treatment. Front Oncol 2013; 3: 128. doi: https://doi.org/10.3389/fonc.2013.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naghavi AO, Johnstone PA, Kim S. Clinical trials exploring the benefit of immunotherapy and radiation in cancer treatment: a review of the past and a look into the future. Curr Probl Cancer 2016; 40: 38–67. doi: https://doi.org/10.1016/j.currproblcancer.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 123.Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol 2015; 25: 54–64. doi: https://doi.org/10.1016/j.semradonc.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015; 1: 1325–32. doi: https://doi.org/10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 125.Sutherland RM. Radiation-enhanced immune response to cancer: workshop, Anaheim, CA, April 17, 2005. Int J Radiat Oncol Biol Phys 2006; 64: 3–5. [DOI] [PubMed] [Google Scholar]
- 126.Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res 2011; 176: 139–57. doi: https://doi.org/10.1667/RR2548.1 [DOI] [PubMed] [Google Scholar]
- 127.Brady D, O'Sullivan JM, Prise KM. What is the role of the bystander response in radionuclide therapies? Front Oncol 2013; 3: 215. doi: https://doi.org/10.3389/fonc.2013.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iyengar P, Westover K, Timmerman RD. Stereotactic ablative radiotherapy (SABR) for non-small cell lung cancer. Semin Respir Crit Care Med 2013; 34: 845–54. doi: https://doi.org/10.1055/s-0033-1358554 [DOI] [PubMed] [Google Scholar]
- 129.Stanic S, Paulus R, Timmerman RD, Michalski JM, Barriger RB, Bezjak A, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early-stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014; 88: 1092–9. doi: https://doi.org/10.1016/j.ijrobp.2013.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014; 88: 254–62. doi: https://doi.org/10.1016/j.ijrobp.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boone JD, Dobbin ZC, Straughn JM, Jr, Buchsbaum DJ. Ovarian and cervical cancer patient derived xenografts: the past, present, and future. Gynecol Oncol 2015; 138: 486–91. doi: https://doi.org/10.1016/j.ygyno.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 132.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 2014; 4: 998–1013. doi: https://doi.org/10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res 2015; 17: 17. doi: https://doi.org/10.1186/s13058-015-0523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zanella ER, Galimi F, Sassi F, Migliardi G, Cottino F, Leto SM, et al. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med 2015; 7: 272ra12. doi: https://doi.org/10.1126/scitranslmed.3010445 [DOI] [PubMed] [Google Scholar]
- 135.Martin P, Stewart E, Pham NA, Mascaux C, Panchal D, Li M, et al. Cetuximab inhibits T790M-mediated resistance to epidermal growth factor receptor tyrosine kinase inhibitor in a lung adenocarcinoma patient-derived xenograft mouse model. Clin Lung Cancer 2016. Epub ahead of print. doi: https://doi.org/10.1016/j.cllc.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 136.Willey CD, Gilbert AN, Anderson JC, Gillespie GY. Patient-derived xenografts as a model system for radiation research. Semin Radiat Oncol 2015; 25: 273–80. doi: https://doi.org/10.1016/j.semradonc.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bristow RG, Berlin A, Dal Pra A. An arranged marriage for precision medicine: hypoxia and genomic assays in localized prostate cancer radiotherapy. Br J Radiol 2014; 87: 20130753. doi: https://doi.org/10.1259/bjr.20130753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lalonde E, Ishkanian AS, Sykes J, Fraser M, Ross-Adams H, Erho N, et al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol 2014; 15: 1521–32. doi: https://doi.org/10.1016/S1470-2045(14)71021-6 [DOI] [PubMed] [Google Scholar]
- 139.Taiakina D, Dal Pra A, Bristow RG. Intratumoral hypoxia as the genesis of genetic instability and clinical prognosis in prostate cancer. Adv Exp Med Biol 2014; 772: 189–204. doi: https://doi.org/10.1007/978-1-4614-5915-6_9 [DOI] [PubMed] [Google Scholar]
- 140.Fung AS, Lee C, Yu M, Tannock IF. The effect of chemotherapeutic agents on tumor vasculature in subcutaneous and orthotopic human tumor xenografts. BMC Cancer 2015; 15: 112. doi: https://doi.org/10.1186/s12885-015-1091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clarkson R, Lindsay PE, Ansell S, Wilson G, Jelveh S, Hill RP, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys 2011; 38: 845–56. doi: https://doi.org/10.1118/1.3533947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang Y, Wang KK, Eslami S, Iordachita II, Patterson MS, Wong JW. Systematic calibration of an integrated X-ray and optical tomography system for preclinical radiation research. Med Phys 2015; 42: 1710–20. doi: https://doi.org/10.1118/1.4914860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tillner F, Thute P, Bütof R, Krause M, Enghardt W. Pre-clinical research in small animals using radiotherapy technology—a bidirectional translational approach. Z Med Phys 2014; 24: 335–51. doi: https://doi.org/10.1016/j.zemedi.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 144.Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol 2011; 56: R55–83. doi: https://doi.org/10.1088/0031-9155/56/12/R01 [DOI] [PubMed] [Google Scholar]
- 145.Zhang P, Lo A, Huang Y, Huang G, Liang G, Mott J, et al. Identification of genetic loci that control mammary tumor susceptibility through the host microenvironment. Sci Rep 2015; 5: 8919. doi: https://doi.org/10.1038/srep08919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kirsch DG. Using genetically engineered mice for radiation research. Radiat Res 2011; 176: 275–9. doi: https://doi.org/10.1667/RRXX35.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016; 16: 234–49. doi: https://doi.org/10.1038/nrc.2016.18 [DOI] [PubMed] [Google Scholar]
- 148.Steel GG. The ESTRO Breur lecture. Cellular sensitivity to low dose-rate irradiation focuses the problem of tumour radioresistance. Radiother Oncol 1991; 20: 71–83. [DOI] [PubMed] [Google Scholar]
- 149.Steel GG, Deacon JM, Duchesne GM, Horwich A, Kelland LR, Peacock JH. The dose-rate effect in human tumour cells. Radiother Oncol 1987; 9: 299–310. [DOI] [PubMed] [Google Scholar]